Abstract
Longevity of mice can be increased by spontaneous or experimentally induced mutations that interfere with the biosynthesis or actions of growth hormone (GH), insulin-like growth factor 1 (IGF-1), or insulin in the adipose tissue. The effects of GH resistance and deficiency of GH (along with thyrotropin and prolactin) on aging and lifespan are the most pronounced and best established of these mutations. Potential mechanisms linking these endocrine deficits with delayed aging and extended longevity include increased stress resistance, alterations in insulin and mammalian target of rapamycin (mTOR) signaling and metabolic adjustments.
Physiological relationships deduced from the extreme phenotypes of long-lived mouse mutants appear to apply broadly, encompassing genetically normal (“wild-type”) mice and other mammalian species. The role of GH in the control of human aging continues to be hotly debated, but recent data indicate that reduced somatotropic signaling provides protection from cancer and other age-related diseases and may promote old age survival.
Keywords: longevity, cancer, insulin-like growth factor 1 (IGF-1), dwarf mice
Introduction
The important involvement of insulin/insulin-like growth factor signaling (IIS) in the control of aging in organisms ranging from yeast to mammals has been thoroughly documented during the last two decades and represents one of the most exciting recent findings in the study of aging. In laboratory populations of mice, deletion of growth hormone (GH) receptors or GH (alone or along with other pituitary hormones) produces secondary suppression of circulating IGF-1 and insulin, robust extension of both median and maximal lifespan and numerous indications of delayed and/or slower aging. Direct targeting of IGF-1 signaling can also extend longevity, although the effects are generally smaller than the effects of GH deficiency or resistance and often limited to females. Furthermore, deletion of insulin receptors in the adipose tissue increases lifespan of mice. The origin and phenotypic characteristics of these long-lived mouse mutants have been reviewed by us and others (Bartke 2006; Russell & Kahn, 2007) and will not be discussed in this article. Instead, we will focus on two questions:
How can serious defects in the endocrine system produce benefits in terms of “healthspan” and survival? and
Which of the findings in mutant mice apply to normal animals and to other species, including our own?
Tentative mechanisms of extended longevity in GH-deficient and GH-resistant mice
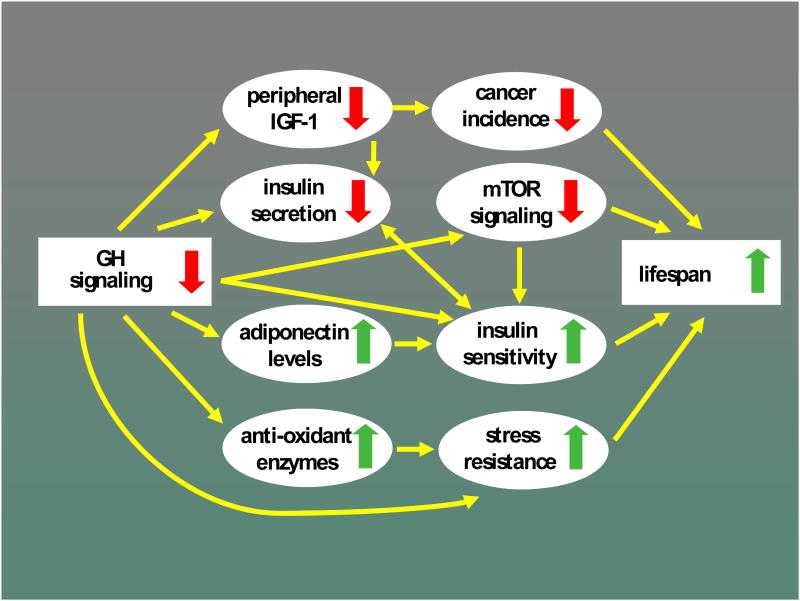
The unexpected and indeed counterinituitive demonstration that hypopituitary Snell dwarf and Ames dwarf mice, as well as GH receptor-deficient “Laron dwarf” mice, remain healthy into advanced age and consistently outlive their normal siblings prompted an intensive search for mechanisms that could account for these associations. Results obtained to date suggest the involvement of multiple, likely interacting mechanisms (Figure 1).
Figure 1.
Mechanisms believed to link reduced growth hormone (GH) signaling with extended longevity in mutant mice and some of the known interactions between these mechanisms. Details and references in text. IGF-1 = insulin-like growth factor 1; mTOR = mammalian target of rapamycin.
Increased stress resistance
Association of extended longevity with enhanced stress resistance is a consistent finding in Caenorhabditis elegans and Drosophila. Resistance to oxidative stress is of particular interest because oxidative damage is generally accepted as a very important and possibly the key mechanism of aging across taxa. Cultured fibroblasts derived from long-lived Snell dwarf, Ames dwarf and GHRKO mice are more resistant to a variety of oxidative, cytotoxic and metabolic stresses than fibroblasts derived from normal animals from the same strains (Harper et al., 2006). Increased stress resistance of these cells is associated with reduced phosphorylation of the stress-activated protein kinases ERK 1 and 2 and enhanced effect of stress on the expression of some ERK-dependent immediate early genes (Sun et al., 2009).
Importantly, in Ames dwarf mice an increased resistance of fibroblasts to oxidative stress induced by paraquat was associated with increased resistance to administration of this toxin in vivo (Bokov et al., 2009). Curiously, resistance to paraquat in vivo was not enhanced in GHRKO mice (in fact, the males were more susceptible) even though dermal fibroblasts derived from these animals were resistant to paraquat in vitro (Harper et al., 2006).
In Ames dwarf mice, oxidative damage to DNA and other cell components in different organs is reduced. This protection from oxidative damage has been linked to reduced production of reactive oxygen species (ROS) (Brown-Borg 2006), increased activity of anti-oxidant enzymes, including catalase, superoxide dismutase and glutathione peroxidase (Brown-Borg 2006) and altered methiomine and glutathione metabolism (Brown-Borg et al., 2005). Enhanced anti-oxidant defense mechanisms in Ames dwarf mice are presumably caused by GH deficiency, because opposite changes were detected in transgenic mice overexpressing GH and in hepatocytes exposed to GH or IGF-1 in vitro (Brown-Borg 2006). However, enhancement of antioxidant defenses in GHRKO mice is less consistent and less pronounced, with differences from normal animals being generally organ- and/or age-specific (Hauck et al., 2002). In the context of increased stress resistance of GH-related mutants, it is of interest that hippocampal slices from Ames dwarf mice exhibit increased resistance to detrimental effects of beta amyloid (Schrag et al., 2008).
Reduced IGF-1 and mTOR signaling
As expected from the well documented impact of GH on IGF-1 expression and the role of IGF-1 as a mediator of many (although not all) GH actions, circulating levels of IGF-1 are drastically suppressed in both GH-deficient and GH-resistant mice. This is due primarily to reduced expression of IGF-1 in the liver. Expression of IGF-1 in some of the other organs is either preserved or only partially suppressed. It is tempting to link extended maintenance of cognitive function in these long-lived mutants (Kinney 2001) to normal expression of IGF-1 in the brain and particularly the hippocampus, where IGF-1 expression levels in young adults in fact exceeds expression levels in normal animals (Sun et al., 2005).
Similarly, preservation of IGF-1 expression and enhanced expression of IGF-1 receptor in the heart of GHRKO mice (Masternak et al., 2006) likely contributes to their remarkable longevity. Reduced levels of IGF-1 in the circulation can be assumed to contribute to reduced incidence and delayed onset of fatal neoplastic disease in hypopituitary and GH-resistant mice (Ikeno et al., 2003; 2009; Alderman et al., 2009). Proliferative and anti-apoptotic effects of IGF-1 as well as the resulting somatic growth have been linked to cancer on the basis of numerous in vitro and epidemiological studies including a recent report correlating IGF-1 levels and cancer mortality in elderly men (Major 2010).
Mammalian target of rapamycin (mTOR) signaling is reduced in the liver of Ames dwarfs (Sharpe & Bartke, 2005) and in the skeletal muscle of GHRKO mice (Bonkowski et al., 2006). This would account for reduced cell size due to reduced protein synthesis and very likely contribute to delayed aging and extended longevity. Significant increases of lifespan were produced in mice by pharmacological suppression of mTOR (Harrison et al., 2009) or by deletion of mTOR-dependent S6 kinase (Selman et al., 2009). Absence of GH actions and reduced mTOR signaling, along with a shift of adipokine profiles away from pro-inflammatory and toward anti-inflammatory are believed to be responsible for enhanced insulin sensitivity of the long-lived dwarf mice (detailed in the next section).
Enhanced insulin sensitivity
Co-existence of reduced insulin levels and enhanced insulin sensitivity is among the most prominent endocrine features shared by Ames dwarf, Snell dwarf and GHRKO mice (Bartke 2006). Studies of interactive effects of Ames dwarfism and Ghr deletion with calorie restriction (CR) reveal clear correspondence between the changes in insulin sensitivity (as measured by insulin tolerance tests) and longevity (Bonkowski et al., 2006; 2009; Masternak et al., 2009). These studies also allowed identification of changes in the expression of genes related to the insulin signaling pathway that accompanied changes in lifespan induced by either GH receptor deletion or by CR (Bonkowski et al., 2009).
Enhanced insulin sensitivity of GH-deficient and GH-resistant mice is consistent with the well documented anti-insulinemic actions of GH, with likely additional effects of the reductions in plasma levels of tumor necrosis factor alpha, interleukin 6 and lipids, the increase in plasma adiponectin and reduction in mTOR signaling. Many of these factors converge on the control of site-specific inhibitory phosphorylation of insulin receptor substrate 1 (IRS-1) (Bonkowski et al., 2009). Aging and survival benefits of reduced insulin levels and enhanced insulin sensitivity can be deduced from detrimental effects of opposite changes, i.e., hyperinsulinemia and insulin resistance in metabolic syndrome and pre-diabetic states.
Metabolic adjustments and other mechanisms
There is considerable evidence that metabolic rate, as well as mitochondrial biogenesis and functions including efficiency, uncoupling and ROS production, are related to aging and longevity, but the specific cause:effect relationships are difficult to identify. At the standard room temperature (approx. 22° C) oxygen consumption per gram of body weight is significantly higher in singly housed Ames dwarf and GHRKO mice than in normal (wild-type) animals from the same strains (Westbrook et al., 2009). These differences are evident in both fasted and fed animals, but it is difficult to determine to what extent they may be due to major differences between mutant and normal animals in the body weight:surface ratios and in body composition. The commonly used method of estimating metabolic body mass is not designed for animals in which reduced body weight is associated with increased adiposity. Measurements of oxygen consumption under conditions of thermoneutrality would aid interpretation of these findings.
Respiratory quotient is reduced in both Ames dwarf and GHRKO mice, suggesting increased reliance on lipids (vs. carbohydrates) as a metabolic fuel (Westbrook et al., 2009). These findings correspond to the various indices of increased beta oxidation of fatty acids in these animals (Al-Regaiey et al., 2005; Brooks et al., 2007), and it is interesting to relate them to the evidence that increased fat burning is associated with extended longevity in C. elegans (Wang et al., 2008). In Snell dwarf mice, endogenous glucose production is reduced during both feeding and fasting (Brooks et al., 2007; Alderman et al., 2009). This is likely due to suppression of gluconeogenesis and glycogenolysis by adiponectin (Brooks et al., 2007) and is also consistent with enhanced insulin sensitivity. Reduced rates of glucose production and utilization in Snell dwarf mice may explain resistance of these animals to cancer (Alderman et al., 2009).
Body temperature is reduced in Snell dwarf, Ames dwarf and GHRKO mice (Hunter et al., 1999; Hauck et al., 2002) in spite of increased food intake and oxygen consumption per unit of body mass. Reduced body temperature likely contributes to their longevity (Conti et al., 2006).
Other mechanisms that have been related to extended lifespan in one or more of the GH signaling-related mutant mice include improved detoxification of xenobiotics and improved genome maintenance, as indicated by reduced levels of pro-inflammatory cytokines, reduced activity of the p38 MAPK stress pathway, improved cardioprotection, increased expression of various micro RNA species, and in the case of Snell and Ames dwarf mice, also severe hypothyroidism that is genetically associated with GH deficiency in these animals. Detailed discussion of these mechanisms is outside the scope of this article.
Can conclusions from studies on GH-related mutants be extrapolated to normal animals?
Extended longevity of hypopituitary and GH-resistant mice implies that normal actions of physiological levels of GH are not optimal for extended survival and must involve significant “costs” in terms of aging and longevity. The conclusion that normal levels and actions of a hormone are somehow “harmful” is counterintuitive and may be hard to accept but is supported by both theoretical/evolutionary perspective and by experimental findings. First of all, evolution focuses on reproductive success rather than longevity; thus it is not surprising that rapid growth, early sexual maturation and high fertility—three phenotypes promoted by GH and IGF-1—would be favored regardless of consequences on the susceptibility to diseases that develop late in life and on the chances for long-term survival. Secondly, some of the relationships between hormonal signaling and aging deduced from the extreme phenotypes of long-lived mutant mice have been shown to apply to normal animals. In many comparisons of different strains of mice and in comparisons of individual animals within a genetically heterogeneous population, adult body size (a biological marker of GH/IGF-1 actions) was negatively correlated to longevity (Rollo 2002; Miller et al., 2002). Yuan and his colleagues recently reported that in a comparison of 31 inbred mouse strains, plasma IGF-1 levels are negatively correlated with median lifespan (Yuan et al., 2009).
The negative association of adult body size and longevity applies to other species including rats, domestic dogs, horses and likely also humans (Samaras 2007; Rollo 2002), although the relationship of human height to longevity continues to be controversial. Pathologic GH excess in the syndromes of acromegaly and gigantism leads to reduced life expectancy, and height has been identified as a cancer risk in a number of large population studies. A recent study reported correlation of plasma IGF-1 levels with cancer mortality in elderly men (Major et al., 2010). However, effects of GH deficiency or resistance on human lifespan are unclear and certainly do not include life extension comparable to what is seen in mice that have corresponding mutations and endocrine syndromes. Individuals afflicted by these hereditary endocrine syndromes fail to grow normally, are considered to be at increased risk for cardiovascular disease and benefit greatly from GH or IGF-1 therapy.
Surprisingly, recent data indicate that an absence of GH actions also exerts some protective effects. While GH deficiency or resistance leads to increased adiposity, lipid profiles believed to constitute increased risk for cardiovascular disease and poor glucose tolerance, recent studies discovered that individuals afflicted with these conditions were not only free of cancer (Shevah & Laron, 2007; V. Longo, personal communication), but also unexpectedly protected from artherosclerosis and diabetes (V. Longo, personal communication, Oliveira et al., 2007). In addition, reduced somatotropic signaling and subclinical hypothryoidism have been linked to reduced mortality in old women and to an increased probability of exceptionally long survival (van Heemst et al., 2005; Gussekloo et al., 2006; Atzmon et al., 2009).
It is interesting to ask why identical defects in somatotropic signaling do not have the same impact on longevity in mice and humans. One possible reason that the impact of suppression of circulating IGF-1 levels and the consequent protection from cancer on lifespan is greater in mice is that cancer is by far the most common cause of death in most populations of laboratory mice while most people die of other causes. Another possibility relates to the likely very different potential of any “intervention” (including altered endocrine function) to affect longevity in species with major differences in the life history. Comparative analysis of lifespans vs. body mass indicates that the mouse is an inherently short-lived species while humans are exceptionally long-lived. Short-lived species mature early, rapidly produce large numbers of progeny (with a relatively small parental investment in each offspring) and thus assure survival of the species in spite of high mortality related to predation and other environmental factors. Somatotropic signaling likely plays a major role in maintaining this type of life history and reproductive strategy by prompting growth, puberty and fecundity. This, in turn, could lead to greater “costs” in terms of susceptibility to early aging and cancer, thus providing a plausible explanation for the clear life-extending effects of GH resistance and GH deficiency in mice and more subtle protective effects of the same endocrine syndromes in humans. Hopefully, future work and novel animal models will allow identifying mechanisms that link aging to specific effects of anabolic hormones, including GH, IGF-1 and insulin in different organs and cell types and at different stages of life history.
Acknowledgments
Work in the author's laboratory was supported by NIA, The Ellison Medical Foundation, The Glenn Foundation for Biomedical Research and SIU Geriatric Research Initiative. Sincere apologies are extended to those whose work pertinent to this topic was not mentioned due to inadvertent omissions or limitations of space.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alderman JM, Flurkey K, Brooks NL, Naik SB, Gutierrez JM, Srinivas U, Ziara KB, Jing L, Boysen G, Bronson R, Klebanov S, Chen X, Swenberg JA, Stridsberg M, Parker CE, Harrison DE, Combs TP. Neuroendocrine inhibition of glucose production and resistance to cancer in dwarf mice. Exper Gerontol. 2009;44:26–33. doi: 10.1016/j.exger.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: Interaction of reduced insulin-like growth factor 1/insulin signaling and caloric restriction. Endocrinology. 2005;146:851–60. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- Atzmon G, Barzilai N, Hollowell JG, Surks MI, Gabriely I. Extreme longevity is associated with increased serum thyrotropin. J Clin Endocrinol Metab. 2009;94:1251–4. doi: 10.1210/jc.2008-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A. Life extension in the dwarf mouse. In: Conn PM, editor. Handbook of models for the study of human aging. Elsevier Academic Press; 2006. pp. 403–414. [Google Scholar]
- Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived ames dwarf mice are resistant to chemical stressors. J Gerontol A Biol Sci Med Sci. 2009;64:819–27. doi: 10.1093/gerona/glp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski MS, Dominici FP, Arum O, Rocha JS, Al Regaiey KA, Westbrook R, Spong A, Panici J, Masternak MM, Kopchick JJ, Bartke A. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS ONE. 2009;4:e4567. doi: 10.1371/journal.pone.0004567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks NL, Trent CM, Raetzsch CF, Flurkey K, Boysen G, Perfetti MT, Jeong YC, Klebanov S, Patel KB, Khodush VR, Kupper LL, Carling D, Swenberg JA, Harrison DE, Combs TP. Low utilization of circulating glucose after food withdrawal in snell dwarf mice. J Biol Chem. 2007;282:35069–35077. doi: 10.1074/jbc.M700484200. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM. Longevity in mice: is stress resistance a common factor? Age (Dordr) 2006;28:145–62. doi: 10.1007/s11357-006-9003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Uthus EO. Growth hormone alters methionine and glutathione metabolism in Ames dwarf mice. Mech Ageing Dev. 2005;126:389–398. doi: 10.1016/j.mad.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, de Lecea L, Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314:825–828. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frolich M, Westendorp RG. Thyroid function, activities of daily living and survival in extreme old age: The ‘Leiden 85-plus study’. Ned Tijdschr Geneeskd. 2006;150:90–6. [PubMed] [Google Scholar]
- Harper JM, Salmon AB, Chang Y, Bonkowski M, Bartke A, Miller RA. Stress resistance and aging: Influence of genes and nutrition. Mech Age Dev. 2006;127:687–694. doi: 10.1016/j.mad.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck SJ, Aaron JM, Wright C, Kopchick JJ, Bartke A. Antioxidant enzymes, free-radical damage, and response to paraquat in liver and kidney of long-living growth hormone receptor/binding protein gene-disrupted mice. Horm Metab Res. 2002:481–486. doi: 10.1055/s-2002-34787. [DOI] [PubMed] [Google Scholar]
- Hunter WS, Croson WB, Bartke A, Gentry MV, Meliska CJ. Low body temperature in long-lived Ames dwarf mice at rest and during stress. Physiology and Behavior. 1999;67:433–437. doi: 10.1016/s0031-9384(99)00098-0. [DOI] [PubMed] [Google Scholar]
- Kinney BA, Coschigano KT, Kopchick JJ, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav. 2001;72:653–660. doi: 10.1016/s0031-9384(01)00423-1. [DOI] [PubMed] [Google Scholar]
- Longo V. personal communication [Google Scholar]
- Major JM, Laughlin GA, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Insulin-like growth factor-i and cancer mortality in older men. J Clin Endocrinol Metab. 95:1054–9. doi: 10.1210/jc.2009-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Jimenez-Ortega V, Panici JA, Bonkowski MS, Kopchick JJ, Wang Z, Bartke A. Caloric restriction and growth hormone receptor knockout: Effects on expression of genes involved in insulin action in the heart. Exper Gerontol. 2006;41:417–429. doi: 10.1016/j.exger.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci. 2009;64:516–21. doi: 10.1093/gerona/glp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harper JM, Galecki A, Burke DT. Big mice die young: Early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell. 2002;1:22–29. doi: 10.1046/j.1474-9728.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- Oliveira JL, Aguiar-Oliveira MH, D'Oliveira A, Jr, Pereira RM, Oliveira CR, Farias CT, Barreto-Filho JA, Anjos-Andrade FD, Marques-Santos C, Nascimento-Junior AC, Alves EO, Oliveira FT, Campos VC, Ximenes R, Blackford A, Parmigiani G, Salvatori R. Congenital growth hormone (GH) deficiency and atherosclerosis: Effects of GH replacement in GH-naive adults. J Clin Endocrinol Metab. 2007;92:4664–70. doi: 10.1210/jc.2007-1636. [DOI] [PubMed] [Google Scholar]
- Rollo CD. Growth negatively impacts the life span of mammals. Evol Dev. 2002;4:55–61. doi: 10.1046/j.1525-142x.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007;8:681–91. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- Samaras TT. Human Body Size and The Laws of Scaling: Physiological, Performance, Growth, Longevity and Ecological Ramifications. 1st. Nova Science Publishers, Inc.; New York: 2007. [Google Scholar]
- Schrag M, Sharma S, Brown-Borg H, Ghribi O. Hippocampus of ames dwarf mice is resistant to beta-amyloid-induced tau hyperphosphorylation and changes in apoptosis-regulatory protein levels. Hippocampus. 2008;18:239–244. doi: 10.1002/hipo.20387. [DOI] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein s6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–4. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp ZD, Bartke A. Evidence for down-regulation of phosphoinositide 3-kinase/akt/mammalian target of rapamycin (pi3k/akt/mtor)-dependent translation regulatory signaling pathways in ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2005;60:293–300. doi: 10.1093/gerona/60.3.293. [DOI] [PubMed] [Google Scholar]
- Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: A preliminary report. Growth Horm IGF Res. 2007;17:54–7. doi: 10.1016/j.ghir.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of gh and igf-1 in the hippocampus of gh-deficient long-lived mice. Neurobiol Aging. 2005;26:929–937. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Sun LY, Steinbaugh MJ, Masternak MM, Bartke A, Miller RA. Fibroblasts from long-lived mutant mice show diminished erk1/2 phosphorylation but exaggerated induction of immediate early genes. Free Radic Biol Med. 2009;47:1753–61. doi: 10.1016/j.freeradbiomed.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heemst D, Beekman M, Mooijaart SP, Heijmans BT, Brandt BW, Zwaan BJ, Slagboom PE, Westendorp GJ. Reduced insulin/IGF-1 signaling and human longevity. Aging Cell. 2005;4:79–85. doi: 10.1111/j.1474-9728.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- Wang MC, O'Rourke EJ, Ruvkun G. Fat metabolism links germline stem cells and longevity in C. elegans. Science. 2008;322:957–960. doi: 10.1126/science.1162011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived ghrko and ames dwarf mice, and short-lived bgh transgenic mice. J Gerontol A Biol Sci Med Sci. 2009;64A:443–451. doi: 10.1093/gerona/gln075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B. Aging in inbred strains of mice: Study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8:277–87. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]