Abstract
The use of biomaterials, such as hydrogels, as neural cell delivery devices is becoming more common in areas of research such as stroke, traumatic brain injury, and spinal cord injury. When reviewing the available research there is some ambiguity in the type of materials used and results are often at odds. This review aims to provide the neuroscience community who may not be familiar with fundamental concepts of hydrogel construction, with basic information that would pertain to neural tissue applications, and to describe the use of hydrogels as cell and drug delivery devices. We will illustrate some of the many tunable properties of hydrogels and the importance of these properties in obtaining reliable and consistent results. It is our hope that this review promotes creative ideas for ways that hydrogels could be adapted and employed for the treatment of a broad range of neurological disorders.
Keywords: polymer, hydrogel, drug delivery, biocompatibility, biomaterial, nervous system, neurodegeneration
Introduction
Millions of individuals in the United States are affected by neurological disorders (Hirtz et al., 2007). From stroke and traumatic brain injury to Alzheimer’s and Parkinson’s diseases, the causes and manifestations of these disorders vary greatly, as do the impacts and outcomes. Likewise, treatments for these disorders are numerous and diverse; however, very few result in complete recovery and many diseases have limited treatment options and no cure.
One of the reasons for the lack of effective therapies for neurological disorders is the limited regenerative abilities of the central nervous system (Gurgo et al., 2002; Case and Tessier-Lavigne, 2005). For example, in individuals who have suffered a stroke, there is a sustained loss of functional tissue and thus treatments are often limited to rehabilitation of lost functions, such as speech or movement. Similarly, the damage to cells and brain structures associated with neurodegenerative disorders such as Parkinson’s and Alzheimer’s diseases, for the most part, are permanent. Current attempts to replace lost neural cells by implantation in disorders like Parkinson’s disease and stroke have had limited success and enormous variability between implanted patients (Freed et al., 1992; Brundin et al., 2000; Kondziolka et al., 2000; Rabinovich et al., 2005; Suarez-Monteagudo et al., 2009; Ma et al., 2010a). That there have been some successful outcomes provides proof of concept that replacing lost neural cells through neural grafting or implantation can be a viable treatment option. However, the large scale loss of grafted cells in the days following implantation is most likely the biggest contributor to their variable success (Barker et al., 1996; Meyer et al., 1998; Bjugstad et al., 2008; Yu et al., 2009; Redmond et al., 2010). To improve the outcomes of cell implantation into the central nervous system (CNS), there needs to be some method of protecting the neural grafts from the host response to the implant procedure and for guiding graft growth and integration. The incorporation of cells into protective biomaterials could enhance and universalize the success of neural implants.
Biomaterials, such as polymer-based hydrogels, can also be used as delivery tools not only for cell grafting, but also to deliver drugs, viral constructs, DNA, growth factors, and other therapeutics, with precise delivery into a defined brain region and with specified temporal release (Brem, 1990; Brondsted and Kopecek, 1991; Bromberg, 1996; Jewell et al., 2005; Burdick et al., 2006; Bencherif et al., 2009; Liechty et al., 2010; Mansour et al., 2010; Salmaso et al., 2010; Sanchis et al., 2010; Magnusson et al., 2011). Considering the diversity of options polymers provide, their application to neurological disorders may be limited only by the creativity of the researcher (Table 1). For example, a multi-delivery hydrogel could be formulated to help treat pediatric neuronal ceroid lipofuscinoses (Batten’s disease). Batten’s disease results from a lysosomal enzyme deficiency (Wong et al., 2010). Previous attempts to treat Batten’s disease included enzyme replacement therapies, gene therapies to transduce functional gene copies, and neural stem cell implants which produce operative enzymes (Wong et al., 2010). Because it is possible to have control over multiple delivery options at once, it is conceivable that a single hydrogel could incorporate all three treatment options providing immediate enzyme delivery to at-risk host cells, to insert grafted cells with functional enzyme, and to induce host cells to produce the enzyme themselves. Through the functional modification and customization of hydrogels with cells and therapeutics, neural tissue engineering strategies could achieve greater levels of success. This review is designed to engage researchers interested in, or who are currently performing, neural transplantation and tissue engineering, by addressing the defining properties of hydrogels, thus encouraging these researchers to develop their own applications.
Table 1.
Theoretical applications of user-defined hydrogels for the treatment of neurological disorders.
| Neurological Disorder | Theoretical Hydrogel Application |
|---|---|
| Spinal Cord Injury, Parkinson’s Disease |
|
| Stroke, Traumatic Brain Injury, Gliomas |
|
| Traumatic Brain Injury, Stroke, Alzheimer’s Disease, Huntington’s Disease, Parkinson’s Disease |
|
| Down Syndrome |
|
| Glutaric Acidemia |
|
Polymers and Hydrogels
Polymers are large chains of repeating structural units called monomers. By this broad definition, DNA and RNA are considered natural polymers with their repeating monomers of amino acids; and, while it is more common to think of polymers as rubber or plastics, their industrial uses are much broader, including skin creams, toothpastes, contact lenses, and joint replacements. Because polymers are endlessly versatile in their composition and formation, research is currently focused on better understanding polymer properties and potential applications. This includes expanding the library of existing materials to explore novel functions and increased control over material properties. Of particular interest for this review are applications of polymers as biomaterials for treating brain disorders, through neural tissue engineering (Place et al., 2009; Bhatia, 2010; Hunt and Grover, 2010; Pettikiriarachchi et al., 2010; Shoichet, 2010; Gumera et al., 2011; Perale et al., 2011), site specific drug delivery (Brem, 1990; Anseth et al., 2002; Burdick et al., 2006; Liechty et al., 2010; Cooke et al., 2011; Lampe et al., 2011), or as carriers for neural cell implantation (Li, 1998; Schlosshauer et al., 2003; Ford et al., 2006; Hynd et al., 2007; Nicodemus and Bryant, 2008; Hunt and Grover, 2010).
Hydrogels are one type of polymer with chemical and physical properties which make them highly suited for use in biological systems. Hydrogel polymers have a high water content (i.e. >90% water) due to their hydrophilic nature and, because of this, have attracted much attention for cell culture and tissue regeneration. Hydrogels can be pliant and flexible like soft tissues, rigid like cartilage or bone, or elastic to mimic skin or blood vessels (Stammen et al., 2001; Bryant and Anseth, 2002; Fromstein and Woodhouse, 2002; Guan et al., 2005; Guan and Wagner, 2005; Kraehenbuehl et al., 2008; Mithieux et al., 2009; Rnjak et al., 2009; Vanderhooft et al., 2009; Chatterjee et al., 2010; Sung et al., 2010; Yang et al., 2010; Young and Engler, 2011). The extracellular matrix (ECM) of the brain – which provides the macroscopic architecture and supports neural cell survival, migration, and differentiation – is formed from a hyaluronic acid-based hydrogel backbone (Ruoslahti, 1996; Lodish et al., 2007; Bonneh-Barkay and Wiley, 2009; Quirico-Santos et al., 2010). Compared to hydrogels composed of only natural materials, however, synthetic hydrogels can be created to more accurately imitate the physical and mechanical characteristics of the ECM. The advantage of synthetic hydrogels is the ability to tightly control the polymerization, degradation, and biocompatibility of the hydrogel. Additionally, cells, drugs, or other therapeutic agents can be incorporated into the synthetic hydrogel to fill a functional need (Lee and Mooney, 2001; Drury and Mooney, 2003; Peppas et al., 2006; Nuttelman et al., 2008; Slaughter et al., 2009; Hunt and Grover, 2010; Mather and Tomlins, 2010; Sanchis et al., 2010).
Hydrogels most often used in neuroscience applications not only have a high water content but also fall under the definition of chemically cross-linked synthetic hydrogels with viscoelastic properties. Synthetic hydrogels, unlike those based on natural materials such as gelatin, agarose, or Matrigel, are better chemically defined and are biologically inert. This reduces data variability between in vitro studies and decreases potential immunorejection when inserted into the brain. Having viscoelastic properties provides the hydrogel with the ability to retain its polymerized shape and still have the strain resistance of a viscous liquid. Retaining the three-dimensional (3D) shape is an important characteristic for tissue engineering and will be discussed in later sections.
This review will cover properties of hydrogels that we believe to be important considerations before using them in the brain, with a focus on 3D neural tissue engineering. Polymerization, or gelation, is the transitioning process from a liquid to a solid. How a hydrogel polymerizes and how quickly this process happens can determine how the hydrogel can be used. Degradation is the process of breaking the polymer network bonds, resulting in the erosion of the hydrogel and the release of monomer and oligomer precursors. Similar to polymerization, how a hydrogel degrades, and how quickly the process occurs, can determine how the hydrogel is used therapeutically. For example, a fast degrading hydrogel could be used to quickly deliver an immunosuppressive drug to brain tissue for immediate action against the inflammatory response, while a slowly degrading hydrogel could protect encapsulated cells from this same initial inflammatory insult and could provide a longer-lasting physical scaffold for rebuilding tissue. A major benefit of using hydrogels is the ability to incorporate many different types of molecules and to encapsulate in 3D a variety of different cell types. The performance of a hydrogel in brain tissue and the release of cells or therapeutics from the hydrogel depend on the mechanical and chemical properties of the hydrogel and how it is defined during polymerization and degradation. Finally, biocompatibility, specifically within the brain, is an especially important consideration, as the brain is a partially immune-privileged site reacting independently of the peripheral immune system. Biocompatibility also includes the viability and function of cells encapsulated within the hydrogel carrier. It is important to note that hydrogels can behave very differently in vitro and in vivo. For example, in vitro degradation is usually dependent on hydrolysis through unlimited access to water and/or a single enzyme-based degradation (e.g. hyaluronidase), whereas in vivo access to water is limited and multiple enzymes could be present to degrade the hydrogel. Thus researchers should consider translational aspects, from in vitro to in vivo, when designing a hydrogel.
Polymerization
Polymerization is the chemical process by which monomer precursors are reacted to form a polymeric structure. This process can be used to create well-hydrated, covalently cross-linked hydrogels from monomer precursors, such as poly(ethylene glycol) (PEG) or hyaluronic acid (HA) (Sawhney et al., 1993; West and Hubbell, 1995; Burdick et al., 2005; Nuttelman et al., 2008; Waters et al., 2010). (It should be noted that in many cases, including the two examples above, the hydrogel precursors are in and of themselves polymers.) Polymerization generates covalent bonds between monomers, called cross-links (Sawhney et al., 1993; West and Hubbell, 1995). Covalent cross-links allow precise control of the final cross-link density – the number of bonds between monomers in the final hydrogel product. The cross-link density can alter the degradation rate, mechanical properties, and functionality of the hydrogel (Lee and Mooney, 2001). The density of functional groups, such as acrylates, thiols, or polyesters, on the monomer backbone also alters the number and space between cross-links made during the process of polymerization. An increase in the density of functional groups will lead to a greater number of cross-links in the hydrogel and tighter average mesh structure. Alternatively, decreasing the length of the cross-linker can also decrease the average mesh size. In addition to chemical cross-linking, another class of hydrogels can be formed by the physical association of components, often by hydrogen-bonding at the molecular level. Examples of physically cross-linked hydrogels include alginate, agarose, Matrigel, collagen, and a recently described two-component protein hydrogel (Lee et al., 2001; Yang et al., 2009; Uemura et al., 2010; Nunamaker et al., 2011; Roberts et al., 2011).
Hydrogel formation can be temperature dependant (thermopolymerization) (Suggs and Mikos, 1999; He et al., 2000; Jeong et al., 2000b; Tate et al., 2001; Ruel-Gariepy and Leroux, 2004; Nguyen and Lee, 2010), pH-dependent (Ruel-Gariepy and Leroux, 2004; Chiu et al., 2009; Nguyen and Lee, 2010), or it can occur with the exposure to visible or ultraviolet (UV) light (photopolymerization) (Sawhney et al., 1993; Mellott et al., 2001; Nuttelman et al., 2002; Bowman and Kloxin, 2008). Both temperature- and pH-dependant polymerization are forms of physical cross-linking and can be reversible. The benefits of a reversible hydrogel are best demonstrated in the field of diabetes research: a pH-dependent hydrogel was created to release insulin in response to excess glucose and gluconic acid, which alter the pH of the tissue surrounding the hydrogel (Farmer et al., 2008). Thus, this hydrogel formulation allows insulin to be released only when it is needed, just as release would occur in normally functioning tissue. Many temperature- and pH-dependant hydrogels are formulated to gel under physiological conditions, which can be ideal for in situ polymerization (Van Tomme et al., 2008). In this process, the un-polymerized hydrogel solution is injected into the tissue site and subsequently polymerizes in response to the difference in body temperature or pH. This can allow the hydrogel to fill an irregularly shaped lesion site or coat a surface. However, if the conditions during hydrogel injection exceed standard physiological conditions, the hydrogel may not polymerize. If the precursors are not adequately controlled before or during delivery, the materials can have unintended behaviors, such as clogging the syringe and/or causing some discomfort to the patient at the site of injection (Nguyen and Lee, 2010). Hydrogels which polymerize in situ can be limited in their ability to encapsulate cells and/or proteins or may fail to provide a sometimes necessary macroscopic architecture. In contrast, chemically cross-linked synthetic polymers provide researchers with highly tunable materials whose gelation behavior, and therefore physical and chemical properties, can be carefully adjusted with intricate control.
Photopolymerization is a common laboratory process because of the rapid bond formation that occurs when exposed to white or UV light. The addition of a photoinitiator allows the hydrogel to polymerize by generating reactive species that catalyze the polymerization process. UV exposure and the generation of reactive species can be a concern, especially when cells are to be incorporated into the hydrogel, as this can, in some cases, result in decreased viability of the cells (Bryant et al., 2000; Williams et al., 2005; Lampe et al., 2009). The addition of lactic acid, a commonly used functional unit in biomaterials, can mitigate some of the effects of the reactive species on neural stem cells (Lampe et al., 2009; Lampe et al., 2010a). Despite this, the advantages of photopolymerization may override any concerns regarding how the hydrogel is polymerized.
Ex vivo polymerization of such materials allows for the precise control over size and shape of the hydrogel generated. For example, the amorphous shape produced by the in vivo polymerization of a hydrogel would not benefit peripheral nerve regeneration, which needs a strand or tubular shape implanted parallel to the nerve to increase axonal outgrowth (Pearson et al., 2003; Schlosshauer et al., 2003). The tubular shape of the hydrogel implant mimics the Schwann cell column which allows axons to grow protected on the inside of the tube or column (Langone et al., 1995; Danielsson et al., 1996; Weber et al., 2000; Schlosshauer et al., 2003). Additionally, culturing Schwann cells onto a grooved inner surface of a polymer tube further enhances peripheral nerve regeneration by guiding the direction of outgrowth and concentrating the adhesion molecules and growth factors inside the tube adjacent to the desired area of neuronal growth (Nichterwitz et al., 2010).
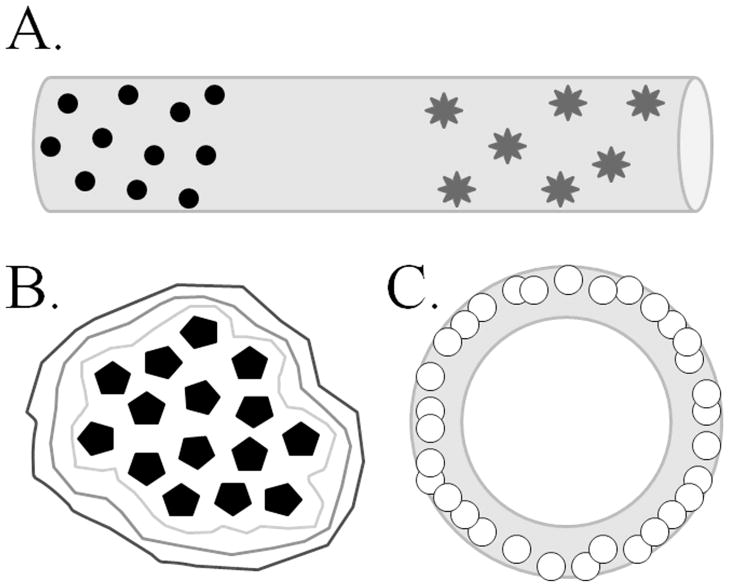
One of the more exciting properties of an ex vivo polymerizing hydrogel is the ability to fabricate a complex or layered hydrogel form (Figure 1, Table 1) (Jessel et al., 2003; Benkirane-Jessel et al., 2004; Michel et al., 2004; Jewell et al., 2005; Mironov et al., 2005; Kim et al., 2007; Wilson et al., 2008; Hu et al., 2009; Lampe et al., 2011). This process can be beneficial if finer control over the spatial or temporal aspects of the hydrogel is desired. For example, in an attempt to control the temporal release of growth factors, Lampe et al (2011), formulated several hydrogel strands in which two distinct microparticle formulations were incorporated into one hydrogel strand. Each type of microparticle carried a different protein and was formulated for a different release rate (Figure 1; see the Drug Delivery section in this review for more details). Some complex hydrogel formations are based on the “layer-by-layer” (LbL) assembly of thin, multiple layered films (Jessel et al., 2003; Benkirane-Jessel et al., 2004; Michel et al., 2004; Jewell et al., 2005; Wilson et al., 2008) (Figure 1). These films can carry DNA, RNA, oligonucleotide constructs, or almost any therapeutic agent for precise spatial exposure of agents to cell surfaces.
Figure 1. Hydrogels can be used to encapsulate cells, microparticles, or therapeutics for multiple delivery purposes.
The hydrogel material should be formulated to provide a specific benefit to the encapsulate or host, such as protection from the immune response for encapsulated cells, spatial seclusion of trophic factors, or temporal release of therapeutic compound. (A) Illustrates the use of a hydrogel strand to deliver two different types of microparticles in a spatially- and temporally-defined manner. Lampe, et al. (2011) employed PLGA-based microparticles loaded with BDNF (circles) or GNDF (stars) with differing release kinetics encapsulated within PEG-based hydrogel strands to demonstrate defined release of protein from the hydrogel into the tissue (see text for more details). (B) Wilson, et al. (2008) used the layer-by-layer formulation technique to encapsulate pancreatic islets (pentagons) within layers of different formulations of hydrogel (encompassing lines). The authors fluorescently labeled the different hydrogel formulations to distinguish layers around the islets. The ability to form multiple unique layers of hydrogel around an encapsulate suggests the potential for each layer to contribute a specific, distinct function for both the encapsulate and the host tissue. (C) Using centrifugal casting methods, Mironov, et al. (2005) used HA-based hydrogel seeded with cadiovascular progenitor cells (small circles) to produce hydrogel tubes lined with cells. The overall shape of hydrogel structures, such as tubes or conduits, allow for the production of specialized tissue structures, such as blood vessels or conduits for axon bundles.
Degradation
Many hydrogels formulated for tissue engineering and/or the release of therapeutics are designed to be biodegradable (or bioresorbable) to reduce the complications of tissue scarring and glia tumor formation from permanent implants, such as those seen around implanted electrodes (Edell et al., 1992; Turner et al., 1999; Szarowski et al., 2003; Biran et al., 2005).
The process of hydrogel degradation is the cleavage of liable bonds within the polymer and the monomer components, resulting in smaller chemical groups which can be cleared from the implant site. Often, the degradation of a hydrogel occurs through the hydrolytic or enzymatic cleavage of bonds made during polymerization (Sawhney et al., 1993; Metters et al., 2000; Anseth et al., 2002; DuBose et al., 2005; Nicodemus and Bryant, 2008; Lampe et al., 2010a; Patterson and Hubbell, 2010). The addition of degradable monomeric units, such as poly(α-hydroxy acids) (i.e. poly(dl-lactic acid) and poly(glycolic acid)), and poly(ε-caprolactone), allow the hydrogel monomers to separate when the bonds are cleaved by water (Sawhney et al., 1993; West and Hubbell, 1995; Ha et al., 1997; Anseth et al., 2002; Nicodemus and Bryant, 2008; Nuttelman et al., 2008; Bencherif et al., 2009; Lampe et al., 2009; Lampe et al., 2010a). In hydrogels with poly(α-hydroxy acids), the ester bonds between poly-lactic or poly-glycolic acid units are hydrolyzed, leading to the production of the simple products of lactic or glycolic acid (Sawhney et al., 1993; Mahoney and Anseth, 2006; Lampe et al., 2009). Hydrogels can also be engineered with enzyme specific cleavage sites by adding sequences of specific peptides between macromers. This allows hydrogel degradation in the presence of an appropriate, tissue specific enzyme (Brondsted and Kopecek, 1991; West and Hubbell, 1998; Lutolf et al., 2003; Rice et al., 2006; Nicodemus and Bryant, 2008; Nuttelman et al., 2008; Lin and Anseth, 2009; Kloxin et al., 2010; Patterson and Hubbell, 2010). For example, Rice, et al. (2006), formulated hydrogels with poly(caprolactone) units which, in addition to hydrolysis, were enzymatically degraded upon addition of exogenous lipase. This allowed the authors to design the hydrogel chemistry with a degradation rate which matches the rate of production of ECM components by encapsulated chondrocytes (Rice et al., 2006; Rice and Anseth, 2007). This type of degradation is often the focus for cell-mediated degradation, especially by encapsulated cells, and cell-specific controlled release of bioactive molecules (West and Hubbell, 1998; Lutolf et al., 2003; Rice and Anseth, 2007; Nuttelman et al., 2008; Katz and Burdick, 2009; Kloxin et al., 2010; Zhu, 2010). Cell-mediated enzymatic degradation may be helpful to encapsulated neural cells which could preferentially degrade the hydrogel material at sites of neurite extension, allowing for increased neuritic growth and pathway formation. A hyaluronic-acid based hydrogel developed by Park, et al. (2010), was designed with matrix metalloproteinase (MMP) sensitivity which allows MMPs produced by encapsulated cells to degrade the hydrogel scaffold. Along with an IKVAV (Ile-Lys-Val-Ala-Val)peptide sequence and brain-derived neurotrophic factor (BDNF), these hydrogels allowed for scaffold remodeling by encapsulated mesenchymal stem cells and stimulated neuronal differentiation and neurite outgrowth (Park et al., 2010). Finally, the rate at which a hydrogel degrades over time can be determined in part by the number of degradable units incorporated by the researcher; typically, the greater number of degradable moieties, the faster the rate of degradation (Sawhney et al., 1993; West and Hubbell, 1995; Anseth et al., 2002; Nuttelman et al., 2002; Rice et al., 2006; Katz and Burdick, 2009; Lampe et al., 2010a; Ma et al., 2010b; Patterson and Hubbell, 2010).
Hydrogel degradation can occur via surface or bulk erosion (Göpferich, 1996; Burkersroda et al., 2002). The type of degradation which occurs is dependent on the amount of incorporated degradable units, cross-link density, and access of water and/or enzymes to the interior of the hydrogel (Burkersroda et al., 2002). Surface degradation occurs when water and enzymes cannot penetrate the interior of the hydrogel, due to high cross-link density or limited access to cleavage points, forcing the surface or exterior bonds to cleave first. Conversely, bulk erosion is degradation that occurs essentially homogeneously throughout the hydrogel, with interior and exterior bonds being cleaved simultaneously. Bulk erosion is common in hydrogels because of their high internal water content and fast diffusion and is even more likely to occur in hydrogels with a lower cross-link density and those with a greater amount of degradable polymer (Göpferich, 1996; Burkersroda et al., 2002). In cases of nerve repair where the structural support is necessary even in a degradable hydrogel, surface erosion can be preferred, as it will allow the hydrogel to continue acting as a conduit scaffold (Subramanian, et al., 2009). Similar to polymerization, the rate of degradation and process by which it occurs influences mechanical properties of the hydrogel and will be further discussed in the next section.
As suggested earlier, degradation can be especially important in decreasing the immunological response to an implanted hydrogel. The temporary presence of a biodegradable hydrogel is most often preferable to a permanent implant, which can encourage a lasting immune response and glial scarring. When designing a hydrogel for a specific application, the degradation rate can be imperative to both the function of the hydrogel and the response of a host. As a scaffold, a slower degradation rate gives the cells the time to develop their own extracellular matrix and to extend processes and reintegrate into the host neural circuitry. However, faster clearance of the hydrogel can be beneficial in vivo, as quick degradation contributes to a reduced immune response. It is necessary to balance the benefit of a hydrogel as a scaffold for developing cells and tissues with the response of a host to a foreign body/material.
Mechanical Properties and Physical Architecture
Mechanical and physical features which are helpful to consider when constructing a hydrogel include the strength and stiffness, the mesh size and porosity, the overall architecture, and physical dimensions of the hydrogel. Many of these characteristics can significantly contribute to the effectiveness of the hydrogel, from cytocompatibility with encapsulated cells, to biocompatibility within tissues, and how therapeutic agents are released from the hydrogel.
The mechanical environment with which cells may interact can affect the viability and behavior of the cells, thus, the use of a hydrogel with specific mechanical properties can affect the function of encapsulated cells or the subsistence of a hydrogel in tissue (Bryant and Anseth, 2002; Flanagan et al., 2002; Engler et al., 2006; Kraehenbuehl et al., 2008; Comolli et al., 2009; Chatterjee et al., 2010; Lampe et al., 2010b; Young and Engler, 2011). As mentioned above, the chemical properties of a hydrogel can determine the cross-link density, which in turn contributes to defining the mechanical and physical properties of a hydrogel – specifically the stiffness of the polymerized product (Figure 2) (Peppas et al., 2006; Vanderhooft et al., 2009; Zhou et al., 2011). The compressive modulus is a measure of mechanical strength or stiffness of a hydrogel and can be most easily varied by changing the percent composition of monomers to directly affect cross-link density (Anseth et al., 1996; Anseth et al., 2002). Alternatively, cross-link density, and thus compressive modulus, can be modified by changing the molecular weight of the monomer or varying the total amount of cross-linker (Sawhney et al., 1993; Anseth et al., 1996; He et al., 2000; Martens et al., 2003; Lin and Anseth, 2009).
Figure 2. Sample parameters for selected hydrogel properties as a function of time (t).
Compressive modulus (solid line) is a measure of the hydrogel strength and increases as the hydrogel polymerizes. During polymerization, cross-links form between monomers, increasing the cross-link density (dotted line). The number of cross-links declines as the bonds are hydrolyzed or cleaved enzymatically during degradation. This also results in a decrease of compressive modulus. The time to degradation (t) is dependent on the chemical composition of the hydrogel and physical properties, such as the incorporation of pores. In most hydrogels, the compressive modulus remains constant during the time between polymerization and degradation. The figure illustrates an example of some sample rates – actual rates depend on a myriad of factors, including chemical and physical properties, incorporations, and external environment.
In general, a higher cross-linking density produces a more rigid hydrogel, such as one that might be suitable for re-engineering bone, while fewer cross-links make for softer hydrogels that are ideal for brain and other soft tissues. When using hydrogels for the engineering of specific tissues, variations in compressive modulus can be critical to how well the hydrogel functions. For instance, using primary neuronal cell cultures, Lampe et al. (2010b), found that the cells survived better in softer hydrogels, with a compressive modulus less than or equal to 3.8kPa, compared to cells in hydrogels with a stiffer compressive modulus (>19kPa). The compressive modulus for brain tissue observed by Lampe et al. and others (Lampe et al., 2010b; Seidlits et al., 2010), ranges from 2.6–5.7kPa. In contrast, a study by Chatterjee, et al. (2010) using osteoblasts, demonstrated that osteogenesis was improved when the cells were grown in hydrogels with a higher compressive modulus, similar to that of mineralizing bone. Tissues such as bone can have a compressive modulus from 100–300kPa, and as high as 1.5×107kPa (Cowin and Doty, 2007; Moffat et al., 2008). These studies suggest there is no single mechanical strength which works best for all cell types and that the stiffness of the culture environment needs to be tuned to the cell type for optimal performance (Figure 3). More specifically, with regards to neural cell types, neurons prefer to grow on substrates with lower compressive moduli (0.1–1.0kPa), compared to relatively stiffer surfaces (0.5–10kPa) preferred by astrocytes and oligodendrocytes; meanwhile the compressive properties ideal for neurite branching and extension are even more limited (Figure 4) (Flanagan et al., 2002; Engler et al., 2006; Georges et al., 2006; Brännvall et al., 2007; Saha et al., 2008; Banerjee et al., 2009; Hynes et al., 2009; Seidlits et al., 2010). The impact of these studies indicates that finely controlled specificity of the mechanical properties of the hydrogel environment is especially important in directing neural cell lineage, proliferation, and growth. To add a final consideration, the compressive modulus of a hydrogel decreases as a function of degradation and the loss of cross-links, resulting in a loss of mechanical integrity (Figure 2) (Metters et al., 2000; Anseth et al., 2002; Hawkins et al., 2011). This can be beneficial if the hydrogel is designed to degrade on the same time scale during which the encapsulated cells produce their own ECM to replace the tissue, theoretically allowing the cells to create their own mechanically robust ECM as the hydrogel degrades.
Figure 3. Survival and fate of peripheral cell types as a function of biomaterial stiffness.
This is a general summary of results obtained across a variety of biomaterials, therefore it is important to remember that biomaterial composition can also have an effect on cell survival and proliferation. (A) Chondrocytes appear to grow on a broad range of stiffnesses, however, the differential expression of collagens and ECM components (ie. aggrecan, glycosaminoglycans) could be a function of the stiffness of the biomaterial (Bryant and Anseth, 2002; Nettles et al., 2004; Chung et al., 2006; Lin et al., 2011; Nguyen et al., 2011). (B) Based on available information, fibroblasts may need a less stiff biomaterial for survival and proliferation, compared to studies that have used chondrocytes (Shu et al., 2004; Burdick et al., 2005; Shu et al., 2006). Based on two in vivo studies, encapsulated fibroblasts inserted subcutaneously survive and produce ECM components in biomaterials with both softer and stiffer characteristics (Shu et al., 2004; Shu et al., 2006). (C) Mesenchymal stem cells (MSC) also appear to survive and proliferate better on softer materials, however, changes in stiffness alters the MSC differentiation. In softer materials, MSC begin to express neuronal markers. As the stiffness increases, MSC may begin to express adipogenic or myogenic markers in addition to increasing their expression of factors important to angiogenesis (i.e. VEGF). At the higher range of stiffness, MSC express osteogenic markers (Engler et al., 2006; Chung et al., 2009; Chung and Burdick, 2009; Seib et al., 2009; Huebsch et al., 2010; Quinchia Johnson et al., 2010; Jha et al., 2011).
Figure 4. Survival and fate of neural stem cells as a function of biomaterial stiffness.
The fate of neural stem cell (NSC) populations grown on (2-dimensional) or in (3-dimensional) a polymer change as result of varying mechanical properties. Neural cells do not survive well in a biomaterial that is very soft (less stiff) or on a very stiff material. However, those which do survive at the lower stiffness tend towards a neuronal cell fate, whereas astrocytes develop more predominantly at higher stiffness. Neuritic extension is also best observed when the stiffness is lower, but NSC migration is more optimal at slightly higher stiffness (Flanagan et al., 2002; Engler et al., 2006; Mahoney and Anseth, 2006; Saha et al., 2008; Hynes et al., 2009; Lampe et al., 2010a; Lampe et al., 2010b; Seidlits et al., 2010). (A) Post-natal day 6 rat neural stem cells (NSC) developed into >50% neurons, ~25% astrocytes, ~15% oligodendrocytes, and ~10% remained undifferentiated (nestin +) at this approximate level of stiffness (Brännvall et al., 2007). (B and C)Sensory spinal neurons had long neurite extensions but branching was favored on the less stiff material (B) compared to the more rigid material (C). Astrocyte survival was poor on both types of materials (Flanagan et al., 2002). (D–F) Embryonic day 13.5 midbrain-derived NSC, at 24 hours post-encapsulation, had ~54% survival with spheres of mixed populations of neuronal and astrocytic cells (D), ~47% survival (E), and ~31% survival (F), with segregated spheres of neuronal and astrocytic cells. At 21 days, no NSC were surviving in the stiffer hydrogel composition (F) (Seidlits et al., 2010).
Additional mechanical measures include elasticity and stretch, measured by the tensile, or Young’s, modulus, and the ability to withstand straining or shearing forces, measured by the shear modulus. The shear and Young’s moduli, along with the compressive modulus, are measures of the elastic properties of the material as defined by Hooke’s law (“Hooke’s Law, Encyclopædia Britannica). Mimicking these mechanical properties in the creation of a hydrogel allows tissue engineers to imitate peripheral tissues, such as skin or blood vessels, which are frequently exposed to these types of mechanical stresses (Pailler-Mattei et al., 2008; Ueki et al., 2010; Yang et al., 2010). In a study which exemplifies the unique tunable characteristics of hydrogels, HA and PEG-based hydrogels designed by Young and Engler (2011) were designed to become stiffer (an increase in elastic modulus) over time in a manner similar to the temporal change in stiffness observed in developing heart muscle. The change in elastic modulus from ~0.5kPa, similar to mesoderm tissues, to ~10kPa, similar to mature heart muscle, induced immature cardiac cells to differentiate into mature cardiomyocytes (Young and Engler, 2011). In adult brain tissue, which is not routinely exposed to stretching and shearing stresses except under extreme conditions, the compressive modulus may be the more critical mechanical characteristic to mimic in the hydrogel. Compressive changes in the brain as a whole can occur with even mild brain trauma, neuroinflammation, and even hypertension (Broder, 2010; Yuh and Dillon, 2010). Highly localized compressive changes can also occur during neural cell migration, which requires remodeling of the ECM (Cayre et al., 2009).
Mesh size describes a nanoscopic physical characteristic which arises from the chemical features of the hydrogel described previously and contributes to mechanical properties, such as hydrogel stiffness. It is defined as the distance between the cross-link points in a hydrogel and is typically measured in angstroms (Å), with sizes sometimes ranging from 10 to 150Å (Bryant and Anseth, 2002; Lin and Anseth, 2009; Lampe et al., 2010b; Lin et al., 2011). Mesh size is especially important to consider when encapsulating cells into a polymer matrix because the cells must be able to exchange nutrients and wastes with the external tissue across the polymer border. Additionally, diffusion rates depend on the size of the molecule and the mesh size of the hydrogel – the smaller the mesh size, the slower diffusion occurs, and vice-versa. The ability for fluids and small molecules to move in and out of a hydrogel depends initially on diffusion, while degradation plays a role in the later movement of larger molecules and cells (Jeong et al., 1997; Bjugstad et al., 2010). Water, a molecule of about 2Å, easily diffuses in and out of hydrogels thus increasing the rate of hydrolysis and contributing to the bulk erosion of the hydrogel (Sawhney et al., 1993; Burkersroda et al., 2002). However, the diffusion of larger molecules, such as proteins – specifically neurotrophic factors – may be limited if a hydrogel is not designed with a suitable mesh size for their free diffusion to cells encapsulated within a hydrogel.
The macroscopic architecture of a hydrogel, such as the incorporation of interconnected pores and the overall shape and size, can determine its function within a tissue. The incorporation of pores in a hydrogel can be an important factor to consider, as some cells and tissues rely on larger spaces within the hydrogel to function properly. While the terms mesh size and pore size (or porosity) are sometimes used interchangeably, pores are defined by their larger size, typically in the micron (μm) range in diameter (Figure 5) (Guan et al., 2005; Keskar et al., 2009b; Namba et al., 2009; Spiller et al., 2010; Lin et al., 2011). Pores can occur naturally depending on hydrogel chemistry (such as molecular weight of monomer precursors) (Lin et al., 2011), or can be created intentionally. The creation of pores can be achieved by constructing a complex hydrogel with regions of polymer with faster degradation kinetics than the rest of the hydrogel, resulting in interconnected pockets within the hydrogel (Namba et al., 2009; Spiller et al., 2010), while other processes include much more complex methods of fabrication (Guan et al., 2005; Stachowiak et al., 2005; Katz and Burdick, 2009; Keskar et al., 2009a; Keskar et al., 2009b). The purposeful incorporation of pores into the hydrogel architecture can serve to drive cell-specific growth and differentiation (Gerecht et al., 2007; Keskar et al., 2009b). For example, neurite extension can be guided by constructing hydrogels with interconnected pores that allow for growth before the hydrogel degrades (Namba et al., 2009; Zhong et al., 2010). This type of polymer architecture may contribute to developing hydrogels for the purpose of neural circuit reconstruction. Similarly, many tissue engineering hydrogel applications, such as producing skeletal or cardiovascular tissues, require the in-growth of host blood vessels to achieve incorporation of the cell-loaded hydrogel into the tissue and to provide oxygen and nutrients to encapsulated cells (Druecke et al., 2004; Ford et al., 2006; Keskar et al., 2009b). Superporous hydrogels, like those developed by Keskar, et al. (2009a; 2009b), demonstrate this permeability by illustrating the in-growth of vascular structures and blood cells within the interconnected pores of the hydrogel.
Figure 5. Hydrogel pore size changes as a function of precursor molecular weight.
Lin, et al. (2011) has demonstrated variation in hydrogel pore size based on the molecular weight of the polymer constituents. Using polyethylene glycol diacrylate (PEGDA) monomer precursors with molecular weights of 3.4kDa (A, box is magnified in a), 6kDa (B, box is magnified in b), 10kDa (C), and 20kDa (D), the authors produced hydrogels with pores (arrows) ranging in size from 9–13μm for hydrogels made from 3.4kDa PEGDA (A), to 38–42μm for 20kDa PEGDA hydrogels (D). The authors observed a similar increase in the nanoscopic property of mesh size depending on molecular weight (45.1Å for 3.4kDa hydrogels and up to 130.9Å for 20kDa hydrogels). These scanning electron microscopy images are of freeze-dried PEGDA hydrogels. It would be unwise to assume that the physical characteristics of the dried hydrogel resemble that of a fully hydrated hydrogel. A fully hydrated hydrogel would be swollen with water and have an amorphous and fibrillary internal meshwork. It is possible that the pore sizes, once hydrated, would be considerable smaller. Scale bar represents 50μm. The above image has been modified from the original by inserting magnification boxes (a, b) to better appreciate the smaller pore sizes. Reproduced with kind permission from Springer Science+Business Media: Pharmaceutical Research, Influence of Physical Properties of Biomaterials on Cellular Behavior, vol. 28, 2011, p.1426, Lin, S., et al.
The overall shape and size of a hydrogel should also be considered. The majority of hydrogels can be formed in any shape desired For example, a hydrogel could be used to fill the amorphous shape of a lesion site, such as a stroke cavity in the brain (Tate et al., 2001; Emerich et al., 2010; Zhong et al., 2010), by implanting before polymerization or by using a hydrogel with very low compressive modulus (and therefore less “solid”). In contrast, a hydrogel formed as a strand could be used to link two remote regions to reconstruct a neural circuit, such as the nigrostriatal pathway in Parkinson’s disease (Lampe et al., 2011). Similarly, the hydrogel strand could be used as a bridge across a glial scar to help repair a spinal cord injury (Hejcl et al., 2008). Size is also important in the brain because of the limited open space within the tissue and the confines presented by the skull. For example, a large volume hydrogel could negatively impact the brain by increasing pressure in the tissue surrounding the implanted hydrogel. Swelling of a hydrogel is an additional consideration in vitro because unconstrained hydrogel degradation results in an increase in the overall size of a hydrogel. Hydrogel studies often describe swell ratios to give an idea of the water and fluid uptake of a hydrogel during degradation, as swell ratio can be a contributing factor to defining degradation rate. Swelling in the brain, however, is less of a concern since the counter-forces of the surrounding tissue and relatively slow degradation confines the increase in overall size due to swelling (Lampe et al., 2011). Size and shape can also impact the biocompatibility of a hydrogel, because increased surface area can contribute to a greater tissue-material interface which increases accessibility to the hydrogel for inflammatory and immune cells (Anderson and Shive, 1997; Fournier et al., 2003).
Drug Delivery
One way to increase the effectiveness of a hydrogel is to incorporate condition-specific drugs or molecules which can function to sustain encapsulated cells or provide support for the tissue surrounding an implanted hydrogel. These can include growth factors and differentiating factors to specify the fate of encapsulated stem cells, or anti-inflammatory agents to suppress the immune system of the host. One well known example of drug delivery via polymers to the CNS is the release of the chemotherapeutic agent, carmustine (BCNU), from implantable polymer wafers to combat brain tumors (Gliadel™ system) (Brem and Gabikian, 2001; Panigrahi et al., 2011). Major advantages of encapsulating carmustine include stabilizing the drug, which normally has a short half-life, and providing precise control over the localization of drug release, limiting collateral damage to healthy brain tissue and reducing side-effects (Brem, 1990; Brem and Gabikian, 2001; Panigrahi et al., 2011). A number of studies have demonstrated the growth and differentiation of neural cells by incorporating neurotrophic factors into polymers and hydrogels. For example, ciliary neurotrophic factor (CNTF) has been incorporated to promote neural cell proliferation, differentiation, and neurite outgrowth (Burdick et al., 2006; Tzeng and Lavik, 2010). Similar results have been seen with neurotrophin-3 (NT3), platelet-derived growth factor (PDGF), glial-derived neurotrophic factor (GDNF), and nerve growth factor (NGF) (Johnson et al., 2010; Wood et al., 2010). Lee, et al. (2010) used a 3D lithographic printing technique to incorporate layers of hydrogel containing neural stem cells on top of hydrogel layers containing vascular endothelial growth factor (VEGF) to demonstrate neural stem cell migration towards the growth factor. Additionally, monomers and peptides, such as a reactive oxygen species-binding polymerizable superoxide dismutase (SOD) mimetic metalloporphyrin macromer (MnTPPyP-Acryl; ref. (Cheung et al., 2008)) or a peptide antagonist to tumor necrosis factor-α (TNFα), can be added to the surface of the hydrogel to impede the host inflammatory system from targeting encapsulated cells (Cheung et al., 2008; Lin et al., 2009).
Though there are still many variables to consider, drug delivery mediated by hydrogel is perhaps the most common hydrogel application. Additionally, some of the first clinical uses of hydrogels were as drug delivery-tools. As suggested, the incorporation of growth factors and trophic molecules into a hydrogel system allows researchers targeted site application along with temporal control over release. Gelatin-based hydrogels were used to deliver dopamine to the striatal region of Parkinsonian rats (Senthilkuma et al., 2007), while the immense variation afforded with synthetic PEG hydrogels have allowed the tailored release of neurotrophic factors over a matter of weeks to months (Burdick et al., 2006). Many proteins and molecules that are difficult to deliver due to stability or kinetics, or compounds that are toxic systemically, have been the focus of research into hydrogel-based drug delivery.
Release of therapeutics from a hydrogel is dependent not only on hydrogel-defined factors, such as degradation and mesh size, but it is also dependent on the therapeutic molecule itself. How the therapeutic molecule is incorporated, for example tethering or encapsulation in the hydrogel or within microparticles, and the actual size of the molecule can define the release profile of the molecule from a hydrogel.
The size and chemical identity of incorporated molecules can affect the release kinetics from the hydrogel. Specifically, a small mesh size hydrogel may prevent larger molecules from readily diffusing from the hydrogel and thus, may require the hydrogel to degrade before they are released. For instance, small molecules, such as the drug diltiazem (used to block calcium channels) at ~5Å in diameter, could readily diffuse out of most hydrogels (Peppas et al., 1999). In contrast, glial-derived neurotrophic factor (GDNF), a rectangular molecule of about 30 × 36 × 80Å (~23kDa), must be incorporated into a hydrogel with a mesh size of 80Å or larger in order for it to easily escape the hydrogel by diffusion (Eigenbrot and Gerber, 1997). Larger molecules and cells often require degradation of the hydrogel before they can progress into the environment (the average neuron soma ranges from 4–100μm, or 40,000–1,000,000Å, in diameter (Chudler, 2011)). Because diffusion can occur more quickly than degradation, molecules incorporated into the hydrogel that are smaller can reach their target more quickly than larger molecules that must wait for the hydrogel to dissolve, resulting in two or more different release rates. These differing release kinetics can be ideal for a hydrogel designed with both encapsulated trophic factors or immunosuppressors and cells, where the diffusible factors can go to work immediately to provide a host environment that is well-suited for, or more closely matched to, the encapsulated cells, which are released later as the hydrogel degrades. Lastly, the polarity of molecules can also affect how they disperse from a hydrogel. Jeong, et al. (2000a), demonstrated that hydrophilic drugs, such as ketoprofen, are released more readily from a hydrogel, where the release rate is determined by diffusion; compared to hydrophobic drugs, such as spironolactone, where release requires hydrogel degradation.
Tethering of molecules to the polymer itself can also greatly impact the release and distribution of molecules from a hydrogel (DuBose et al., 2005). In the case of tethered molecules, release kinetics are dependent on the degradation (hydrolysis or enzymatic cleavage) of the bonds between the therapeutic molecule and hydrogel backbone. Tethering can be accomplished much in the same way that the hydrogel is formed – by introducing the molecule during the polymerization process, the same bonds that connect hydrogel monomers can connect drugs and other proteins (i.e. ester bonds between thiol and ether groups) (DuBose et al., 2005). Certain molecules can also be used to tether drugs to the hydrogel, such as heparin, which binds PEG backbones and has been shown to have a reversible affinity with a number of growth factors (Lin and Anseth, 2009). Tethering can also be achieved through enzyme-sensitive oligopeptide tethers, such as matrix metalloproteinase-sensitive tethers bound to vascular endothelial growth factor (VEGF), which when released induces angiogenesis (Zisch et al., 2003). However, it is important to consider that tethered molecules must withstand the polymerization procedures and the tethers themselves should be biocompatible, as the activity of the molecule could be decreased if the bonding or tethering molecules block active sites or remain attached to the drug post-release (Lin and Anseth, 2009).
Incorporating drugs first into a smaller polymer structures, or microparticles, which are then incorporated into a larger hydrogel structure, is another way to control drug release (Anderson and Shive, 1997; Elisseeff et al., 2001; Burdick et al., 2006; Hou et al., 2008; Guo et al., 2010; Spiller et al., 2010; Lampe et al., 2011). Microparticles can be used to carry trophic factors and a variety of small molecule drugs, proteins, and peptides, such as siRNAs. Interesting examples of the use of microparticles include encapsulating antigens for the development of systemic immunity (Eldridge et al., 1991; Ermak et al., 1995; Thomasin et al., 1996). In the central nervous system, microparticles have been used to deliver dopamine and norepinephrine into the striatum of rats to suppress the symptoms of Parkinson’s disease (McRae et al., 1992). Microparticles (or microspheres) are often made of polymer materials and are subject to variation in polymerization and degradation chemistry and kinetics, particle size, and loading density. Like hydrogels and other degradable polymers, polymer microparticles can be designed with varying rates of degradation. This can be advantageous for applications in which molecule release from a microparticle is designed to occur at a different time point than hydrogel degradation. Such design might be warranted if a hydrogel were being used to implant cells but there was need for extended release of supporting factors – the hydrogel would be degraded, allowing the cells to incorporate into the surrounding tissue while still receiving trophic support from factors incorporated into microparticles.
Microparticles can also be used when more than one therapeutic agent is needed and each needs to be released in its own time and location. In a recent study, hydrogel strands carrying two formulations of poly(lactic-co-glycolic acid) (PLGA)-based microparticles were implanted into the rat brain (Lampe et al., 2011). One group of PLGA-based microparticles were loaded with brain-derived neurotrophic factor (BDNF) and designed to degrade slowly. The other formulation of PLGA-microparticles was loaded with glial cell-derived neurotrophic factor (GDNF) and designed to degrade more quickly. The fast releasing microparticles released all the GDNF within a 28 day window, whereas the slow releasing microparticles released BDNF consistently for at least 2 months. The study demonstrated that the rate of protein release can be controlled by altering the rate of degradation of the microparticles, without changing the properties of the overall hydrogel strand (Lampe et al., 2011). This could be beneficial in treating Parkinson’s disease where BDNF release from slower degrading microparticles into the striatum could encourage neurite outgrowth (Østergaard et al., 1996; Yurek et al., 1996), while GDNF release from faster degrading microparticles into the substantia nigra from the same hydrogel strand could provide immediate cell support for grafted neurons (Lin et al., 1993; Ai et al., 2003).
Biocompatibility
The term biocompatibility can be ambiguous and is often used in a variety of contexts. For this review, we will consider biocompatibility in two contexts: the histocompatibility of an implanted hydrogel with regards to the local and/or systemic response of the host and the cytocompatibility of a hydrogel with encapsulated cells, both in vitro and in vivo. We have also chosen to consider biocompatibility of hydrogels with special attention placed on the inflammatory and immune reaction of the brain.
Immune Response of the Brain
Because the brain is mostly isolated from the periphery by the blood-brain-barrier, it has a similar but slightly different response to tissue damage and foreign materials implanted than peripheral tissues. Brain tissue damage produced during the implantation process can trigger a limited infiltration of macrophages and foreign-body giant cells of the peripheral immune system from damaged blood vessels, however, the bulk of the neuroimmune response is carried by the resident microglia and astrocytes (Stichel and Müller, 1998; Aloisi, 2001; Squire et al., 2003; Kim et al., 2004; Hanisch and Kettenmann, 2007; Bjugstad et al., 2010). The acute neuroinflammatory response is initiated by microglia; this includes antigen presentation and the initiation of cytotoxic pathways occuring over the first few days following injury (Aloisi, 2001; Fournier et al., 2003; Hanisch and Kettenmann, 2007; Bjugstad et al., 2010). It is during this time that some healthy cells will become collateral damage, overwhelmed by the cytotoxic forces of the microglia (Barker et al., 1996; Emgård et al., 1999; Darsalia et al., 2011). In the days to weeks that follow the acute response, the astrocyte population increases in an attempt to repair damage and to isolate any offending materials in contact with the tissue by building a glial scar (Stichel and Müller, 1998; Fournier et al., 2003; Laird et al., 2008). This glial scar is similar to the fibroblast scar tissue that develops in peripheral tissues, however, in the brain and spinal cord, the glial scar becomes a barrier to neuritic and axonal extensions (Stichel and Müller, 1998; Fournier et al., 2003; Fitch and Silver, 2008; Afshari et al., 2009). In the days and weeks following tissue damage, cytokine activation in both microglia and astrocytes turns from cytotoxicity to neuroprotection, through the secretion of anti-inflammatory agents (i.e. TGF-β, TNF-α, and thrombospondin) and neurotrophic factors (BDNF, GDNF, nerve growth factor (NGF)) (Fournier et al., 2003; Hanisch and Kettenmann, 2007; Laird et al., 2008).
Because of the differences in the neuroimmune response in the brain compaired to other tissues, certain considerations should be attended to when designing or using a hydrogel that will be implanted into the brain. Interestingly, many studies of hydrogel biocompatibility with the CNS suggest that the immune reaction is in direct response to the mechanical trauma of implantation and that the material itself does not contribute to the immediate immune response (Figure 6) (Fournier et al., 2003; Bjugstad et al., 2010). As such, this characteristic is advantageous when using hydrogels to implant encapsulated cells into the brain because the hydrogels can be used to increase graft survival during the immediate, implantation-induced neuroimmune response (Grabowski et al., 1994; Cruise et al., 1999; Lin et al., 2009; Zhong et al., 2010). The long term compatibility of the material with the host tissue contributes to the effectivness of the implantation while a subsequent immune reaction may depend on the degradation by-products, therapeutic agents, and/or cells the hydrogel delivers. Particularily, hydrogel chemistry and mechanical properties significantly contribute to the biocompatibilty and must be tuned appropriately for the tissue type being engineered. Fortunately, most synthetic hydrogels have been developed to be biologically inert and thus chemically biocompatible.
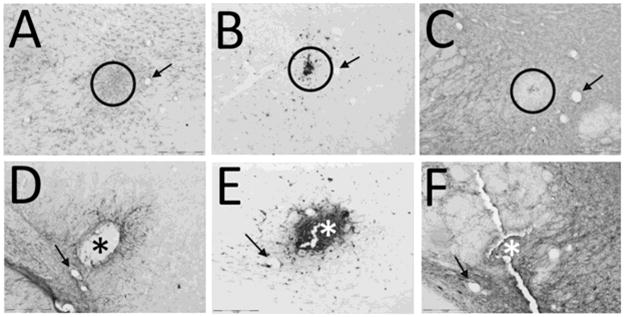
Figure 6. These PEG-PLA based hydrogels implanted into the rat brain induced a long term neuroimmune response similar to that observed in sham brains penetrated with a needle.
Two months after needle penetration (A–C) or hydrogel implant (D–F), a glial response remains. GFAP+ astrocytes (A, D) appear to be more abundant in the tissue surrounding the needle penetration (circled in A) whereas there are fewer astrocytes in the tissue surrounding the hydrogel implant (D). There does appear to be an increase in astrocytes at the hydrogel-brain interface with some GFAP+ processes piercing the hydrogel (D, *). Microglial cells (B, E), identified by positive CD68 reactivity, remain in both the sham brain (B) and in the hydrogel implanted brain (E). While there is not an observable difference in the number of microglia in the surrounding tissues, there is a profusion of microglia that have infiltrated the hydrogel (*). MAP2, labeling identifying neurons (C, F), shows a paucity of MAP2 presence in the area surrounding the needle penetration (circled in C) whereas in the hydrogel brain, MAP2+ cells and neurites can be found closely positioned to the hydrogel (*). Circles in A–C indicate the center of the needle penetration. * in D–F, indicate the center of the hydrogel implant. Arrows (A–C and D–F) indicate corresponding blood vessels found in the adjacent tissue sections. Images were taken between the cerebral peduncle and the subthalamic nucleus. Scale bar indicates 200μm. Quantified data from this study can be found in detail in Bjugstad et al. (2010).
Chemical and Mechanical Contributions to Biocompatibility
Hydrogel chemisty is important in determining the biocompatibility of a hydrogel. The high water content of hydrogels contributes to their biocompatibility and is one major innate advantage of using these types of polymers as biomaterials. The hydrogel by-products, resulting from the degradation process, are probably the most important chemical considerations. Degradation by-products arise as the bonds within the hydrogel are cleaved: hydrolysis and enzymatic cleavage results in the hydrogel breaking down into the various individual monomer or oligomer units from which the hydrogel is comprised. Some studies have demonstrated the adverse effects of toxic degradation by-products, such as poly(methylidene malonate 2.1.2) and poly(propylene fumarate), in various tissues (Williams, 1987; Fournier et al., 2003; Hedberg et al., 2005; Fournier et al., 2006). Recently, however, many groups using hydrogels for tissue engineering have considered the biocompatibility of their by-products when formulating the polymer constituents and, as such, most hydrogels in use today as biomaterials are considered chemically non-toxic. The use of poly(α-hydroxy acids), such as lactic and glycolic acids, as degradable units are a good choice because they have been shown to be biocompatible and can be metabolically recycled by the body (Sawhney et al., 1993; Lampe et al., 2009; Lampe et al., 2010a). Likewise, the use of hyaluronate and poly(ethylene glycol) as polymer backbones can be beneficial because hyaluronate is a natural component of the ECM (Drury and Mooney, 2003; Vanderhooft et al., 2009) and poly(ethylene glycol), though synthetic, is biologically inert (Sawhney et al., 1993). Most synthetic hydrogels have minimal cell adhesion and low protein adsorption, which limits their recognition by immune cells, increasing their biocompatibility (Shoichet, 2010).
Some chemical additions can contribute to the cell-containing functionality of the hydrogel, such as enhancing cell adhesion or encapsulation. Cell adhesion can be controlled by supplementing the hydrogel chemistry with oligopeptide sequences and tethering monomers. For example, the commonly used, fibronectin-derived Arginine-Glycine-Aspartic acid (RGD) peptide sequence, when incorporated into the hydrogel, has been shown to improve cell viability and migration (Elbert and Hubbell, 1996; Mann et al., 2001; Burdick and Anseth, 2002; Hersel et al., 2003; Bryant et al., 2008; Zhu, 2010). Additionally, as RGD sequence density in the hydrogel increases, neural stem cell adherance and neurite outgrowth increases (Schense and Hubbell, 2000; Gunn et al., 2005). Even the addition of electrically charged monomers, such as sodium methacrylate, have been shown to impact cell behavior (including differentiation) through the interaction of the cells with a charged hydrogel surface (Dadsetan et al., 2009; Dadsetan et al., 2011). While hydrogels can be formulated with additional proteins, such as collagen, to further mimic the ECM (Weber et al., 2008; Liu et al., 2010), encapsulated cells themselves have been shown to secrete their own environmental molecules, such as fibronectin and collagen (Bryant et al., 2003; Mahoney and Anseth, 2006; Lin et al., 2011). Chondrocytes especially, have been shown to function well within hydrogels, with evidence that extended in vitro culture in PEG-based hydrogels can lead to formation of complete cartilage-like tissues (Elisseeff et al., 2000; Lin et al., 2011).
In addition to the significant effects hydrogel chemistry can have on encapsulated cells, most cell types also perform better in hydrogel environments in which the mechanical properties (i.e. stiffness, elasticity, etc.) more closely resemble their host tissue. Indeed, mechanical environment can play a key role in cell survival and tissue formation (Engler et al., 2006), and as discussed above, neural cell populations remain viable longer in/on hydrogels which mimic the compressive modulus of the brain, maintaining their neuronal, glial, and progenitor phenotypes (Figure 4). Furthermore, increasing the compressive modulus to 20kPa and higher and exceeding the modulus observed in brain tissue with a stiffness closer to that of tissue culture polystyrene, induces a preferential survival of astrocytes and an increase in gene expression for ECM proteins associated with glial scar formation (Lampe et al., 2010b). As well, complete degradation of the hydrogel over time can be desireable in preventing a permanent neuroimmune response.
One aspect of the host environment that also should be mimicked is the 3D nature of tissue. Throughout the review, the words “encapsulated” or “incorporated” have been used to describe neural cell populations that reside within a 3D hydrogel composition, as compared to cells which are grown on the surface of a hydrogel composition (two-dimensional; 2D). A study by Lampe et al., (2010b) showed that when the mechanical and chemical properties of a hydrogel are all equal, neural cells survive and thrive better when grown in a 3D environment as compared to 2D. Even when the compressive modulus exceeds that which is preferred by neural stem cells, in the range of 15–20kPa, neural cells grown in 3D environments survive significantly better than those on a 2D environment with a compressive modulus closer that that of the brain (e.g. 1.4–3.8 kPa) (Lampe et al., 2010b).
Conclusion
While further development of these systems for neural cells and tissue is still needed, hydrogels provide a promising avenue for tissue engineering and cell transplantation, especially in the brain where the regenerative abilities can be limited. In regards to the brain and CNS, this review attempted to convey to the neuroscience community some basic but important considerations in the use of hydrogels. There are also advanced topics which were not included in this review, which should be considered once the concepts presented here are better understood and appreciated.
The methods of polymerization and degradation of these biomaterials contribute to the general functionality of the hydrogel. These two processes are directly affected by the chemical characteristics of the hydrogel, which also govern the mechanical and physical characteristics, as well as the overall shape of a hydrogel. The properties of a synthetic hydrogel can be easily modified to mimic the ECM of most tissues of the body, including the brain. Lastly, all of these characteristics will directly influence the capacity of the material to perform the task it was designed – for whether it be drug delivery, cell encapsulation for implantation, circuit reconstruction, or a combination of these therapies.
From cross-link density and mesh size, to degradation rate and erosion mechanism, the chemical and mechanical properties of the hydrogel determine the successful integration of neural cells into the host brain tissue and the release profiles of molecules or drugs. This can be beneficial for timed release of therapeutics – for example, the immediate release of fast acting compounds contributing to the attenuation of the inflammatory reaction or the more gradual release of trophic factors acting to support long-term cell growth and survival. By incorporating cells, drugs, and/or trophic factors, researchers can be one step closer to developing tissues ex vivo or in situ for brain repair. This allows for multiple levels of complexity to be achieved with a hydrogel, including the potential for directed cell differentiation and vascularization within the hydrogel and formation of composite neural tissues. Three-dimensional tissue engineering allows for the advanced study of biological and physiological processes in a way heretofore unexplored. As well, advanced hydrogel technologies are allowing for new methods of cell-based drug screening and localized drug delivery to human tissues. The first long-term results of hydrogel implementation are being realized and show great promise for the future of this technology – in the brain and beyond.
Highlights.
Hydrogels can be used in brain tissue engineering for neurological disorders
Chemical, mechanical, and physical properties are important considerations
Polymerization and degradation can determine hydrogel biocompatibility
Hydrogels can have multiple uses for delivery of therapeutics
Acknowledgments
The authors of this review would like to acknowledge the authors of Lin et al. (2011), for their kind permission to use the images found in Figure 5 of this article. We would also like to acknowledge Angela Rachubinski for her assistance with the preparation of this manuscript.
Funding
Linda Crnic Institute for Down Syndrome; NIH/NINDS R01NS052597
Glossary
- Polymer
chains of repeating units (monomers); can be naturally occurring (i.e. DNA, hyaluronan) or synthetic (i.e. poly(ethylene glycol))
- Hydrogel
hydrophilic polymers (natural or synthetic) with high water content and viscoelastic properties; the extracellular matrix of most tissues is an example of a naturally occurring hydrogel
- Polymerization
the process of bond formation between monomers and/or smaller polymers leading to the formation of a larger polymer
- Degradation
the process of cleaving the liable bonds within the polymer structure; most commonly occurs hydrolytically or enzymatically
- Mesh Size
the distance between cross-links in a hydrogel; typically measured in Angstroms (Å)
- Pores
macroscopic features of the hydrogel, pores are defined by their larger size (typically measured in microns (μm)); can be naturally occurring or created intentionally and can be interconnected
- Mechanical Properties
the properties that reveal the reaction of a biomaterial to an applied stress or force, i.e. stiffness, elasticity
- Compressive Modulus
a measure of mechanical strength or stiffness of a hydrogel; typically expressed in pascals or kilopascals (Pa; kPa)
- Biocompatibility
in reference to the brain, this is the neuroimmune response to a biomaterial; it is independent of a peripheral immune response and involves the reaction of astrocytes and microglia
- Microparticle
small polymer structures typically used to encapsulate drugs and therapeutics to be incorporated into a hydrogel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afshari FT, Kappagantula S, Fawcett JW. Extrinsic and intrinsic factors controlling axonal regeneration after spinal cord injury. Expert Rev Mol Med. 2009;11:e37. doi: 10.1017/S1462399409001288. [DOI] [PubMed] [Google Scholar]
- Ai Y, Markesbery W, Zhang ZM, Grondin R, Elseberry D, Gerhardt GA, Gash DM. Intraputamenal infusion of GDNF in aged rhesus monkeys: Distribution and dopaminergic effects. J Comp Neurol. 2003;461:250–261. doi: 10.1002/cne.10689. [DOI] [PubMed] [Google Scholar]
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- Anseth KS, Bowman CN, Brannon-Peppas L. Mechanical properties of hydrogels and their experimental determination. Biomaterials. 1996;17:1647–1657. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- Anseth KS, Metters AT, Bryant SJ, Martens PJ, Elisseeff JH, Bowman CN. In situ forming degradable networks and their application in tissue engineering and drug delivery. J Control Release. 2002;78:199–209. doi: 10.1016/s0168-3659(01)00500-4. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Arha M, Choudhary S, Ashton RS, Bhatia SR, Schaffer DV, Kane RS. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials. 2009;30:4695–4699. doi: 10.1016/j.biomaterials.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker RA, Dunnett SB, Faissner A, Fawcett JW. The Time Course of Loss of Dopaminergic Neurons and the Gliotic Reaction Surrounding Grafts of Embryonic Mesencephalon to the Striatum. Exp Neurol. 1996;141:79–93. doi: 10.1006/exnr.1996.0141. [DOI] [PubMed] [Google Scholar]
- Bencherif SA, Sheehan JA, Hollinger JO, Walker LM, Matyjaszewski K, Washburn NR. Influence of cross-linker chemistry on release kinetics of PEG-co-PGA hydrogels. J Biomed Mater Res A. 2009;90A:142–153. doi: 10.1002/jbm.a.32069. [DOI] [PubMed] [Google Scholar]
- Benkirane-Jessel N, Schwinte P, Falvey P, Darcy R, Haikel Y, Schaaf P, Voegel JC, Ogier J. Build-up of polypeptide multilayer coatings with anti-inflammatory properties based on the embedding of piroxicam-cyclodextrin complexes. Adv Funct Mater. 2004;14:174–182. [Google Scholar]
- Bhatia SK. Tissue engineering for clinical applications. Biotechnol J. 2010;5:1309–1323. doi: 10.1002/biot.201000230. [DOI] [PubMed] [Google Scholar]
- Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 2005;195:115–126. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Bjugstad KB, Lampe K, Kern DS, Mahoney M. Biocompatibility of poly(ethylene glycol)-based hydrogels in the brain: An analysis of the glial response across space and time. J Biomed Mater Res A. 2010;95A:79–91. doi: 10.1002/jbm.a.32809. [DOI] [PubMed] [Google Scholar]
- Bjugstad KB, Teng YD, Redmond DE, Elsworth JD, Roth RH, Cornelius SK, Snyder EY, Sladek JR. Human neural stem cells migrate along the nigrostriatal pathway in a primate model of Parkinson’s disease. Exp Neurol. 2008;211:362–369. doi: 10.1016/j.expneurol.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Wiley CA. Brain Extracellular Matrix in Neurodegeneration. Brain Pathol. 2009;19:573–585. doi: 10.1111/j.1750-3639.2008.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman CN, Kloxin CJ. Toward an Enhanced Understanding and Implementation of Photopolymerization Reactions. AIChE J. 2008;54:2775–2795. [Google Scholar]
- Brännvall K, Bergman K, Wallenquist U, Svahn S, Bowden T, Hilborn J, Forsberg-Nilsson K. Enhanced neuronal differentiation in a three-dimensional collagen-hyaluronan matrix. J Neurosci Res. 2007;85:2138–2146. doi: 10.1002/jnr.21358. [DOI] [PubMed] [Google Scholar]
- Brem H. Polymers to treat brain tumours. Biomaterials. 1990;11:699–701. doi: 10.1016/0142-9612(90)90030-t. [DOI] [PubMed] [Google Scholar]
- Brem H, Gabikian P. Biodegradable polymer implants to treat brain tumors. J Control Release. 2001;74:63–67. doi: 10.1016/s0168-3659(01)00311-x. [DOI] [PubMed] [Google Scholar]
- Broder JS. Head Computed Tomography Interpretation in Trauma: A Primer. Psychiatr Clin North Am. 2010;33:821–854. doi: 10.1016/j.psc.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Bromberg L. Crosslinked poly(ethylene glycol) networks as reservoirs for protein delivery. J Appl Polym Sci. 1996;59:459–466. [Google Scholar]
- Brondsted H, Kopecek J. Hydrogels for Site-Specific Oral-Drug Delivery - Synthesis and Characterization. Biomaterials. 1991;12:584–592. doi: 10.1016/0142-9612(91)90056-g. [DOI] [PubMed] [Google Scholar]
- Brundin P, Pogarell O, Hagell P, Piccini P, Widner H, Schrag A, Kupsch A, Crabb L, Odin P, Gustavii B, Björklund A, Brooks DJ, David Marsden C, Oertel WH, Quinn NP, Rehncrona S, Lindvall O. Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson’s disease. Brain. 2000;123:1380–1390. doi: 10.1093/brain/123.7.1380. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Durand KL, Anseth KS. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. J Biomed Mater Res A. 2003;67A:1430–1436. doi: 10.1002/jbm.a.20003. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Nicodemus GD, Villanueva I. Designing 3D photopolymer hydrogels to regulate biomechanical cues and tissue growth for cartilage tissue engineering. Pharm Res. 2008;25:2379–2386. doi: 10.1007/s11095-008-9619-y. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Nuttelman CR, Anseth K. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed. 2000;11:439–457. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- Burdick JA, Chung C, Jia XQ, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6:386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick JA, Ward M, Liang E, Young MJ, Langer R. Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials. 2006;27:452–459. doi: 10.1016/j.biomaterials.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Burkersroda Fv, Schedl L, Göpferich A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials. 2002;23:4221–4231. doi: 10.1016/s0142-9612(02)00170-9. [DOI] [PubMed] [Google Scholar]
- Case LC, Tessier-Lavigne M. Regeneration of the adult central nervous system. Curr Biol. 2005;15:R749–R753. doi: 10.1016/j.cub.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Cayre M, Canoll P, Goldman JE. Cell migration in the normal and pathological postnatal mammalian brain. Prog Neurobiol. 2009;88:41–63. doi: 10.1016/j.pneurobio.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee K, Lin-Gibson S, Wallace WE, Parekh SH, Lee YJ, Cicerone MT, Young MF, Simon CG., Jr The effect of 3D hydrogel scaffold modulus on osteoblast differentiation and mineralization revealed by combinatorial screening. Biomaterials. 2010;31:5051–5062. doi: 10.1016/j.biomaterials.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CY, McCartney SJ, Anseth KS. Synthesis of Polymerizable Superoxide Dismutase Mimetics to Reduce Reactive Oxygen Species Damage in Transplanted Biomedical Devices. Adv Funct Mater. 2008;18:3119–3126. [Google Scholar]
- Chiu Y-L, Chen S-C, Su C-J, Hsiao C-W, Chen Y-M, Chen H-L, Sung H-W. pH-triggered injectable hydrogels prepared from aqueous N-palmitoyl chitosan: In vitro characteristics and in vivo biocompatibility. Biomaterials. 2009;30:4877–4888. doi: 10.1016/j.biomaterials.2009.05.052. [DOI] [PubMed] [Google Scholar]
- Chudler EH. Brain Facts and Figures Web. 2011 Sep 01; http://faculty.washington.edu/chudler/facts.html.
- Chung C, Beecham M, Mauck RL, Burdick JA. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials. 2009;30:4287–4296. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C, Burdick JA. Influence of Three-Dimensional Hyaluronic Acid Microenvironments on Mesenchymal Stem Cell Chondrogenesis. Tissue Eng Part A. 2009;15:243–254. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C, Mesa J, Randolph MA, Yaremchuk M, Burdick JA. Influence of gel properties on neocartilage formation by auricular chondrocytes photoencapsulated in hyaluronic acid networks. J Biomed Mater Res A. 2006;77A:518–525. doi: 10.1002/jbm.a.30660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comolli N, Neuhuber B, Fischer I, Lowman A. In vitro analysis of PNIPAAm-PEG, a novel, injectable scaffold for spinal cord repair. Acta Biomater. 2009;5:1046–1055. doi: 10.1016/j.actbio.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke MJ, Wang Y, Morshead CM, Shoichet MS. Controlled epi-cortical delivery of epidermal growth factor for the stimulation of endogenous neural stem cell proliferation in stroke-injured brain. Biomaterials. 2011;32:5688–5697. doi: 10.1016/j.biomaterials.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Cowin SC, Doty SB. Tissue Mechanics. 1. Springer; New York: 2007. p. 360. [Google Scholar]
- Cruise GM, Hegre OD, Lamberti FV, Hager SR, Hill R, Scharp DS, Hubbell JA. In vitro and in vivo performance of porcine islets encapsulated in interfacially photopolymerized poly(ethylene glycol) diacrylate membranes. Cell Transplant. 1999;8:293–306. doi: 10.1177/096368979900800310. [DOI] [PubMed] [Google Scholar]
- Dadsetan M, Knight AM, Lu L, Windebank AJ, Yaszemski MJ. Stimulation of neurite outgrowth using positively charged hydrogels. Biomaterials. 2009;30:3874–3881. doi: 10.1016/j.biomaterials.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadsetan M, Pumberger M, Casper ME, Shogren K, Giuliani M, Ruesink T, Hefferan TE, Currier BL, Yaszemski MJ. The effects of fixed electrical charge on chondrocyte behavior. Acta Biomater. 2011;7:2080–2090. doi: 10.1016/j.actbio.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson P, Dahlin L, Povlsen B. Tubulization increases axonal outgrowth of rat sciatic nerve after crush injury. Exp Neurol. 1996;139:238–243. doi: 10.1006/exnr.1996.0097. [DOI] [PubMed] [Google Scholar]
- Darsalia V, Allison SJ, Cusulin C, Monni E, Kuzdas D, Kallur T, Lindvall O, Kokaia Z. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J Cereb Blood Flow Metab. 2011;31:235–242. doi: 10.1038/jcbfm.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druecke D, Langer S, Lamme E, Pieper J, Ugarkovic M, Steinau HU, Homann HH. Neovascularization of poly(ether ester) block-copolymer scaffolds in vivo: Long-term investigations using intravital fluorescent microscopy. J Biomed Mater Res A. 2004;68A:10–18. doi: 10.1002/jbm.a.20016. [DOI] [PubMed] [Google Scholar]
- Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- DuBose JW, Cutshall C, Metters AT. Controlled release of tethered molecules via engineered hydrogel degradation: Model development and validation. J Biomed Mater Res A. 2005;74A:104–116. doi: 10.1002/jbm.a.30307. [DOI] [PubMed] [Google Scholar]
- Edell DJ, Toi VV, McNeil VM, Clark LD. Factors influencing the biocompatibility of insertable silicon microshafts in cerebral cortex. IEEE Trans Biomed Eng. 1992;39:635–643. doi: 10.1109/10.141202. [DOI] [PubMed] [Google Scholar]
- Eigenbrot C, Gerber N. X-ray structure of glial cell-derived neurotrophic factor at 1.9 angstrom resolution and implications for receptor binding. Nat Struct Biol. 1997;4:435–438. doi: 10.1038/nsb0697-435. [DOI] [PubMed] [Google Scholar]
- Elbert DL, Hubbell JA. Surface treatments of polymers for biocompatibility. Annu Rev Mater Sci. 1996;26:365–394. [Google Scholar]
- Eldridge JH, Staas JK, Meulbroek JA, Tice TR, Gilley RM. Biodegradable and biocompatible poly(DL-lactide-co-glycolide) microspheres as an adjuvant for staphylococcal enterotoxin B toxoid which enhances the level of toxin-neutralizing antibodies. Infect Immun. 1991;59:2978–2986. doi: 10.1128/iai.59.9.2978-2986.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164–171. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Elisseeff J, McIntosh W, Fu K, Blunk T, Langer R. Controlled-release of IGF-I and TGF-β1 in a photopolymerizing hydrogel for cartilage tissue engineering. J Orthop Res. 2001;19:1098–1104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Silva E, Ali O, Mooney D, Bell W, Yu SJ, Kaneko Y, Borlongan C. Injectable VEGF Hydrogels Produce Near Complete Neurological and Anatomical Protection Following Cerebral Ischemia in Rats. Cell Transplant. 2010;19:1063–1071. doi: 10.3727/096368910X498278. [DOI] [PubMed] [Google Scholar]
- Emgård M, Karlsson J, Hansson O, Brundin P. Patterns of Cell Death and Dopaminergic Neuron Survival in Intrastriatal Nigral Grafts. Exp Neurol. 1999;160:279–288. doi: 10.1006/exnr.1999.7198. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Ermak TH, Dougherty EP, Bhagat HR, Kabok Z, Pappo J. Uptake and Transport of Copolymer Biodegradable Microspheres by Rabbit Peyer-Patch M-Cells. Cell Tissue Res. 1995;279:433–436. doi: 10.1007/BF00318501. [DOI] [PubMed] [Google Scholar]
- Farmer TG, Edgar TF, Peppas NA. In Vivo Simulations of the Intravenous Dynamics of Submicrometer Particles of pH-Responsive Cationic Hydrogels in Diabetic Patients. Ind Eng Chem Res. 2008;47:10053–10063. doi: 10.1021/ie070957b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport. 2002;13:2411–2415. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MC, Bertram JP, Hynes SR, Michaud M, Li Q, Young M, Segal SS, Madri JA, Lavik EB. A macroporous hydrogel for the coculture of neural progenitor and endothelial cells to form functional vascular networks in vivo. Proc Natl Acad Sci U S A. 2006;103:2512–2517. doi: 10.1073/pnas.0506020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier E, Passirani C, Colin N, Sagodira S, Menei P, Benoit J-P, Montero-Menei CN. The brain tissue response to biodegradable poly(methylidene malonate 2.1.2)-based microspheres in the rat. Biomaterials. 2006;27:4963–4974. doi: 10.1016/j.biomaterials.2006.04.045. [DOI] [PubMed] [Google Scholar]
- Fournier E, Passirani C, Montero-Menei CN, Benoit JP. Biocompatibility of implantable synthetic polymeric drug carriers: focus on brain biocompatibility. Biomaterials. 2003;24:3311–3331. doi: 10.1016/s0142-9612(03)00161-3. [DOI] [PubMed] [Google Scholar]
- Freed CR, Breeze RE, Rosenberg NL, Schneck SA, Kriek E, Qi J-x, Lone T, Zhang Y-b, Snyder JA, Wells TH, Ramig LO, Thompson L, Mazziotta JC, Huang SC, Grafton ST, Brooks D, Sawle G, Schroter G, Ansari AA. Survival of Implanted Fetal Dopamine Cells and Neurologic Improvement 12 to 46 Months after Transplantation for Parkinson’s Disease. N Engl J Med. 1992;327:1549–1555. doi: 10.1056/NEJM199211263272202. [DOI] [PubMed] [Google Scholar]
- Fromstein JD, Woodhouse KA. Elastomeric biodegradable polyurethane blends for soft tissue applications. J Biomater Sci Polym Ed. 2002;13:391–406. doi: 10.1163/156856202320253929. [DOI] [PubMed] [Google Scholar]
- Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with Compliance Comparable to that of Brain Tissue Select Neuronal over Glial Growth in Mixed Cortical Cultures. Biophys J. 2006;90:3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerecht S, Townsend SA, Pressler H, Zhu H, Nijst CLE, Bruggeman JP, Nichol JW, Langer R. A porous photocurable elastomer for cell encapsulation and culture. Biomaterials. 2007;28:4826–4835. doi: 10.1016/j.biomaterials.2007.07.039. [DOI] [PubMed] [Google Scholar]
- Göpferich A. Mechanisms of polymer degradation and erosion. Biomaterials. 1996;17:103–114. doi: 10.1016/0142-9612(96)85755-3. [DOI] [PubMed] [Google Scholar]
- Grabowski M, Johansson BB, Brundin P. Survival of Fetal Neocortical Grafts Implanted in Brain Infarcts of Adult Rats: The Influence of Postlesion Time and Age of Donor Tissue. Exp Neurol. 1994;127:126–136. doi: 10.1006/exnr.1994.1086. [DOI] [PubMed] [Google Scholar]
- Guan J, Fujimoto KL, Sacks MS, Wagner WR. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials. 2005;26:3961–3971. doi: 10.1016/j.biomaterials.2004.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J, Wagner WR. Synthesis, Characterization and Cytocompatibility of Polyurethaneurea Elastomers with Designed Elastase Sensitivity. Biomacromolecules. 2005;6:2833–2842. doi: 10.1021/bm0503322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumera C, Rauck B, Wang YD. Materials for central nervous system regeneration: bioactive cues. J Mater Chem. 2011;21:7033–7051. [Google Scholar]
- Gunn JW, Turner SD, Mann BK. Adhesive and mechanical properties of hydrogels influence neurite extension. J Biomed Mater Res A. 2005;72A:91–97. doi: 10.1002/jbm.a.30203. [DOI] [PubMed] [Google Scholar]
- Guo X, Park H, Young S, Kretlow JD, van den Beucken JJ, Baggett LS, Tabata Y, Kasper FK, Mikos AG, Jansen JA. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater. 2010;6:39–47. doi: 10.1016/j.actbio.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgo RD, Bedi KS, Nurcombe V. Current concepts in central nervous system regeneration. J Clin Neurosci. 2002;9:613–617. doi: 10.1054/jocn.2002.1080. [DOI] [PubMed] [Google Scholar]
- Ha J-H, Kim S-H, Han S-Y, Sung Y-K, Lee Y-M, Kang I-K, Cho C-S. Albumin release from bioerodible hydrogels based on semi-interpenetrating polymer networks composed of poly([epsilon]-caprolactone) and poly(ethylene glycol) macromer. J Control Release. 1997;49:253–262. doi: 10.1016/s0378-5173(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Hanisch U-K, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hawkins AM, Milbrandt TA, Puleo DA, Hilt JZ. Synthesis and analysis of degradation, mechanical and toxicity properties of poly([beta]-amino ester) degradable hydrogels. Acta Biomater. 2011;7:1956–1964. doi: 10.1016/j.actbio.2011.01.024. [DOI] [PubMed] [Google Scholar]
- He S, Yaszemski JM, Yasko AW, Engel PS, Mikos AG. Injectable biodegradable polymer composites based on poly(propylene fumarate) crosslinked with poly(ethylene glycol)-dimethacrylate. Biomaterials. 2000;21:2389–2394. doi: 10.1016/s0142-9612(00)00106-x. [DOI] [PubMed] [Google Scholar]
- Hedberg EL, Kroese-Deutman HC, Shih CK, Crowther RS, Carney DH, Mikos AG, Jansen JA. In vivo degradation of porous poly(propylene fumarate)/poly(DL-lactic-co-glycolic acid) composite scaffolds. Biomaterials. 2005;26:4616–4623. doi: 10.1016/j.biomaterials.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Hejcl A, Lesny P, Pradny M, Michalek J, Jendelova P, Stulik J, Sykova E. Biocompatible Hydrogels in Spinal Cord Injury Repair. Physiol Res. 2008;57:S121–S132. doi: 10.33549/physiolres.931606. [DOI] [PubMed] [Google Scholar]
- Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- “Hooke’s law.” . Encyclopædia Britannica Online. Encyclopædia Britannica; 2011. Encyclopædia Britannica. Web. 02 Sep. 2011. http://www.britannica.com/EBchecked/topic/271336/Hookes-law. [Google Scholar]
- Hou QP, Chau DYS, Pratoomsoot C, Tighe PJ, Dua HS, Shakesheff KM, Rose F. In situ gelling hydrogels incorporating microparticles as drug delivery carriers for regenerative medicine. J Pharm Sci. 2008;97:3972–3980. doi: 10.1002/jps.21310. [DOI] [PubMed] [Google Scholar]
- Hu M, Kurisawa M, Deng R, Teo C-M, Schumacher A, Thong Y-X, Wang L, Schumacher KM, Ying JY. Cell immobilization in gelatin-hydroxyphenylpropionic acid hydrogel fibers. Biomaterials. 2009;30:3523–3531. doi: 10.1016/j.biomaterials.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt N, Grover L. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol Lett. 2010;32:733–742. doi: 10.1007/s10529-010-0221-0. [DOI] [PubMed] [Google Scholar]
- Hynd MR, Turner JN, Shain W. Applications of hydrogels for neural cell engineering. J Biomater Sci Polym Ed. 2007;18:1223–1244. doi: 10.1163/156856207782177909. [DOI] [PubMed] [Google Scholar]
- Hynes SR, Rauch MF, Bertram JP, Lavik EB. A library of tunable poly(ethylene glycol)/poly(L-lysine) hydrogels to investigate the material cues that influence neural stem cell differentiation. J Biomed Mater Res A. 2009;89A:499–509. doi: 10.1002/jbm.a.31987. [DOI] [PubMed] [Google Scholar]
- Jeong B, Bae YH, Kim SW. Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. J Control Release. 2000a;63:155–163. doi: 10.1016/s0168-3659(99)00194-7. [DOI] [PubMed] [Google Scholar]
- Jeong B, Bae YH, Kim SW. In situ gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions and degradation thereof. J Biomed Mater Res. 2000b;50:171–177. doi: 10.1002/(sici)1097-4636(200005)50:2<171::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Jeong B, Bae YH, Lee DS, Kim SW. Biodegradable block copolymers as injectable drug-delivery systems. Nature. 1997;388:860–862. doi: 10.1038/42218. [DOI] [PubMed] [Google Scholar]
- Jessel N, Atalar F, Lavalle P, Mutterer J, Decher G, Schaaf P, Voegel JC, Ogier J. Bioactive coatings based on a polyelectrolyte multilayer architecture functionalized by embedded proteins. Adv Mater. 2003;15:692–695. [Google Scholar]
- Jewell CM, Zhang JT, Fredin NJ, Lynn DM. Multilayered polyelectrolyte films promote the direct and localized delivery of DNA to cells. J Control Release. 2005;106:214–223. doi: 10.1016/j.jconrel.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Jha AK, Xu X, Duncan RL, Jia X. Controlling the adhesion and differentiation of mesenchymal stem cells using hyaluronic acid-based, doubly crosslinked networks. Biomaterials. 2011;32:2466–2478. doi: 10.1016/j.biomaterials.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PJ, Tatara A, Shiu A, Sakiyama-Elbert SE. Controlled Release of Neurotrophin-3 and Platelet-Derived Growth Factor From Fibrin Scaffolds Containing Neural Progenitor Cells Enhances Survival and Differentiation Into Neurons in a Subacute Model of SCI. Cell Transplant. 2010;19:89–101. doi: 10.3727/096368909X477273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JS, Burdick JA. Hydrogel mediated delivery of trophic factors for neural repair. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:128–139. doi: 10.1002/wnan.10. [DOI] [PubMed] [Google Scholar]
- Keskar V, Gandhi M, Gemeinhart EJ, Gemeinhart RA. Initial evaluation of vascular ingrowth into superporous hydrogels. J Tissue Eng Regen Med. 2009a;3:486–490. doi: 10.1002/term.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskar V, Marion NW, Mao JJ, Gemeinhart RA. In Vitro Evaluation of Macroporous Hydrogels to Facilitate Stem Cell Infiltration, Growth, and Mineralization. Tissue Eng Part A. 2009b;15:1695–1707. doi: 10.1089/ten.tea.2008.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee K-W, Hefferan TE, Currier BL, Yaszemski MJ, Lu L. Synthesis and Evaluation of Novel Biodegradable Hydrogels Based on Poly(ethylene glycol) and Sebacic Acid as Tissue Engineering Scaffolds. Biomacromolecules. 2007;9:149–157. doi: 10.1021/bm700924n. [DOI] [PubMed] [Google Scholar]
- Kim Y-T, Hitchcock RW, Bridge MJ, Tresco PA. Chronic response of adult rat brain tissue to implants anchored to the skull. Biomaterials. 2004;25:2229–2237. doi: 10.1016/j.biomaterials.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Kloxin AM, Kloxin CJ, Bowman CN, Anseth KS. Mechanical Properties of Cellularly Responsive Hydrogels and Their Experimental Determination. Adv Mater. 2010;22:3484–3494. doi: 10.1002/adma.200904179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, Jannetta P, DeCesare S, Elder EM, McGrogan M, Reitman MA, Bynum L. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55:565–569. doi: 10.1212/wnl.55.4.565. [DOI] [PubMed] [Google Scholar]
- Kraehenbuehl TP, Zammaretti P, Van der Vlies AJ, Schoenmakers RG, Lutolf MP, Jaconi ME, Hubbell JA. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: Systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials. 2008;29:2757–2766. doi: 10.1016/j.biomaterials.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Laird MD, Vender JR, Dhandapani KM. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals. 2008;16:154–164. doi: 10.1159/000111560. [DOI] [PubMed] [Google Scholar]
- Lampe KJ, Bjugstad KB, Mahoney MJ. Impact of Degradable Macromer Content in a Poly(Ethylene Glycol) Hydrogel on Neural Cell Metabolic Activity, Redox State, Proliferation, and Differentiation. Tissue Eng Part A. 2010a;16:1857–1866. doi: 10.1089/ten.tea.2009.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe KJ, Kern DS, Mahoney MJ, Bjugstad KB. The administration of BDNF and GDNF to the brain via PLGA microparticles patterned within a degradable PEG-based hydrogel: Protein distribution and the glial response. J Biomed Mater Res A. 2011;96A:595–607. doi: 10.1002/jbm.a.33011. [DOI] [PubMed] [Google Scholar]
- Lampe KJ, Namba RM, Bjugstad KB, Mahoney MJ. Effect of macromer weight percent on neural cell growth in 2D and 3D nondegradable PEG hydrogel culture. J Biomed Mater Res A. 2010b;94A:1162–1171. doi: 10.1002/jbm.a.32787. [DOI] [PubMed] [Google Scholar]
- Lampe KJ, Namba RM, Silverman TR, Bjugstad KB, Mahoney MJ. Impact of Lactic Acid on Cell Proliferation and Free Radical-Induced Cell Death in Monolayer Cultures of Neural Precursor Cells. Biotechnol Bioeng. 2009;103:1214–1223. doi: 10.1002/bit.22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langone F, Lora S, Veronese FM, Caliceti P, Parnigotto PP, Valenti F, Palma G. Peripheral-Nerve Repair Using a Poly(organo)phosphazene Tubular Prosthesis. Biomaterials. 1995;16:347–353. doi: 10.1016/0142-9612(95)93851-4. [DOI] [PubMed] [Google Scholar]
- Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int J Pharm. 2001;221:1–22. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- Lee KY, Mooney DJ. Hydrogels for Tissue Engineering. Chem Rev. 2001;101:1869–1880. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- Lee Y-B, Polio S, Lee W, Dai G, Menon L, Carroll R, Yoo S-S. Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp Neurol. 2010;223:645–652. doi: 10.1016/j.expneurol.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Li RH. Materials for immunoisolated cell transplantation. Adv Drug Deliv Rev. 1998;33:87–109. doi: 10.1016/s0169-409x(98)00022-2. [DOI] [PubMed] [Google Scholar]
- Liechty WB, Kryscio DR, Slaughter BV, Peppas NA. Polymers for Drug Delivery Systems. Annu Rev Chem Biomol Eng. 2010;1:149–173. doi: 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-C, Metters AT, Anseth KS. Functional PEG-peptide hydrogels to modulate local inflammation inducedby the pro-inflammatory cytokine TNF[alpha] Biomaterials. 2009;30:4907–4914. doi: 10.1016/j.biomaterials.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Anseth KS. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm Res. 2009;26:631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LFH, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF – a Glial-Cell Line Derived Neurotrophic Factor for Midbrain Dopaminergic-Neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lin S, Sangaj N, Razafiarison T, Zhang C, Varghese S. Influence of Physical Properties of Biomaterials on Cellular Behavior. Pharm Res. 2011;28:1422–1430. doi: 10.1007/s11095-011-0378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SQ, Tian Q, Hedrick JL, Po Hui JH, Rachel Ee PL, Yang YY. Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage. Biomaterials. 2010;31:7298–7307. doi: 10.1016/j.biomaterials.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Lodish H, Berk A, Matsudaira P, Kaiser C, Krieger M, Scott M, Zipursky S, Darnell J, editors. Molecular Cell Biology. 6. W.H. Freeman & Company; New York: 2007. pp. 820–833. [Google Scholar]
- Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Tang C, Chaly T, Greene P, Breeze R, Fahn S, Freed C, Dhawan V, Eidelberg D. Dopamine Cell Implantation in Parkinson’s Disease: Long-Term Clinical and 18F-FDOPA PET Outcomes. J Nucl Med. 2010a;51:7–15. doi: 10.2967/jnumed.109.066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Nelson DM, Hong Y, Wagner WR. Thermally Responsive Injectable Hydrogel Incorporating Methacrylate-Polylactide for Hydrolytic Lability. Biomacromolecules. 2010b;11:1873–1881. doi: 10.1021/bm1004299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson JP, Saeed AO, Fernandez-Trillo F, Alexander C. Synthetic polymers for biopharmaceutical delivery. Polym Chem. 2011;2:48–59. [Google Scholar]
- Mahoney MJ, Anseth KS. Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials. 2006;27:2265–2274. doi: 10.1016/j.biomaterials.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Mann BK, Gobin AS, Tsai AT, Schmedlen RH, West JL. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22:3045–3051. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- Mansour HM, Sohn M, Al-Ghananeem A, DeLuca PP. Materials for Pharmaceutical Dosage Forms: Molecular Pharmaceutics and Controlled Release Drug Delivery Aspects. Int J Mol Sci. 2010;11:3298–3322. doi: 10.3390/ijms11093298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens PJ, Bryant SJ, Anseth KS. Tailoring the Degradation of Hydrogels Formed from Multivinyl Poly(ethylene glycol) and Poly(vinyl alcohol) Macromers for Cartilage Tissue Engineering. Biomacromolecules. 2003;4:283–292. doi: 10.1021/bm025666v. [DOI] [PubMed] [Google Scholar]
- Mather ML, Tomlins PE. Hydrogels in regenerative medicine: towards understanding structure-function relationships. Regen Med. 2010;5:809–821. doi: 10.2217/rme.10.32. [DOI] [PubMed] [Google Scholar]
- McRae A, Hjorth S, Dahlstrom A, Dillon L, Mason D, Tice T. Dopamine Fiber Growth Induction by Implantation of Synthetic Dopamine-Containing Microspheres in Rats with Experimental Hemiparkinsonism. Mol Chem Neuropathol. 1992;16:123–141. doi: 10.1007/BF03159965. [DOI] [PubMed] [Google Scholar]
- Mellott MB, Searcy K, Pishko MV. Release of protein from highly cross-linked hydrogels of poly(ethylene glycol) diacrylate fabricated by UV polymerization. Biomaterials. 2001;22:929–941. doi: 10.1016/s0142-9612(00)00258-1. [DOI] [PubMed] [Google Scholar]
- Metters AT, Anseth KS, Bowman CN. Fundamental studies of a novel, biodegradable PEG-b-PLA hydrogel. Polymer (Guildf) 2000;41:3993–4004. [Google Scholar]
- Meyer M, Widmer HR, Wagner B, Guzman R, Evtouchenko L, Seiler RW, Spenger C. Comparison of mesencephalic free-floating tissue culture grafts and cell suspension grafts in the 6-hydroxydopamine-lesioned rat. Exp Brain Res. 1998;119:345–355. doi: 10.1007/s002210050350. [DOI] [PubMed] [Google Scholar]
- Michel M, Vautier D, Voegel JC, Schaaf P, Ball V. Layer by layer self-assembled polyelectrolyte multilayers with embedded phospholipid vesicles. Langmuir. 2004;20:4835–4839. doi: 10.1021/la049736q. [DOI] [PubMed] [Google Scholar]
- Mironov V, Kasyanov V, Zheng Shu X, Eisenberg C, Eisenberg L, Gonda S, Trusk T, Markwald RR, Prestwich GD. Fabrication of tubular tissue constructs by centrifugal casting of cells suspended in an in situ crosslinkable hyaluronan-gelatin hydrogel. Biomaterials. 2005;26:7628–7635. doi: 10.1016/j.biomaterials.2005.05.061. [DOI] [PubMed] [Google Scholar]
- Mithieux SM, Tu Y, Korkmaz E, Braet F, Weiss AS. In situ polymerization of tropoelastin in the absence of chemical cross-linking. Biomaterials. 2009;30:431–435. doi: 10.1016/j.biomaterials.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Moffat KL, Sun W-HS, Pena PE, Chahine NO, Doty SB, Ateshian GA, Hung CT, Lu HH. Characterization of the structure function relationship at the ligament-to-bone interface. Proc Natl Acad Sci U S A. 2008;105:7947–7952. doi: 10.1073/pnas.0712150105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba RM, Cole AA, Bjugstad KB, Mahoney MJ. Development of porous PEG hydrogels that enable efficient, uniform cell-seeding and permit early neural process extension. Acta Biomater. 2009;5:1884–1897. doi: 10.1016/j.actbio.2009.01.036. [DOI] [PubMed] [Google Scholar]
- Nettles DL, Vail TP, Morgan MT, Grinstaff MW, Setton LA. Photocrosslinkable Hyaluronan as a Scaffold for Articular Cartilage Repair. Ann Biomed Eng. 2004;32:391–397. doi: 10.1023/b:abme.0000017552.65260.94. [DOI] [PubMed] [Google Scholar]
- Nguyen LH, Kudva AK, Saxena NS, Roy K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials. 2011;32:6946–6952. doi: 10.1016/j.biomaterials.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Nguyen MK, Lee DS. Injectable Biodegradable Hydrogels. Macromol Biosci. 2010;10:563–579. doi: 10.1002/mabi.200900402. [DOI] [PubMed] [Google Scholar]
- Nichterwitz S, Hoffmann N, Hajosch R, Oberhoffner S, Schlosshauer B. Bioengineered glial strands for nerve regeneration. Neurosci Lett. 2010;484:118–122. doi: 10.1016/j.neulet.2010.08.028. [DOI] [PubMed] [Google Scholar]
- Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:149–165. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunamaker EA, Otto KJ, Kipke DR. Investigation of the material properties of alginate for the development of hydrogel repair of dura mater. J Mech Behav Biomed Mater. 2011;4:16–33. doi: 10.1016/j.jmbbm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Nuttelman CR, Henry SM, Anseth KS. Synthesis and characterization of photocrosslinkable, degradable poly(vinyl alcohol)-based tissue engineering scaffolds. Biomaterials. 2002;23:3617–3626. doi: 10.1016/s0142-9612(02)00093-5. [DOI] [PubMed] [Google Scholar]
- Nuttelman CR, Rice MA, Rydholm AE, Salinas CN, Shah DN, Anseth KS. Macromolecular monomers for the synthesis of hydrogel niches and their application in cell encapsulation and tissue engineering. Prog Polym Sci. 2008;33:167–179. doi: 10.1016/j.progpolymsci.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard K, Jones SA, Hyman C, Zimmer J. Effects of Donor Age and Brain-Derived Neurotrophic Factor on the Survival of Dopaminergic Neurons and Axonal Growth in Postnatal Rat Nigrostriatal Cocultures. Exp Neurol. 1996;142:340–350. doi: 10.1006/exnr.1996.0203. [DOI] [PubMed] [Google Scholar]
- Pailler-Mattei C, Bec S, Zahouani H. In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med Eng Phys. 2008;30:599–606. doi: 10.1016/j.medengphy.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Panigrahi M, Das PK, Parikh PM. Brain tumor and Gliadel wafer treatment. Indian J Cancer. 2011;48:11–17. doi: 10.4103/0019-509X.76623. [DOI] [PubMed] [Google Scholar]
- Park J, Lim E, Back S, Na H, Park Y, Sun K. Nerve regeneration following spinal cord injury using matrix metalloproteinase-sensitive, hyaluronic acid-based biomimetic hydrogel scaffold containing brain-derived neurotrophic factor. J Biomed Mater Res A. 2010;93A:1091–1099. doi: 10.1002/jbm.a.32519. [DOI] [PubMed] [Google Scholar]
- Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31:7836–7845. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- Pearson RG, Molino Y, Williams PM, Tendler SJB, Davies MC, Roberts CJ, Shakesheff KM. Spatial confinement of neurite regrowth from dorsal root ganglia within nonporous microconduits. Tissue Eng. 2003;9:201–208. doi: 10.1089/107632703764664675. [DOI] [PubMed] [Google Scholar]
- Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv Mater. 2006;18:1345–1360. [Google Scholar]
- Peppas NA, Keys KB, Torres-Lugo M, Lowman AM. Poly(ethylene glycol)-containing hydrogels in drug delivery. J Control Release. 1999;62:81–87. doi: 10.1016/s0168-3659(99)00027-9. [DOI] [PubMed] [Google Scholar]
- Perale G, Rossi F, Sundstrom E, Bacchiega S, Masi M, Forloni G, Veglianese P. Hydrogels in Spinal Cord Injury Repair Strategies. ACS Chem Neurosci. 2011;2:336–345. doi: 10.1021/cn200030w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettikiriarachchi JTS, Parish CL, Shoichet MS, Forsythe JS, Nisbet DR. Biomaterials for Brain Tissue Engineering. Aust J Chem. 2010;63:1143–1154. [Google Scholar]
- Place ES, George JH, Williams CK, Stevens MM. Synthetic polymer scaffolds for tissue engineering. Chem Soc Rev. 2009;38:1139–1151. doi: 10.1039/b811392k. [DOI] [PubMed] [Google Scholar]
- Quinchia Johnson B, Fox R, Chen X, Thibeault S. Tissue regeneration of the vocal fold using bone marrow mesenchymal stem cells and synthetic extracellular matrix injections in rats. Laryngoscope. 2010;120:537–545. doi: 10.1002/lary.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirico-Santos T, Fonseca CO, Lagrota-Candido J. Brain sweet brain Importance of sugars for the cerebral microenvironment and tumor development. Arq Neuropsiquiatr. 2010;68:799–803. doi: 10.1590/s0004-282x2010000500024. [DOI] [PubMed] [Google Scholar]
- Rabinovich SS, Seledtsov VI, Banul NV, Poveshchenko OV, Senyukov VV, Astrakov SV, Samarin DM, Taraban VY. Cell therapy of brain stroke. Bull Exp Biol Med. 2005;139:126–128. doi: 10.1007/s10517-005-0229-y. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Weiss S, Elsworth JD, Roth RH, Wakeman DR, Bjugstad KB, Collier TJ, Blanchard BC, Teng YD, Synder EY, Sladek JR. Cellular Repair in the Parkinsonian Nonhuman Primate Brain. Rejuvenation Res. 2010;13:188–194. doi: 10.1089/rej.2009.0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice MA, Anseth KS. Controlling cartilaginous matrix evolution in hydrogels with degradation triggered by exogenous addition of an enzyme. Tissue Eng. 2007;13:683–691. doi: 10.1089/ten.2006.0142. [DOI] [PubMed] [Google Scholar]
- Rice MA, Sanchez-Adams J, Anseth KS. Exogenously Triggered, Enzymatic Degradation of Photopolymerized Hydrogels with Polycaprolactone Subunits: Experimental Observation and Modeling of Mass Loss Behavior. Biomacromolecules. 2006;7:1968–1975. doi: 10.1021/bm060086+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rnjak J, Li Z, Maitz PKM, Wise SG, Weiss AS. Primary human dermal fibroblast interactions with open weave three-dimensional scaffolds prepared from synthetic human elastin. Biomaterials. 2009;30:6469–6477. doi: 10.1016/j.biomaterials.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Roberts JJ, Earnshaw A, Ferguson VL, Bryant SJ. Comparative study of the viscoelastic mechanical behavior of agarose and poly(ethylene glycol) hydrogels. J Biomed Mater Res B Appl Biomater. 2011;99B:158–169. doi: 10.1002/jbm.b.31883. [DOI] [PubMed] [Google Scholar]
- Ruel-Gariepy E, Leroux JC. In situ-forming hydrogels - review of temperature-sensitive systems. Eur J Pharm Biopharm. 2004;58:409–426. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Brain extracellular matrix. Glycobiology. 1996;6:489–492. doi: 10.1093/glycob/6.5.489. [DOI] [PubMed] [Google Scholar]
- Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate Modulus Directs Neural Stem Cell Behavior. Biophys J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmaso S, Bersani S, Scomparin A, Mastrotto F, Caliceti P. Supramolecular Bioconjugates for Protein and Small Drug Delivery. Isr J Chem. 2010;50:160–174. [Google Scholar]
- Sanchis J, Canal F, Lucas R, Vicent MJ. Polymer-drug conjugates for novel molecular targets. Nanomedicine. 2010;5:915–935. doi: 10.2217/nnm.10.71. [DOI] [PubMed] [Google Scholar]
- Sawhney AS, Pathak CP, Hubbell JA. Bioerodible Hydrogels Based on Photopolymerized Poly(ethylene glycol)-co-Poly(alpha-hydroxy acid) Diacrylate Macromers. Macromolecules. 1993;26:581–587. [Google Scholar]
- Schense JC, Hubbell JA. Three-dimensional migration of neurites is mediated by adhesion site density and affinity. J Biol Chem. 2000;275:6813–6818. doi: 10.1074/jbc.275.10.6813. [DOI] [PubMed] [Google Scholar]
- Schlosshauer B, Müller E, Schröder B, Planck H, Müller H-W. Rat Schwann cells in bioresorbable nerve guides to promote and accelerate axonal regeneration. Brain Res. 2003;963:321–326. doi: 10.1016/s0006-8993(02)03930-6. [DOI] [PubMed] [Google Scholar]
- Seib FP, Prewitz M, Werner C, Bornhäuser M. Matrix elasticity regulates the secretory profile of human bone marrow-derived multipotent mesenchymal stromal cells (MSCs) Biochem Biophys Res Commun. 2009;389:663–667. doi: 10.1016/j.bbrc.2009.09.051. [DOI] [PubMed] [Google Scholar]
- Seidlits SK, Khaing ZZ, Petersen RR, Nickels JD, Vanscoy JE, Shear JB, Schmidt CE. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials. 2010;31:3930–3940. doi: 10.1016/j.biomaterials.2010.01.125. [DOI] [PubMed] [Google Scholar]
- Senthilkuma KS, Saravanan KS, Chandra G, Sindhu KM, Jayakrishnan A, Mohanakumar KP. Unilateral implantation of dopamine-loaded biodegradable hydrogel in the striatum attenuates motor abnormalities in the 6-hydroxydopamine model of hemi-parkinsonism. Behav Brain Res. 2007;184:11–18. doi: 10.1016/j.bbr.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Shoichet MS. Polymer Scaffolds for Biomaterials Applications. Macromolecules. 2010;43:581–591. [Google Scholar]
- Shu XZ, Ahmad S, Liu YC, Prestwich GD. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J Biomed Mater Res A. 2006;79A:902–912. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- Shu XZ, Ghosh K, Liu Y, Palumbo FS, Luo Y, Clark RA, Prestwich GD. Attachment and spreading of fibroblasts on an RGD peptide–modified injectable hyaluronan hydrogel. J Biomed Mater Res A. 2004;68A:365–375. doi: 10.1002/jbm.a.20002. [DOI] [PubMed] [Google Scholar]
- Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in Regenerative Medicine. Adv Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller KL, Holloway JL, Gribb ME, Lowman AM. Design of semi-degradable hydrogels based on poly(vinyl alcohol) and poly(lactic-co-glycolic acid) for cartilage tissue engineering. J Tissue Eng Regen Med. 2010;5:636–647. doi: 10.1002/term.356. [DOI] [PubMed] [Google Scholar]
- Squire LR, Bloom FE, McConnell SK, Roberts JL, Spitzer NC, Zigmond MJ, editors. Fundamental Neuroscience. 2. Academic Press; San Diego: 2003. pp. 67–69. [Google Scholar]
- Stachowiak AN, Bershteyn A, Tzatzalos E, Irvine DJ. Bioactive hydrogels with an ordered cellular structure combine interconnected macroporosity and robust mechanical properties. Adv Mater. 2005;17:399–403. [Google Scholar]
- Stammen JA, Williams S, Ku DN, Guldberg RE. Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials. 2001;22:799–806. doi: 10.1016/s0142-9612(00)00242-8. [DOI] [PubMed] [Google Scholar]
- Stichel CC, Müller HW. The CNS lesion scar: new vistas on an old regeneration barrier. Cell Tissue Res. 1998;294:1–9. doi: 10.1007/s004410051151. [DOI] [PubMed] [Google Scholar]
- Suarez-Monteagudo C, Hernandez-Ramirez P, Alvarez-Gonzalez L, Garcia-Maeso I, de la Cuetara-Bernal K, Castillo-Diaz L, Bringas-Vega ML, Martinez-Aching G, Morales-Chacon LM, Baez-Martin MM, Sanchez-Catasus C, Carballo-Barreda M, Rodriguez-Rojas R, Gomez-Fernandez L, Alberti-Amador E, Macias-Abraham C, Balca ED, Rosales LC, Perez LD, Ferrer BBS, Gonzalez RM, Bergado JA. Autologous bone marrow stem cell neurotransplantation in stroke patients. An open study. Restor Neurol Neurosci. 2009;27:151–161. doi: 10.3233/RNN-2009-0483. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Krishnan UM, Sethuraman S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J Biomed Sci. 2009;16:108. doi: 10.1186/1423-0127-16-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs LJ, Mikos AG. Development of poly(propylene fumarate-co-ethylene glycol) as an injectable carrier for endothelial cells. Cell Transplant. 1999;8:345–350. doi: 10.1177/096368979900800402. [DOI] [PubMed] [Google Scholar]
- Sung JH, Hwang M-R, Kim JO, Lee JH, Kim YI, Kim JH, Chang SW, Jin SG, Kim JA, Lyoo WS, Han SS, Ku SK, Yong CS, Choi H-G. Gel characterisation and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int J Pharm. 2010;392:232–240. doi: 10.1016/j.ijpharm.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, Turner JN, Shain W. Brain responses to micro-machined silicon devices. Brain Res. 2003;983:23–35. doi: 10.1016/s0006-8993(03)03023-3. [DOI] [PubMed] [Google Scholar]
- Tate MC, Shear DA, Hoffman SW, Stein DG, LaPlaca MC. Biocompatibility of methylcellulose-based constructs designed for intracerebral gelation following experimental traumatic brain injury. Biomaterials. 2001;22:1113–1123. doi: 10.1016/s0142-9612(00)00348-3. [DOI] [PubMed] [Google Scholar]
- Thomasin C, Corradin G, Men Y, Merkle HP, Gander B. Tetanus toxoid and synthetic malaria antigen containing poly(lactide)/poly(lactide-co-glycolide) microspheres: Importance of polymer degradation and antigen release for immune response. J Control Release. 1996;41:131–145. [Google Scholar]
- Turner JN, Shain W, Szarowski DH, Andersen M, Martins S, Isaacson M, Craighead H. Cerebral Astrocyte Response to Micromachined Silicon Implants. Exp Neurol. 1999;156:33–49. doi: 10.1006/exnr.1998.6983. [DOI] [PubMed] [Google Scholar]
- Tzeng SY, Lavik EB. Photopolymerizable nanoarray hydrogels deliver CNTF and promote differentiation of neural stem cells. Soft Matter. 2010;6:2208–2215. [Google Scholar]
- Ueki Y, Sakamoto N, Sato M. Direct measurement of shear strain in adherent vascular endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun. 2010;394:94–99. doi: 10.1016/j.bbrc.2010.02.115. [DOI] [PubMed] [Google Scholar]
- Uemura M, Refaat MM, Shinoyama M, Hayashi H, Hashimoto N, Takahashi J. Matrigel supports survival and neuronal differentiation of grafted embryonic stem cell-derived neural precursor cells. J Neurosci Res. 2010;88:542–551. doi: 10.1002/jnr.22223. [DOI] [PubMed] [Google Scholar]
- Van Tomme SR, Storm G, Hennink WE. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int J Pharm. 2008;355:1–18. doi: 10.1016/j.ijpharm.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Vanderhooft JL, Alcoutlabi M, Magda JJ, Prestwich GD. Rheological Properties of Cross-Linked Hyaluronan-Gelatin Hydrogels for Tissue Engineering. Macromol Biosci. 2009;9:20–28. doi: 10.1002/mabi.200800141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters DJ, Engberg K, Parke-Houben R, Hartmann L, Ta CN, Toney MF, Frank CW. Morphology of Photopolymerized End-Linked Poly(ethylene glycol) Hydrogels by Small-Angle X-ray Scattering. Macromolecules. 2010;43:6861–6870. doi: 10.1021/ma101070s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber LM, Hayda KN, Anseth KS. Cell-Matrix Interactions Improve beta-Cell Survival and Insulin Secretion in Three-Dimensional Culture. Tissue Eng Part A. 2008;14:1959–1968. doi: 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber RA, Breidenbach WC, Brown RE, Jabaley ME, Mass DP. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000;106:1036–1045. doi: 10.1097/00006534-200010000-00013. [DOI] [PubMed] [Google Scholar]
- West JL, Hubbell JA. Photopolymerized Hydrogel Materials for Drug-Delivery Applications. React Polym. 1995;25:139–147. [Google Scholar]
- West JL, Hubbell JA. Polymeric Biomaterials with Degradation Sites for Proteases Involved in Cell Migration. Macromolecules. 1998;32:241–244. [Google Scholar]
- Williams CG, Malik AN, Kim TK, Manson PN, Elisseeff JH. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 2005;26:1211–1218. doi: 10.1016/j.biomaterials.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Williams DF. Tissue-biomaterial interactions. J Mater Sci. 1987;22:3421–3445. [Google Scholar]
- Wilson JT, Cui WX, Chaikof EL. Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation. Nano Lett. 2008;8:1940–1948. doi: 10.1021/nl080694q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AMS, Rahim AA, Waddington SN, Cooper JD. Current therapies for the soluble lysosomal forms of neuronal ceroid lipofuscinosis. Biochem Soc Trans. 2010;38:1484–1488. doi: 10.1042/BST0381484. [DOI] [PubMed] [Google Scholar]
- Wood MD, MacEwan MR, French AR, Moore AM, Hunter DA, Mackinnon SE, Moran DW, Borschel GH, Sakiyama-Elbert SE. Fibrin matrices with affinity-based delivery systems and neurotrophic factors promote functional nerve regeneration. Biotechnol Bioeng. 2010;106:970–979. doi: 10.1002/bit.22766. [DOI] [PubMed] [Google Scholar]
- Yang C-Y, Song B, Ao Y, Nowak AP, Abelowitz RB, Korsak RA, Havton LA, Deming TJ, Sofroniew MV. Biocompatibility of amphiphilic diblock copolypeptide hydrogels in the central nervous system. Biomaterials. 2009;30:2881–2898. doi: 10.1016/j.biomaterials.2009.01.056. [DOI] [PubMed] [Google Scholar]
- Yang JJ, Chen YM, Kurokawa T, Gong JP, Onodera S, Yasuda K. Gene expression, glycocalyx assay, and surface properties of human endothelial cells cultured on hydrogel matrix with sulfonic moiety: Effect of elasticity of hydrogel. J Biomed Mater Res A. 2010;95A:531–542. doi: 10.1002/jbm.a.32875. [DOI] [PubMed] [Google Scholar]
- Young JL, Engler AJ. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials. 2011;32:1002–1009. doi: 10.1016/j.biomaterials.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Neeley WL, Pritchard CD, Slotkin JR, Woodard EJ, Langer R, Teng YD. Blockade of Peroxynitrite-Induced Neural Stem Cell Death in the Acutely Injured Spinal Cord by Drug-Releasing Polymer. Stem Cells. 2009;27:1212–1222. doi: 10.1002/stem.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuh EL, Dillon WP. Intracranial Hypotension and Intracranial Hypertension. Neuroimaging Clin N Am. 2010;20:597–617. doi: 10.1016/j.nic.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Lu W, Hipkens S, Wiegand SJ. BDNF Enhances the Functional Reinnervation of the Striatum by Grafted Fetal Dopamine Neurons. Exp Neurol. 1996;137:105–118. doi: 10.1006/exnr.1996.0011. [DOI] [PubMed] [Google Scholar]
- Zhong J, Chan A, Morad L, Kornblum HI, Guoping Fan Carmichael ST. Hydrogel Matrix to Support Stem Cell Survival After Brain Transplantation in Stroke. Neurorehabil Neural Repair. 2010;24:636–644. doi: 10.1177/1545968310361958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Ma G, Shi S, Yang D, Nie J. Photopolymerized water-soluble chitosan-based hydrogel as potential use in tissue engineering. Int J Biol Macromol. 2011;48:408–413. doi: 10.1016/j.ijbiomac.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–4656. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmökel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA. Cell-demanded release of VEGF from synthetic, biointeractive cell-ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17:2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]