Abstract
Neuroendocrine (NE) carcinoma of the breast is extremely rare and constitutes less than 0.1% of all breast tumors. Only a few studies are currently available in the literature and a standard approach to treating this tumor has yet to be established. The aim of this study was to apply pathological treatment modalities in clinical practice and to select the most appropriate treatment accordingly. Six female patients were diagnosed with primary NE carcinoma of the breast. The patients underwent modified radical mastectomy with axillary dissection. Pathological specimens were stained with hematoxylin and eosin and an immunohistochemical panel of antibodies [neuron-specific enolase (NSE), chromogranin, synoptophysin, estrogen and progesterone receptor, c-erbB2 and Ki-67]. The results showed that tumor size ranged from 2 to 4.5 cm in diameter. Lymph node metastasis was detected in 4 (67%) patients. Estrogen and progesterone receptor expression was found in 5 (83%) patients. None of the patients expressed c-erbB2. Chromogranin was found to be positive in 5 (83%) patients. Synoptophysin expression was detected in 5 (83%) patients. NSE was stained in 4 (67%) patients. An intraductal component was found in 5 (83%) patients. Lymphovascular invasion was found in 5 (83%) patients. Adjuvant chemotherapy was administered to patients with a Ki-67 index of ≥10%. Radiotherapy was administered to 4 (67%) patients, and 4 (67%) patients received hormonal therapy. The mean follow-up time was 31.1 months (range 12-52). All 6 patients survived, although following chemotherapy and tamoxifen, the disease progressed in 1 patient who received second-line hormonal therapy. In conclusion, NE carcinoma of the breast is a distinct entity. Management of this rare tumor may include surgery and radiotherapy depending on the size of the tumor and lymph node status. However, the exact role of chemotherapy and hormonal therapy has yet to be established. Adjuvant chemotherapy is recommended for patients with a Ki-67 index of ≥10%, and hormonal treatment appears to be feasible in patients who are positive for estrogen and/or progesterone receptor.
Introduction
Neuroendocrine (NE) tumors originate from NE cells that are present throughout the body. Most of these cells are located in the gastrointestinal and bronchopulmonary system. NE carcinoma of the breast is rare and constitutes less than 0.1% of all breast tumors (1). In 1977, Cubilla and Woodruff first reported a carcinoid tumor of the breast, presented with argyrophila and cytoplasmic dense granules (2). In 2003, primary NE carcinoma of the breast was identified as a distinct entity by the World Health Organization (WHO) classification of tumors. The WHO classification defines primary NE carcinoma of the breast as tumors that express 50% or more of NE markers (3). Scattered NE cells can be detected in 10-50% of breast tumors depending on the detection methods employed (4). NE-differentiated breast cancer identifies a group of tumors that co-express apocrine phenotype with NE markers (5).
Primary NE carcinoma of the breast comprises solid NE carcinoma, atypical carcinoid tumors, small cell/oat cell carcinoma and large cell carcinoma. The limited number of cases available make it difficult to adequately assess prognosis of this rare tumor. In addition, a standard approach to the management of this disease has yet to be established, since only a few case reports have indicated therapeutic options (2,6-8). Therefore the aim of this study was to investigate management of NE carcinoma of the breast.
Patients and methods
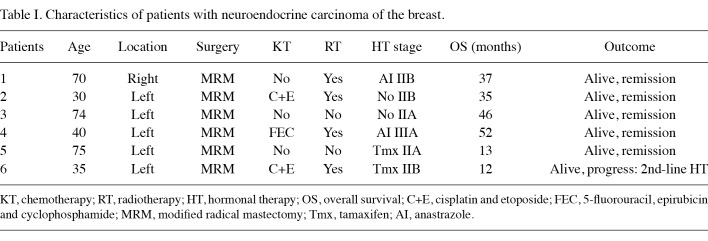
Patients. Between 2006 and 2010, 6 female patients with primary NE carcinoma were diagnosed and treated at the Cumhuriyet University Hospital's Department of General Surgery. The median age of the 6 female patients was 55 years (range 30-75). Patient characteristics are shown in Table I.
Table 1. Characteristics of patients with neuroendocrine carcinoma of the breast.
All 6 patients were admitted to the hospital with the complaint of breast mass. The tumor was unilaterally located in all 6 cases. Breast mass was evaluated by mammography and breast ultrasound. Prior to modified radical mastectomy, fine needle aspiration biopsy was used in 2 patients. Four patients underwent excisional biopsy. Final diagnosis was made by pathological examination of the mastectomy specimens. A chest X-ray, abdominal ultrasound and bone scintigraphy were performed to evaluate distant spread of the tumor. Patients were evaluated by a tomography scan if a suspected lesion was detected on the chest X-ray or abdominal ultrasound.
Pathology. Recent surgical specimens obtained from the 6 patients were fixed in formalin and routinely processed. The materials were stained with hematoxylin and eosin. If a NE component was suspected, an immunohistochemical examination was carried out by the avidin-biotin method using chromogranin, synoptophysin or neuron-specific enolase (NSE) antibodies. Selection criteria for NE carcinoma was >50% staining with at least two of the above NE markers, i.e., chromogranin, synoptophysin or NSE. An immunohistochemical analysis of steroid receptors was performed on formalin-fixed tissues using specific monoclonal antibodies to detect estrogen and progesterone receptors. C-erbB2 expression was also evaluated immunohistochemically. Mitotic activity was measured using the Ki-67 proliferative index.
Results
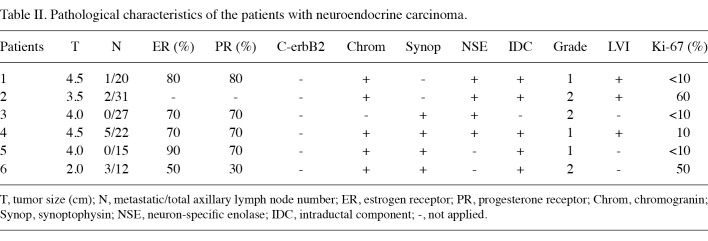
Six female patients presenting with primary NE carcinoma of the breast were included in this study. Surgical specimens obtained from the 6 patients were examined. Tumor size ranged from 2 to 4.5 cm in diameter. The pathological characteristics of the patients are shown in Table II. Four patients were diagnosed as having solid NE carcinoma of the breast (Fig. 1). The diagnosis of the remaining 2 patients was cellular mucinous NE carcinoma of the breast (Fig. 2) and large cell NE carcinoma of the breast, respectively. Lymph node metastasis was detected in 4 (67%) patients. Estrogen and progesterone receptor expression was found in 5 (83%) patients. None of the patients expressed c-erbB2. Chromogranin was found to be positive in 5 (83%) patients (Fig. 3). Synoptophysin expression was detected in 5 (83%) patients (Fig. 4). An intraductal component was found in 5 (83%) patients and lymphovascular invasion in 5 (83%) patients.
Table 2. Pathological characteristics of the patients with neuroendocrine carcinoma.
Figure 1. Tumor cells forming trabecular structure and rosettes (hemato-xylin and eosin; magnification, x10).
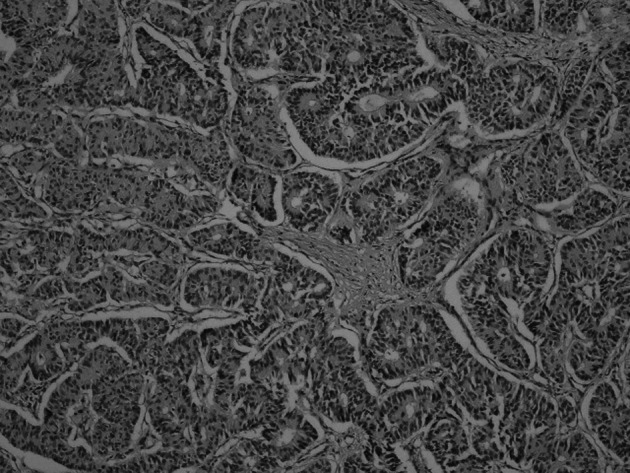
Figure 2. Cellular mucinous NE carcinoma of the breast (hematoxylin and eosin; magnification, x20).
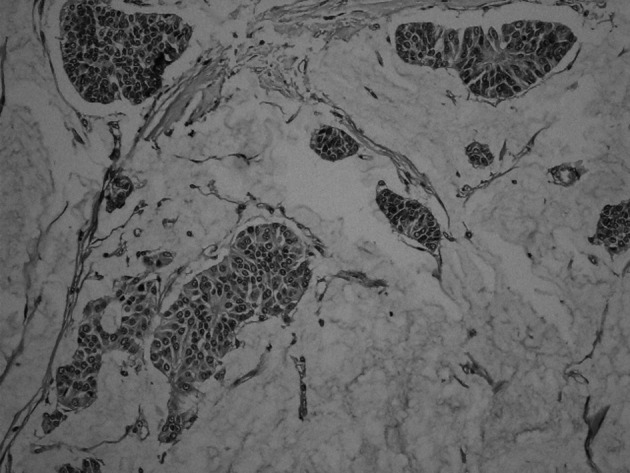
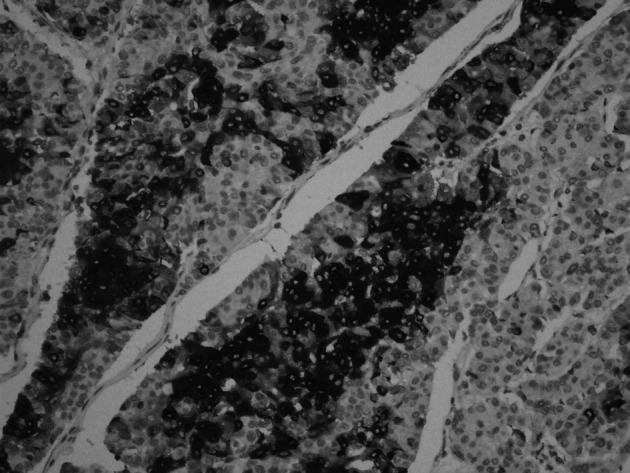
Figure 3. Chromogranin positivity (magnification, x10).
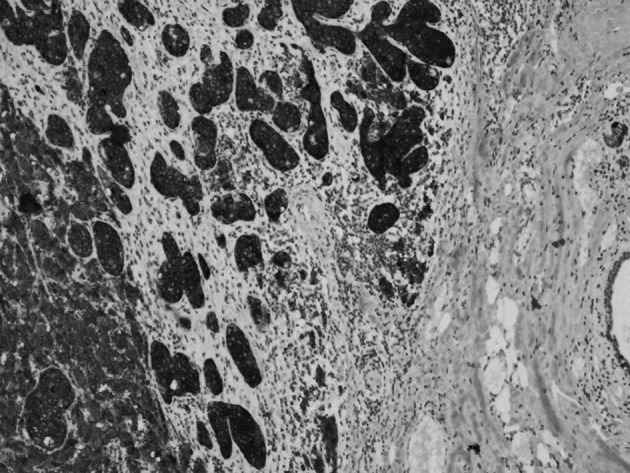
Figure 4. Diffuse synoptophysin staining (magnification, x20).
Treatment and outcome. All 6 patients underwent modified radical mastectomy and axillary dissection. Adjuvant chemotherapy was administered to 3 (50%) patients. Cisplatin and etoposide combination therapy was administered to 2 patients. One patient received an anthracycline-containing regimen. Radiotherapy was administered to 4 (67%) patients, while 4 (67%) patients received hormonal therapy. The mean follow-up time was 31.1 months (range 12-52). Two patients had recently been diagnosed. All 6 patients survived, although the disease progressed in 1 patient who received second-line hormonal therapy.
Discussion
Primary NE carcinoma of the breast is a distinct entity and constitutes less than 1% of NE carcinomas (9). These tumors are more common in elderly women of 60-80 years of age (10). In the present study, the median age of diagnosis was 59 years. NE tumors of the breast have no specific presenting signs or symptoms. In the present study, the most common presenting symptom was a breast mass. No bilateral tumor was observed and the majority of the tumors were diagnosed in the left breast. The radiological features are non-specific. However, findings of certain studies have revealed that NE-differentiated tumors of the breast present as dense round or irregular masses with spiculated or lobular margins on the mammogram (5,11). Definitive diagnosis is made with core needle biopsy, allowing for the immunohistochemical evaluation of the specimen for the NE markers.
Differential diagnosis of NE carcinoma of the breast from metastatic tumor is essential. The ductal carcinoma in situ component is the only proof of the primary nature of the tumor (1). However, a number of cases found in the literature did not exhibit this feature (12,13). Tomography screening should be carried out if metastatic disease is suspected. In the present study, patients were principally evaluated with a chest X-ray and abdominal ultrasound. A tomography scan was preferred whenever a suspected lesion was detected in these studies. Somatostatin receptor scintigraphy (SRS) has markedly improved the visualization of NE tumors at other sites (14). Scaramuzzi et al used SRS for diagnosis in patients with NE carcinoma of the breast (14). SRS is usually valid for well-differentiated NE carcinomas. Although use of positron emission tomography (PET) for the evaluation of NE tumors has been limited, tumors with moderate or high proliferative activity can be identified by fluorodeoxyglucose (FDG) PET (15). However, SRS and PET are not available in our clinic. Thus, the patients in the present study were not evaluated using these techniques. In concordance with other studies, diagnosis of patients was made by pathological examination (4,5,16).
In pathology, NE carcinoma of the breast is defined as tumors having specific morphological features and as expressing NE markers, particularly those of chromogranin or synoptophysin in ≥50% of the cells (5). NSE is another NE marker used in the study of argyrophilic breast carcinoma cells. However, its specificity is lower than that of chromogranin and synoptophysin (17). This study of primary NE carcinoma of the breast differs from prior retrospective studies (4,5,14,16). First, previous studies, included patients with mixed tumors or tumors with NE differentiation (5,14). The present study included patients presenting with NE carcinomas that expressed at least two NE markers (synoptophysin, chromogranin or NSE). Patients with NE-differentiated breast adenocarcinoma were not included. Second, in former studies, management of the disease had not been sufficiently clarified in that the role of chemotherapy and hormonal treatment was not adequately evaluated (4,5,14). The present study focused on both clinicopathological characteristics of the tumor and management of the patients.
Local treatment of this rare tumor remains controversial. Mastectomy with axillary dissection is the preferred method for larger, multifocal or retroareolar tumors (14). In our study, all 6 patients underwent modified radical mastectomy with axillary dissection.
The aim of adjuvant chemotherapy is to eliminate microscopic disease, particularly in tumor cells with higher proliferative capacity, thereby preventing metastatic disease. In order to clarify the need for adjuvant chemotherapy, proliferative activity and aggressiveness of the tumor should be determined. Ki-67 protein is a proliferation antigen, which is present in the different phases of the cell cycle. The mean Ki-67 value among well-differentiated pancreatic NE tumors was found to be 6.4%. However, poorly differentiated tumors had a higher Ki-67 index (26.1%) (18). The Ki-67 index has been used to predict clinical behavior in NE carcinoma of the digestive tract. Clarke et al established a cut-off value of ≥10% as a significant predictor of metastasis in pancreatic NE tumors (19). Oberg et al proposed a treatment algorithm based on the Ki-67 index in NE carcinoma of the digestive tract (15). In this algorithm, chemotherapy is preferred in patients with tumors with a Ki-67 expression of ≥10% (15). For anaplastic tumors with a Ki-67 index of >15%, combination chemotherapy with cisplatin and etoposide has been suggested. This regimen has been useful, with a response rate of >60% (20,21). Three of our patients had a Ki-67 index of ≥10%. Following surgical treatment, adjuvant chemotherapy was administered to these patients. The cisplatin-etoposide combination was used in 2 patients with a Ki-67 index of >15%. An anthracycline-containing regimen was used in patients with a Ki-67 value of 10%. In a previous case report, epirubicin was used in patients with metastatic NE-differentiated carcinoma of the breast, and a partial response was observed (6).
Detection of a positive expression of estrogen and progesterone receptor is considered to be a useful tool for prognosis (2,7). Sapino et al suggested that estrogen and progesterone receptor expression correlates with favorable prognosis (5). The role of hormonal treatment in this tumor has yet to be determined. In certain studies, patients with NE carcinoma of the breast were treated with tamoxifen or aromatase inhibitors (2,6,7). In the present study, hormonal therapy was planned for all of the patients who positively expressed estrogen and/or progesterone receptor. However, 1 patient refused hormonal therapy. In this patient (Patient 6), disease progression was observed 6 months following treatment with tamoxifen, and this patient went on to receive second-line hormonal treatment. The prognostic significance of c-erbB2 expression in NE carcinoma of the breast remains to be determined. In the present study, none of the patients with NE carcinoma expressed c-erbB2.
The role of radiotherapy in the management of NE carcinoma of the breast is controversial. In the present study, radiotherapy was used to treat patients with larger tumors and/or three or more axillary lymph node metastases.
In conclusion, NE carcinoma of the breast is a distinct entity. The treatment approach may include surgery and radiotherapy depending on tumor size and the lymph node status. Adjuvant chemotherapy may be recommended to patients with a Ki-67 index of ≥10%. Hormonal treatment appears to be feasible in patients who exhibit a positive expression of estrogen and/or progesterone receptor.
Contributor Information
Yesim Yildirim, Department of Medical Oncology, Acibadem University, Soyak Yenisehir Selale Evleri, 34770 Umraniye, Istanbul, Turkey.
Sahende Elagoz, Department of Pathology, Cumhuriyet University, Sivas, Turkey.
Ayhan Koyuncu, Department of General Surgery, Cumhuriyet University, Sivas, Turkey.
Cengiz Aydin, Department of General Surgery, Cumhuriyet University, Sivas, Turkey.
Kursat Karadayi, Department of General Surgery, Cumhuriyet University, Sivas, Turkey.
References
- Ogawa H, Nishio A, Satake H, et al: Neuroendocrine tumors in the breast. Radiat Med 26: 28-32, 2008. [DOI] [PubMed]
- Cubilla AL and Woodruff JM: Primary carcinoid tumor of the breast: a report of eight patients. Am J Surg Pathol 1: 283-292, 1977.
- Anonymous: Tumors of the breast: neuroendocrine tumors. In: World Health Organization Classification of the Tumors, Pathology and Genetics of the Tumors of the Breast and Female Genital Organs. Tavassoli FA and Devilee P (eds). IARC Press, Lyon, pp32-34, 2003.
- Zekioglu O, Erhan Y, Ciris M and Bayramoglu H: Neuro-endocrine differentiated carcinomas of the breast: a distinct entity. Breast 12: 251-257, 2003. [DOI] [PubMed]
- Sapino A, Papotti M, Righi L, et al: Clinical significance of neuroendocrine carcinoma of the breast. Ann Oncol 12 (Suppl 2): 115-117, 2001. [DOI] [PubMed]
- Berruti A, Saini A, Leonardo E, et al: Management of neuroendocrine differentiated breast carcinoma. The Breast 13: 527-529, 2004. [DOI] [PubMed]
- Yaren A, Kelten C, Akbulut M, Teke Z, Duzcan E and Erdem E: Primary neuroendocrine carcinoma of the breast: a case report. Tumori 93: 496-498, 2007. [DOI] [PubMed]
- Kinoshita S, Hirano A, Komine K, et al: Primary small-cell neuroendocrine carcinoma of the breast. Surg Today 38: 734-738, 2008. [DOI] [PubMed]
- Upalakalin JN, Collins LC, Tawa N and Parangi S: Carcinoid tumors in the breast. Am J Surg 191: 799-805, 2006. [DOI] [PubMed]
- Pagotti M, Marci L, Finzi G, et al: Neuroendocrine differentiation in carcinoma of the breast: a study of 51 cases. Semin Diagn Pathol 6: 174-188, 1989. [PubMed]
- Bilgen-Gunhan I, Zekioglu O, Ustun EE, Memis A and Erhan Y: Neuroendocrine differentiated breast carcinoma: imaging features correlated with clinical and histopathological findings. Eur Radiol 13: 788-793, 2003. [DOI] [PubMed]
- Rosen PP: Rosen's Breast Pathology. Lippincott-Raven, Philadelphia, pp437-439, 1997.
- Richardson RL and Weiland LH: Undifferentiated small-cell carcinomas in extrapulmonary sites. Semin Oncol 9: 484-496, 1982. [PubMed]
- Scaramuzzi G, Murgo RM, Cuttitta A and Ciuffreda L: Il carcinoma neuroendocrine della mamella. Nostra esperienza e proposta di un algoritmo terapeutico per un tumore raro. G Chir 29: 203-206, 2008. [PubMed]
- Oberg K: Management of neuroendocrine tumors. Ann Oncol 15 (Suppl 4): 293-294, 2004. [DOI] [PubMed]
- Lopez-Bonet E, Alonso-Ruano M, Barazza G, et al: Solid neuroendocrine breast carcinomas: Incidence, clinicopathological features and immunohistochemical profiling. Oncol Rep 20: 1369-1374, 2008. [PubMed]
- Ingelman-Sundber H, Wiktröm B, Stromby N, et al: Immunohistochemical reactivity of breast tissue with antibodies to neuron specific enolase and an adenocarcinoma-associated gylcolipid antigen. Virchows Arch 415: 539-544, 1989. [DOI] [PubMed]
- La Rosa S, Sessa F, Capella C, et al: Prognostic criteria in nonfunctioning pancreatic endocrine tumors. Virchows Archiv 429: 323-333, 1996. [DOI] [PubMed]
- Clarke MR, Baker EE, Weyant RJ, et al: Proliferative activity in pancreatic endocrine tumors: association with function, metastases and survival. Endocr Pathol 8: 181-187, 1997. [DOI] [PubMed]
- Oberg K: Advances in chemotherapy and biotherapy of endocrine tumors. Curr Opin Oncol 10: 58-65, 1998. [PubMed]
- Oberg K: Chemotherapy and biotherapy in the treatment of neuroendocrine tumours. Ann Oncol 12 (Suppl 2): 111-114, 2001. [DOI] [PubMed]