Abstract
Approximately 40% of males with low Gleason grade clinically localized prostate cancer (PCa) at biopsy were finally diagnosed with high Gleason grade PCa at radical prostatectomy (RP). Therefore, a more reliable assessment of the Gleason grade prior to RP is required. Readily available modalities such as circulating biomarkers may be useful for this purpose. The aim of this study was to evaluate the ability of preoperative interleukin 6 (IL‑6) and its soluble receptor (sIL‑6R), as well as urokinase-type plasminogen activator (u-PA), its receptor (u-PAR) and the inhibitor (PAI-1) to predict Gleason score upgrading. A total of 51 PCa patients with biopsy Gleason score ≤7 were studied. IL‑6 and sIL‑6R, uPA, uPAR and PAI-1 preoperative serum levels were determined. Differences in the median and mean values of the preoperative blood levels of all biomarkers between patients with and without Gleason score upgrading were tested. The prognostic performance of each biomarker was further assessed by means of receiver operating characteristic (ROC) curves. The results showed the sIL‑6R and sIL‑6R/IL-6 ratio median levels to be significantly higher in patients who had Gleason score upgrading from ≤7 at biopsy to >7 at RP (p=0.024 and p=0.011, respectively). The ROC curve revealed that sIL‑6R and the sIL‑6R/IL‑6 ratio identified subjects at a high risk of upgrading [area under curve (AUC)=0.80 and AUC=0.83, respectively] with similar sensitivity and higher specificity for the ratio. The findings suggest that preoperative sIL‑6R and sIL‑6R/IL‑6 ratio determination in serum are useful as prognostic biomarkers in PCa patients.
Introduction
Patients with clinically localized PCa are classified as low, intermediate and high risk, according to prostate-specific antigen (PSA) levels, tumor clinical staging and Gleason score (GS). Patients with GS >7 belong to the high-class risk category (1,2). Treatment options depend on the recurrence risk. Although radical prostatectomy (RP) is recommended for the low to intermediate risk categories, a high-risk class category requires aggressive local radiation combined with 2-3 years of androgen-deprivation therapy (ADT) (1,2).
GS upgrading from ≤6 at biopsy to ≥7 at RP has been reported to be approximately 40% (3,4). Thus factors predictive of GS upgrading at RP are required. A promising modality may be represented by molecules involved in PCa progression, which are detectable in blood. These molecules include interleukin 6 (IL-6) as well as its soluble receptor (sIL-6R) and urokinase-type plasminogen system members. IL-6 stimulates cell proliferation (5) and increases angiogenesis and invasion in tumor cells (6,7). The soluble form of the membrane IL‑6 receptor (mIL-6R) binds the cytokine, and their complex is capable of activating the transmembrane protein, gp130, which promotes signal transduction in cells that do not express mIL‑6R (IL-6 trans-signaling) (8). Certain authors observed a strong association between circulating sIL-6R levels prior to prostatectomy and the higher probability of developing metastases (9,10) and disease relapse (11).
The urokinase‑type plasminogen system includes the activator (uPA), the receptor (uPAR) and the inhibitors (PAIs). uPA binds to its receptor uPAR, stimulating cleavage of plasminogen in plasmin and promoting cell motility, invasion, proliferation and survival in various human malignancies (12). The uPA system is negatively regulated by PAIs, which neutralize the proteolytic activity of uPA (13). The upregulation of uPA correlates with the aggressive phenotype and poor prognosis of PCa (14-16).
This study evaluated the ability of preoperative blood levels of IL‑6 and its soluble receptor (sIL‑6R), as well as uPA, uPAR and PAI-1, to predict GS upgrading from ≤7 at biopsy to >7 at RP. This GS upgrading classification is significant to allow clinicians to select appropriate treatment at initial diagnosis.
Patients and methods
Patients. During a one‑year period, a total of 51 subjects with PCa, ranging from 51 to 75 years of age (median 64 years), were enrolled at our institution. Informed consent was obtained from the subjects. Inclusion criteria were: no evidence of active infection or inflammatory disease, no neo-adjuvant androgen therapy, no 5α-reductase inhibitor treatment, PSA ≤20 ng/ml, T-clinical stage ≤2c (according to the 2009 TNM system). Between 12 and 18 needle biopsy cores were obtained under transrectal ultrasound (TRUS) guidance: 40 patients (74%) had 12 cores obtained and 11 had 14-18 cores in glands ≥50 cm3, including transition zone (TZ) cores. Primary and secondary GSs were assigned by a single pathologist. The prostatectomy specimens were processed according to the Stanford protocol and were also graded according to the Gleason system. GS upgrading was defined as a Gleason sum increase between biopsy and RP from ≤7 to >7.
Serum biomarkers. Preoperative serum samples were collected prior to digital rectal examination (DRE) and TRUS. Blood was collected into non-heparinized tubes and serum was separated within 1 h of blood collection. The serum was stored at -80˚C and then thawed just prior to testing. Serum levels of PSA, free‑PSA and IL‑6 were measured using the Immulite 2000 automated assay (DPC, Los Angeles, CA, USA). The concentrations of sIL‑6R (R&D Systems, Minneapolis, MN, USA), uPA, uPAR and PAI-1 (Assaypro, Winfield, MO, USA) in serum were determined according to the manufacturer's instructions using the ELISA test. Every sample was run in duplicate and the mean was used. The differences between the two measurements were minimal.
Data analysis and statistics. Data for continuous variables were the mean ± standard deviation (SD) and the median with the interquartile range. Data for categorical variables are presented as frequencies and percentages. Differences in median values of the preoperative blood levels of all of the biomarkers between patients with and without significant GS upgrading were tested using the exact Wilcoxon-Mann‑Whitney test (or Wilcoxon rank-sum test). The difference in medians (95% CI) was based on the Hodges‑Lehmann estimator. The prognostic performance of each biomarker was further assessed by means of ROC curves. Tests were two‑sided and p<0.05 was considered to be statistically significant. Statistical analyses were performed using the R environment by the R Foundation, version 2.12.0.
Results
Patient characteristics are shown in Table I. Approximately 90% of patients had PSA <10 ng/ml and approximately 80% had % free PSA <15. The values of the analyzed biomarkers in samples classified on the basis of GS upgrading are shown in Table II.
Table 1. Patient characteristics.
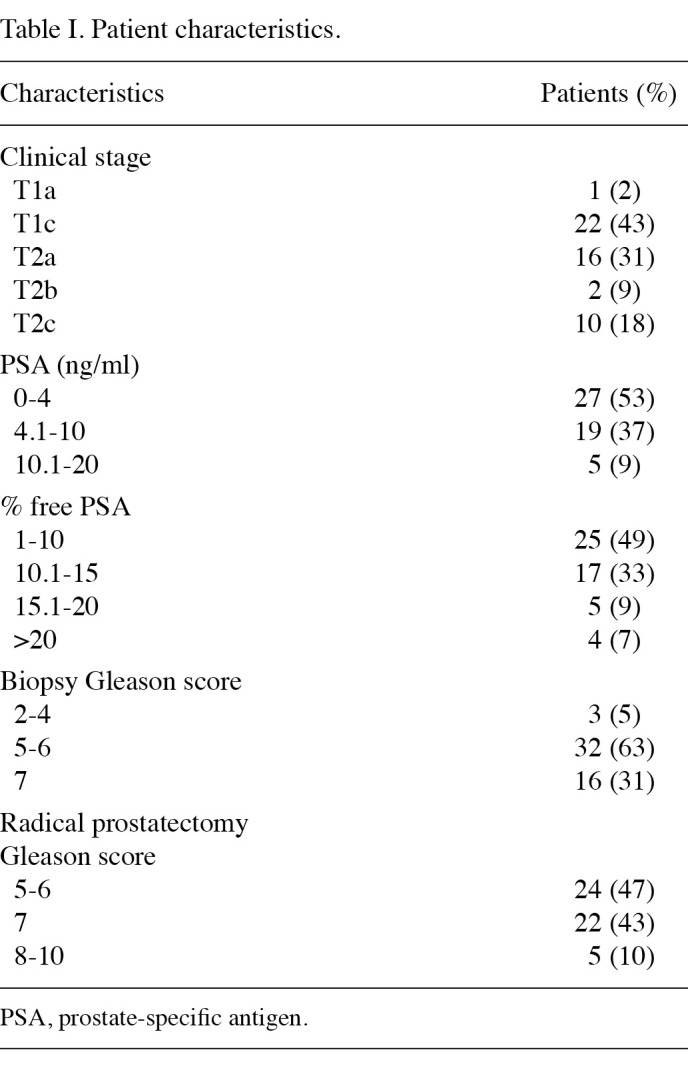
Table 2. Descriptive statistics of measured variables in serum of patients stratified by Gleason score upgrade (GSU).
GS upgrading was defined as a Gleason sum increase between biopsy and RP from ≤7 to >7, since this is important for the therapeutic strategy. On this basis, an upgrade was noted in 5 (10%) samples.
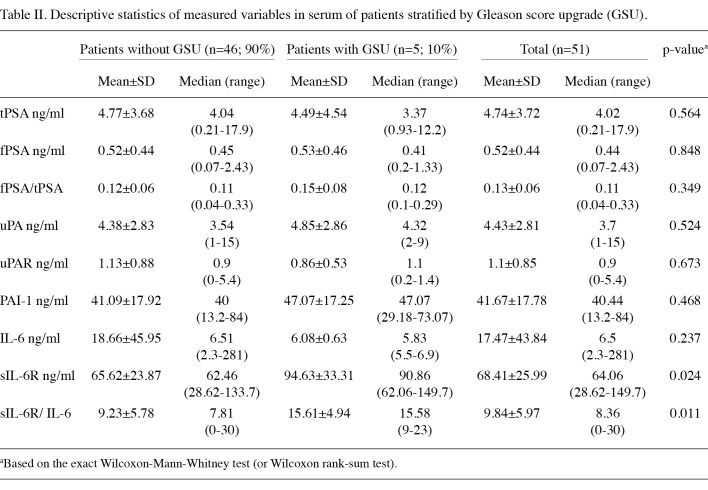
Median sIL-6R values were found to be significantly higher in patients with GS upgrading (difference in medians, 28.40 ng/ml; 95% CI, 5.60-49.44; p=0.024). The association between sIL-6R and GS upgrading became more significant by using the sIL-6R/IL-6 ratio (difference in medians, 7.77; 95% CI, 1.72-11.28; p=0.011). Sensitivity and specificity of sIL-6R and the sIL-6R/IL-6 ratio were explored by ROC curve analysis (Fig. 1). The results indicated an extremely good ability to predict the probability of biopsy Gleason sum upgrading of the two parameters (sIL‑6R: AUC=0.80; 95% CI, 0.63-0.97; p=0.026. sIL-6R/IL-6 ratio: AUC=0.84; 95% CI, 0.69-0.98; p=0.014). The optimal cut-off point for sIL-6R was 76.5 ng/ml, providing a sensitivity of 80% and a specificity of 76%, whereas for the sIL-6R/IL-6 ratio the optimal cut-off point was 13.5 with comparable sensitivity (80%), but higher specificity (83%).
Figure 1. ROC analysis comparing sIL-6R (--) and sIL-6R/IL-6 ratio (–).
Discussion
The Gleason grading system remains one of the most powerful prognostic factors for prostate cancer, and plays a crucial role in the prediction of metastatic progression after RP (2,17). The National Comprehensive Cancer Network (NCCN) guidelines suggested that PCa diagnosed with a pathological Gleason sum >7 requires aggressive local radiation and no RP (1). Clinicians are in need of simple diagnostic modalities, available at initial diagnosis, which may be employed to estimate the clinical significance of cancer and guide their treatment decisions. Subsequently, we evaluated the ability of preoperative blood levels of IL-6, sIL-6R , uPA, uPAR and PAI-1 to predict the magnitude of the risk of patients with a biopsy GS of ≤7 to harbor a higher‑grade cancer.
High sIL‑6R levels were found to be significantly associated with high-grade cancer diagnosed at RP and this relationship was strengthened by calculating the sIL‑6R/IL‑6 ratio. This finding regarding the association between sIL‑6R circulating values and features of PCa aggressiveness and metastases is supported by the literature (9,10,18). Moreover, certain authors provided in vitro and in vivo evidence of the contribution of IL‑6 to the development of androgen independence (19-21). It was found that the serum concentration of sIL‑6R is within a range higher than IL‑6. This observation suggested that sIL‑6R is crucial for the IL‑6 pro-tumorigenic effect and IL-6 trans-signaling may be a relevant target in PCa treatment. This conclusion was strengthened by the ROC curve analysis outcomes indicating that the sIL-6R/IL-6 ratio had a higher specificity in the prediction of a high RP Gleason sum compared to sIL-6R. Previous reports indicated that IL‑6 trans‑signaling may play a significant role in tumor progression by stabilizing IL‑6 and enhancing IL‑6‑mediated signaling, which is capable of affecting the expression of genes involved in survival, proliferation, differentiation, osteogenesis/osteolysis, angiogenesis and immunomodulation (22-24). Jostock et al suggested that soluble gp130 (sgp130) is a natural specific inhibitor of IL‑6 trans-signaling and may be used as a valuable therapeutic tool in disease states (25). In this regard, studies on the usefulness of sgp130 combined with IL‑6 and sIL‑6R blood levels to predict worse PCa prognosis are noteworthy.
Nevertheless, this study is limited by the small number of patients included in the study population. Therefore, the findings may not be extended in clinical practice prior to further evaluation, ideally in a multicenter study on a larger population, in order to confirm the use of the sIL‑6R/IL‑6 ratio as a routine marker in PCa patient management.
sIL‑6R may be a significant circulating biomarker employed to predict GS upgrading in patients with a biopsy GS ≤7. Moreover, this ability appears to be enhanced by calculating the sIL‑6R/IL‑6 ratio. These findings may provide an additional 'staging tool' for PCa patients, which may be of great significance in guiding clinicians' treatment choices, according to current guidelines.
Acknowledgments
This study has been partially supported by Grant 2008CF2873 from the Italian Ministry of University and Research in Rome.
Contributor Information
Daniela Terracciano, Department of Cellular and Molecular Biology and Pathology ‘L. Califano’, University of Naples ‘Federico II’, I-80131 Naples, Italy.
Dario Bruzzese, Department of Preventive Medical Sciences, University of Naples ‘Federico II’, I-80131 Naples, Italy.
Matteo Ferro, Department of Urology, University of Naples ‘Federico II’, I-80131 Naples, Italy.
Riccardo Autorino, Urology Clinic, Second University of Naples, I-80138 Naples, Italy.
Giuseppe di Lorenzo, Department of Molecular and Clinical Endocrinology and Oncology, University of Naples ‘Federico II’, I-80131 Naples, Italy.
Carlo Buonerba, Department of Molecular and Clinical Endocrinology and Oncology, University of Naples ‘Federico II’, I-80131 Naples, Italy.
Angela Mariano, Department of Cellular and Molecular Biology and Pathology ‘L. Califano’, University of Naples ‘Federico II’, I-80131 Naples, Italy.
Vincenzo Macchia, Department of Cellular and Molecular Biology and Pathology ‘L. Califano’, University of Naples ‘Federico II’, I-80131 Naples, Italy.
Vincenzo Altieri, Department of Urology, University of Naples ‘Federico II’, I-80131 Naples, Italy.
Angelina di Carlo, Department of Medical Surgical Sciences and Biotechnologies, University of Rome ‘La Sapienza’, I-00161 Rome, Italy.
References
- Mohler JL: The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw 8: 145, 2010. [DOI] [PubMed]
- Epstein JI: An update of the Gleason grading system. J Urol 183: 433-440, 2010. [DOI] [PubMed]
- Moussa AS, Li J, Soriano M, Klein EA, Dong F and Jones JS: Prostate biopsy clinical and pathological variables that predict significant grading changes in patients with intermediate and high grade prostate cancer. BJU Int 103: 43-48, 2009. [DOI] [PubMed]
- Hong SK, Han BK, Lee ST, Kim SS, Min KE, Jeong SJ, Jeong H, Byun SS, Lee HJ, Choe G and Lee SE: Prediction of Gleason score upgrading in low-risk prostate cancers diagnosed via multi (> or = 12)-core prostate biopsy. World J Urol 27: 271-276, 2009. [DOI] [PubMed]
- Ogata A, Chauhan D, Teoh G, Treon SP, Urashima M, Schlossman RL and Anderson KC: IL-6 triggers cell growth via the Ras-dependent mitogen-activated protein kinase cascade. J Immunol 159: 2212-2221, 1997. [PubMed]
- Dankbar B, Padro T, Leo R, Feldmann B, Kropff M, Mesters RM, Serve H, Berdel WE and Kienast J: Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood 95: 2630-2636, 2000. [PubMed]
- Huang SP, Wu MS, Shun CT, Wang HP, Lin MT, Kuo ML and Lin JT: Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci 11: 517-527, 2004. [DOI] [PubMed]
- Rose-John S: Interleukin‑6 biology is coordinated by membrane bound and soluble receptors. Acta Biochim Pol 50: 603-611, 2003. [PubMed]
- Kattan MW, Shariat SF, Andrews B, Zhu K, Canto E, Matsumoto K, Muramoto M, Scardino PT, Ohori M, Wheeler TM and Slawin KM: The addition of interleukin-6 soluble receptor and transforming growth factor β1 improves a preoperative nomogram for predicting biochemical progression in patients with clinically localized prostate cancer. J Clin Oncol 21: 3573-3579, 2003. [DOI] [PubMed]
- Shariat SF, Kattan MW, Traxel E, Andrews B, Zhu K, Wheeler TM and Slawin KM: Association of pre- and postoperative plasma levels of transforming growth factor β(1) and interleukin 6 and its soluble receptor with prostate cancer progression. Clin Cancer Res 10: 1992-1999, 2004. [DOI] [PubMed]
- Shariat SF, Karam JA, Walz J, Roehrborn CG, Montorsi F, Margulis V, Saad F, Slawin KM and Karakiewicz PI: Improved prediction of disease relapse after radical prostatectomy through a panel of preoperative blood-based biomarkers. Clin Cancer Res 14: 3785-3791, 2008. [DOI] [PubMed]
- Blasi F and Sidenius N: The urokinase receptor: focused cell surface proteolysis, cell adhesion and signaling. FEBS Lett 584: 1923-1930, 2010. [DOI] [PubMed]
- Smith HW and Marshall CJ: Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol 11: 23-36, 2010. [DOI] [PubMed]
- Helenius MA, Saramaki OR, Linja MJ, Tammela TL and Visakorpi T: Amplification of urokinase gene in prostate cancer. Cancer Res 61: 5340-5344, 2001. [PubMed]
- Gavrilov D, Kenzior O, Evans M, Calaluce R and Folk WR: Expression of urokinase plasminogen activator and receptor in conjunction with the ets family and AP-1 complex transcription factors in high grade prostate cancers. Eur J Cancer 37: 1033‑1040, 2001. [DOI] [PubMed]
- Shariat SF, Roehrborn CG, McConnell JD, Park S, Alam N, Wheeler TM and Slawin KM: Association of the circulating levels of the urokinase system of plasminogen activation with the presence of prostate cancer and invasion, progression, and metastasis. J Clin Oncol 25: 349-355, 2007. [DOI] [PubMed]
- Porter CR, Suardi N, Kodama K, Capitanio U, Gibbons RP, Correa R, Jeldres C, Perrotte P, Montorsi F and Karakiewicz PI: A nomogram predicting metastatic progression after radical prostatectomy. Int J Urol 15: 889-894, 2008. [DOI] [PubMed]
- Shariat SF, Andrews B, Kattan MW, Kim J, Wheeler TM and Slawin KM: Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology 58: 1008-1015, 2001. [DOI] [PubMed]
- Spiotto MT and Chung TD: STAT3 mediates IL-6-induced neuroendocrine differentiation in prostate cancer cells. Prostate 42: 186-195, 2000. [DOI] [PubMed]
- Deeble PD, Murphy DJ, Parsons SJ and Cox ME: Interleukin-6‑ and cyclic AMP-mediated signaling potentiates neuroendocrine differentiation of LNCaP prostate tumor cells. Mol Cell Biol 21: 8471-8482, 2001. [DOI] [PMC free article] [PubMed]
- Wallner L, Dai J, Escara-Wilke J, Zhang J, Yao Z, Lu Y, Trikha M, Nemeth JA, Zaki MH and Keller ET: Inhibition of interleukin-6 with CNTO328, an anti-interleukin-6 monoclonal antibody, inhibits conversion of androgen-dependent prostate cancer to an androgen-independent phenotype in orchiectomized mice. Cancer Res 66: 3087-3095, 2006. [DOI] [PubMed]
- Hong DS, Angelo LS and Kurzrock R: Interleukin-6 and its receptor in cancer: implications for Translational Therapeutics. Cancer 110: 1911-1928, 2007. [DOI] [PubMed]
- Ara T and Declerck YA: Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer 46: 1223-1231, 2010. [DOI] [PMC free article] [PubMed]
- Santer FR, Malinowska K, Culig Z and Cavarretta IT: Interleukin-6 trans-signalling differentially regulates proliferation, migration, adhesion and maspin expression in human prostate cancer cells. Endocr Relat Cancer 17: 241-253, 2010. [DOI] [PMC free article] [PubMed]
- Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Voltz N, Fischer M, Neurath MF and Rose-John S: Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem 268: 160-167, 2001. [DOI] [PubMed]