Abstract
The three main complications of hepatitis B virus (HBV) infection are chronic active hepatitis (CAH), liver cirrhosis, and hepatocellular carcinoma (HCC). The aim of this study was to identify differentially expressed serum proteins among the three liver complications in patients with HBV infection. Differentially expressed proteins have been shown to be potential biomarkers for disease diagnosis, prognosis and therapy guidance. Two-dimensional polyacrylamid gel electrophoresis (2DE) combined with liquid chromatography tandem mass spectrometry (LC-MS/MS) was performed on sera from CAH, cirrhosis and HCC patients with HBV infection, as well as those obtained from healthy individuals. Of 54 differentially expressed (≥1.5-fold and p<0.05) protein spots, 35 spots were identified by LC-MS/MS. The identified spots correlated to 13 proteins. The proteins included haptoglobolin α-2 and β isoforms, haptoglobin cleaved β isoforms, retinol-binding protein, transthyretin, ficolin, leucine-rich-α-2-glycoprotein, α-1-antitrypsin and clusterin. Of particular interest is the significant increase of haptoglobin α-2 isoforms in HCC patients compared to cirrhosis ones. In contrast, a significant decrease of the isoforms was noted among cirrhosis patients.
Introduction
Hepatocellular carcinoma (HCC) is the seventh most common and third most fatal cancer worldwide (1). More than 50% of HCC cases are related to hepatitis B virus (HBV) infection, a condition responsible for a wide spectrum of clinical manifestations ranging from a healthy carrier state to acute and chronic infection. Chronic HBV infection may result in serious complications, notably CAH, liver cirrhosis and HCC (2). At present, more than 350 million individuals suffer from chronic HBV infection worldwide, of which 20% are likely to develop cirrhosis and/or HCC. HCC usually occurs in a cirrhotic liver. The incidence rate of HCC has been estimated to be 2.5-3% in HBV-infected patients with cirrhosis and 0.5-1% in non-cirrhotic carriers. HCC is up to three times more common in males than in females and is especially prevalent in Eastern Asia and central Africa due to the high frequency of chronic infection with HBV (1). In Iran, the incidence of HCC has yet to be clarified. However, 80% of HCC patients are HBV-positive, rendering this virus the most common cause of HCC in Iran (3).
Differentially expressed proteins are potential biomarkers for disease diagnosis, progression and therapy. Two-dimensional polyacrylamid gel electrophoresis (2DE) followed by mass spectrometry (MS) have been extensively utilized to uncover differentially expressed proteins between different stages of disease, as well as those in healthy individuals, in a wide variety of biological systems and body fluids (4). Cancer cells release a variety of proteins into the extracellular fluids, which may contain biological information on processes related to tumorigenesis. Some of these products can end up in the blood stream and thus serve as potential serum biomarkers (5). In this respect, serum has become the sample of choice in the search for inexpensive and easily applied cancer biomarkers.
The most fatal complication of HBV infection is HCC, which unlike other types of cancer is predictable in HBV-infected patients with cirrhosis who are routinely screened for the disease. α-fetoprotein (AFP) is the only widely used serum HCC biomarker, but has low sensitivity for HCC detection at the early stages (4). Putative serum biomarkers with potential clinical applications for HCC detection in general or detection of disease progression to HCC in HBV‑infected patients have been reported using proteomics technology (6,7). In this regard, among the Chinese population, upregulation of serum HSP 27, HSP 90 and haptoglobin was reported by Feng et al, Sun et al, and Shu et al, respectively, in HCC compared to cirrhosis patients (8-10). C3 and Apo AI were also reported by He et al, for identification of liver inflammation in Chinese chronic HBV patients (11).
It is generally accepted that data obtained from different ethnic backgrounds clarify the importance of currently identified biomarkers in particular demographic contexts, information that may be fundamental for establishing widespread use of biomarkers. No studies investigating differentially expressed proteins among CAH, liver cirrhosis and HCC in Iranian patients with HBV infection are currently available. Accordingly, the aim of the current study was to identify differentially expressed proteins among the three stages of HBV infection.
Materials and methods
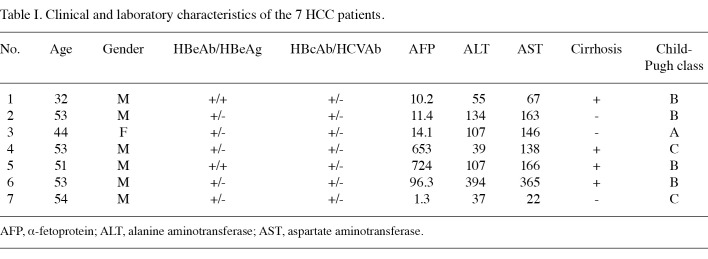
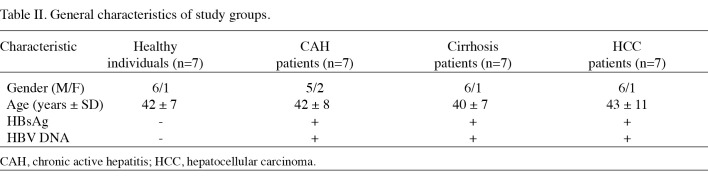
Subjects. Patients (n=21) were recruited from the Gastro-entrohepatology and Liver Transplant Research Centers at the Namazi Hospital in Shiraz, Iran, between September 2007 and April 2009. All 21 patients were HBV positive; 7 patients had CAH, 7 had cirrhosis and 7 had HCC. Patient diseases were confirmed by biochemical, virological, imaging and pathological examinations. The clinical and laboratory characteristics of the 7 HCC patients are shown in Table I. Seven healthy individuals with no history of liver disease, HBV laboratory signals, malignancy or recent or chronic infectious disease were also included in this study. These individuals were of the same age as the patients and resided in the same region (Table II). The study was approved by the Ethics Committee of Shiraz University of Medical Sciences and written informed consent was obtained from each participant prior to sampling.
Table 1. Clinical and laboratory characteristics of the 7 HCC patients.
Table 2. General characteristics of study groups.
Serum samples. A blood sample of 5 ml was drawn from each participant and allowed to clot for 2 h. Blood samples were then spun at 3000 rpm for 10 min and serum was separated, aliquoted and stored at -70˚C until required for testing. To increase serum protein resolution, high-abundant proteins such as albumin and immunoglobulin G (IgG), were depleted from 60 µl of serum by an Arum protein mini-kit (Bio-Rad, Hercules, CA, USA). The protein concentration of depleted sera was determined by the Bradford protein assay using albumin as the standard.
Two-dimensional polyacrylamid gel electrophoresis (2DE). Briefly, approximately 100 µg of proteins were loaded onto immobilized pH gradient (IPG) strips, pH 3-10, linear (Bio-Rad) in the first dimensional isoelectric focusing. Rehydration solution contained 8 M urea, 3% CHAPS, 2% IPG buffer, pH 3-10, 50 mM dithiothreitol (DTT) and trace amount of bromophenol blue. Strips were focused at 80,000 Vh. Focused strips were equilibrated and reduced in 10 ml equilibration buffer [50 mM Tris pH 8.8, 6 M urea, 30% (w/v) glycerol and 2% (w/v) SDS] containing 1% (w/v) DTT for 15 min. The strips were then alkylated in another 10 ml equilibration buffer containing 2.5% (w/v) iodoacetamide for 15 min. Strips were then sealed on top of a 12.5% SDS gel by 0.5% agarose gel electrophoresis. 2DE was performed in the Protean II xi cell (Bio-Rad). Electrophoresis was run at 10 mA per gel for 30 min followed by 25 mA per gel until the tracking dye settled to the bottom of the gels.
Gels were visualized by a complete protocol of a silver staining method for analytical gels. For preparative gels, the method was modified to produce the standard protocol compatible with MS analysis (12).
The silver-stained gels were scanned using a GS-800 calibrated densitometer (Bio-Rad). To determine the differentially expressed proteins the gel images were analyzed by Progenesis software (Nonlinear, Newcastle-upon-Tyne, UK) according to the manufacturer's instructions. Protein spots whose normalized volumes were changed >1.5-fold and p<0.05 were removed from the gels stained with the MS-compatible method.
In-gel digestion of protein spots. The obtained spots were destained with 30 mM potassium ferricyanide and 100 mM sodium thiosulfate (1:1 v/v) and then rinsed with ultra-pure water. The destained gels were reduced twice in 5 mM DTT for 10 min, alkylated in 10 mM iodoacetamide for 40 min and dehydrated twice in 100% acetonitrile (ACN) for 30 min. Digestion was performed in 10 µg/µl of sequencing grade modified trypsin (Promega, Madison, WI, USA) in 50 mM amonium bicarbonate, 5 mM DTT and 30% ACN, at 30˚C overnight. The resulting peptides were extracted with 50 µl of 30% ACN, 50 mM ammonium bicarbonate and 5 mM DTT. The extracted peptides were lyophilized using a vacuum drier.
LC-MS/MS analysis. Lyophilized samples were resuspended in 0.1% formic acid (FA) prior to LC-MS/MS analysis. An Agilent 1100 LC/MSD trap XCT was used for HPLC and MS/MS. Solutions used were water/0.1% FA and ACN/0.1% FA. A trap column (G 1375-87320, 105 mm, 25 µm; Agilent, Germany) was connected to a standard column (Zorbax 300 SB-C18, 3.5 µm, 0.3x75 mm). Peptides (12 µl) were loaded on a trapping column and desalted. The elution program was 2-60% B for 55 min, 80% B for 8 min and re-equlibration of 2% B for 10 min, for a total run of 78 min. The MS was operated in standard scan mode for MS analysis and in ultra scan mode for MS/MS analysis. The MS/MS data were analyzed with Spectrum Mill (Agilent) against the SWISS-PROT database (released May 2010). The filters used after database searching were: peptide score 8, peptide SPI >70% and protein score 10.
Results
In the present study, 21 HBV-positive patients (7 with CAH, 7 with cirrhosis and 7 with HCC) and 7 healthy individuals were compared reagarding their serum proteomes.
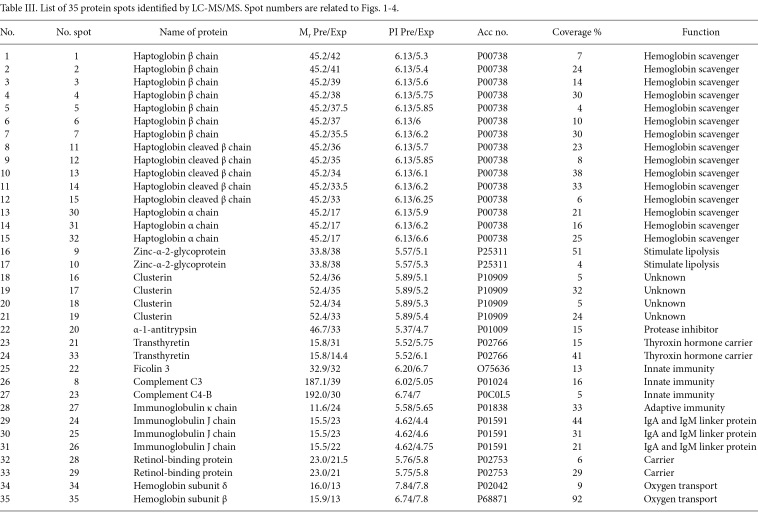
Silver staining of 2D gels revealed more than 300 spots in each gel. Among the four groups of patients, 54 protein spots were differentially expressed (≥1.5-fold and p<0.05). Of these, 35 spots were identified by MS. Their positions in the gels are shown in Fig. 1. Table III indicates the molecular mass (Mr), pI and accession numbers of the identified proteins. The identified spots corresponded to 13 proteins. The proteins HP (15 spots), clusterin (CLU) (4 spots), α-1-antitrypsin (AAT) (2 spots), ficolin 3, transthyretin (TTR) (2 spots), complement C3 and C4-B, Ig κ chain, Ig J chain (3 spots), retinol binding protein (RBP) (2 spots), hemoglobin (Hb)-δ, Hb-β and Zinc-α-2-glycoprotein (2 spots) were differentially expressed among the groups studied (Table III).
Figure 1. Differentially expressed proteins among sera from (A) healthy individuals, (B) CAH, (C) cirrhosis, and (D) HCC associated with HBV infection are labeled on 2D gels. Spot numbers are for cross-reference with Table I and Figs. 2, 3 and 4.
Table 3. List of 35 protein spots identified by LC-MS/MS. Spot numbers are related to Figs. 1-4.
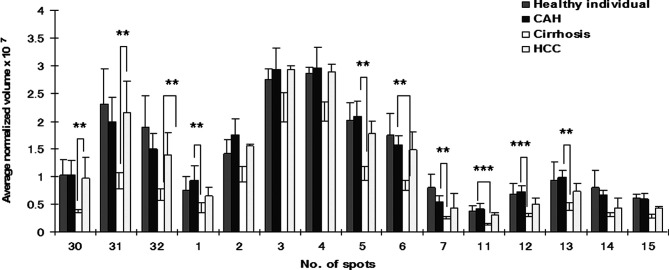
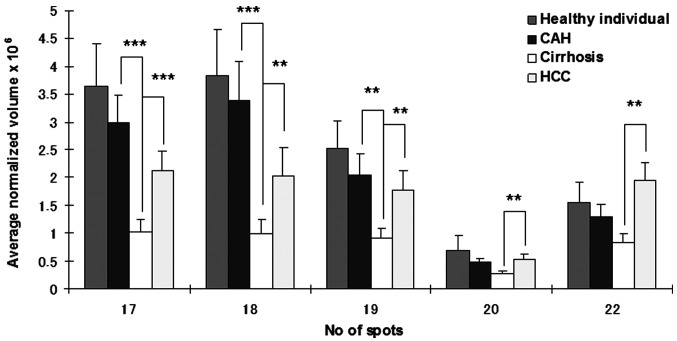
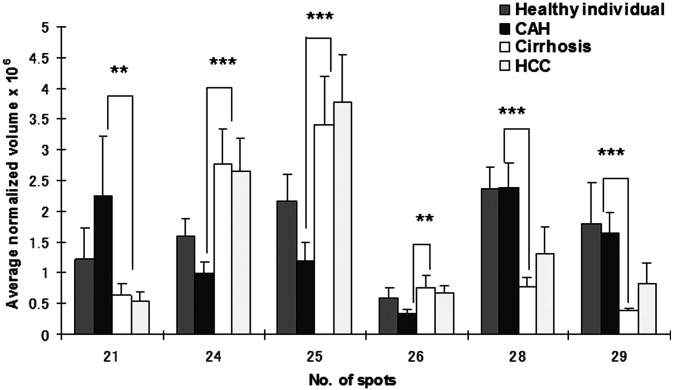
In our study, 15 spots were identified as different isoforms of HP. Three of these were HP-α, HP-β and cleaved β chain. HP-α chain spots were significantly decreased (p<0.05 and >2-fold) between CAH and cirrhosis stages, but conversely increased (p<0.05 and >2-fold) in HCC patients (Fig. 2). HP-β chain spots, although significantly decreased (p<0.05 and >2-fold) in cirrhosis compared to CAH stages, were not significantly increased in HCC patients (only spot Nos. 2 and 5 increased 1.5- and 1.7-fold, respectively). In addition to HP α-2 chains, ficolin, AAT and CLU were differentially expressed (>2-fold) between HBV-HCC and cirrhosis patients (Fig. 3). A differential expression (>2-fold) of Ig J chain, RBP and TTR between CAH and cirrhosis cases was also found (Fig. 4).
Figure 2. Comparison of average normalized volumes of haptoglobin α-2 (spot Nos. 30-32), β (spot Nos. 1-7) and cleaved-β chain (spot Nos. 11-15) spots among healthy individuals, and CAH, cirrhosis and HCC patients. Only spots corresponding to HP α chain were significantly increased (p<0.01 and >2-fold) in the HCC group as compared to cirrhosis patients but HP α chain spots and certain spots of HP β chain including spot Nos. 1, 5, 6, 7, 11, 12, 13 were significantly decreased (p<0.05 and >2-fold) in cirrhosis compared to CAH. ***p<0.001.
Figure 3. Comparison of average normalized volumes of clusterin (spot Nos. 17-19), α-1 antitrypsin (spot No. 20) and ficolin (spot No. 22) among healthy individuals, and CAH, cirrhosis and HCC patients. **p<0.01 and ***p<0.001, respectively.
Figure 4. Comparison of average normalized volumes of transthyretin (spot No. 21), Ig J chain (spot Nos. 24-26) and retinol binding protein (spot Nos. 28 and 29) among healthy individuals, and CAH, cirrhosis and HCC patients. These spots were significantly different (>2-fold) between chronic and cirrhosis patients. **p<0.01 and ***p<0.001, respectively.
Discussion
The 2DE technique has been used for protein separation even in the light of recent developments in off-gel approaches. It remains particularly useful for highlighting post-translational modifications. Genomic and proteomic studies showed certain patterns of specificities among HCCs with different etiologies (13,14). To minimize multifactorial interference in patient groups, our study was limited to patients infected with HBV. Using 2DE-LC-MS/MS, the serum proteomes of healthy individuals and HBV-related CAH, cirrhosis and HCC patients were compared. Results showed that differentially expressed proteins between different stages of a disease are promising diagnostic, prognostic and therapeutic biomarkers.
Isoforms of HP were markedly differentially expressed protein spots. HP is involved in Hb scavenging, inflammatory responses, immune suppression and angiogenesis (15). HP is mainly produced and secreted by liver cells, and partly by intestinal, seminiferous and endometrial epithelia (16). Moreover, it has been reported that HP is produced by cancer cells such as malignant ovarian epithelium, renal cell carcinoma and HCC cells (17). Increased serum HP is frequently reported in cancer, and is thought to be a marker of inflammation and tissue damage, mainly triggered by interleukins such as IL-6 and TNF-α (15). Mounting evidence supports the use of serum HP (either its level or modifications) as a biomarker for cancer diagnosis irrespective of cancer type. HP has also been used to study various liver diseases, including HBV and HCC. Both the amount and oligosaccharide moiety of HP change in liver diseases. It has been reported that the HP serum level changes significantly in cirrhosis and HCC patients. In their study, Ang et al showed alterations of specific HP glycoform sialylation and fucosylation in the sera of HCC patients and suggested that HPs with different degrees of glycosylation are produced by HCC tissue, whereas other HP glycoforms are produced by normal host bodies (17).
HP molecules contain two types of polypeptide chains, β (heavy, 40 kDa) and α (light, α -1, 8.9 kDa or α -2, 16 kDa), resulting in three HP phenotypes, HP1-1, HP 2-1 and HP 2-2 (18). These phenotypes are correlated to susceptibility to various diseases ranging from heart and infectious diseases to cancer. The HP serum level varies among different phenotypes, since the lowest amount is present in HP 2-2 and the highest in HP 1-1 patients (18). Notably, evidence reveals specific profiles of HP-α chain in sera from breast, ovary and head and neck cancers (15,17,19). Our findings showed that three spots of HP α-2 chain were upregulated in HCC patients compared to cirrhosis patients (Fig. 2). Although the cause and consequence of this elevation in HCC is not clear, these isoforms may have a unique biological function and their occurrence may be related to an alteration of unknown intracellular processes. Elevated serum levels of dissociated fragments of HP in HCC, such as HP α-2 chain, were considered to be a factor inhibiting the native HP-Hb complex and affecting the immune cellular response through its potent immunosuppressant activity (17,20). Numerous studies (11,21) are available regarding the increased expression of intact HP in HCC compared to cirrhosis patients. However, in the case of HP α-2 chain, Shu et al recently reported an association between HP α-2 chain and HCC (10).
In contrast to the upregulation of HP α-2 spots in HCC compared to cirrhosis, these spots, together with HP-β spots (intact HP), were significantly decreased in cirrhosis patients compared to those with CAH. In concordance with our data, decreased expression of HP has been reported in HBV- and HCV-related liver cirrhosis (11,22). A negative correlation of HP with the progression of liver fibrosis has been shown, which may be due to the destruction of parenchyma in cirrhotic livers. As observed in experimental fibrosis, an increased expression of hepatocyte growth factor may account for the unexpected decrease in TGF-β reduction, resulting in a decrease in HP (23). It has been reported that HP level is altered in CAH patients compared to healthy individuals, a finding that is also in agreement with our data (Fig. 2) (11).
Other overexpressed spots, of more than 2-fold, in HCC compared to cirrhosis were ficolin, CLU and AAT (Fig. 3). CLU is a glycoprotein involved in numerous biological processes, such as cell death and proliferation (24). In HCC, CLU may play a significant role in tumorigenesis, progression and metastasis (25). Increased serum levels of CLU have been reported in lung cancer, breast cancer, ovarian cancer and HCC (8,24,26-28). Ficolin belongs to the serum lectins and plays a crucial role in innate immunity. Moreover, ficolin specifically binds to apoptotic cells and is involved in the clearance of apoptotic cells (29). The serum level of this protein has been reported to be increased in lung cancer (26), ovarian cancer (30) and melanoma (31) patients. Elevated levels of this protein in our case series have been reported for the first time in HBV-associated HCC patients. AAT is a powerful inhibitor of apoptosis and caspase activation and protects normal tissues during periods of stress, such as inflammation, by inhibiting a number of the proteases that are released from dead cells (32). On the other hand, deficiency of AAT activity has been closely associated with liver diseases (33). Considering that AAT is a single chain protein of 394 amino acids with a molecular mass of 52 kDa, the identified protein in our samples contained only amino acid sequences of the N-terminus region of the molecule and shows a lower Mr (33 kDa), leading to the conclusion that this protein may be an N-terminal fragment resulting from a specific cleavage pathway (34). An increased expression of the AAT fragment has been reported in human cancers of the pancreas, lung, prostate, breast and liver (11,17,26).
In this study, a differential expression (>2-fold) of RBP, TTR, CLU and Ig J chain between CAH and cirrhosis stage was also found (Fig. 4). These molecules may be useful as biomarkers for follow-up of the disease progression from CAH to cirrhosis. Further studies are required to clarify the molecular mechanism involved in these changes.
In conclusion, this study has compared, for the first time, the proteomic profiling of sera from cirrhotic, CAH and HCC patients related to HBV in Iran. Some of our identified proteins were previously known to be differentially expressed at particular stages of liver diseases among other populations, while other findings were novel. Further studies are required to clarify the significance of these molecules as biomarkers for different stages of HBV infection.
Acknowledgments
This study was supported by grants from the Tehran University of Medical Sciences (No. 86-448), Shiraz Institute for Cancer Research (No. ICR-87-503), and Kiban Kenkyu Hi from Yamaguchi University Graduate School of Medicine.
Contributor Information
Jamal Sarvari, Institute for Cancer Research, Shiraz University of Medical Sciences, Shiraz, Iran.
Zahra Mojtahedi, Institute for Cancer Research, Shiraz University of Medical Sciences, Shiraz, Iran.
Yasuhiro Kuramitsu, Department of Biochemistry and Functional Proteomics, Yamaguchi University Graduate School of Medicine, Yamaguchi, Japan.
Seyed-Ali Malek‑Hosseini, Transplant Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
Mahmoud Shamsi Shahrabadi, Department of Virology, Tehran University of Medical Sciences, Hemat Highway, Tehran 1449614535, Iran.
Abbas Ghaderi, Institute for Cancer Research, Shiraz University of Medical Sciences, Shiraz, Iran.
Kazuyuki Nakamura, Department of Biochemistry and Functional Proteomics, Yamaguchi University Graduate School of Medicine, Yamaguchi, Japan.
References
- Yang JD and Roberts LR: Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol 7: 448-458, 2010. [DOI] [PMC free article] [PubMed]
- Elgouhari HM, Abu-Rajab Tamimi TI and Carey WD: Hepatitis B virus infection: understanding its epidemiology, course, and diagnosis. Cleve Clin J Med 75: 881-889, 2008. [DOI] [PubMed]
- Merat S, Malekzadeh R, Rezvan H, Khatibian M: Hepatitis B in Iran. Arch Iran Med 3: 192-201, 2000.
- Sherman M, Peltekian KM and Lee C: Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology 22: 432-438, 1995. [PubMed]
- Sheu JC, Sung JL, Chen DS, et al: Early detection of hepatocellular carcinoma by real-time ultrasonography. A prospective study. Cancer 56: 660-666, 1985. [DOI] [PubMed]
- Hanash S: Disease proteomics. Nature 422: 226-232, 2003. [DOI] [PubMed]
- Pieper R, Gatlin CL, Makusky AJ, et al: The human serum proteome: display of nearly 3700 chromatographically separated protein spots on two-dimensional electrophoresis gels and identification of 325 distinct proteins. Proteomics 3: 1345-1364, 2003. [DOI] [PubMed]
- Feng JT, Liu YK, Song HY, et al: Heat-shock protein 27: a potential biomarker for hepatocellular carcinoma identified by serum proteome analysis. Proteomics 5: 4581-4588, 2005. [DOI] [PubMed]
- Sun Y, Zang Z, Xu X, et al: Differential proteomics identification of HSP90 as potential serum biomarker in hepatocellular carcinoma by two-dimensional electrophoresis and mass spectrometry. Int J Mol Sci 11: 1423-1433, 2010. [DOI] [PMC free article] [PubMed]
- Shu H, Kang X, Guo K, et al: Diagnostic value of serum haptoglobin protein as hepatocellular carcinoma candidate marker complementary to α fetoprotein. Oncol Rep 24: 1271-1276, 2010. [DOI] [PubMed]
- He QY, Lau GK, Zhou Y, et al: Serum biomarkers of hepatitis B virus infected liver inflammation: a proteomic study. Proteomics 3: 666-674, 2003. [DOI] [PubMed]
- Yan JX, Wait R, Berkelman T, et al: A modified silver staining protocol for visualization of proteins compatible with matrix-assisted laser desorption/ionization and electrospray ionization-mass spectrometry. Electrophoresis 21: 3666-3672, 2000. [DOI] [PubMed]
- Ura S, Honda M, Yamashita T, et al: Differential microRNA expression between hepatitis B and hepatitis C leading disease progression to hepatocellular carcinoma. Hepatology 49: 1098‑1112, 2009. [DOI] [PubMed]
- Kim W, Oe Lim S, Kim JS, et al: Comparison of proteome between hepatitis B virus- and hepatitis C virus-associated hepatocellular carcinoma. Clin Cancer Res 9: 5493-5500, 2003. [PubMed]
- Ahmed N, Barker G, Oliva KT, et al: Proteomic-based identification of haptoglobin-1 precursor as a novel circulating biomarker of ovarian cancer. Br J Cancer 91: 129-140, 2004. [DOI] [PMC free article] [PubMed]
- Ye B, Cramer DW, Skates SJ, et al: Haptoglobin-alpha subunit as potential serum biomarker in ovarian cancer: identification and characterization using proteomic profiling and mass spectrometry. Clin Cancer Res 9: 2904-2911, 2003. [PubMed]
- Ang IL, Poon TC, Lai PB, et al: Study of serum haptoglobin and its glycoforms in the diagnosis of hepatocellular carcinoma: a glycoproteomic approach. J Proteome Res 5: 2691-2700, 2006. [DOI] [PubMed]
- Carter K and Worwood M: Haptoglobin: a review of the major allele frequencies worldwide and their association with diseases. Int J Lab Hematol 29: 92-110, 2007. [DOI] [PubMed]
- Di Bisceglie AM and Hoofnagle JH: Elevations in serum alpha-fetoprotein levels in patients with chronic hepatitis B. Cancer 64: 2117-2120, 1989. [DOI] [PubMed]
- Chen CB, Su YC, Huang TT, et al: Differentially expressed serum haptoglobin alpha chain isoforms with potential application for diagnosis of head and neck cancer. Clin Chim Acta 398: 48-52, 2008. [DOI] [PubMed]
- Shu H, Kang XN, Li M, et al: [Comparison of the serum proteomes of pathological stages during hepatocarcinogenesis]. Zhonghua Gan Zang Bing Za Zhi 17: 520-525, 2009. [PubMed]
- Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y and Poynard T: Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 357: 1069-1075, 2001. [DOI] [PubMed]
- Ueki T, Kaneda Y, Tsutsui H, et al: Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med 5: 226-230, 1999. [DOI] [PubMed]
- Rizzi F and Bettuzzi S: The clusterin paradigm in prostate and breast carcinogenesis. Endocr Relat Cancer 17: R1-R17, 2010. [DOI] [PubMed]
- Kang YK, Hong SW, Lee H and Kim WH: Overexpression of clusterin in human hepatocellular carcinoma. Hum Pathol 35: 1340-1346, 2004. [DOI] [PubMed]
- Okano T, Kondo T, Kakisaka T, et al: Plasma proteomics of lung cancer by a linkage of multi-dimensional liquid chromatography and two-dimensional difference gel electrophoresis. Proteomics 6: 3938-3948, 2006. [DOI] [PubMed]
- Redondo M, Villar E, Torres-Munoz J, Tellez T, Morell M and Petito CK: Overexpression of clusterin in human breast carcinoma. Am J Pathol 157: 393-399, 2000. [DOI] [PMC free article] [PubMed]
- Lau SH, Sham JS, Xie D, et al: Clusterin plays an important role in hepatocellular carcinoma metastasis. Oncogene 25: 1242‑1250, 2006. [DOI] [PubMed]
- Kuraya M, Ming Z, Liu X, Matsushita M and Fujita T: Specific binding of L-ficolin and H-ficolin to apoptotic cells leads to complement activation. Immunobiology 209: 689-697, 2005. [DOI] [PubMed]
- Andersen JD, Boylan KL, Xue FS, et al: Identification of candidate biomarkers in ovarian cancer serum by depletion of highly abundant proteins and differential in-gel electrophoresis. Electrophoresis 31: 599-610, 2010. [DOI] [PMC free article] [PubMed]
- Greco M, Mitri MD, Chiriaco F, Leo G, Brienza E and Maffia M: Serum proteomic profile of cutaneous malignant melanoma and relation to cancer progression: association to tumor derived alpha-N-acetylgalactosaminidase activity. Cancer Lett 283: 222-229, 2009. [DOI] [PubMed]
- Van Molle W, Denecker G, Rodriguez I, Brouckaert P, Vandenabeele P and Libert C: Activation of caspases in lethal experimental hepatitis and prevention by acute phase proteins. J Immunol 163: 5235-5241, 1999. [PubMed]
- Durr R and Caselmann WH: Carcinogenesis of primary liver malignancies. Langenbecks Arch Surg 385: 154-161, 2000. [DOI] [PubMed]
- Dengler R, Eger G, Lottspeich F, Plewan A, Ogilvie A and Emmerich B: Proteolytic inactivation of alpha 1-proteinase inhibitor in vivo: detection, characterization and quantitation of the main fragment excreted in the urine of leukemia patients. Biol Chem Hoppe Seyler 373: 581-588, 1992. [DOI] [PubMed]