‘Impact’, the ability to have a long lasting significant influence in the field, has not only become the focus of the NIH and NSF, but of science in general. The paper in this issue “Optical Coherent Tomographic Analysis of In-stent Neo-atherosclerosis after Drug–Eluting Stent Implantation” deals with two high-impact areas of cardiology: extending the diagnostic capabilities of cardiovascular optical coherence tomography (OCT) and the etiology of late drug eluting stent (DES) failure1. With respect to the latter, the article looks at atherosclerotic progression after DES placement and late development of different plaque morphologies, predominately at the stent site. It suggests variability of plaque progression in the peri-stent area as a potential etiology for failure. The literature on this topic is reviewed in the article, so the cardiovascular OCT aspects of the paper, mainly the strengths and weaknesses of the technology, will be the focus of the remainder of this editorial. The primary topic will be in particular the effectiveness of OCT for plaque lipid identification.
As cardiovascular OCT now enters its 17th year, it is still widely held that its greatest potential for impact are in risk stratifying thin-cap fibroatheromas (TCFA) and reducing stent placement failures2, 3. The S-J Kang et. al. paper addresses both these areas1. More broadly, as with other studies done over the last several years, the paper represents a new direction in the OCT field. With the recent commercialization of OCT imaging systems, we are seeing a new era of cardiovascular OCT studies directed by skilled clinical scientists at hypotheses that will affect patient outcomes (i.e. high impact) and are not simply observational studies4.
OCT’s progress in the last 17 years has been dramatic, from the original embodiment that had a penetration of less than 500 µm, a frame rate of 40 seconds/image, and inability to be performed through a catheter2. The S-J Kang et. al. paper, however, indirectly draws attention to two major obstacles facing OCT in cardiology1. The first is that while recent studies have provided insight into why late occlusions occur, markers predicting poor outcomes at the time of intervention are essentially non-existent5, 6. This is related in part to the paucity of studies in the previous decade designed to address this question.
But, the Kang et. al. study also draws attention to an issue I find even more critical: the limitations of current OCT embodiments for characterizing plaque1. While it does not significantly detract from the conclusions of the manuscript, the plaque characterizations at times overstate the abilities of current OCT, feeding off of misconceptions in the literature (or at least, in my opinion, poorly validated previous studies).
To prevent acute coronary syndromes (ACS), it is likely that the culprit plaques need to be identified and treated. ACS occurs secondary to the rupture of small thin-walled, lipid-filled plaques in the coronary arteries. When these plaques rupture, they release thrombogenic factors into the blood, which leads to a cascading sequence of events, resulting in clot formation and vessel occlusion7, 8. These plaques are beyond the resolution of essentially all in vivo non-OCT imaging technologies. The AHA classification of plaque uses broad criteria and has recently further subclassified plaque based on the likelihood of rupture 8. The plaques most relevant to ACS are the thin-cap fibroatheromas (TCFA). TCFA are characterized by a thin fibrous cap (< 75 µm thickness), infiltration by macrophages/lymphocytes at some points, neovascularization into the intima, and few cap collagen bundles. The plaques leading to ACS are therefore structurally weak and thereby vulnerable to mechanical pressure such as shear stress or rupture of intimal angiogenic vessels. But, the problem is further complicated by the fact most TCFAs do not lead to ACS7, 8, which is consistent with results in the Kang, et.al. paper stating, “many of the OCT neointimal ruptures and thrombi were small,… [but] were not responsible for clinical instability.” Kang et. al. confidently label plaques as having a lipid core, although OCT’s current ability to differentiate lipid from non-lipid has been highly questionable. This has implications for studies characterizing plaque with OCT in vivo, like the one in this issue.
The major TCFA marker examined effectively to date by OCT is intimal cap thickness, as was done by Kang et. al. At autopsy, 95% of ruptured plaques have a thin fibrous cap. In our initial study of thin-walled plaques 17 years ago, we demonstrated the ability of OCT to identify in vitro plaque intimal cap thickness below 40 µm with histological comparisons2. These intimal cap measurements were confirmed ex vivo a decade later (r=0.90, p<0.001)9. The ability to demonstrate intimal cap thickness is clearly the best demonstrated strength of OCT for identifying TCFA.
But the ability of OCT to identify lipid based on diffuse borders (or signal decay) comes from small data sets and, at the very least, needs to be confirmed (and at worst are incorrect). Aside from the thin intimal cap, it is also critical to know when deciding on intervention if the plaque has a lipid core; if the plaque has a solid core, the likelihood of rupture would be small. Some groups have suggested that in an OCT image, a diffuse border (non-sharp) between the intima and the core is indicative of lipid 10. This criterion was used extensively in the current paper for plaque characterization and is being used in many clinical studies, making this a conclusion that should be well tested. However, we have previously suggested that the diffuse boundary was due to heavy scattering in the cap, likely from either calcium deposits or lipid crystals, and not due to core composition2, 11. Consequently, this raises a concern regarding current interpretations of plaque during in vivo studies. Yabushita, et al. in 2002 measured the sensitivity and specificity of OCT in vitro ranging from 71% to 98% for fibrous plaques, 95% to 97% for fibrocalcific plaques, and 90% to 94% for lipid-rich plaques among two OCT observers with good inter-observer variability (kappa = 0.83–0.84)10. Similar results were obtained by a second group in 2006, but two additional studies were performed the same year that had less promising results; one of these studies reported only a 45% sensitivity and 83% specificity for identifying lipid-filled plaques12–14. This study had difficulty in distinguishing lipid and calcified plaque, which is consistent with our concern that intimal scattering is being measured rather than lipid.
Groups previously supporting the diffuse border have now suggested exponential signal decay at the intimal-cap boundary as a means of identifying lipid, emphasizing the concern over using diffuse borders as a marker15. But again, this decay can be from high cap scattering of light and is therefore independent of the core composition.
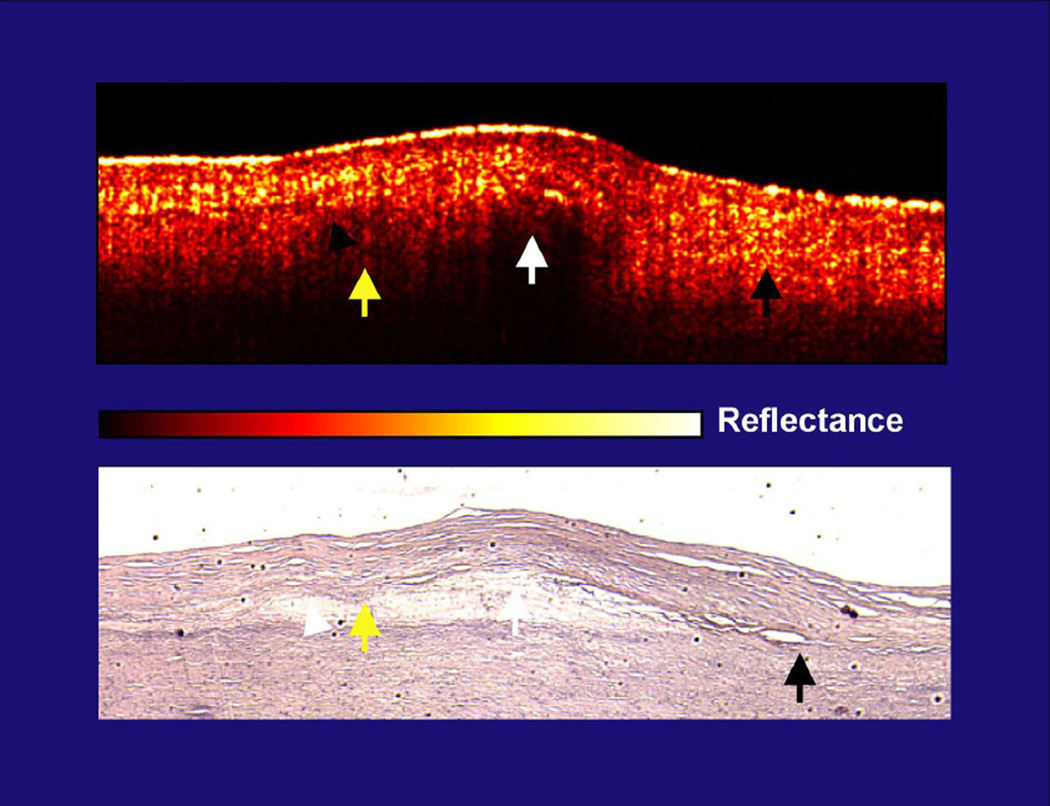
This problem of using diffuse borders to identify plaque lipid is illustrated in the figure 1 from our original OCT plaque characterization paper (but is also seen in figure 1 of the S-J Kang et. al. paper as is discussed in the legend).
Figure 1. Lack of correlation in diffuse boundaries and lipid content.
This figure is from the initial Circulation publication from 19963. The yellow arrows demonstrate the cap-lipid interface that is diffuse and is covered by a highly scattering cap (yellow reflections in cap). The white arrow, which is also over lipid, identifies a cap with lower scattering and the cap-lipid interface is sharply defined. Both are over the same lipid collection. Finally, the black arrow shows the intimal-elastic layer interface (no lipid present) that is diffuse, with an intima that is highly scattering. In figure 1B, E, and F of the S-J Kang et. al. paper, there are diffuse border plaques, but the caps over them are highly scattering, which is potentially the etiology. The concern is therefore whether the lipid interface is diffuse in some OCT images because of the core composition, as others have previously suggested, or high scattering from the cap, as we have previously suggested. It is possible that highly scattering caps correlate with lipid plaques, but it is also possible that this scattering is the etiology for the variations in sensitivity and specificity. As clinical studies are now being performed in large numbers due to the availability of the technology, like the paper in this issue, OCT’s lipid characterizing ability needs to be more firmly established.
There is little solid experimental data that supports diffuse boundaries being used for lipid identification accurately, in spite of the fact that this criteria is being widely used in current in vivo human studies. But, this editorial is not suggesting that OCT ultimately will not be able to identify high risk TFCA. Much of the effort in vivo in the last decade surrounded pushing OCT forward as a ‘clinically friendly’ high resolution structural imaging technology. This made it available for studies like the S-J Kang et. al. paper in this issue. But in parallel, adjuvant techniques were developed such as polarization sensitive OCT (PS-OCT) for the assessment of collagen, elastography for assessing the tensile strength of intimal caps, image processing for improved contrast, Doppler for neovascularization, and absorption/dispersion analysis (such as second order correlation approaches) for lipid detection16–20. These OCT techniques will likely greatly enhance plaque characterization. PS-OCT looks particularly promising for assessing intimal cap collagen and is being successfully used in other organ system in vivo (such as measuring cartilage collagen content)16.
Currently, cardiovascular OCT can neither reliably risk stratify plaque nor predict late occlusion after DES placement. The S-J Kang et. al. paper in this issue is part of a new era where clinical scientists now have access to commercial OCT systems and are producing hypothesis driven studies with potentially high impact to patient management. This is critical, for example, in identifying markers of eminent stent failure. In addition, more in vitro and ex vivo work is needed for both interpreting OCT images of plaque in vivo, as well as developing adjuvant techniques for plaque characterization. That being said, as someone in the field since the original paper describing its utility, OCT has great potential to be a high impact technology in the management of cardiovascular disease.
Acknowledgments
This research is sponsored by the National Institutes of Health, contracts R01-AR44812 (Brezinski), R01-EB000419 (Brezinski), R01 AR46996 (Brezinski), R01-HL55686 (Brezinski), and R01-EB002638 (Brezinski).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
I receive royalties on OCT imaging catheters.
References
- 1.Kang SJ, Mintz GS, Akasaka T, et al. Optical Coherent Tomographic Analysis of In-stent Neo-atherosclerosis after Drug-Eluting Stent Implantation. (CIRCULATIONAHA/2010/988436) [Google Scholar]
- 2.Brezinski ME. Optical Coherence Tomography: Principle and Practice. Burlington: Academic Press; 2006. [Google Scholar]
- 3.Brezinski ME, Tearney GJ, Bouma BE, Izatt JA, Hee MR, Swanson EA, Southern JF, Fujimoto JG. Optical coherence tomography for optical biopsy - properties and demonstration of vascular pathology. Circulation. 1996;93:1206–1213. doi: 10.1161/01.cir.93.6.1206. [DOI] [PubMed] [Google Scholar]
- 4.St. Jude Medical, St. Paul Minnesota. http://www.sjmprofessional.com /Products/US/Imaging-Diagnostics/OCT-Technology.aspx.
- 5.Tanigawa J, Barlis P, Dimopoulos K, Dalby M, Moore P, Di Mario C. The influence of strut thickness and cell design on immediate apposition of drug-eluting stents assessed by optical coherence tomography. International Journal Of Cardiology. 2009;134:180–188. doi: 10.1016/j.ijcard.2008.05.069. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki Y, Ikeno F, Koizumi T, Tio F, Yeung AC, Yock PG, Fitzgerald PJ, Fearon WF. In vivo comparison between optical coherence tomography and intravascular ultrasound for detecting small degrees of in-stent neointima after stent implantation. JACC-Cardiovascular Interventions. 2008;1:168–173. doi: 10.1016/j.jcin.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death - a comprehensive morphological classification scheme for atherosclerotic lesions. Arteriosclerosis Thrombosis And Vascular Biology. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 8.Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. New England Journal Of Medicine. 1997;336:1276–1282. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- 9.Kume T, Akasaka T, Kawamoto T, Okura H, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y, Yoshida K. Measurement of the thickness of the fibrous cap by optical coherence tomography. American Heart Journal. 2006;152:755.e1. doi: 10.1016/j.ahj.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Yabushita H, Bourna BE, Houser SL, Aretz T, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Kang DH, Halpern EF, Tearney GJ. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106:1640–1645. doi: 10.1161/01.cir.0000029927.92825.f6. [DOI] [PubMed] [Google Scholar]
- 11.Brezinski ME. Optical coherence tomography for identifying unstable coronary plaque. International Journal Of Cardiology. 2006;107:154–165. doi: 10.1016/j.ijcard.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 12.Rieber J, Meissner O, Babaryka G, Reim S, Oswald M, Koenig A, Schiele TM, Shapiro M, Theisen K, Reiser MF, Klauss V, Hoffmann U. Diagnostic accuracy of optical coherence tomography and intravascular ultrasound for the detection and characterization of atherosclerotic plaque composition in ex-vivo coronary specimens: A comparison with histology. Coronary Artery Disease. 2006;17:425–430. doi: 10.1097/00019501-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Kume T, Akasaka T, Kawamoto T, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y, Yoshida K. Assessment of coronary arterial plaque by optical coherence tomography. American Journal Of Cardiology. 2006;97:1172–1175. doi: 10.1016/j.amjcard.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 14.Manfrini O, Mont E, Leone O, Arbustini E, Eusebi V, Virmani R, Bugiardini R. Sources of error and interpretation of plaque morphology by optical coherence tomography. American Journal Of Cardiology. 2006;98:156–159. doi: 10.1016/j.amjcard.2006.01.097. [DOI] [PubMed] [Google Scholar]
- 15.van Soest G, Goderie T, Regar E, Koljenovic S, van Leenders G, Gonzalo N, van Noorden S, Okamura T, Bouma BE, Tearney GJ, Oosterhuis JW, Serruys PW, van der Steen AFW. Atherosclerotic tissue characterization in vivo by optical coherence tomography attenuation imaging. Journal Of Biomedical Optics. 15:011105. doi: 10.1117/1.3280271. [DOI] [PubMed] [Google Scholar]
- 16.Giattina SD, Courtney BK, Herz PR, Harman M, Shortkroff S, Stamper DL, Liu B, Fujimoto JG, Brezinski ME. Assessment of coronary plaque collagen with polarization sensitive optical coherence tomography (PS-OCT) International Journal Of Cardiology. 2006;107:400–409. doi: 10.1016/j.ijcard.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 17.Liu B, MacDonald EA, Stamper DL, Brezinski ME. Group velocity dispersion effects with water and lipid in 1.3 µm optical coherence tomography system. Physics In Medicine And Biology. 2004;49:923–930. doi: 10.1088/0031-9155/49/6/004. [DOI] [PubMed] [Google Scholar]
- 18.Brezinski ME, Liu B. Nonlocal quantum macroscopic superposition in a high-thermal low-purity state. Physical Review A. 2008;78:063824. doi: 10.1103/PhysRevA.78.063824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogowska J, Patel N, Plummer S, Brezinski ME. Quantitative optical coherence tomographic elastography: Method for assessing arterial mechanical properties. British Journal Of Radiology. 2006;79:707–711. doi: 10.1259/bjr/22522280. [DOI] [PubMed] [Google Scholar]
- 20.Rollins AM, Yazdanfar S, Barton JK, Izatt JA. Real-time in vivo colors Doppler optical coherence tomography. Journal Of Biomedical Optics. 2002;7:123–129. doi: 10.1117/1.1428291. [DOI] [PubMed] [Google Scholar]