SYNOPSIS
Evidence indicates that protein kinases play an important role in the regulation of epithelial tight junctions. In the present study, we investigated the role of PKCζ in tight junction regulation in Caco-2 and MDCK cell monolayers. Inhibition of PKCζ by a specific PKCζ-pseudosubstrate peptide results in redistribution of occludin and ZO-1 from the intercellular junctions and disruption of barrier function without affecting cell viability. Reduced expression of PKCζ by antisense oligonucleotide or shRNA also results in compromised tight junction integrity. Inhibition or knock down of PKCζ delays calcium-induced assembly of tight junctions. Tight junction disruption by PKCζ-pseudosubstrate is associated with the dephosphorylation of occludin and ZO-1 on Ser and Thr residues. PKCζ directly binds to the C-terminal domain of occludin and phosphorylates it on Thr residues. T403, T404, T424 and T438 in occludin C-terminal domain are the predominant sites of PKCζ-dependent phosphorylation. T424A or T438A mutation in full length occludin delays its assembly into the tight junctions. Inhibition of PKCζ also induces redistribution of occludin and ZO-1 from the tight junctions and dissociates these proteins from the detergent-insoluble fractions in mouse ileum. This study demonstrates that PKCζ phosphorylates occludin on specific Thr residues and promotes assembly of epithelial tight junctions.
Keywords: Protein kinase, ZO-1, epithelium, barrier function, cell-cell adhesion, phosphothreonine
INTRODUCTION
Tight junctions in different epithelia form a barrier to the diffusion of macromolecules across the epithelial monolayer [1]. In the gastrointestinal (GI) tract, lung and kidney the epithelial tight junctions prevent the diffusion of allergens, toxins and pathogens from the external environment into the tissues. Disruption of tight junctions and the attenuation of epithelial barrier function are involved in the pathogenesis of GI, pulmonary and renal diseases [2-4]. Tight junctions also play a fence function in the epithelium to maintain the distinct molecular identities of the apical and basolateral membranes, thus determining the polarity of epithelial cells. A sustained loss of tight junction integrity leads to epithelial to mesenchymal transition.
Tight junctions are formed by the assembly of a multi-protein complex at the apical end of the cells. The transmembrane proteins of tight junctions include occludin, claudin (Cldn), junctional adhesion molecule and tricellulin [1, 5, 6]. The intracellular C-terminal domains of these proteins interact with the scaffold proteins, ZO-1, ZO-2, ZO-3 [7], which in turn interact with other soluble proteins (7H6, cingulin, symplekin, etc) [8, 9] and the actin cytoskeleton [10]. Dissociation of protein-protein interactions or disassembly of actin cytoskeleton leads to disruption of tight junctions and increase in paracellular permeability in different epithelia. Similarly, disruption of tight junctions by inflammatory mediators such as hydrogen peroxide [11], acetaldehyde [12, 13], tumor necrosis factor-α [14-16] and interferon-γ [17, 18] also results in dissociation of tight junction protein complexes.
A significant body of evidence indicates that the assembly and the maintenance of tight junctions are regulated by several components of intracellular signaling pathways [11]. Pharmacologic and molecular studies demonstrated that tight junctions are regulated by protein tyrosine kinases such as c-Src c-Yes [19-23] and protein Ser/Thr kinases such proteins kinase C (PKC) [13, 24-29], MAP kinase [30] and protein kinase A [31, 32]. A number of these signaling proteins are localized at the tight junctions and seem to interact directly with the tight junction proteins.
Occludin spans the membrane four times to form two extracellular loops, one intracellular loop and the N-terminal and C-terminal domains project into the intracellular compartment. At the C-terminal domain it interacts with ZO-1 and this interaction is essential for the assembly of tight junctions. Occludin is highly phosphorylated on Ser/Thr residues and the phosphorylated occludin is associated with the actin-rich, detergent-insoluble fractions, suggesting that phosphorylated occludin is associated with the intact tight junction [33]. Disruption of tight junctions by calcium depletion [34], phorbol ester [35] and pathogens [36] results in dephosphorylation of occludin on Ser/Thr residues. Therefore, dynamic phosphorylation of occludin seems to play an important role in the assembly and disassembly of tight junctions. Very little is known about the protein kinases and protein phosphatases involved in this regulation of occludin phosphorylation. While recent studies showed that protein Ser/Thr phosphatases, PP2A and PP1, play an important role in occludin dephosphorylation and destabilization of tight junctions [34], PKCη is involved in occludin phosphorylation and assembly of tight junctions [26]. Atypical PKCs, PKCζ and PKCλ/τ, are closely associated with the tight junctions and implicated in the assembly of tight junctions [37-39]. However, the precise role of these PKC isoforms in assembly or maintenance of tight junctions is unknown.
The present study, using Caco-2 and MDCK cell monolayers investigated the role of PKCζ in the regulation of epithelial tight junctions. Results show that, 1) inhibition or knockdown of PKCζ leads to disruption of tight junctions and prevents tight junction assembly in both Caco-2 and MDCK cell monolayers, 2) PKCζ activity is involved in Ser/Thr phosphorylation of occludin and ZO-1, 3) PKCζ directly interacts with the C-terminal domain of occludin and phosphorylates it on specific Thr residues, 4) phosphorylation of occludin on T424 and T438 is required for its optimal assembly into the junctions, and 5) inhibition of PKCζ disrupts tight junctions also in mouse ileum.
METHODS
Chemicals
Cell culture and transfection reagents were purchased from GIBCO-BRL (Grand Island, NY). Fluorescein isothiocyanate (FITC)-inulin, GSH (glutathione), leupeptin, aprotinin, bestatin, pepstatin A, PMSF, GSH-agarose, Triton X100, vanadate and protein-A Sepharose were purchased from Sigma Chemical Company (St Louis, MO). All other chemicals were of analytical grade and purchased either from Sigma Chemical Company or Fisher Scientific (Tustin, CA). PKCζ pseudosubstrate peptide with the sequence, Myr-SIYRRGARRWRKL (PKCζ-PS) and control peptide with scrambled sequence (Myr-SRIYRGRARWRLK) were custom synthesized by GenScript Corp (Piscataway, NJ).
Expression constructs and recombinant proteins
pEGFP-Occludin (human) construct was generated as described previously [26]. The cytoplasmic C-terminal region of human occludin encoding 378-522 (Ocl-C) was amplified from the pEGFP-Occludin vector, and was cloned into pGEX-2T vector (Amersham, Arlington Heights, IL). Point mutations of T400, T403, T404, T424, and T438 to Ala were introduced in wild-type GFP-occludin or GST-Ocl-C nucleotide sequence as described before. Recombinant Ocl-C was produced as GST fusion protein (GST-Ocl-C) in E. coli BL21DE cells and purified using GSH-agarose as described before [40]. The cDNA clone for human occludin was a kind gift from Dr. Van Italie and James M. Anderson (University of North Carolina, Chapel Hill, NC).
Antibodies
Mouse monoclonal anti-PKCζ and HRP-conjugated anti-GST antibodies were purchased from BD Transduction Laboratories (San Jose, CA). Mouse monoclonal anti-occludin, rabbit polyclonal anti-ZO-1, HRP-conjugated anti-occludin, and rabbit polyclonal anti-p-Thr (phospho-threonine) and anti-p-Ser (phospho-serine) antibodies were purchased from Zymed laboratories (San Francisco, CA). AlexaFluor 488-conjugated anti-mouse IgG and anti-rabbit IgG antibody were obtained from Molecular Probes (Eugene, OR). Cy3-conjugated anti-rabbit IgG, HRP-conjugated anti-mouse IgG, anti-β-actin, rabbit polyclonal anti-PKCζ and HRP-conjugated anti-rabbit IgG antibodies purchased from Sigma chemical company (St Louis, MO).
Antisense oligos and shRNA for PKCζ
A vector-based short hairpin RNA (shRNA) method was used to silence gene expression of human PKCζ. Two targeting sequences were chosen against the nucleotide sequence of human (Z15108.1) and canine PKCζ (XM_536716) genes using the Dharmacon web site [Target: CCATGAAAGTGGTGAAGAA (nucleotide position, 818-836 in human and 693-711 in canine)]. The sequences were further verified by BLAST search on the known human genome databases, and no matches were found other than PKCζ, confirming the uniqueness of these sequences. It was a hundred percent match for human and canine PKCζ sequence, whereas less than 50% match with human PKCλ/τ. To construct the shRNA vectors, a pair of oligonucleotides containing the antisense sequence, a hairpin loop region (TTGATATCCG) and the sense sequence with cohesive BamHI and HindIII sites was synthesized (Sigma Genosys, St Louis, MO) as follows: Top strand1, 5′-GGATCCCGCCATGAAAGTGGTGAAGAATTGATATCCGTTCTTCACCACTTTCATGGT TTTTTCCAAAAGCTT-3′ and bottom strand, 5′-AAGCTTTTGGAAAAAACCATGAAAGTG GTGAAGAACGGATATCAATTCTTCACCACTTTCATGGCGGGATCC-3′. The top and bottom strands were annealed and cloned into BamHI and HindIII sites of the pRNAtin-H1.2/neo vector (GenScript Corp., Piscataway, NJ), which induces expression of shRNA by H1.2 promoter and AcGFP protein by CMV promoter. Successful insertion of the shRNA constructs into the vector was confirmed by releasing the oligonucleotides by digesting with BamHI and HindIII, and by nucleotide sequencing.
We designed two antisense oligonucleotides against the nucleotide sequence of human PKCζ. Target sequences were compared to nucleotide sequences of other PKC isoforms, using the CLUSTAL W program, which aligned the nucleotide sequences of PKCs to enable us to pinpoint areas in the nucleotide sequences of the enzyme, which were unique and did not match the sequences in other PKC isoforms. The antisense sequences for selected sense sequences were used for synthesis of oligonucleotides as follows: AS-1, CTCTCTTTGGGGTCCTTATT (to the PKCζ target sequence 1331-1350) and AS-2, TGCCGTAGTCTGTGAGCTTG (to the PKCζ target sequence 1641-1660). We also designed a control, missense oligonucleotide with mutated sequence of AS-1 (CTCTATTTGCGGTTCTGATT). BLAST analysis indicated that AS-1 sequence has 94% match and 100% coverage with PKCζ sequence of canine gene, whereas AS-2 has only 50% match. This is reflected in their ability to knockdown PKCζ in MDCK cells; AS-1 is more effective than AS-2 in reducing PKCζ expression in MDCK cells.
Cell culture
Caco-2 cells (American Type Cell Collection, Rockville, MD) were grown under standard cell culture conditions as described before [13, 26]. Cells were grown on polyester membranes in Transwell inserts (6.5 mm, 12 mm or 24 mm; Costar, Cambridge, MA), and experiments were conducted on day 11-13 (6.5 or 12 mm Transwells) or day 17-19 (24 mm Transwell) post-seeding. Type II MDCK cells were grown on polyester membranes in Transwell inserts as above and experiments were conducted on day 7-9 (6.5 or 12 mm Transwells) or day 13-15 (24 mm Transwells) post seeding.
Tight junction assembly by calcium switch
Caco-2/MDCK cells were grown on transwells. Extra cellular calcium was depleted in Caco-2 cell monolayers by incubation with EGTA (4 mM) for 30 min. MDCK cell monolayers were incubated with low calcium medium (2 μM calcium) for 16 hours to disassemble tight junctions. Regular medium, containing 0.6 mM calcium was placed to restore calcium concentration and allowed to recover for 3-5 hrs. tight junction integrity was assessed by measuring TER, inulin flux and by immunofluorescence localization of occludin and ZO-1.
Transfection of antisense oligos and shRNA
Caco-2 cells (125,000 cells/well) were incubated in 1 ml antibiotic-free DMEM containing 10% FBS, 1 μg DNA plasmid (Empty vector or shRNA) or oligonucleotides (MS, AS-1 or AS-2), 1 μl Plus reagent, and 3 μl Lipofectamine-LTX for each well. After 20 hours, the cell monolayers were trypsinized and seeded on to 6.5 mm transwells. Experiments were conducted on day 3, 4 and 5 after transfection. Reduction in PKCζ protein expression was verified by immunoblot analysis.
Cell viability assessment
Cell viability was assessed by measuring lactate dehydrogenase (LDH) activity in the incubation medium 3 hours after incubation with or without PKCζ-PS. LDH activity measured using a kit from Sigma Chemical Company (St Louis, MO). The metabolic activity of cells was evaluated by using WST-1 cell proliferation assay reagent from Clontech (Mountain View, CA) according to vendor’s instructions.
Measurement of transepithelial electrical resistance (TER)
TER was measured as described previously [13, 26] using a Millicell-ERS Electrical Resistance System (Millipore, Bedford, MA). TER was calculated as Ω●cm2 by multiplying it with the surface area of the monolayer. The resistance of the polyester membrane in Transwells (approximately 30 Ω●cm2) was subtracted from all readings.
Unidirectional flux of inulin
Transwells with the cell monolayers were incubated under different experimental conditions in the presence of FITC-inulin (0.5 mg/ml) in the basal well. At varying times during the experiment, 50 μl of apical and basal media were withdrawn, and the fluorescence was measured using a fluorescence plate reader (BioTEK Instruments, Winooski, Vermont). The flux into the apical well was calculated as the percent of total fluorescence administered into the basal well per hour per cm2 surface area.
Immunofluorescence Microscopy
Cell monolayers were washed with PBS and fixed in acetone: methanol (1:1) at 0°C for 5 min. Fixed cell monolayers were blocked in 3% non-fat milk in TBST (20 mM Tris, pH 8.0, containing 150 mM NaCl and 0.5% Tween 20) and incubated for one hour with primary antibodies; rabbit polyclonal anti-ZO-1 and mouse monoclonal anti-occludin, followed by incubation for one hour with secondary antibodies, goat AlexaFluor 488-conjugated anti-mouse IgG and Cy3-conjugated anti-rabbit IgG antibodies. The fluorescence was visualized using a Zeiss LSM 5 Laser Scanning Confocal Microscope, and images from Z-series sections (1 μm) collected by using Zeiss LSM 5 Pascal, the confocal microscopy software (Release 3.2). Images were stacked using the software, Image J (NIH), and processed by Adobe Photoshop (Adobe Systems Inc., San Jose, CA).
Preparation of detergent-insoluble fractions
Triton-insoluble fractions were prepared as previously described [33]. Ileal mucosa was incubated for 5 min with lysis buffer-CS (50 mM Tris buffer, pH 7.4, containing 1.0% Triton-X100, 5 mM EGTA, 10 mM sodium fluoride, 2 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml bestatin, 10 μg/ml pepstatin-A, 1 mM vanadate and 1 mM PMSF). Cell lysates were centrifuged at 15,600 × g for 4 min at 4°C to sediment the high-density actin-rich fraction. The pellet was suspended in 200 μl of lysis buffer-D. Protein concentration in different fractions was measured by the BCA method (Pierce Biotechnology, Inc., Rockford, IL). Triton-insoluble and Triton-soluble fractions were mixed with equal volume of Laemmli’s sample buffer (2x concentrated) and heated at 100°C for 10 min.
Immunoprecipitation
Caco-2 cell monolayers (24 mm transwells) were washed with ice-cold PBS and proteins extracted in immunoprecipitation buffer (50 mM Tris buffer, pH 7.4, containing 1% NP40, 2 mM EDTA, 2 mM EGTA, 10 mM sodium fluoride, 1 mM vanadate and protease inhibitor cocktail as described above). Protein extracts (1.0 mg protein/ml) were incubated with 2 μg of anti-occludin antibodies at 4°C for 16 hr. Immune complexes were isolated by precipitation using protein-A Sepharose (for 1 h at 4°C). For immunoprecipitation of p-Thr and p-Ser, proteins was extracted in lysis buffer D and heated for 10 min at 100°C, followed by centrifugation. The clear supernatant was used for immunoprecipitation.
Immunoblot analysis
Proteins were separated by 7% SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes. Membranes were blotted for different proteins by using specific antibodies in combination with HRP-conjugated anti-mouse IgG or HRP-conjugated anti-rabbit IgG antibodies. HRP-conjugated anti-GST antibody was used for immunoblot analysis of GST or GST-Ocl-C. The blot was developed using ECL chemiluminescence method (Amersham, Arlington Heights, IL).
Pair wise binding assay
To determine the direct interaction between occludin and PKCζ, GST-Ocl-C (10 μg) was prepared as described before [40] and incubated with recombinant, His-tagged, PKCζ (30-500 ng) in PBS containing 0.2% Triton X100, 1 mM vanadate, and 10 mM sodium fluoride for 16 hours at 4°C on an inverter. GST-Ocl-C was pulled down by binding to 20 μl of 50% GSH-agarose slurry at 4°C for 1 hour. The amounts of PKCζ bound to GSH-agarose pull down were determined by immunoblot analysis. Nonspecific binding was determined by carrying out the binding with GST, instead of GST-Ocl-C.
Occludin phosphorylation in vitro
GST-Ocl-C (10 μg) was incubated with 500 ng of active PKCζ in 20 mM MOPS, pH 7.2, containing 25 mM β-glycerophosphate, 2.25 mM MgCl2, 0.2 mM ATP and 1 mM dithiothreitol, or with 500 ng of PKCη as described before [26]. Following 3-hour incubation at 30°C reaction mixture was immunoblotted for p-Thr.
Statistics
Comparison between two groups was made by Student’s t-tests for grouped data. Significance in all tests was set at 95% or greater confidence level.
RESULTS
PKCζ-PS disrupts tight junctions in Caco-2 cell monolayers
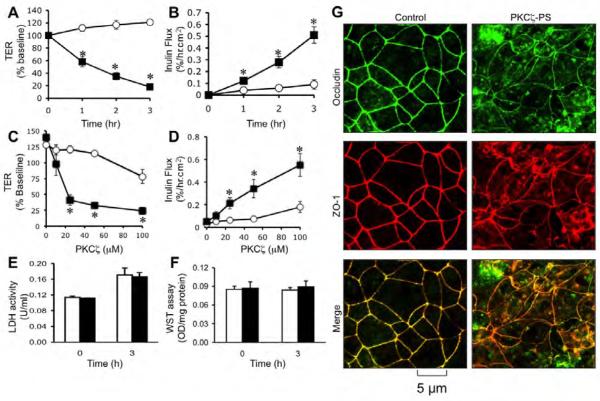
To determine the role of PKCζ activity in the maintenance of tight junction integrity we evaluated the effect of a PKCζ-PS on tight junction integrity in Caco-2 cell monolayers. Administration of PKCζ-PS decreased TER (Fig. 1A) and increased inulin permeability (Fig. 1B) in a time (Fig. A & B) and dose-dependent manner (Fig. C & D). Control peptide up to 50 μM did not cause significant change in TER or inulin permeability; however, at 100 μM concentration, the control peptide slightly, but significantly increased inulin permeability and reduced TER. PKCζ-PS did not increase LDH activity in the incubation medium (Fig. 1E) or reduced the mitochondrial activity (Fig. 1F). Confocal microscopy showed that occludin and ZO-1 are co-localized at the intercellular junctions in control cell monolayers (Fig. 1G). PKCζ-PS induced redistribution of occludin and ZO-1 from the intercellular junctions into the intracellular compartment.
Fig. 1. Inhibition of PKCζ activity disrupts tight junctions in Caco-2 cell monolayers.
A-D: Caco-2 cell monolayers were incubated with 50 μM PKCζ-PS (■) or control peptide (○) for varying times (A, B) or with varying concentrations of peptide for 2 hours (C, D). Barrier function was evaluated by measuring TER (A, C) and unidirectional flux of FITC-inulin (B, D). Values are mean ± sem (n = 6). Asterisks indicate the values that are significantly (P<0.05) different from corresponding control values. E, F: The cell viability assessed by measuring LDH activity in the incubation medium (E) or metabolic activity in the cell by WST assay (F) at 3 hour after PKCζ-PS (100 μM) administration. Values are mean ± s.e.m. (n = 6). G: Caco-2 cell monolayers incubated with or without 50 μM PKCζ-PS for 2 hr were fixed and stained for occludin and ZO-1 by immunofluorescence method. Images collected by confocal microscopy.
PKCζ-PS delays calcium-induced tight junction assembly in Caco-2 cell monolayers
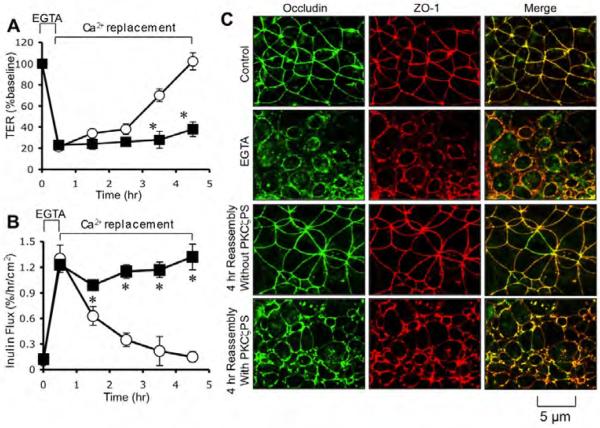
To determine the requirement of PKCζ activity for the assembly of tight junctions we evaluated the effect of PKCζ-PS on the development of barrier function during calcium-induced tight junction reassembly. Treatment of cell monolayers with EGTA rapidly reduced TER (Fig. 2A) and increased inulin permeability (Fig. 2B). Replacement of calcium gradually increased TER and reduced inulin permeability. Calcium-induced increase in TER and decrease in inulin permeability were significantly attenuated by the presence of PKCζ-PS. EGTA treatment induced redistribution of occludin and ZO-1 from the intercellular junctions into the intracellular compartments (Fig. 2C), which were reassembled back at the intercellular junctions during calcium replacement. The presence of PKCζ-PS prevented calcium-induced reassembly of occludin and ZO-1 into intercellular junctions (Fig. 2C).
Fig. 2. PKCζ-PS prevents calcium-induced assembly of tight junctions in Caco-2 cells.
A, B: Caco-2 cell monolayers were treated with 3 mM EGTA for 30 min to deplete extracellular calcium. Regular medium containing calcium with (■) or without (○) PKCζ-PS (10 μM) was then replaced. TER (A) and inulin permeability (B) were measured at varying times. Values are mean ± sem (n = 6). Asterisks indicate the values that are significantly (P<0.05) different from corresponding control values. C: Cell monolayers at various stages of tight junction assembly were fixed and stained for occludin and ZO-1 by immunofluorescence method. Images collected by confocal microscopy.
PKCζ-PS disrupts tight junctions and delays tight junction assembly in MDCK cells
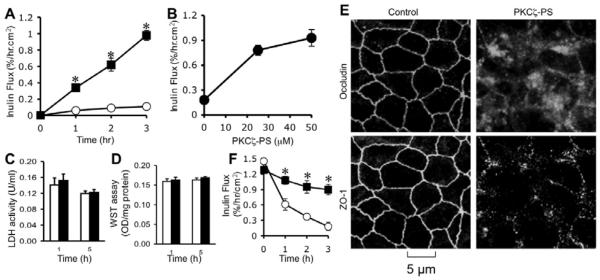
Studies described above indicate that PKCζ activity plays a critical role in the assembly and maintenance of tight junctions in Caco-2 cell monolayers (an intestinal epithelium). To investigate whether a similar mechanism exists in another epithelium we evaluated the effect of PKCζ-PS on the maintenance of tight junction integrity and the de novo assembly of tight junctions in MDCK cell monolayers (renal tubular epithelium). PKCζ-PS increased inulin permeability in a time (Fig. 3A) and dose (Fig. 3B)-dependent manner without inducing LDH release (Fig. 3C) or loss of cell viability (Fig. 3D). PKCζ-PS-mediated disruption of barrier to inulin permeability was associated with a redistribution of occludin and ZO-1 from the intercellular junctions (Fig. 3E). Presence of PKCζ-PS also delayed the calcium-induced reassembly of tight junctions in MDCK cells (Fig. 3F).
Fig. 3. Inhibition of PKCζ activity disrupts barrier function and delays tight junction assembly in MDCK cell monolayers.
A, B: MDCK cell monolayers were incubated with (■) or without (○) 50 μM PKCζ-PS for varying times (A) or with varying concentrations of PKCζ-PS for 2 hours (B). Barrier function was evaluated by measuring unidirectional flux of FITC-inulin. Values are mean ± s.e.m. (n = 6). Asterisks indicate the values that are significantly (P<0.05) different from corresponding control values. C, D: The cell viability assessed by measuring LDH activity in the incubation medium (C) or metabolic activity in the cell by WST assay (D) at 3 hour after 50 μM PKCζ-PS (black bars) or control peptide (white bars) administration. Values are mean ± sem (n = 6). E: MDCK cell monolayers incubated with or without 50 μM PKCζ-PS for 2 hr were fixed and stained for occludin and ZO-1 by immunofluorescence method. Images collected by confocal microscopy. F: Cell monolayers were incubated in low calcium medium for 16 hours to deplete extracellular calcium. Regular medium containing calcium with (■) or without (○) PKCζ-PS (10 μM) was then replaced. Inulin permeability (B) was measured at varying times after calcium replacement. Values are mean ± sem (n = 6). Asterisks indicate the values that are significantly (P<0.05) different from corresponding values for control cells.
PKCζ-PS disrupts adherens junctions in Caco-2 cell monolayers
To determine the role of PKCζ in the maintenance of adherens junctions we analyzed the effect of PKCζ-PS on distribution of E-cadherin and β-catenin. E-cadherin and β-catenin were co-localized at the intercellular junctions in control cell monolayers (Fig. 4). PKCζ-PS administration induced a redistribution of E-cadherin and β-catenin from the intercellular junctions into the intracellular compartment in a dose-dependent manner.
Fig. 4. Inhibition of PKCζ activity disrupts adherens junctions in Caco-2 cell monolayers.
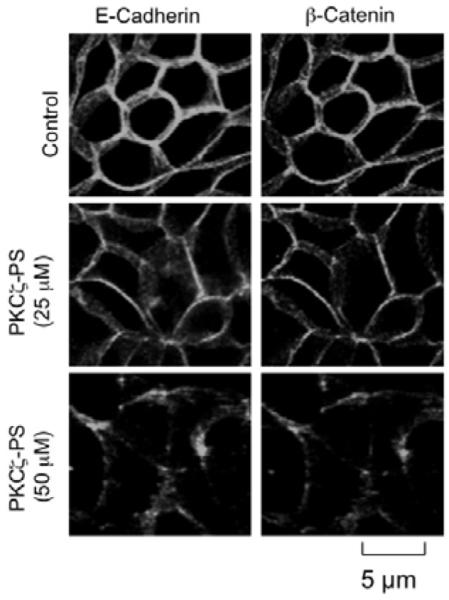
Caco-2 cell monolayers were incubated with or without PKCζ-PS (50 μM) for 2 hours. Cell monolayers were fixed and stained for E-cadherin and β-catenin by immunofluorescence method. Images collected by confocal microscopy.
Knockdown of PKCζ attenuates tight junction integrity in Caco-2 cell monolayers
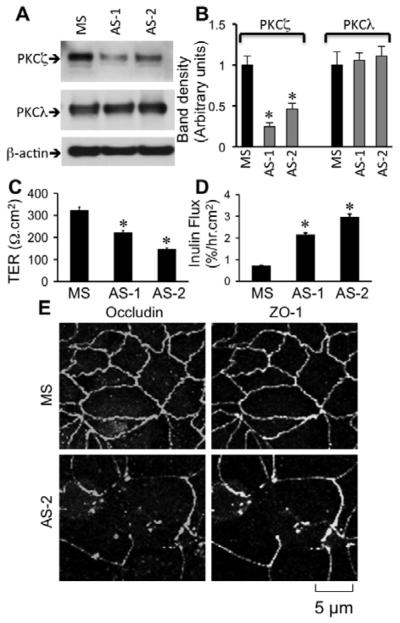
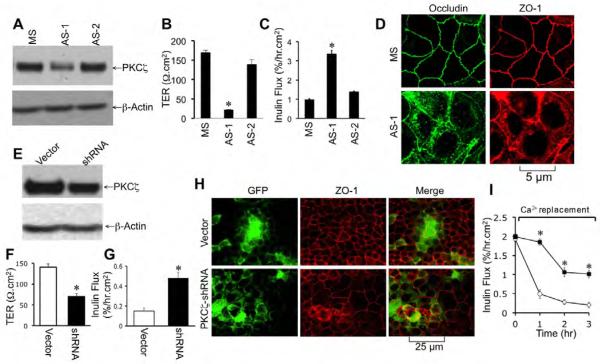
PKCζ-PS is likely to inhibit PKCλ also [41], and therefore, we designed antisense oligos specific for the nucleotide sequence of human PKCζ (AS-1 and AS-2). Caco-2 cells were transfected with AS-1, AS-2 or missense oligos. Transfection with AS-1 and AS-2 reduced the levels of PKCζ in Caco-2 cells without affecting the levels of PKCλ (Fig. 5A & 5B). Tight junction integrity was evaluated three days after transfection. Both AS-1 and AS-2 significantly reduced TER (Fig. 5C) and enhanced inulin permeability (Fig. 5D). Knockdown of PKCζ by AS-2 caused redistribution of occludin and ZO-1 from intercellular junctions into the intracellular compartments (Fig. 5E), indicating a delayed assembly of tight junctions in the absence of optimal level of PKCζ.
Fig. 5. Reduced expression of PKCζ by antisense oligos attenuates tight junction integrity in Caco-2 cells.
A & B: Caco-2 cells were transfected with missense oligo (MS) or two different antisense oligos (AS-1, AS-2), and the levels of PKCζ and PKCλ were measured by immunoblot analysis. Band densities evaluated by densitometric analysis (B). Values are mean ± sem (n = 6). Asterisks indicate the values that are significantly (P<0.05) different from corresponding values for MS-transfected cells. C & D: Barrier function (C) and inulin permeability (D) were measured on day 3 after seeding. Values are mean ± sem (n = 6). Asterisks indicate the values that are significantly (P<0.05) different from corresponding values for MS-transfected cells. E: Caco-2 cell monolayers transfected with MS or AS-2 were fixed and stained for occludin and ZO-1 by immunofluorescence method.
Transfection with AS-1 significantly reduced the level of PKCζ in MDCK cells (Fig. 6A). AS-1 reduced TER (Fig. 6B) and increased inulin permeability (Fig. 6C). Knockdown of PKCζ by AS-1 also resulted in redistribution of occludin and ZO-1 from the intercellular junctions into the intracellular compartments (Fig. 6D), indicating a delayed assembly of tight junctions.
Fig. 6. Reduced expression of PKCζ attenuates tight junction integrity in MDCK cell monolayers.
A-D: MDCK cells were transfected with missense oligo (MS) or two different antisense oligos (AS-1, AS-2), and the levels of PKCζ and PKCλ were measured by immunoblot analysis (A). Barrier function (B) and inulin permeability (C) were measured on day 3 after seeding. Values are mean ± sem (n = 6). Asterisks indicate the values that are significantly (P<0.05) different from corresponding values for MS-transfected cells. MDCK cell monolayers transfected with MS or AS-1 were fixed and stained for occludin and ZO-1 by immunofluorescence method (D). E-I: shRNA specific for PKCζ in pRNAtinH1.2 vector or the empty vector was transfected into MDCK cells. PKCζ expression was determined by immunoblot analysis (E). Barrier function was evaluated by measuring TER (F) and inulin permeability (G) on day 3 after seeding. Values are mean ± sem (n = 6). Asterisks indicate the values that are significantly (P<0.05) different from corresponding values for vector-transfected cells. Fixed cell monolayers were stained for GFP and ZO-1 (H). tight junction assembly in transfected cells was evaluated by calcium switch method (I). Inulin permeability measured during calcium-induced reassembly in vector-transfected (○) and shRNA-transfected cell monolayers (■). Values are mean ± sem (n = 6). Asterisks indicate the values that are significantly (P<0.05) different from corresponding values for vector-transfected cells.
We further designed an shRNA specific for the nucleotide sequence of canine PKCζ gene (also showed 100% match with human PKCζ gene sequence) and inserted it into pRNAtinH1.2/neo vector that also contained a gene for AcGFP, so that transfected cells could be distinguished from non-transfected cells in the same cell monolayer. Transfection of shRNA into MDCK cells resulted in significant reduction in the level of PKCζ (Fig. 6E). Cell monolayers transfected with shRNA exhibited significantly low TER (Fig. 6F) and high inulin permeability (Fig. 6G) compared to those in vector-transfected cell monolayers, indicating that reduced expression of PKCζ leads to delayed development of barrier function. Immunofluorescence microscopy showed the presence of GFP in about 35% of cells in both vector and shRNA-transfected cells (Fig. 6H). Co-immunostaining for GFP and ZO-1 showed that ZO-1 was localized at the intercellular junctions in vector-transfected cells, in both GFP-positive and negative cells. On the other hand, in shRNA-transfected cells, GFP-positive cells showed intracellular distribution of ZO-1 (Fig. 6H), whereas in GFP-negative cells, ZO-1 was localized predominantly at the intercellular junctions. Calcium-induced development of barrier function was significantly delayed in shRNA-transfected cells compared to that in vector-transfected cells (Fig. 6I).
PKCζ activity is involved in Ser/Thr-phosphorylation of occludin and ZO-1
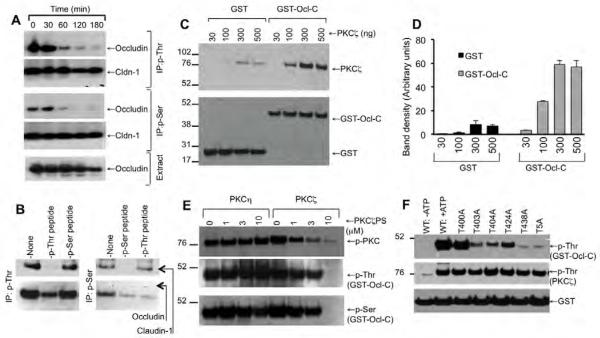
To determine the effect of PKCζ activity on Ser and Thr phosphorylation of occludin we immunoprecipitated p-Ser and p-Thr from denatured protein extracts from control and PKCζ-PS-treated Caco-2 cell monolayers and immunoblotted them for occludin and Cldn-1. The Thr and Ser phosphorylation of occludin remained unaffected during the incubation time in the absence of PKCζ-PS (data not shown). The presence of PKCζ-PS caused a rapid reduction in the levels of Ser/Thr-phosphorylated occludin and ZO-1 (Fig. 7A). Results also show that Cldn-1 is phosphorylated on both Ser and Thr residues. However, Cldn-1 phosphorylation was unaffected by PKCζ-PS (Fig. 7A). We previously showed that anti-p-Thr and anti-p-Ser antibodies, under our experimental conditions, are quite specific in detecting p-Thr and p-Ser [34]. We performed an additional experiment to rule out the non-specific pull down of occludin and claudin-1 during immunoprecipitation. Immunoprecipitation of Thr-phosphorylated occludin and claudin-1 using anti-p-Thr antibody was prevented by the inclusion of excessive amounts (25 μM) of p-Thr-peptide, but not by p-Ser-peptide, indicating a specific pull down of Thr-phosphorylated occludin and claudin-1 (Fig. 7B). Similarly, immunoprecipitation of Ser-phosphorylated claudin-1 was prevented by p-Ser-peptide, but not by p-Thr-peptide. Immunoprecipitation of occludin using anti-p-Ser antibody however was reduced by both p-Thr-peptide and p-Ser-peptide. This indicates that anti-p-Ser antibodies may be specific only for certain proteins. Therefore, results obtained from experiments using anti-p-Ser antibodies should be viewed with caution. On the other hand, anti-p-Thr antibody was much more specific for Thr-phosphorylated proteins.
Fig. 7. PKCζ directly binds to the C-terminal domain of occludin and phosphorylates it on Ser/Thr residues.
A: Caco-2 cell monolayers were incubated with PKCζ-PS (50 μM) for varying times. P-Thr or p-Ser was immunoprecipitated from the denatured protein extracts. Immunocomplexes were then immunoblotted for occludin, ZO-1 and Cldn-1. B: To determine the specificity of the antibodies, immunoprecipitation of p-Thr and p-Ser were performed using corresponding antibodies in the presence or absence of 25 μM p-Thr-peptide or p-Ser-peptide. Immunocomplexes were then immunoblotted for occludin and claudin-1. C & D: Recombinant GST-Ocl-C or GST (10 μg) was incubated with varying amounts of recombinant PKCζ. The GSH-agarose pull down from these samples was then immunoblotted for PKCζ and GST. Values on the left margin of the blots represent the molecular weights of marker proteins. Densitometric evaluation of the bands is summarized in panel D. Values are mean ± sem (n = 3). E: GST-Ocl-C was incubated with PKCζ (500 ng) or PKCη (500 ng) in the presence of varying concentrations of PKCζ-PS and 0.5 mM ATP. Samples were then immunoblotted for p-Thr and GST. F: Wild type and Thr-mutants of GST-Ocl-C were incubated with PKCζ in the presence of ATP for 3 hours. Phosphorylation was assessed by immunoblot analysis for p-Thr.
PKCζ directly interacts with the C-terminal domain of occludin
Previous study showed that PKCζ is localized at the vicinity of tight junctions in MDCK cell monolayers [25]. However, the direct interaction of PKCζ with tight junction proteins and its involvement in the phosphorylation of tight junction proteins is unknown. To determine the direct interaction of PKCζ with occludin we prepared recombinant C-terminal domain of human occludin (C-terminal 150 amino acids) as a GST-fusion protein (GST-Ocl-C). GST-Ocl-C was then incubated with varying concentrations of recombinant PKCζ and the direct binding was evaluated by GST pull down assay. Results show that PKCζ binds to GST-Ocl-C in a dose-dependent manner (Fig. 7C & 7D). Densitometric analysis indicated that only trace amounts of PKCζ bound to GST (Fig. 7D).
PKCζ phosphorylates C-terminal tail of occludin on specific Thr residues
To determine the direct role of PKCζ activity in phosphorylation of occludin, we incubated GST-Ocl-C with recombinant PKCζ in the presence of ATP. PKCζ induced phosphorylation of GST-Ocl-C on Ser and Thr residues (Fig. 7E). Presence of PKCζ-PS in the assay mixture reduced PKCζ-mediated phosphorylation of GST-Ocl-C in a dose-dependent manner. Previous study indicated that PKCη also phosphorylates occludin on Thr residues [16]. The present study shows that PKCη-mediated occludin phosphorylation was unaffected by PKCζ-PS (Fig. 7E), indicating the specificity of PKCζ-PS. We focused our further study on Thr-phosphorylation of occludin.
To determine the site of phosphorylation we induced point mutation to Thr residues (selected on the basis of evolutionary conservation) in GST-Ocl-C and tested for in vitro phosphorylation by PKCζ. Mutation of T400 did not influence PKCζ-mediated Thr phosphorylation, while T403A, T404A, T424A or T438A mutations resulted in partial reduction in Thr-phosphorylation (Fig. 8F); mutation of T438 almost completely attenuated Thr-phosphorylation of GST-Ocl-C.
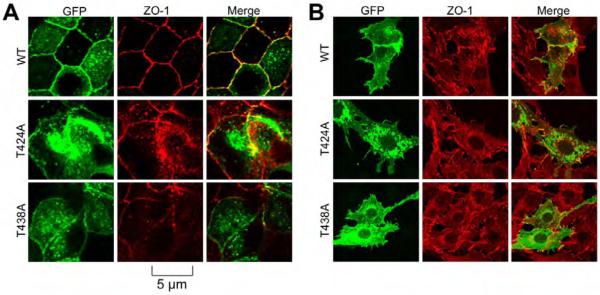
Fig. 8. Mutation of T424 and T348 in occludin attenuates its assembly into tight junctions.
Wild type and Thr-mutants of full-length human occludin in pEGFP vector were transfected to MDCK (A) or Rat-1 (B) cells. Transfected cells were fixed and stained for GFP and ZO-1. Images were collected using a confocal microscope.
Phosphorylation of T424 and T438 is required for the assembly of occludin into tight junctions
Our previous study showed that mutation of T403 and T404 results in delayed assembly of occludin into tight junctions. To determine the role of occludin phosphorylation on T424 and T438 we mutated these residues in full length occludin and expressed them as GFP fusion proteins in MDCK epithelial cells and Rat-1 fibroblasts. Cell monolayers fixed during calcium-induced tight junction assembly and stained for GFP and ZO-1. Results show that GFP-OclWT was recruited to the intercellular junctions during calcium-induced tight junction assembly in MDCK cells (Fig. 8A). However, GFP-OclT424A and GFP-OclT438A were localized predominantly in the intracellular compartments. In Rat-1 fibroblasts, GFP-OclWT was located in the plasma membranes and at the intercellular junctions and co-localized with ZO-1 (Fig. 8B). GFP-OclT424A and GFP-OclT438A on the other hand was localized predominantly in the intracellular compartment.
PKCζ-PS disrupts tight junctions in mouse ileum
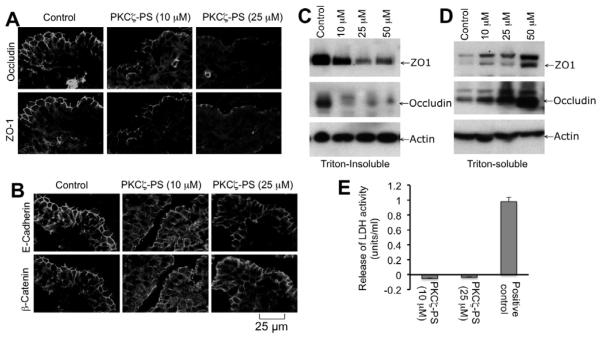
To confirm the physiological relevance of the observation made in cultured cell monolayers we examined the effect of PKCζ-PS on tight junction integrity in mouse ileum. Mouse ileal strips were incubated with varying concentrations of PKCζ-PS for one hour. Confocal microscopy showed that occludin and ZO-1 are co-localized at the intercellular junctions of epithelium in the untreated ileum (Fig. 9A). Treatment with PKCζ-PS disrupted the junctional organization of occludin and ZO-1 in a dose-dependent manner (Fig. 9A). The PKCζ-PS also induced redistribution of E-cadherin and β-catenin in a dose-dependent manner (Fig. 9B). The PKCζ-PS-mediated disruption of tight junctions was associated with a decrease in the level of Triton-insoluble fractions of occludin and ZO-1 (Fig. 9C) with a corresponding increase in the levels of Triton-soluble fractions (Fig. 9D). To evaluate the viability of mouse intestinal tissues during incubation with PKCζ-PS we analyzed the incubation for released LDH activity. Incubation with PKCζ-PS for 2 hours did not increase LDH activity in the medium (Fig. 9E), whereas, tissue damage induced by detergent dramatically increased LDH activity in the incubation medium, indicating that PKCζ-PS did not cause loss of tissue viability.
Fig. 9. PKCζ-PS attenuates tight junction integrity in mouse ileum.
Mouse ileal strips were incubated with or without varying doses of PKCζ-PS for one hour. A, B: Cryosections of tissues were stained for occludin and ZO-1 (A) or E-cadherin and β-catenin (B) by immunofluorescence method. C, D: Triton-insoluble (C) and Triton-soluble (D) fractions prepared from mucosal scrapings of ileum were immunoblotted for different proteins. E: At the end of incubations with PKCζ-PS medium was analyzed for LDH activity. For positive control tissues were incubated with 0.1% Triton X100. LDH values for tissues incubated with no inhibitor or Triton X100 were subtracted from experimental values. Values are mean ± sem (n = 4).
DISCUSSION
A significant body of evidence indicates that epithelial tight junctions are regulated by intracellular signaling elements including protein kinases. Evidence suggests that atypical PKCs are involved in stabilization of epithelial polarity in embryonic tissue [37, 39] and in epidermal barrier regulation [38]. The present study provides evidence to the role of PKCζ in the assembly and maintenance of tight junctions in Caco-2 and MDCK cell monolayers. This study also indicates that PKCζ directly interacts with occludin and induces phosphorylation of occludin on specific Thr residues.
PKCζ-PS (a cell permeable peptide) has been previously shown to inhibit PKCζ activity in various types of cells [42]. The present study shows that administration of PKCζ-PS to Caco-2 and MDCK cell monolayers result in rapid disruption of barrier function as shown by the decrease in TER and increase in inulin permeability. This barrier disruption was not associated with any loss of cell viability as evidenced by no change in LDH release or mitochondrial activity in the cell after 3 hr incubation with PKCζ-PS. On the other hand, barrier dysfunction induced by inhibition of PKCζ was associated with a redistribution of occludin and ZO-1 from the intercellular junctions into the intracellular compartments, indicating that PKCζ-PS caused disruption of tight junctions. In both Caco-2 and MDCK cell monolayers calcium-induced reassembly of tight junctions was prevented by the presence of PKCζ-PS. Therefore, these results indicate that PKCζ activity is required for the maintenance of tight junction integrity in both Caco-2 And MDCK epithelial cell monolayers. However, PKCζ-PS is likely to inhibit the activity of PKCλ also [43]. Therefore, possibility exists that both atypical PKCs are involved in tight junction regulation.
PKCζ-PS also caused redistribution of E-cadherin and β-catenin from the intercellular junctions into the intracellular compartments, indicating that the adherens junctions are disrupted by the inhibition of atypical PKCs. Although adherens junctions do not form a physical barrier to the diffusion of macromolecules across the epithelium, it indirectly regulates the integrity of tight junctions. Disruption of adherens junctions by calcium depletion is well known to disrupt tight junctions [44]. A recent study demonstrated that inhibition of PKCη by a specific pseudosubstrate inhibitor disrupts tight junctions without affecting the adherens junctions [26]. Therefore, PKCζ may regulate the integrity of both tight junctions and adherens junctions, whereas PKCη influences only tight junctions. It is however not clear if both junctions are disrupted simultaneously of one is affected prior to the other.
To distinguish the role of PKCζ from PKCλ in tight junction regulation we designed antisense oligonucleotides and shRNA to selectively knockdown PKCζ. Sequence of both antisense oligos and shRNA showed less than 40% match with the nucleotide sequence of PKCλ. Accordingly, antisense oligos knocked down PKCζ without altering PKCλ levels. Knockdown of PKCζ disrupted barrier function, which was associated with a redistribution of tight junction proteins from the intercellular junctions into intracellular compartments. PKCζ-specific shRNA was transfected in pRNAtinH1.2/neo vector, which also contained AcGFP gene. Similar to antisense oligos, shRNA attenuated the barrier function of MDCK cell monolayers. The expression of AcGFP in these cells allowed us to compare the GFP-positive, transfected cells with the GFP-negative, non-transfected cells in the same monolayer. The results of this study confirmed that junctional distribution of ZO-1 is disrupted in GFP-positive cells, whereas ZO-1 distribution was intact at the intercellular junctions in GFP-negative cells. Antisense oligo sequences were designed against nucleotide sequence of human PKCζ, whereas shRNA was designed against canine PKCζ. AS-1 sequence is 94% match with canine PKCζ sequence, and accordingly, AS-1 significantly reduced the level of PKCζ in MDCK cells, while AS-2 was ineffective in these cells. The shRNA sequence is 100% match for both human and canine PKCζ gene sequences.
Loss of tight junction integrity in epithelial monolayers by calcium depletion [34], oxidative stress [45], acetaldehyde [13], phorbol esters [35] and pathogen [36] is known to be associated with dephosphorylation of occludin on Ser and/or Thr residues. The precise mechanism involved in this process is unclear. The present study shows that disruption of tight junctions by the inhibition of PKCζ is associated with a rapid dephosphorylation of occludin on Ser and Thr residues. PKCζ may maintain the tight junction integrity by preserving the phosphorylation state of occludin. It is likely that Ser/Thr-phosphorylation of other tight junction proteins may also be involved. Therefore, multiple mechanisms may be associated with tight junction regulation. In the present study, we focused our attention to Thr-phosphorylation of occludin. The results show that PKCζ directly interacts with C-terminal domain of occludin and phosphorylates it on Thr residues.
Our previous study showed that PKCη phosphorylates occludin on specific Thr residues [16]. In the present study we show that PKCζ predominantly phosphorylates occludin C-terminal domain on T438, T403, T404 and T424. This is somewhat different from PKCη-mediated phosphorylation, where T403, T404 and T438 are phosphorylated, but not T424 [26]. Both PKCζ and PKCη may phosphorylate occludin on T403 and T404 residues, while only PKCζ is involved in phosphorylation of T424. Previous study showed that phosphorylation of T403 and T404 is required for optimal assembly of occludin into the tight junctions [26]. To determine the role of T424 and T438 phosphorylation in occludin assembly into the tight junctions, we induced T424A and T438A mutations in GFP-tagged full length occludin and expressed them in MDCK and Rat-1 fibroblasts. Wild type occludin was localized at the intercellular junctions of MDCK cell monolayers, which co-localized with ZO-1. On the other hand, T424A and T438A mutants were localized predominantly in the intracellular compartments. In Rat-1 cells, that do not express occludin, GFP-Occludin was incorporated into the plasma membrane and newly formed intercellular junctions, whereas T424A and T438A mutants were distributed in the intracellular compartments. These results indicate that phosphorylation of T424 and T438 is also required for the normal assembly of occludin into the tight junctions. There was a difference in the intracellular distribution of T438A mutant in MDCK and Rat-1 fibroblasts, the explanation for which is unclear at this time. However, it should be noted that MDCK cells express occludin, whereas Rat-1 cells do not. In MDCK cells, T438A mutant may form dimers with the endogenous occludin and contribute to difference in the distribution.
Caco-2 and MDCK cell monolayers have been extensively applied in studies toward understanding the structure and regulation of epithelial tight junctions, and the information derived from such studies has been extended to animal tissues. The present study shows that incubation of mouse ileum with PKCζ-PS result in disruption of tight junctions. PKCζ-PS induces redistribution of occludin and ZO-1 from the intercellular junctions and reduces the levels of detergent-insoluble fractions of occludin and ZO-1. These results demonstrate that PKCζ activity is required for the maintenance of tight junction integrity in mouse ileum and confirm the physiologic relevance of the observation made in Caco-2 and MDCK cell monolayers.
This study therefore, demonstrates that PKCζ activity is required for the maintenance of epithelial tight junction integrity. The mechanism of this tight junction regulation may involve PKCζ-mediated phosphorylation of occludin on specific Thr residues.
ACKNOWLEDGEMENTS
This study was supported by National Institute of Health grants R01-DK55532 and R01-AA12307 (RKR).
Abbreviations
- AS-1 and AS-2
antisense oligonucleotides specific for PKCζ
- Caco-2
colon adenocarcinoma cell line
- CMV promoter
cytomegalovirus promoter
- EGTA
Ethylene glycol tetra acetic acid
- EDTA
ethylenediamine tetraacetic acid
- FITC
fluorescein isothiocyanate
- GFP
green fluorescence protein
- GSH
glutathione
- GST
glutathione S-transferase
- GST-Ocl-C
C-terminal tail region of occludin fused with GST
- HRP
horse radish peroxidase
- MDCK
Madine Darby canine kidney epithelial cell line
- MS
missense oligonucleotide
- PBS
phosphate buffered saline
- PKC
protein kinase C
- PKCζ-PS
PKCζ-specific pseudo substrate peptide inhibitor
- PMSF
phenyl methyl sulfonyl fluoride;
- p-Ser
phospho-serine
- p-Thr
phospho-threonine
- SDS
sodium dodecyl sulfate
- shRNA
short hairpin RNA
- TER
transepithelial electrical resistance
- ZO-1, ZO-2 & ZO-3
zona occludens 1, 2 & 3
- Cldn
claudin
Footnotes
Author contribution:
Jain conducted most of the experiments and participated in organizing results. Suzuki contributed by designing and preparing the shRNA constructs and occludin mutants. Seth and Samak participated in some of the cell experiments. Rao is responsible for designing the experiments, organizing results and writing the manuscript.
REFERENCES
- 1.Van Itallie CM, Anderson JM. The molecular physiology of tight junction pores. Physiology (Bethesda) 2004;19:331–338. doi: 10.1152/physiol.00027.2004. [DOI] [PubMed] [Google Scholar]
- 2.Edelblum KL, Turner JR. The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol. 2009;9:715–720. doi: 10.1016/j.coph.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee DB, Huang E, Ward HJ. Tight junction biology and kidney dysfunction. Am J Physiol Renal Physiol. 2006;290:F20–34. doi: 10.1152/ajprenal.00052.2005. [DOI] [PubMed] [Google Scholar]
- 4.Tieu DD, Kern RC, Schleimer RP. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:37–42. doi: 10.1016/j.jaci.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol. 1999;10:1337–1345. doi: 10.1681/ASN.V1061337. [DOI] [PubMed] [Google Scholar]
- 6.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279:G250–254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Mariscal L, Betanzos A, Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol. 2000;11:315–324. doi: 10.1006/scdb.2000.0178. [DOI] [PubMed] [Google Scholar]
- 8.Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274:F1–9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 9.Citi S, Cordenonsi M. Tight junction proteins. Biochim Biophys Acta. 1998;1448:1–11. doi: 10.1016/s0167-4889(98)00125-6. [DOI] [PubMed] [Google Scholar]
- 10.Turner JR. ‘Putting the squeeze’ on the tight junction: understanding cytoskeletal regulation. Semin Cell Dev Biol. 2000;11:301–308. doi: 10.1006/scdb.2000.0180. [DOI] [PubMed] [Google Scholar]
- 11.Rao R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front Biosci. 2008;13:7210–7226. doi: 10.2741/3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1280–1288. doi: 10.1152/ajpgi.2001.280.6.G1280. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki T, Seth A, Rao R. Role of phospholipase Cgamma-induced activation of protein kinase Cepsilon (PKCepsilon) and PKCbetaI in epidermal growth factor-mediated protection of tight junctions from acetaldehyde in Caco-2 cell monolayers. J Biol Chem. 2008;283:3574–3583. doi: 10.1074/jbc.M709141200. [DOI] [PubMed] [Google Scholar]
- 14.Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422–430. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- 15.Mazzon E, Cuzzocrea S. Role of TNF-alpha in ileum tight junction alteration in mouse model of restraint stress. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1268–1280. doi: 10.1152/ajpgi.00014.2008. [DOI] [PubMed] [Google Scholar]
- 16.Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–172. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 17.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–933. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 18.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen YH, Lu Q. Association of nonreceptor tyrosine kinase c-yes with tight junction protein occludin by coimmunoprecipitation assay. Methods Mol Biol. 2003;218:127–132. doi: 10.1385/1-59259-356-9:127. [DOI] [PubMed] [Google Scholar]
- 20.Chen YH, Lu Q, Goodenough DA, Jeansonne B. Nonreceptor tyrosine kinase c-Yes interacts with occludin during tight junction formation in canine kidney epithelial cells. Mol Biol Cell. 2002;13:1227–1237. doi: 10.1091/mbc.01-08-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nusrat A, Chen JA, Foley CS, Liang TW, Tom J, Cromwell M, Quan C, Mrsny RJ. The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction. J Biol Chem. 2000;275:29816–29822. doi: 10.1074/jbc.M002450200. [DOI] [PubMed] [Google Scholar]
- 22.Basuroy S, Sheth P, Kuppuswamy D, Balasubramanian S, Ray RM, Rao RK. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J Biol Chem. 2003;278:11916–11924. doi: 10.1074/jbc.M211710200. [DOI] [PubMed] [Google Scholar]
- 23.Sheth P, Basuroy S, Li C, Naren AP, Rao RK. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem. 2003;278:49239–49245. doi: 10.1074/jbc.M305654200. [DOI] [PubMed] [Google Scholar]
- 24.Deli MA. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim Biophys Acta. 2009;1788:892–910. doi: 10.1016/j.bbamem.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Stuart RO, Nigam SK. Regulated assembly of tight junctions by protein kinase C. Proc Natl Acad Sci U S A. 1995;92:6072–6076. doi: 10.1073/pnas.92.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R, Rao R. PKC eta regulates occludin phosphorylation and epithelial tight junction integrity. Proc Natl Acad Sci U S A. 2009;106:61–66. doi: 10.1073/pnas.0802741106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avila-Flores A, Rendon-Huerta E, Moreno J, Islas S, Betanzos A, Robles-Flores M, Gonzalez-Mariscal L. Tight-junction protein zonula occludens 2 is a target of phosphorylation by protein kinase C. Biochem J. 2001;360:295–304. doi: 10.1042/0264-6021:3600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, Tabuse Y, Kemphues KJ, Ohno S. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol. 1998;143:95–106. doi: 10.1083/jcb.143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mullin JM, Kampherstein JA, Laughlin KV, Clarkin CE, Miller RD, Szallasi Z, Kachar B, Soler AP, Rosson D. Overexpression of protein kinase C-delta increases tight junction permeability in LLC-PK1 epithelia. Am J Physiol. 1998;275:C544–554. doi: 10.1152/ajpcell.1998.275.2.C544. [DOI] [PubMed] [Google Scholar]
- 30.Basuroy S, Seth A, Elias B, Naren AP, Rao R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J. 2006;393:69–77. doi: 10.1042/BJ20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Souza T, Agarwal R, Morin PJ. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J Biol Chem. 2005;280:26233–26240. doi: 10.1074/jbc.M502003200. [DOI] [PubMed] [Google Scholar]
- 32.Kohler K, Louvard D, Zahraoui A. Rab13 regulates PKA signaling during tight junction assembly. J Cell Biol. 2004;165:175–180. doi: 10.1083/jcb.200312118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seth A, Sheth P, Elias BC, Rao R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J Biol Chem. 2007;282:11487–11498. doi: 10.1074/jbc.M610597200. [DOI] [PubMed] [Google Scholar]
- 35.Clarke H, Soler AP, Mullin JM. Protein kinase C activation leads to dephosphorylation of occludin and tight junction permeability increase in LLC-PK1 epithelial cell sheets. J Cell Sci. 2000;113(Pt 18):3187–3196. doi: 10.1242/jcs.113.18.3187. [DOI] [PubMed] [Google Scholar]
- 36.Simonovic I, Arpin M, Koutsouris A, Falk-Krzesinski HJ, Hecht G. Enteropathogenic Escherichia coli activates ezrin, which participates in disruption of tight junction barrier function. Infect Immun. 2001;69:5679–5688. doi: 10.1128/IAI.69.9.5679-5688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bossinger O, Klebes A, Segbert C, Theres C, Knust E. Zonula adherens formation in Caenorhabditis elegans requires dlg-1, the homologue of the Drosophila gene discs large. Dev Biol. 2001;230:29–42. doi: 10.1006/dbio.2000.0113. [DOI] [PubMed] [Google Scholar]
- 38.Helfrich I, Schmitz A, Zigrino P, Michels C, Haase I, le Bivic A, Leitges M, Niessen CM. Role of aPKC isoforms and their binding partners Par3 and Par6 in epidermal barrier formation. J Invest Dermatol. 2007;127:782–791. doi: 10.1038/sj.jid.5700621. [DOI] [PubMed] [Google Scholar]
- 39.Manabe N, Hirai S, Imai F, Nakanishi H, Takai Y, Ohno S. Association of ASIP/mPAR-3 with adherens junctions of mouse neuroepithelial cells. Dev Dyn. 2002;225:61–69. doi: 10.1002/dvdy.10139. [DOI] [PubMed] [Google Scholar]
- 40.Kale G, Naren AP, Sheth P, Rao RK. Tyrosine phosphorylation of occludin attenuates its interactions with ZO-1, ZO-2, and ZO-3. Biochem Biophys Res Commun. 2003;302:324–329. doi: 10.1016/s0006-291x(03)00167-0. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki A, Ishiyama C, Hashiba K, Shimizu M, Ebnet K, Ohno S. aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J Cell Sci. 2002;115:3565–3573. doi: 10.1242/jcs.00032. [DOI] [PubMed] [Google Scholar]
- 42.Alexander DR, Graves JD, Lucas SC, Cantrell DA, Crumpton MJ. A method for measuring protein kinase C activity in permeabilized T lymphocytes by using peptide substrates. Evidence for multiple pathways of kinase activation. Biochem J. 1990;268:303–308. doi: 10.1042/bj2680303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki T, Seth A, Rao R. Role of phospholipase Cgamma-induced activation of protein kinase Cepsilon (PKCepsilon) and PKCbetaI in epidermal growth factor-mediated protection of tight junctions from acetaldehyde in Caco-2 cell monolayers. J Biol Chem. 2008;283:3574–3583. doi: 10.1074/jbc.M709141200. [DOI] [PubMed] [Google Scholar]
- 44.Capaldo CT, Macara IG. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2007;18:189–200. doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheth P, Samak G, Shull JA, Seth A, Rao R. Protein phosphatase 2A plays a role in hydrogen peroxide-induced disruption of tight junctions in Caco-2 cell monolayers. Biochem J. 2009;421:59–70. doi: 10.1042/BJ20081951. [DOI] [PMC free article] [PubMed] [Google Scholar]