Abstract
The present study was undertaken to characterize carbon and iron ion radiation-induced adverse biological effects in terms of toxicity and transformation in vitro. HTori-3 human thyroid epithelial cells were irradiated with 0.3-GeV/n (13.6 KeV/µm) carbon ions and 1-GeV/n (150 KeV/µm) iron ions, both of which represent high-mass, high atomic number (Z) and high-energy particles known as HZE particles, as well as γ-rays. The survival of the irradiated cells was determined by a clonogenic survival assay. The yield of colonies growing in soft agar was used as a surrogate endpoint biomarker for transformation in vitro. The results showed that HZE particles and γ-ray radiations are effective in increasing the yield of anchorage-independent colonies. Based on the relative biological effectiveness (RBE) values in the clonogenic survival assays, 0.3-GeV/n carbon ions and 1-GeV/n iron ions were 2.9 and 2.4 times, respectively, as effective as γ-rays at killing the irradiated HTori-3 cells. At a dose of 200 cGy, 0.3-GeV/n carbon ions and 1-GeV/n iron ions were found to be 3.5 and 7.3 times, respectively, as effective as γ-rays at inducing anchorage-independent growth. These results suggest that the carcinogenic potential of 0.3-GeV/n carbon ions, as represented by the ability to induce anchorage-independent growth, may be lower than that of 1-GeV/n iron ions.
Introduction
As previously reviewed (1), the main components of radiation in interplanetary space are galactic cosmic rays (GCR) and solar cosmic radiation. GCR originate from outside of the Solar System and consist of 2% electrons and 98% baryons, which in turn are composed of 87% protons (hydrogen nuclei), 12% α particles (helium nuclei) and approximately 1% of heavier nuclei with atomic numbers (Z) up to 92 (uranium). The heavier nuclei include highly energetic, heavy and charged particles, also known as HZE particles. Although iron ions, as a specific type of HZE particle, only account for less than 1% of the GCR particle fluxes, they contribute significantly to the total radiation dose received by individual cells exposed to GCR due to the fact that the dose to an individual cell is proportional to the square of the particle's energy dependent effective charge (2). Thus, iron ion radiation is of special interest in space radiation research.
Exposure to space radiation may place astronauts at significant risk of developing both acute and long-term radiation-induced adverse biological effects. Acute effects arising from exposure to solar particle event (SPE) radiation includes radiation sickness (nausea and/or vomiting), skin injury, changes in hematopoietic and immune system functions and fatigue. Exposure to either SPE or GCR radiation results in long-term effects, such as the induction of cancer. Depending on the radiation dose, dose rate and quality, exposure to radiation during space missions may immediately affect the probability for successful mission completion (mission critical) or result in late radiation effects in individual astronauts (1).
Although iron ion radiation is of special interest in space radiation research, carbon ion radiation is more widely used for cancer radiotherapy. Compared to protons, which have increasingly become an accepted part of radiation therapy, carbon ions have higher Linear Energy Transfer (LET) than protons, which allows carbon ions to kill tumor cells more efficiently (3).
Previously, we evaluated the adverse biological effects of γ-rays and iron ions in MCF10 human breast epithelial cells and HTori-3 human thyroid epithelial cells (4,5). The present investigation extended the previous studies using three different types of radiation, i.e., γ-rays and two types of different HZE particles (0.3-GeV/n carbon ions and 1-GeV/n iron ions).
Materials and methods
Cells and cell culture. HTori-3 cells are a human thyroid epithelial cell line immortalized by transfecting primary cultures of human thyroid epithelial cells with an origin-defective SV40 genome. This cell line is not tumorigenic and unirradiated HTori-3 cells form colonies in soft agar with a relatively low efficacy of approximately 0.3% (6). In this study, HTori-3 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 7% fetal bovine serum (FBS). The cells were dissociated by trypsin-EDTA treatment and sub-cultured as required.
Radiation sources and dose. The radiation experiments were performed using a Cesium-137 γ-ray source, which emits 0.662-MeV γ-rays, carbon ions (0.3-GeV/n with LET of 13.6 keV/µm) or iron ions (1-GeV/n with LET of 150 keV/µm) generated by the Alternating Gradient Synchrotron (AGS) at the Brookhaven National Laboratory (BNL).
Cell clonogenic survival assay. The effect of HZE particle radiation on cell survival was evaluated by clonogenic survival assays. HTori-3 human thyroid epithelial cells were used for these experiments. To determine the cell survival levels following radiation exposure, the cells were irradiated with γ-rays, 1-GeV/n iron ions or 0.3-GeV/n carbon ions at a single dose of 0 (sham radiation control), 10, 20, 40, 80, 125, 200 or 400 cGy. After the radiation exposure, the cells were dissociated by treatment with trypsin-EDTA, resuspended in medium, plated in T-25 tissue culture flasks at 320-800 cells per flask for the γ-ray and carbon ion radiation experiments, or 320-2,400 cells per flask for the iron ion radiation experiment, and cultured for 6 days. At the end of the incubation period, the cell colonies were fixed and stained with crystal violet and methylene blue dissolved in 90% ethanol and counted under a dissection microscope. The number of cell colonies was divided by the number of cells plated to calculate the clonogenic survival for each flask. The surviving fraction data were plotted against the radiation doses to calculate radiation sensitivity constants according to the multitarget theory (7) using the equation S = ne-kD, where S is the surviving fraction, n represents the number of targets, -k is the radiation sensitivity constant and D is the dose of radiation (cGy).
Soft agar colony formation assay. The transformation of HTori-3 cells (6) irradiated with γ-ray, iron ion and carbon ion radiation was quantitated by a soft agar colony formation assay that measures the ability of the cells to grow under anchorage-independent conditions. Sham-irradiated cells were included as controls. HTori-3 cells were previously adapted for studies of radiation transformation (8,9). Anchorage-independent growth is a phenotypic change associated with the ability of these cells to form tumors in animals; tumor formation was previously reported within 7-20 weeks after irradiated HTori-3 cells were transplanted into athymic nude mice (8).
To carry out the experiments, HTori-3 cells were irradiated with γ-rays or carbon ions at a single dose of 10, 20, 40 or 200 cGy or iron ions at a single dose of 200 cGy. Sham-irradiated cells were included in each radiation experiment as a control. To determine the yield of anchorage-independent colonies of HTori-3 cells growing in soft agar, irradiated and sham radiation control cells were treated with trypsin and suspended in growth medium containing 0.8% methyl cellulose and plated in 24 multi-well polystyrene plates at a density of 8,000 cells/well. The bottoms of wells were pre-coated with an agar layer prepared by adding 1.8% agar to 2X DMEM without supplements to yield a final agar concentration of 0.9%. The plates were incubated at 37˚C and the medium was changed twice a week. Four or 8 wells per treatment group were stained with Neutral Red at 3-4 weeks after plating.
Data and statistical analyses. The surviving fraction was calculated by dividing the number of colonies counted in tissue culture flasks by the number of cells seeded into the tissue culture flasks. The yield of anchorage-independent colonies was calculated by dividing the number of colonies counted in soft agar by the number of viable cells plated in soft agar.
The mean surviving fraction and the yield of anchorage-independent colonies were calculated for each treatment group and compared among different treatment groups by one-way ANOVA followed by Tukey's test. The statistical analyses were performed using Prism version statistical software (version 2.0; GraphPad Software, San Diego, CA, USA).
Results
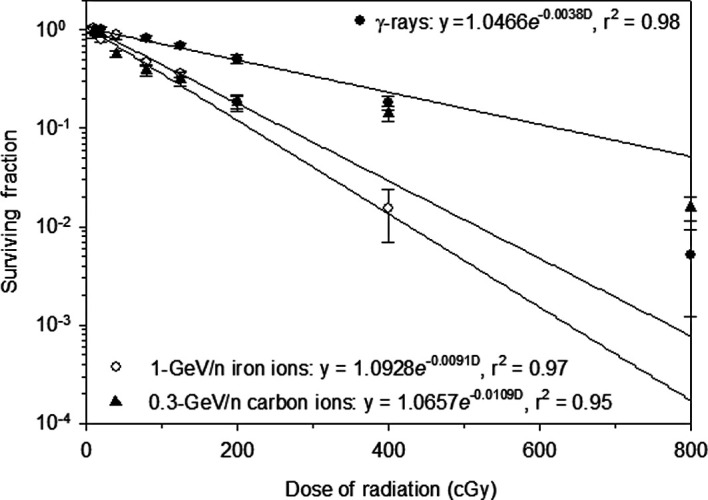
In the present study, the effect of γ-ray and HZE particle radiation on cell survival was determined by clonogenic survival assays using HTori-3 human thyroid epithelial cells. Exposure to γ-ray, carbon ion or iron ion radiation resulted in a dose-dependent decrease in the clonogenic survival of the irradiated HTori-3 cells (Fig. 1). The radiation sensitivity constants (-k) for the cells irradiated with γ-rays, carbon ions and iron ions were 0.0038, 0.0109 and 0.0091, respectively. The corresponding D37 values (1/-k) for the γ-ray, carbon ion and iron ion radiation were 263, 92 and 110 cGy, respectively. These results indicate a RBE value of 2.9 (263/92) for the carbon ion radiation and a RBE of 2.4 (263/110) for the iron ion radiation in the clonogenic survival assay.
Figure 1. Survival curve of HTori-3 cells irradiated with γ-rays, carbon ions or iron ions. HTori-3 cells were irradiated with γ-rays, 0.3-GeV/n carbon ions or 1-GeV/n iron ions at different doses. Each data point is the mean of a minimum of 5 replicate flasks from one experiment. The standard deviation is indicated by the error bars.
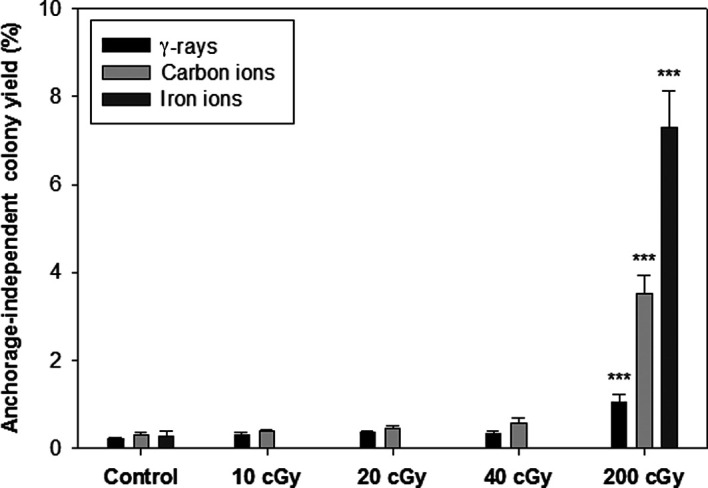
The ability to form colonies in soft agar was regarded as the surrogate endpoint biomarker for the radiation-induced malignant transformation of HTori-3 cells in this study. The yield of anchorage-independent colonies of the sham-irradiated HTori-3 cells and HTori-3 cells exposed to γ-ray or carbon ion radiation at a single dose up to 40 cGy ranged from 0.21 to 0.59%, and the difference did not reach statistical significance (Fig. 2). The yield of anchorage-independent colonies of the HTori-3 cells irradiated with γ-rays, carbon ions and iron ions at a single dose of 200 cGy was 1.0, 3.50 and 7.30%, respectively, which were significantly higher than the yield observed for the respective sham radiation treatment groups (p<0.001).
Figure 2. Soft agar colony formation efficiency of HTori-3 cells irradiated with γ-rays, carbon ions or iron ions. HTori-3 cells were irradiated with γ-rays, 0.3-GeV/n carbon ions or 1-GeV/n iron ions at a single dose of 200 cGy. Each data point is the mean of 8 replicate flasks from one experiment with γ-ray or carbon ion radiation, or 4 replicate flasks from one experiment with iron ion radiation.
Discussion
In a previous study, we evaluated the effect of γ-ray and iron ion radiation on the clonogenic survival of MCF10 human breast epithelial cells and HTori-3 human thyroid epithelial cells (4,5). The present investigation extended the previous study using three different types of radiation, i.e., γ-rays and two types of HZE particles (0.3-GeV/n carbon ions and 1-GeV/n iron ions), to produce cytotoxic effects and induce anchorage-independent growth in HTori-3 cells in vitro. Based on the RBE values determined in the clonogenic survival experiments, 0.3-GeV/n carbon ions and 1-GeV/n iron ions were approximately 2.9 and 2.4 times, respectively, as effective as γ-rays at killing the irradiated HTori-3 cells. The D37 values determined in the present study for γ-rays and 1-GeV/n iron ion radiation were 263 and 110 cGy, respectively, which were similar to the D37 values previously determined for HTori-3 cells (5).
Exposure to γ-ray, carbon ion and iron ion radiation at a single radiation dose of 200 cGy was shown to induce HTori-3 cell transformation, as demonstrated by the increased yield of anchorage-independent colonies observed for the irradiated cells, and the carbon ion and iron ion radiation were found to be 3.5 and 7.3 times, respectively, as effective as γ-rays at inducing anchorage-independent growth in the irradiated HTori-3 cells. It should be noted that anchorage-independent growth was only evaluated when a single radiation dose of 200 cGy was utilized. Thus, the magnitudes of differences observed between the γ-ray radiation and carbon ion or iron ion radiation should not be considered as RBE values for this biological endpoint. Nevertheless, the difference between the abilities of carbon ion and iron ion radiation to induce anchorage-independent growth of the surviving population of the irradiated cells suggests a possible difference in the mechanism(s) by which the different HZE particles affect the carcinogenic process in the irradiated cells. Other investigators noted major differences in the RBE values for transformation induced by radiation from different heavy ions, with the RBEs varying in a LET-dependent manner up to 80-120 keV/µm (10) or 100-200 keV/µm (11). The LET of the carbon ions (13.6 kev/µm) was less than one tenth of that of the iron ions (150 kev/µm) used in this study. The relatively low efficiency of the carbon ion radiation to induce anchorage-independent growth of HTori-3 cells may be related to its low LET.
The efficiency of ionizing radiation to produce a given biological change is known to depend on both the LET of the radiation and the nature of the biological endpoint being affected by the radiation exposure (12). In general, it is expected that for various types of ionizing radiations, including heavy ions, the RBE is likely to increase with LET for cytotoxic, mutagenic and carcinogenic effects, reaching a maximum at approximately 100 keV/µm (11,13). The findings presented for the cytotoxicity results, in which the effects of carbon ions, with a LET of 13.6 KeV/µm, are comparable to those of iron ions, with a LET of 150 KeV/µm, are noteworthy surprising given the expected relationship between RBE and LET. These findings may have particular significance for the use of heavy ions in clinical radiotherapy. Several different types of heavy ions have been used in the clinic for cancer therapy (14). Carbon ions have been generated for use in clinical radiotherapy by the BEVALAC accelerator in Berkeley, CA, USA (14), the Heavy Ion Medical Accelerator in Chiba (HIMAC) at the National Institute of Radiological Sciences in Japan (15,16) and the GSI SIS accelerator in Darmstadt, Germany (17). The clinical use of carbon ions for radiotherapy in the near future is planned for other sites as well. Since 1994, over 3,000 cancer patients have been treated by heavy ions produced by the HIMAC accelerator, and the results have established that carbon ions are one of the most effective radiations for radiotherapy (15,16).
Carbon beams are of great interest in clinical radiotherapy because they have beneficial physical depth-dose properties, making them appropriate for use in tumor therapy, as well as a significant high LET component, which is presumably responsible for their efficacy in tumor cell killing. The results presented here suggest other possible benefits for carbon ion radiotherapy. In particular, carbon ions may have the same beneficial cytotoxic effects in tumor cells as iron ions with less potential for inducing a second malignancy in normal tissues exposed to the carbon ion radiation during cancer radiotherapy.
Acknowledgments
This study was supported by a grant from NASA (NAG9‑1517). The authors thank the staff members of the Brookhaven National Laboratory for their help in the performance of these studies. In particular, they acknowledge the contributions of Dr Adam Rusek and Dr I. Hung Chiang to the research investigations described here.
Contributor Information
Zhaozong Zhou, Department of Radiation Oncology, The University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA.
Jeffrey H. Ware, Department of Radiation Oncology, The University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA
Ann R. Kennedy, Department of Radiation Oncology, The University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA
References
- Hellweg CE and Baumstark-Khan C: Getting ready for the manned mission to Mars: the astronauts' risk from space radiation. Naturwissenschaften 94: 517-526, 2007. [DOI] [PubMed]
- Katz R, Ackerson B, Homayoonfar M and Sharma SC: Inactivation of cells by heavy ion bombardment. Radiat Res 47: 402-425, 1971. [PubMed]
- Jongen Y: Radiotherapy systems using proton and carbon beams. Academie Royale de Medecine de Belgique 163: 471-478, 2008. [PubMed]
- Kennedy AR, Ware JH, Guan J, et al: Selenomethionine protects against adverse biological effects induced by space radiation. Free Rad Biol Med 36: 259-266, 2004. [DOI] [PubMed]
- Kennedy AR, Zhou Z, Donahue JJ and Ware JH: Protection against space radiation induced adverse biological effects by the Bowman-Birk inhibitor and antioxidants. Radiat Res 166: 327-332, 2006. [DOI] [PubMed]
- Lemoine NR, Mayall ES, Jones T, et al: Characteristics of human thyroid epithelial cells immortalized in vitro by simian virus 40 DNA transfection. Brit J Caner 60: 897-903, 1989. [DOI] [PMC free article] [PubMed]
- Casarett AP: Radiation Biology. Prentice-Hall, Inc., Englewood, New Jersey, 1968.
- Riches AC, Herceg Z, Bryant PE and Wynford-Thomas D: Radiation-induced transformation of SV40-immortalized human thyroid epithelial cells by single and fractionated exposure to γ-irradiation in vitro. Intl J Radiat Biol 66: 757-765, 1994. [PubMed]
- Riches AC, Herceg Z, Bryant PE, Stevens DL and Goodhead DT: Radiation-induced transformation of SV40-immortalized human thyroid epithelial cells by single exposure to plutonium alpha-particles in vitro. Intl J Radiat Biol 72: 515-521, 1997. [DOI] [PubMed]
- Hei TK, Komatsu K, Hall EJ and Zaider M: Oncogenic transformation by charged particles of defined LET. Carcinogenesis 9: 747-750, 1988. [DOI] [PubMed]
- Yang TC, Craise LM, Mei MT and Tobias CA: Neoplastic cell transformation by heavy charged particles. Radiat Res 8: S177-S187, 1985. [PubMed]
- Johns HE and Cunningham JR: The Physics of Radiology. Charles C. Thomas, Springfield, IL, 1983.
- Cucinotta FA, Schimmerling W, Wilson JW, et al: Space radiation cancer risks and uncertainties for Mars missions. Radiat Res 156: 682-688, 2001. [DOI] [PubMed]
- Castro JR: Results of heavy ion radiotherapy. Radiat Environ Biophys 34: 45-48, 1995. [DOI] [PubMed]
- Kitagawa A, Muramatsu M, Sasaki N, et al: Multiply charged carbon-ion production for medical application. Review of Scientific Instruments 79: 02C303, 2008. [DOI] [PubMed]
- Tsujii H, Mizoe J, Kamada T, et al: Clinical results of carbon ion radiotherapy at NIRS. J Radiat Res 48: A1-A13, 2007. [DOI] [PubMed]
- Schulz-Ertner D, Haberer T, Scholz M, et al: Acute radiation-induced toxicity of heavy ion radiotherapy delivered with intensity modulated pencil beam scanning in patients with base of skull tumors. Radiother Oncol 64: 189-195, 2002. [DOI] [PubMed]


