Abstract
Excision repair cross‑complementing rodent repair deficiency, complementation group 5 (ERCC5, XPG) is a key molecule in DNA damage repair. We analyzed the contribution of ERCC5 rs751402 polymorphism in increased susceptibility to hepatocellular carcinoma (HCC). A total of 96 patients diagnosed with HCC and 336 healthy controls provided blood samples for analysis of rs751402 genotypes. Demographic data and information on habitual use of tobacco and alcohol were collected. After adjusting for covariates, rs751402 homozygocity for allele C was found to confer a statistically significant protection [adjusted odds ratio (AOR)=0.56; 95% CI, 0.35‑0.89; p=0.01] against HCC, whereas rs751402 T alleles were associated with increased risk (AOR=1.69; 95% CI, 0.74‑3.87). Individuals with the inherited ERCC rs751402 CC genotype may experience significant protection against HCC, whereas individuals with T alleles appear to be exposed to higher risk.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver. It is the seventh most common cause of cancer‑related deaths in men and the ninth in women (1). Potential risk factors for HCC include infection with hepatitis viruses B and C, as well as exposure to carcinogens from tobacco, including smokeless tobacco and areca nut mixtures, and alcohol use (2‑4). A significant correlation between dose and response was found involving the risk of HCC and habitual substance use (3,4).
However, only a fraction of tobacco and alcohol users develop HCC, suggesting inter‑individual variation in susceptibility (2‑4). Although no concrete evidence of familial aggregation of HCC exists, certain subsets of individuals are prone to developing cancer if their inherent ability to repair DNA has been compromised.
A number of carcinogens are produced by tobacco, which convert to reactive metabolites and bind to cellular DNA to form adducts (5). These DNA adducts are removed by the DNA repair mechanism, which restores genomic integrity (5). Evidence suggests that genetic variations in critical DNA repair genes, such as the excision repair cross‑complementing rodent repair deficiency, complementation group 5 (ERCC5, XPG) may contribute to increased cancer risk (6‑11). Our hypothesis is that specific allelotypes of rs751402 exist in the promoter region of ERCC5, controlling its expression and, thus, its DNA damage repair capacity. Individuals with genetic variants that suboptimize the function of ERCC5 may be more susceptible to developing HCC.
Patients and methods
Patients. A hospital‑based case‑control study was conducted after obtaining the appropriate institutional review board (IRB) approval. A total of 432 patients were enrolled in the study at Chung Shan Medical University Hospital in Taichung, Taiwan, between 2007 and 2009. Of these, 96 patients had a histologically confirmed diagnosis of HCC. A total of 336 non‑cancer healthy individuals attending the Department of Family Medicine, Chung Shan Medical University Hospital, Taiwan, for an annual physical examination were enrolled as controls. The subjects were interviewed using a structured questionnaire to obtain information on sociodemographic characteristics (age, gender and race/ethnicity), tobacco use (current smoker vs. non‑ or past smoker) and alcohol consumption [current heavy drinker, defined by the centers for disease control (CDC) as consuming an average of more than 2 drinks per day vs. not current heavy drinker]. Relevant medical information including stage of HCC, HBsAg, anti‑hepatitis C virus (HCV), liver cirrhosis history, Child‑Pugh grade, α‑fetoprotein (AFP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) was also collected from patients by medical chart review. Peripheral blood samples were collected from both HCC patients and controls utilizing a standard venipuncture technique and stored at ‑80˚C. Informed consent was obtained from all of the subjects prior to the commencement of the study.
Genotyping. Genomic DNA was extracted from the blood by QIAamp DNA blood mini kits (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. DNA was dissolved in TE buffer [10 mMTris (pH 7.8) and 1 mM EDTA] and then quantitated by a measurement of OD260. The final preparation was stored at ‑20˚C and used as templates in polymerase chain reaction (PCR).
Genotyping of the ERCC5 C/T (rs751402) polymorphism was carried out using Taq Man® SNP genotyping assays (Applied Biosystems, Foster City, CA, USA): C_924624_20. The final volume for each reaction was 10 µl, containing 5 µl Taq Man Universal PCR Master Mix (Applied Biosystems), 0.25 µl primers/Taq Man probe mix, and 10 ng genomic DNA. Real‑time PCR consisted of an initial denaturation step at 95˚C for 10 min, followed by 40 cycles, each consisting of 92˚C for 15 sec and 60˚C for 1 min. The fluorescence level was measured with Applied Biosystems® StepOne™ real‑time PCR system (Applied Biosystems). Allele frequencies were determined by ABI SDS software.
Statistical analysis. With regard to statistical analysis, the study aimed primarily to assess the correlation between HCC risk and ERCC5 rs751402 genotype variants, while simultaneously controlling for known confounders such as age, gender, alcohol, tobacco or other pertinent medical parameters. Standard methods of analysis of case‑control studies were applied (12). Odds ratios (ORs) were estimated using the Mantel‑Haenszel method (13) with 95% confidence intervals (95% CI) computed using the Robins, Greenland and Breslow method (14). All tests of statistical significance were 2‑sided. Multivariate logistic regression analyses were performed to control simultaneously for all stratification variables as described by Breslow and Day (15). Multivariate models were adjusted for gender, age, tobacco smoking, alcohol consumption, HBsAg, anti‑HCV status, liver cirrhosis disease history, Child‑Pugh grade, tumor size, lymph node metastasis, distant metastasis and HCC clinical stage. Gene environment interaction testing was also conducted, where the interaction term was tested along with the main effects. In addition to the p‑value of the interaction term, the likelihood ratio test statistic was used to evaluate goodness‑of‑fit of the final model.
Results
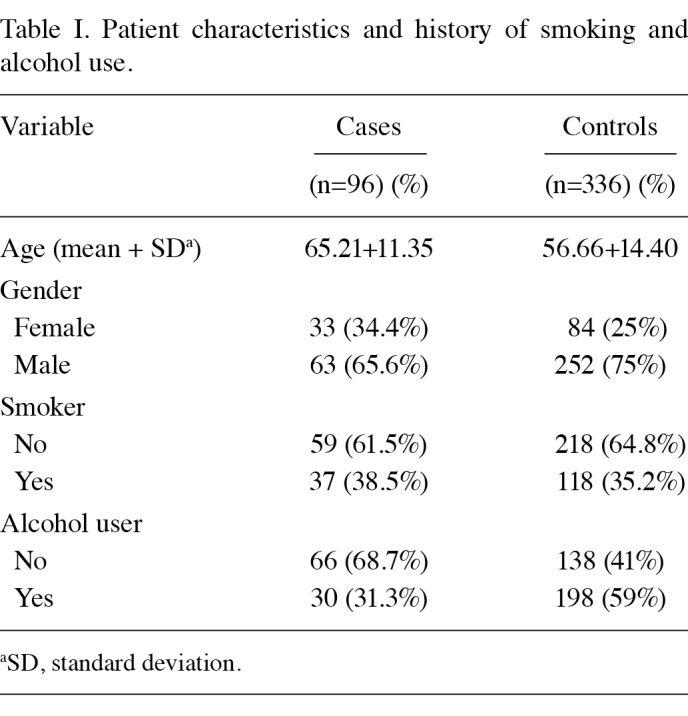
A total of 432 patients participated in this study. Of these, 96 patients had histologically confirmed HCC and 336 were healthy controls. Males were over‑represented, especially among the HCC‑positive cases. The demographic details of patients and information on their habitual use of tobacco and alcohol are shown in Table I. The difference in the frequency of tobacco and alcohol use HCC cases and controls was not pronounced.
Table 1. Patient characteristics and history of smoking and alcohol use.
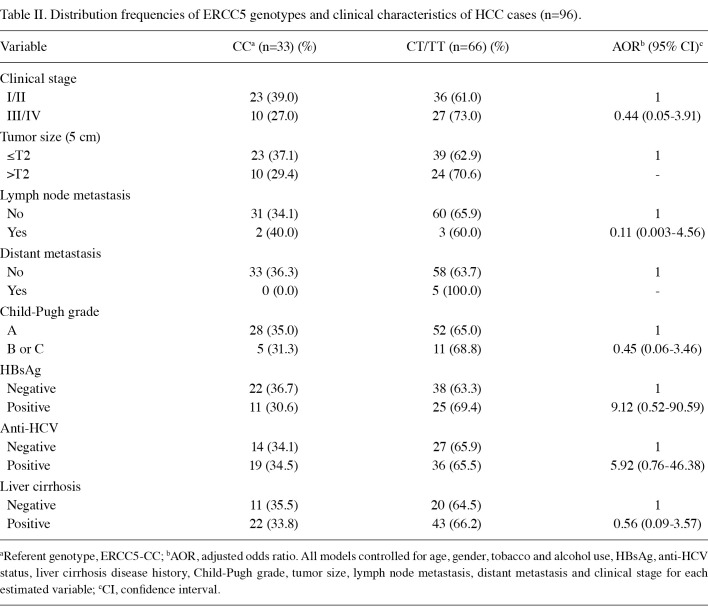
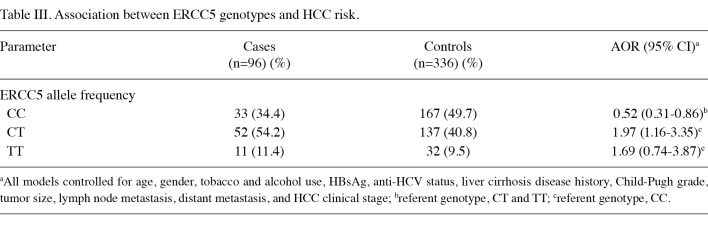
Distribution frequencies of clinicopathological parameters of ERCC5 genetic variants in HCC patients are shown in Table II. HCC patients with T alleles tended to be positive for hepatitis viruses B and C [adjusted odds ratio (AOR)=9.1, 95% CI, 0.52‑90.59; AOR=5.92, 95% CI, 0.76‑46.38, respectively). With regard to ERCC5 genotypes, the most common variant among HCC patients was the heterozygote CT type, with a frequency of 54.2%, followed by ERCC5‑CC (34.4%) and ERCC5‑TT (11.4%), as shown in Table III. The most common variant of the control group was ERCC5‑CC (49.7%), followed by ERCC5‑CT (40.8%) and ERCC5‑TT (9.5%). The frequency of ERCC5‑CC homozygotes was found to be higher in the controls, whereas the frequency of ERCC5‑TT was higher in the HCC patients. In the bivariate statistical analysis, the Chi‑square test for ERCC5‑CC was found to be statistically significant (p=0.04), a fact that was maintained in the adjusted analysis. In the multivariate logistic regression model, after controlling for age, gender, tobacco and alcohol use, as well as various clinical parameters, ERCC5‑CC showed significant protection against cancer with an AOR of 0.52 (95% CI, 0.31‑0.86). In a multivariate model controlling for the above‑named variables where ERCC5‑CC was used as the basis for comparing the effects of the other two genotypic variants, a trend of increased risk was noted for individuals with the T allele. The OR of ERCC5‑CT was 1.97 (95% CI, 1.16‑3.35), whereas the odds ratio for ERCC5‑TT was 1.69 (95% CI, 0.74‑3.87). These results suggest that the CC genotype confers significant protection against HCC.
Table 2. Distribution frequencies of ERCC5 genotypes and clinical characteristics of HCC cases (n=96).
Table 3. Association between ERCC5 genotypes and HCC risk.
Discussion
Our study results suggest that individuals who are carriers of the ERCC5-CC allotype are less susceptible to HCC compared to those with one or two copies of T allele, after adjusting for other covariates. However, having one copy of C allele (CT allotype) did not confer a lower risk (OR of 1.97) when compared with the TT allotype (OR of 1.69). It is possible that the lack of association between HCC risk and the copy number of C alleles is due to the relatively low number of patients with TT genotype (n=11).
ERCC5 is a key component in DNA repair. DNA damage may be induced by carcinogens from tobacco and alcohol, which are known risk factors of HCC (2‑4). Reactive chemicals such as polycyclic aromatic hydrocarbons, nitrosamines and aromatic amines from tobacco smoking can directly bind DNA and form bulky DNA adducts (4). Alcohol, besides being directly responsible for causing liver cirrhosis (4), serves as a solvent for and promotes absorption of a number of ingested toxins (4). Alcohol is also known to induce xenobiotic‑metabolizing enzymes that activate procarcinogens (16). These chemical mutagens from tobacco and alcohol bound to DNA are removed mainly by the nucleotide excision repair (NER) mechanism (11).
ERCC5 (XPG) is the central component of NER and its expression is upregulated during DNA damage response (6). Polymorphisms at the promoter region of ERCC5 can lead to decreased transcription, thereby reducing overall DNA repair capacity (9). The short nucleotide polymorphism we studied (rs751402) is in the E2F/YY1 binding and response site located in the proximal promoter region of ERCC5 (6). CEBPG (CCAAT/enhancer‑binding protein γ), another transcription factor located in the region of the ERCC5 promoter, contributes to the upregulation of ERCC5 expression (9). CEBPG binds to the ERCC5 promoter region and its action is modified by the binding of E2F1/YY1 in the nearby region. Additionally, E2F1 collaborates with CEBPG to upregulate ERCC5, whereas the YY1 element ensures optimal regulation of transcription (9). Polymorphisms in rs751402 (E2F/YY1 binding and response site) contributes to genetic variation with regard to repairing tobacco‑ and alcohol‑induced DNA damage via the modulation of ERCC5 expression (6,11). Since the ability to repair DNA damage is critical for maintaining genomic stability and for preventing carcinogenesis after exposure to carcinogens (7,11), polymorphisms that jeopardize the expression levels of ERCC5 may result in an increased risk of cancer initiation and progression (9). Such genetic variation also explains individual variations in susceptibility of developing cancer (11).
Similar findings were observed in studies of head and neck squamous cell carcinoma, in which individuals who inherited the ERCC5 rs751402 CC genotype demonstrated significant resistance to cancer, whereas individuals with T alleles were exposed to a higher risk (unpublished data). In other studies, rs751402 A allele was associated with a decreased expression of ERCC5 in individuals with lung cancer (6). Moreover, variation in the rs751402 allelotype was associated with altered oxaliplatin response and progression‑free survival in colorectal cancer (10).
Our study is limited by the small sample size, which may be responsible for the decreased cancer risk observed for the TT allotype compared to the CT allotype. A further limitation was the lack of detailed information regarding patients' alcohol and tobacco habits, which were collected using binary coding of the 'current user' vs. 'not current user'. As a result, we were not able to perform a more in‑depth analysis by stratifying individuals based on amount, length of use and past history of alcohol and tobacco consumption. Nevertheless, our findings suggest that the rs751402 polymorphism has important phenotypic consequences for ERCC5 expression, DNA repair and cancer risk. Further investigation is required to validate the exact role of ERCC5 in the carcinogenic process of HCC in diverse population groups. Following further validation, the result may have significant clinical utility in HCC risk assessment modality.
Contributor Information
Angela J. Yoon, College of Dental Medicine, Columbia University, New York City, NY, USA
Wu‑Hsien Kuo, Department of Medicine, Armed‑Force Taichung General Hospital, Taichung, Taiwan, R.O.C..
Chiao‑Wen Lin, Institute of Biochemistry and Biotechnology, Taichung, Taiwan, R.O.C..
Shun‑Fa Yang, Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan, R.O.C..
References
- Bosch FX, Ribes J, Cleries R and Diaz M: Epidemiology of hepatocellular carcinoma. Clin Liver Dis 9: 191‑211, 2005. [DOI] [PubMed]
- Farazi PA and DePinho RA: Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 6: 674‑687, 2006. [DOI] [PubMed]
- Wen CP, Tsai SP, Cheng TY, Levy DT, Yang HJ and Eriksen MP: Uncovering the relation between betal quid chewing and cigarette smoking in Taiwan. Tobacco Control 14: i16‑i22, 2005. [DOI] [PMC free article] [PubMed]
- Wang LY, You SL, Lu SN, et al: Risk of hepatocellular carcinoma and habits of alcohol drinking, betel quid chewing and cigarette smoking: a cohort of 2416 HBsAg‑eropositive and 9421 HBsAg‑seronegative male residents in Taiwan. Cancer Causes Control 14: 241‑250, 2003. [DOI] [PubMed]
- Zienolddiny S, Campa D, Lind H, et al: Polymorphisms of DNA repair genes and risk of non‑small cell lung cancer. Carcinogenesis 27: 560‑567, 2006. [DOI] [PubMed]
- Blomquist TM, Crawford EL and Willey JC: Cis‑acting genetic variation at an E2F1/YY1 response site and putative p53 site is associated with altered allele‑specific expression of ERCC5 (XPG) transcript in normal human bronchial epithelium. Carcinogenesis 31: 1242‑1250, 2010. [DOI] [PMC free article] [PubMed]
- Mu D, Hsu DS and Sancar A: Reaction mechanism of human DNA repair excision nuclease. J Biol Chem 271: 8285‑8249, 1996. [DOI] [PubMed]
- Blomquist T, Crawford EL, Mullins D, et al: Pattern of antioxidant and DNA repair gene expression in normal airway epithelium associated with lung cancer diagnosis. Cancer Res 69: 8629‑8635, 2009. [DOI] [PMC free article] [PubMed]
- Crawford EL, Blomquist T, Mullins DN, et al: CEBPG regulates ERCC5/XPG expression in human bronchial epithelial cells and this regulation is modified by E2F1/YY1 interactions. Carcinogenesis 28: 2552‑2559, 2007. [DOI] [PubMed]
- Chen J, Xie F, Chen K, et al: ERCC5 promoter polymorphisms at ‑763 and +25 predict the response to oxaliplatin‑based chemotherapy in patients with advanced colorectal cancer. Cancer Biol Ther 8: 1424‑1430, 2009. [DOI] [PubMed]
- Abbasi R, Ramroth H, Becher H, Dietz A, Schmezer P and Popanda O: Laryngeal cancer risk associated with smoking and alcohol consumption is modified by genetic polymorphisms in ERCC5, ERCC6, and RAD23B but not by polymorphisms in five other nucleotide excision repair genes. Int J Cancer 125: 1431‑1439, 2009. [DOI] [PubMed]
- Selvin S: Statistical analysis of epidemiologic data. Oxford University Press, New York, 1991.
- Rothman KJ: Modern epidemiology, 2nd ed. Lippincott‑Raven, Philadelphia, 1998.
- Robins J, Greenland S and Breslow NE: A general estimator for the variance of the Mantel‑Haenszel odds ratio. Am J Epidemiol 124: 719‑23, 1986. [DOI] [PubMed]
- Breslow NE and Day NE: Statistical methods in cancer research, vol. I. The analysis of case‑control studies. IARC 32: 335‑338, 1980. [PubMed]
- Lieber CS: Alcohol: its metabolism and interaction with nutrients. Ann Rev Nutrition 20: 395‑430, 2000. [DOI] [PubMed]