Abstract
This is the first study from Central America to analyze genetic mutations and histopathological features associated with gastrointestinal stromal tumors (GIST). Mutations found in the tyrosine kinase membrane receptors c-kit and pdgfra are associated with clinical and pathological characteristics of GIST. New drugs that inhibit the expression of these oncogenes at the molecular level substantially improve the quality of life for patients with this tumor. It is therefore essential for patient care in Panama that genetic analysis of GIST tumors continues to develop from the pilot study presented herein into routine clinical use. This study evaluated 39 cases of GIST in Panama, using samples archived at the Instituto Oncológico Nacional from 1994 to 2004. DNA from paraffin‑embedded tumor tissues was isolated and amplified for the exons of c-kit and pdgfra associated with a high frequency of mutations. Direct PCR sequencing of specific exons was performed, and those with different alleles were cloned and re-sequenced. Amino acid sequences were inferred from DNA and aligned to Genbank reference sequences to determine the position and type of mutation. The highest frequency of mutations was found in exon 11 of the c-kit gene (70%). Mutations found in this exon were heterogeneous, while only one type of mutation (p.A502_Y503dup) was observed in c-kit exon 9. Mutations in the pdgfra gene constituted several substitutions, with the deletion p.D842V being observed most frequently. The observed GIST-associated mutations were previously described. Four patients with mutations associated with familial GIST were also found. The majority (66%) of patients with mutations in exon 11 (residues 550-591) were considered to be at high risk and 75% of patients with mutations specifically within residues 556-560 (exon 11) were considered to have high-risk GIST. This is the first molecular study of GIST in Central America. It was performed to gain a better understanding of the cancer-associated mutations of KIT and platelet‑derived growth factor receptor α (PDGFRA) receptors. This may aid in the prediction of clinical evolution and guide the use of specific drug treatments in patients with GIST in Panama.
Introduction
The incidence of gastrointestinal stromal tumors (GIST) is estimated to be approximately 10-20 per million individuals per year worldwide with a malignancy rate of 20-30% (1-3). Although the biology of GIST is now well understood (4), the precise incidence of GIST is unknown due to the incomplete definition and classification of the tumor (5). GIST, mastocytosis syndromes and certain types of leukemia are associated with the presence of mutations in c-kit and pdgfra genes (6-9). These genes encode the KIT protein [stem cell factor (SCF) receptor] and platelet‑derived growth factor receptor α (PDGFRA), which are membrane receptors with tyrosine kinase activity; both proteins are involved in essential cellular signaling pathways that promote cell growth and proliferation (10-12).
Previous studies showed that drugs including STI-571 (Imatinib, Novartis, Basel, Switzerland) have an antitumor effect that prevents tyrosine kinase over-activation (13). Such drugs have an efficacy of 50-70% on tumor regression and 85-90% on tumor arrest. Clinicopathological parameters, together with patient drug responses, are associated with the type (substitution, duplication, deletion or insertion) and position of observed mutations (9). Tumor cells responding to imatinib develop alternative resistance mechanisms (second mutations in other exons or over-activation of alternative tyrosine kinase receptors), which cause treatment failure when using tyrosine kinase inhibitors (14). Resistance to imatinib, for example, is present in 14% of the patients after 6 months of treatment and in 50% of patients after 2 years of treatment (15,16).
Since the intrinsic processes of activation and autoinhibition in protein receptors may be modified by site-specific mutations, the ability to detect these DNA changes may be translated into more effective treatments for GIST patients. This is the first study in Central America to evaluate gastrointestinal stromal tumors at the molecular level, and is consistent with new approaches to personalized treatment for cancer patients. Few Latin American studies exist that aim to evaluate the mutational status of the KIT/PDGFRA oncoproteins and the utility of identifying these mutations in predicting the clinical response of patients, or which aim to identify subsets of patients who may be sensitive or resistant to treatment.
In the present study, the histopathological features of paraffin-embedded tumor tissues and the mutations in c-kit and pdgfra genes from 39 cases from Panama archived between 1994 and 2004, were evaluated. The results of this study may facilitate the identification of a more effective treatment for Panamanian patients based on molecular analysis.
Materials and methods
Patients. Representative tissue specimens from 39 patients with GIST were obtained from the Department of Pathology, Instituto Oncológico Nacional, Panama. The parameters of gender, age, tumor size and tumor location were recorded for each case. The 39 patients included 20 males and 19 females, ranging in age from 29 to 85 years. The samples were otherwise anonymous and without any identifying personal information. Surgical excision was the primary treatment in all of the cases and complete follow-up was recorded for 33 of the 39 patients. The follow-up period commenced at the date of primary surgery and the end-point case summaries included any observed relapse (local recurrence and/or distant metastasis) of GIST. Patients were assessed clinically and radiographically, with CT imaging, after surgery. Risk stratification for each patient was performed according to the method of Fletcher et al (17).
Immunohistochemistry. H&E-stained sections of each tumor were evaluated for cell shape. Tumor cells were classified as predominantly spindle, predominantly epithelioid or a combination of the two types. Mitotic count was measured per 50 high-power fields (HPF) at a magnification of x400. Immunohistochemical staining was performed using the following primary antibodies: KIT (CD117), CD34, α-smooth muscle actin (SMA), desmin and S100 protein.
Molecular studies. Total nucleic acid was extracted from formalin-fixed paraffin-embedded tissues using the standard phenol-chloroform method. Somatic mutations in exons 9, 11, 13 and 17 of c-kit were evaluated using PCR-based assays as previously described (13,14). PCR products were electrophoresed on 2% agarose gels. Bands of the appropriate, expected size were purified from the gel by standard agarose DNA purification methods. Direct cycle sequencing was performed on each clean PCR reaction. Patient samples with non-mutated c-kit were then evaluated for pdgfra gene mutations (exons 18, 12, 14 and 10). Not all exons per patient were screened per sample due to the observation by other researchers that mutations in these exons are mutually exclusive (18,19). Samples where chromatograms revealed heterozygosity were cloned using the pGEM-T Easy Vector System (Promega, Madison, WI, USA) to separate the various alleles present. Ten samples were selected for screening for mutant alleles, and plasmid DNA was sequenced using a M13 forward primer. Mutated amino acids within the sequences were determined based on a comparison to Genbank reference sequences using MacClade software (20).
Results
The molecular and clinicopathological parameters for each of the 39 GIST cases are shown in Table I. The primary tumor sites were the stomach (59%), small bowel (25%), colon/rectum (2%) and omentum/mesenterium (13%). The median tumor size was 11 cm (range 3-37). Of the primary tumors for which tumor size data were available (n=25), 15 tumors (60%) were found to be <10 cm in diameter and 10 tumors (40%) were ≥10 cm. Based on visual observation of H&E-stained sections of tumor tissue, the primary cell type, present in 30 cases (77%), was spindle cells. Epithelioid cells were present in 6 cases (15%) and 3 cases (8%) exhibited a combination of the two cell types. In addition, the mitotic rate was <5/50 HPF in 15 cases (38%) and ≥5/50 HPF in 24 cases (62%).
Table 1. Clinical and pathological characteristics of patients according to observed c-kit and pdgfra mutations.
As determined by immunohistochemistry, 36 cases were strongly positive for KIT (CD117) expression and only 3 had weak KIT staining. Tumors with weak staining for KIT were primary tumors from the stomach and predominantly of the epithelioid cell type. Tumor cells were positive for CD34 in 78% of tumors, for SMA in 53% of tumors and for desmin in 2% of the tumors tested. All tumors were negative for S100 protein.
Risk stratification of primary GIST based on tumor size and mitotic index was considered to be low in 7 cases (18%), intermediate in 6 cases (15%) and high‑risk in 26 cases (67%). Complete follow-up was obtained for 33 of the 39 patients. The follow‑up period for the 18 patients without metastases ranged from 5 to 50 months (median 23). Of the 15 patients with metastases, 8 were detected at the time of diagnosis, whereas 7 had metastases between 2 and 30 months after surgical treatment.
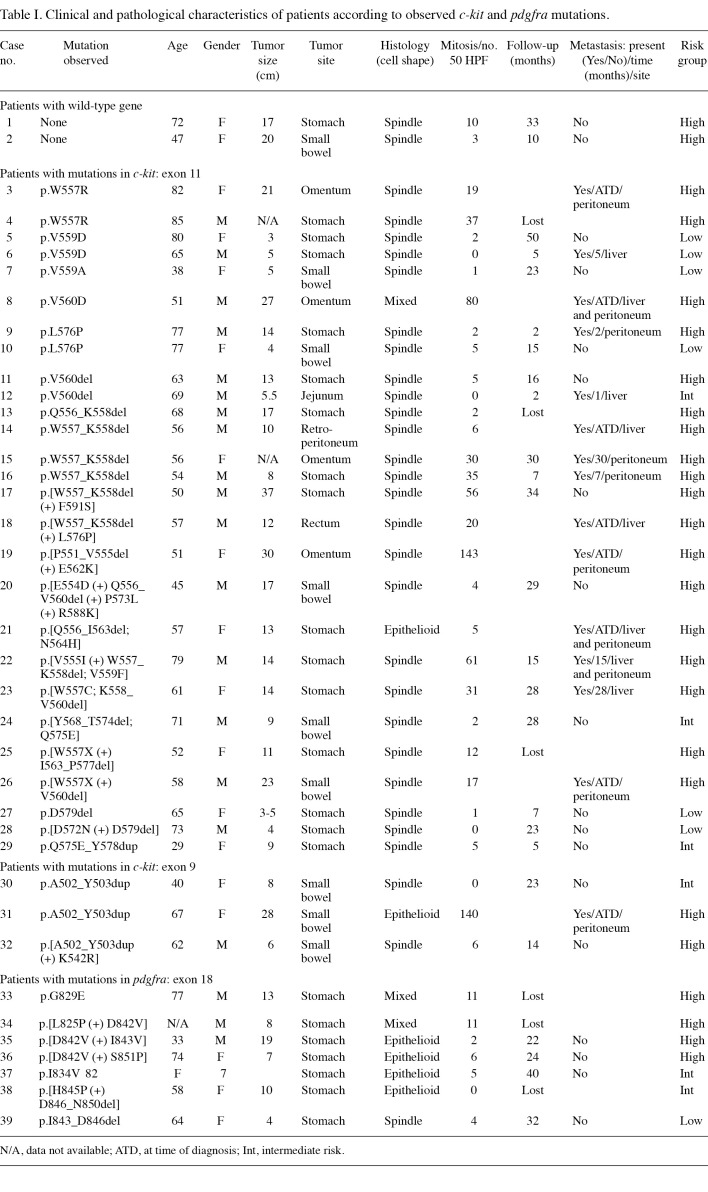
Of the 39 GIST cases examined by molecular studies, 30 cases (77%) of c-kit mutations and 7 cases (18%) of pdgfra mutations were observed. Only 2 cases (5%) were wild-type for the studied c-kit and pdgfra exons (Table I). The majority of c-kit mutations were observed in exon 11 (27 cases, 69%) and in exon 9 (3 cases, 8%). No mutations were observed in exons 13 and 17. High heterogeneity in exon 11 was observed at the 5' region, translating to amino acids 550-560. Across exon 11, we observed 7 simple deletions, 8 simple substitutions, 1 duplication and 11 substitutions, followed or preceded by a deletion (Fig. 1A).
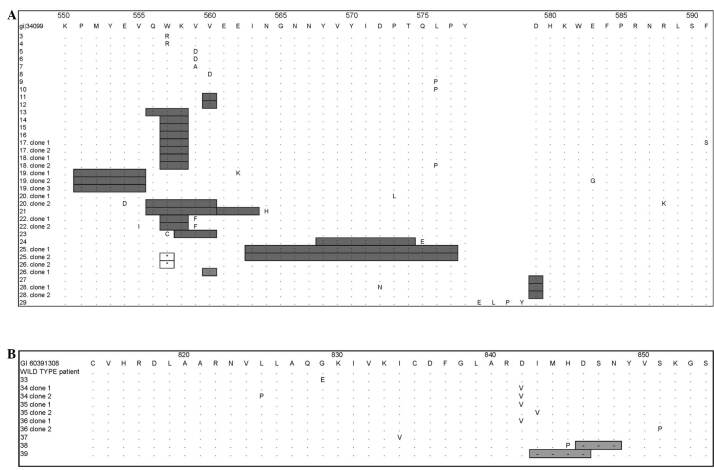
Figure 1. Inferred amino acid sequence showing the type and position of primary tumor mutations within exon 11 in (A) c-kit and exon 18 in (B) pdgfra compared to a consensus reference sequence (gi34099 and gi60391308, respectively, top sequence). Letters in the amino acid sequence for each clone indicate substitutions; insertions are shown by the addition of the appropriate amino acids in the insertion site; boxed asterisks indicate stop codons; gray boxes show amino acid deletions.
Deletions ranging from 1 to 15 codons were observed at the 3' region of exon 11. However, the most common simple amino acid deletion in exon 11 (n=4) was tryptophan and lysine at codons 557 and 558 (p.W557_558Kdel) (patients 13 to 16). Tumors with this type of mutation were all spindle cell‑type and classified as high risk. Patients 17, 18, 20, 21 and 22 also demonstrated deletions over the 557-558 region, but in conjunction with other deletions or substitutions. These patients were all considered to have high-risk GIST and, with the exception of patient 21, had spindle cell tumors.
Mutations resulting in amino acid substitutions were observed throughout the entire exon, but patients with single amino acid substitutions encoded by the c-kit exon 11 exclusively involved codons 557, 559, 560 or 576. These substitutions were p.W557R (n=2), p.V559D (n=2), p.V559A (n=1), p.V560D and p.L576P (n=2), respectively. Patient 29 demonstrated a substitution followed by duplication (p.Q575E_Y578dup) in exon 11.
Exon 9 mutations were identified in 3 cases, all of which consisted of tandem duplication of six nucleotides encoding alanine and tyrosine at codons 502-503. All of these cases were primary tumors from the small bowel.
Mutations of pdgfra were observed in only 7 of the 39 tumors, all in exon 18. Three cases demonstrated a substitution of valine for aspartic acid at codon 842 (p.D842V). The 3 cases were primary tumors from the stomach, 2 epithelioid cell type and 1 was mixed cell‑type. The remaining 4 tumors had deletions or combined deletions and substitutions involving codons 829-851. Notably, weak staining was observed for KIT. At the nucleotide level, 9 of 11 patient samples sequenced for the pdgfra gene had single nucleotide deletions in intron 17, at 42 bp from the beginning of exon 18.
Discussion
This study is the first in Central America to analyze genetic mutations and histopathological characteristics associated with GISTs. A total of 39 archival cases of GIST in Panama were screened for mutations in the c-kit and pdgfra genes. GIST‑associated mutations in c-kit exons 9 and 11 and pdgfra exon 18 that have frequently been commonly observed in other studies were also identified in this study. This finding indicates that routine genetic screening of GIST tumors using standard protocols may be useful in optimizing tyrosine kinase inhibitors and other therapies for cancer patients in Panama. The results obtained for c-kit and pdgfra in GIST also validate our laboratory's developing program of personalized mutational analysis using epidermal growth factor receptor (egfr) and v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (kras) for lung and colon cancers, respectively.
GIST predominantly occurs in patients of middle or older age. Over 90% of GISTs occur in adults over 40 years of age, with a median age at diagnosis of 63 years (21). However, GIST cases have been reported in all ages, including children (1). The incidence rate is not affected by gender, although one study reported a slight predominance of GIST in males (22). In the present study, the age range was between 29 and 85 years, with a mean of 62 years (in 36 of 39 cases). There was no predominance of males (n=20) over females (n=19). No association was found between the incidence rate of GIST and geographic location, ethnicity, race or occupation. However, Tran et al observed a higher incidence rate among males and in individuals of African descent (5).
The most common location of GIST is the stomach (50-60%) and small intestine (30-40%), while 5-10% of GISTs arise in the colon and rectum and 5% are located in the esophagus (23). A large study of 1,765 GIST cases by Miettinen et al revealed that the most common location of GIST is the stomach, followed by the colorectum and small intestine (22). In the present study of 39 cases, the primary tumor location was found to be the stomach (59%), followed by the small bowel (25%), colon/rectum (2%) and omentum/mesenterium (13%). Another study of 1,458 cases found that 51% were located in the stomach, 36% in the small intestine, 7% in the colon, 5% in the rectum and 1% in the esophagus (5). Other less common locations are those outside of the GI tract, which include the mesentery, retroperitoneum and omentum. There are also rare cases reported in the gallbladder, pancreas, liver and urinary bladder (23).
Tumor size and mitotic activity are among the most powerful prognostic factors. Miettinen et al demonstrated that tumor size was the only independent prognostic factor in multivariate analysis for disease-specific survival (22). Additionally, Wong et al demonstrated that mitotic rate was also an independent factor in multivariate analysis (24). In a study analyzing 1,765 cases of GIST, the tumor size varied from 0.5 to 44 cm, with a median of 6.0 cm (22). In the present study, the tumor size ranged from 3 to 37 cm, with the median tumor size nearly double (11 cm) the median size reported by Miettinen et al (22). The mitotic rate observed from tissue sections was <5/50 HPF in 15 cases (38%) and ≥5/50 HPF in 24 cases (62%). According to the consensus report of GIST by Fletcher et al, the malignant potential of GIST depends on the tumor size and mitotic count (17).
In the present study, the malignant risk according to mitotic count and tumor size was low in 7 cases, intermediate in 6 cases and high risk in 26 cases. Other microscopic findings were compatible with previous studies (21,22,25,26). In general, it was observed that the spindle cell type predominated over the epithelioid cell type (77% vs. 15% of the cases, respectively). A combination of the two cell types was found in 8% of the cases.
The immunohistochemistry data revealed that 36 (92%) tumors were strongly positive for KIT (CD117) expression and only 3 (8%) cases had weak KIT staining. Large case studies have shown that in more than 90% of GIST cases KIT and/or CD34 is expressed. However, there are KIT-negative cases of GIST, and in those cases a genetic analysis is a prerequisite (21,25). Epithelioid GISTs biologically differ from spindle cell tumors by having PDGFRA more frequently than KIT mutations (22).
Results of the present study showed that more GIST tumors harbor mutations in the c-kit gene (77%) than in the pdgfra gene (18%), as previously reported by other investigators (27,28). Most c-kit mutations in GIST were found in exon 11; the majority of these mutations involved the proximal section of the juxtamembrane domain (69%) and were diverse in mutation type. Single substitutions in c-kit exon 11 exclusively involved the codons 557, 559, 560 or 576. The most common substitutions were p.W557R (n=2), p.V559D (n=2) and p.L576P (n=2).
The majority (66%) of patients with mutations in exon 11 (residues 550-591) were considered to be at high risk and 75% of patients with mutations specifically within residues 556-560 (exon 11) were considered to have high-risk GIST. The effect of the mutations in this region is possibly to disrupt the hydrophobic (W557, V559 and V560) or electrostatic (Q556) interactions that enable the autoinhibited conformation of KIT (29).
The most common simple deletions in exon 11 were observed at codons 557 and 558 (p.W557_K558del) in patients classified as high risk and exhibiting spindle cell‑type tumors. All 3 patients with this simple mutation had metastases in the peritoneum or liver. Eleven other patients showed other types of deletions or substitutions involving residues 557 and 558. Data were only available for 8 of the patients, but 6 patients from this group also had metastases in the peritoneum or liver (patients 3, 18, 21, 22, 23 and 26). It has been suggested that loss of W557 and K558 may be associated with poor clinical outcome (30). Studies at the protein level have revealed that mutations of these amino acids maintain the KIT receptors constitutively activated, since this specific region corresponds to the domain that regulates dimerization, activation and signal transduction (29,31-33). Patient 24 with mutation p.[Y568_T574del; Q575E] had residues Y568 and Y570 deleted, which are the primary sites of phosphorylation in KIT that initiate protein enzymatic activation as demonstrated in in vitro studies (29). Notably, Y568 and Y570 recruit intracellular proteins src, APS, Sch and CHK, which are associated with the cellular proliferation processes (34-38).
In this study, four patients were identified with mutations p.W557R, p.V559A and p.D579del that were associated with familial GIST (patients 3, 4, 7 and 27) (33,39-41). Notably, in this group, only one mutation, p.D579del, was outside the region that is crucial for protein regulation and activation. Neither this patient nor the patient harboring the conservative p.V559A mutation had high-risk tumors. Mutations p.D579del and p.V559A affect the activity of the intracellular kinase domain (33).
Mutations in exon 9, encoding the end of the extracellular domain of c-kit, are the second most commonly involved. These mutations were associated with small bowel GISTs (3/3 cases). All exon 9 mutations represent insertion/duplication of A502 and Y503, previously associated with GIST (30,42,43). KIT proteins with these mutations exhibit a constitutively activated autophosphorylation in the absence of SCF ligand stimuli (27).
pdgfra mutations were observed in stomach GIST (7/7 cases) with epithelioid morphology in 4 of 7 cases and mixed morphology in 2 of 7 cases. All cases presented mutations in exon 18 (Fig. 1B). Using protein expression experiments, Heinrich et al (18) determined that when PDGFRA harbors mutations p.D842V, p.D842_845H and p.[H845_848N (+) N848P], it activates phosphorylation cascades independently of the presence of the ligand. These authors also found that the pdgfra mutation p.D842V is analogous in protein function to the mutation p.D816V in c-kit. The mutation p.D816V in KIT was also previously identified in mastocytosis tumors (44), in patients with mononuclear cells that have hematopoietic mastocytosis disorders (7) and in germ line cells (45).
In conclusion, this investigation identified new mutations in the c-kit and pdgfra genes, which require future study, along with numerous mutations of c-kit (exons 9 and 11) and pdgfra (exon 18) previously found to be associated with GIST. The sites and types of these mutations maintain receptors KIT and PDGFRA constitutively activated in GIST, as previously reported. Mutations in other exons of c-kit are responsible for the constitutive enzymatic activation of KIT in other diseases (44-49). Our results contribute to understanding the incidence of mutant forms of c-kit and pdgfra in patients with GIST, and have helped us lay the foundations for personalized cancer medicine in Panama.
Acknowledgments
The authors thank the patients and doctors from the Instituto Oncológico Nacional for their assistance in obtaining the samples and clinical data necessary to perform this study, and co-workers from the Department of Genomics and Proteomics of the Gorgas Memorial Institute for Health Studies for their assistance with molecular analysis. This study was partially supported by funds from Novartis Pharma Panama and from the Gorgas Memorial Institute for Health Studies.
Contributor Information
Yaxelis Mendoza, Department of Genomics and Proteomics, Gorgas Memorial Institute for Health Studies, P.O. Box 0816-02593, Panama.
Carlos Singh, Department of Genomics and Proteomics, Gorgas Memorial Institute for Health Studies, P.O. Box 0816-02593, Panama.
Juan Castillo Mewa, Department of Genomics and Proteomics, Gorgas Memorial Institute for Health Studies, P.O. Box 0816-02593, Panama.
Evelise Fonseca, Department of Genomics and Proteomics, Gorgas Memorial Institute for Health Studies, P.O. Box 0816-02593, Panama.
Rebecca Smith, Department of Genomics and Proteomics, Gorgas Memorial Institute for Health Studies, P.O. Box 0816-02593, Panama.
Juan M. Pascale, Department of Genomics and Proteomics, Gorgas Memorial Institute for Health Studies, P.O. Box 0816-02593, Panama
References
- Kim KM, Kang DW, Moon WS, et al: Gastrointestinal stromal tumors in Koreans: its incidence and the clinical, pathologic and immunohistochemical findings. J Korean Med Sci 20: 977-984, 2005. [DOI] [PMC free article] [PubMed]
- Tryggvason G, Gislason HG, Magnusson MK and Jonasson JG: Gastrointestinal stromal tumors in Iceland, 1990-2003: the Icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer 117: 289-293, 2005. [DOI] [PubMed]
- Goettsch WG, Bos SD, Breekveldt-Postma N, Casparie M, Herings RM and Hogendoorn PC: Incidence of gastrointestinal stromal tumours is underestimated: results of a nation-wide study. Eur J Cancer 41: 2868-2872, 2005. [DOI] [PubMed]
- Liegl-Atzwanger B, Fletcher JA and Fletcher CD: Gastrointestinal stromal tumors. Virchows Arch 456: 111-127, 2010. [DOI] [PubMed]
- Tran T, Davila JA and El-Serag HB: The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol 100: 162-168, 2005. [DOI] [PubMed]
- Ikeda H, Kanakura Y, Tamaki T, Kuriu A, Kitayama H, Ishikawa J, Kanayama Y, Yonezawa T, Tarui S and Griffin JD: Expression and functional role of the proto-oncogene c-kit in acute myeloblastic leukemia cells. Blood 78: 2962-2968, 1991. [PubMed]
- Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y and Metcalfe DD: Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci USA 92: 10560-10564, 1995. [DOI] [PMC free article] [PubMed]
- Kimura A, Nakata Y, Katoh O and Hyodo H: c-kit point mutation in patients with myeloproliferative disorders. Leuk Lymphoma 25: 281-287, 1997. [DOI] [PubMed]
- Corless CL, Fletcher JA and Heinrich MC: Biology of gastrointestinal stromal tumors. J Clin Oncol 22: 3813-3825, 2004. [DOI] [PubMed]
- Yarden Y, Escobedo JA, Kuang WJ, et al: Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature 323: 226-232, 1986. [DOI] [PubMed]
- Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U and Ullrich A: Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J 6: 3341-3351, 1987. [DOI] [PMC free article] [PubMed]
- Roskoski R Jr: Structure and regulation of Kit protein-tyrosine kinase – the stem cell factor receptor. Biochem Biophys Res Commun 338: 1307-1315, 2005. [DOI] [PubMed]
- Debiec-Rychter M, Dumez H, Judson I, et al: Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 40: 689-695, 2004. [DOI] [PubMed]
- Heinrich MC, Corless CL, Blanke CD, et al: Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol 24: 4764-4774, 2006. [DOI] [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al: Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347: 472-480, 2002. [DOI] [PubMed]
- Verweij J, Casali PG, Zalcberg J, et al: Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 364: 1127-1134, 2004. [DOI] [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al: Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 33: 459-465, 2002. [DOI] [PubMed]
- Heinrich MC, Corless CL, Duensing A, et al: PDGFRA activating mutations in gastrointestinal stromal tumors. Science 299: 708-710, 2003. [DOI] [PubMed]
- Medeiros F, Corless CL, Duensing A, Hornick JL, Oliveira AM, Heinrich MC, Fletcher JA and Fletcher CD: KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol 28: 889-894, 2004. [DOI] [PubMed]
- Madison D and Madison W: MacClade 4: analysis of phylogeny and character evolution. Version 4.08. Sinauer Associates, Sunderland, Massachusetts, 2001.
- Miettinen M and Lasota J: Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 130: 1466-1478, 2006. [DOI] [PubMed]
- Miettinen M, Sobin LH and Lasota J: Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 29: 52-68, 2005. [DOI] [PubMed]
- Miettinen M and Lasota J: Gastrointestinal stromal tumors – definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 438: 1-12, 2001. [DOI] [PubMed]
- Wong NA, Young R, Malcomson RD, Nayar AG, Jamieson LA, Save VE, Carey FA, Brewster DH, Han C and Al-Nafussi A: Prognostic indicators for gastrointestinal stromal tumours: a clinicopathological and immunohistochemical study of 108 resected cases of the stomach. Histopathology 43: 118-126, 2003. [DOI] [PubMed]
- Hirota S and Isozaki K: Pathology of gastrointestinal stromal tumors. Pathol Int 56: 1-9, 2006. [DOI] [PubMed]
- Singer S, Rubin BP, Lux ML, Chen CJ, Demetri GD, Fletcher CD and Fletcher JA: Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol 20: 3898-3905, 2002. [DOI] [PubMed]
- Hirota S, Nishida T, Isozaki K, Taniguchi M, Nakamura J, Okazaki T and Kitamura Y: Gain-of-function mutation at the extracellular domain of KIT in gastrointestinal stromal tumours. J Pathol 193: 505-510, 2001. [DOI] [PubMed]
- Duensing A, Medeiros F, McConarty B, Joseph NE, Panigrahy D, Singer S, Fletcher CD, Demetri GD and Fletcher JA: Mechanisms of oncogenic KIT signal transduction in primary gastrointestinal stromal tumors (GISTs). Oncogene 23: 3999-4006, 2004. [DOI] [PubMed]
- Mol CD, Dougan DR, Schneider TR, Skene RJ, Kraus ML, Scheibe DN, Snell GP, Zou H, Sang BC and Wilson KP: Structural basis for the autoinhibition and STI-571 inhibition of c-Kit tyrosine kinase. J Biol Chem 279: 31655-31663, 2004. [DOI] [PubMed]
- Martin J, Poveda A, Llombart-Bosch A, et al: Deletions affecting codons 557-558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS). J Clin Oncol 23: 6190-6198, 2005. [DOI] [PubMed]
- Heinrich MC, Corless CL, Demetri GD, et al: Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 21: 4342-4349, 2003. [DOI] [PubMed]
- Kang HJ, Nam SW, Kim H, Rhee H, Kim NG, Hyung WJ, Noh SH, Kim JH, Yun CO and Liu ET: Correlation of KIT and platelet-derived growth factor receptor alpha mutations with gene activation and expression profiles in gastrointestinal stromal tumors. Oncogene 24: 1066-1074, 2005. [DOI] [PubMed]
- Tarn C, Merkel E, Canutescu AA, Shen W, Skorobogatko Y, Heslin MJ, Eisenberg B, Birbe R, Patchefsky A, Dunbrack R, Arnoletti JP, von Mehren M and Godwin AK: Analysis of KIT mutations in sporadic and familial gastrointestinal stromal tumors: therapeutic implications through protein modeling. Clin Cancer Res 11: 3668-3677, 2005. [DOI] [PubMed]
- Linnekin D, DeBerry CS and Mou S: Lyn associates with the juxtamembrane region of c-Kit and is activated by stem cell factor in hematopoietic cell lines and normal progenitor cells. J Biol Chem 272: 27450-27455, 1997. [DOI] [PubMed]
- Price DJ, Rivnay B, Fu Y, Jiang S, Avraham S and Avraham H: Direct association of Csk homologous kinase (CHK) with the diphosphorylated site Tyr568/570 of the activated c-KIT in megakaryocytes. J Biol Chem 272: 5915-5920, 1997. [DOI] [PubMed]
- Kozlowski M, Larose L, Lee F, Le DM, Rottapel R and Siminovitch KA: SHP-1 binds and negatively modulates the c-Kit receptor by interaction with tyrosine 569 in the c-Kit juxtamembrane domain. Mol Cell Biol 18: 2089-2099, 1998. [DOI] [PMC free article] [PubMed]
- Wollberg P, Lennartsson J, Gottfridsson E, Yoshimura A and Ronnstrand L: The adapter protein APS associates with the multifunctional docking sites Tyr-568 and Tyr-936 in c-Kit. Biochem J 370: 1033-1038, 2003. [DOI] [PMC free article] [PubMed]
- Roskoski R Jr: Src protein-tyrosine kinase structure and regulation. Biochem Biophys Res Commun 324: 1155-1164, 2004. [DOI] [PubMed]
- Hirota S, Okazaki T, Kitamura Y, O'Brien P, Kapusta L and Dardick I: Cause of familial and multiple gastrointestinal autonomic nerve tumors with hyperplasia of interstitial cells of Cajal is germline mutation of the c-kit gene. Am J Surg Pathol 24: 326-327, 2000. [DOI] [PubMed]
- Maeyama H, Hidaka E, Ota H, et al: Familial gastrointestinal stromal tumor with hyperpigmentation: association with a germline mutation of the c-kit gene. Gastroenterology 120: 210-215, 2001. [DOI] [PubMed]
- Li FP, Fletcher JA, Heinrich MC, Garber JE, Sallan SE, Curiel‑Lewandrowski C, Duensing A, van de Rijn M, Schnipper LE and Demetri GD: Familial gastrointestinal stromal tumor syndrome: phenotypic and molecular features in a kindred. J Clin Oncol 23: 2735-2743, 2005. [DOI] [PubMed]
- Lux ML, Rubin BP, Biase TL, Chen CJ, Maclure T, Demetri G, Xiao S, Singer S, Fletcher CD and Fletcher JA: KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol 156: 791-795, 2000. [DOI] [PMC free article] [PubMed]
- Wardelmann E, Merkelbach-Bruse S, Pauls K, Thomas N, Schildhaus HU, Heinicke T, Speidel N, Pietsch T, Buettner R, Pink D, Reichardt P and Hohenberger P: Polyclonal evolution of multiple secondary KIT mutations in gastrointestinal stromal tumors under treatment with imatinib mesylate. Clin Cancer Res 12: 1743-1749, 2006. [DOI] [PubMed]
- Furitsu T, Tsujimura T, Tono T, et al: Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest 92: 1736-1744, 1993. [DOI] [PMC free article] [PubMed]
- Tian Q, Frierson HF Jr, Krystal GW and Moskaluk CA: Activating c-kit gene mutations in human germ cell tumors. Am J Pathol 154: 1643-1647, 1999. [DOI] [PMC free article] [PubMed]
- Nakata Y, Kimura A, Katoh O, Kawaishi K, Hyodo H, Abe K, Kuramoto A and Satow Y: c-kit point mutation of extracellular domain in patients with myeloproliferative disorders. Br J Haematol 91: 661-663, 1995. [DOI] [PubMed]
- Hongyo T, Li T, Syaifudin M, Baskar R, Ikeda H, Kanakura Y, Aozasa K and Nomura T: Specific c-kit mutations in sinonasal natural killer/T-cell lymphoma in China and Japan. Cancer Res 60: 2345-2347, 2000. [PubMed]
- Tang X, Boxer M, Drummond A, Ogston P, Hodgins M and Burden AD: A germline mutation in KIT in familial diffuse cutaneous mastocytosis. J Med Genet 41: e88, 2004. [DOI] [PMC free article] [PubMed]
- Miettinen M and Lasota J: KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol 13: 205-220, 2005. [DOI] [PubMed]