Abstract
Purpose of review
Swallowing disorders impact the health and quality of millions of lives of patients across the age spectrum. The broad scope of the problem is in contrast to the volume of methods that we have to treat the problem. Investigators are testing interventions that go beyond the swallowing system and are targeting those that cross or overlap with swallowing function. This review will highlight the potential clinical implications of respiratory–swallowing cross-system interaction in health and disease.
Recent findings
A collection of current studies demonstrates a tight neural coupling between the central control of respiration and swallowing. Results from recent studies suggest that this neural coupling may be altered under certain conditions of development, age, disease, and eating/swallowing tasks.
Summary
The functional significance of cross-system neural control on respiratory–swallowing coordination is far from understood. Preliminary data, however, show destabilization of respiratory–swallowing patterns in various neurological diseases and in head and neck cancer. These findings suggest the need to develop a line of research that tests the effects of therapeutic strategies that transcend swallowing and include cross-system interactions such as respiratory–swallow phase patterning.
Keywords: apnea, deglutition, dysphagia, respiration, swallowing
Introduction
Swallowing is an array of synergistic interdependent movements, initiated by a complex set of sensory inputs that generate pressures and forces for propelling ingested materials through the upper aerodigestive tract and simultaneously protect the upper airway. The pharynx is a specialized, common conduit through which food and liquid pass during swallowing and gases flow during respiration and airway clearance maneuvers, that is, coughing, throat clearing, belching. The functional conflict of the pharynx, therefore, must require fine coordination at the levels of neural control to ensure that the peripheral structures produce the intended target behavior. Evidence in support of a tight neural linkage between respiration and swallowing is derived from the work of many neurophysiologists who have identified and detailed specialized neural networks in the brainstem and cortex. Recent findings have delineated single neurons within these medullary networks that demonstrate multifunctionality in the control of both respiratory and swallowing behaviors [1].
Multifunctionality could also adequately describe the structures of the upper aerodigestive tract for the purposes of respiration and swallowing. Muscles of the lips, face, tongue, pharynx, larynx, and esophagus are active during breathing and swallowing and have purposes of airway patency, airway protection, and bolus propulsion. Swallowing disorders (i.e., dysphagia) and their causes of varying types aim at these muscles and surrounding connective tissues with functional consequences of impaired bolus flow and airway invasion. Behavioral therapeutic approaches often include compensatory strategies, such as postural alteration, maneuvers that promote airway protection and bolus clearance, and regimens of strengthening exercises [2]. Each of these approaches involves some modifications in respiratory behavior and may alter the otherwise coordinated breathing and swallowing pattern. These alterations may have deleterious effects on learning the behavioral strategy and, hence, on the swallowing recovery of patients. These suspicions have led researchers to explore the normal coordinative relationships between these life-sustaining behaviors.
Normal breathing and swallowing coordination
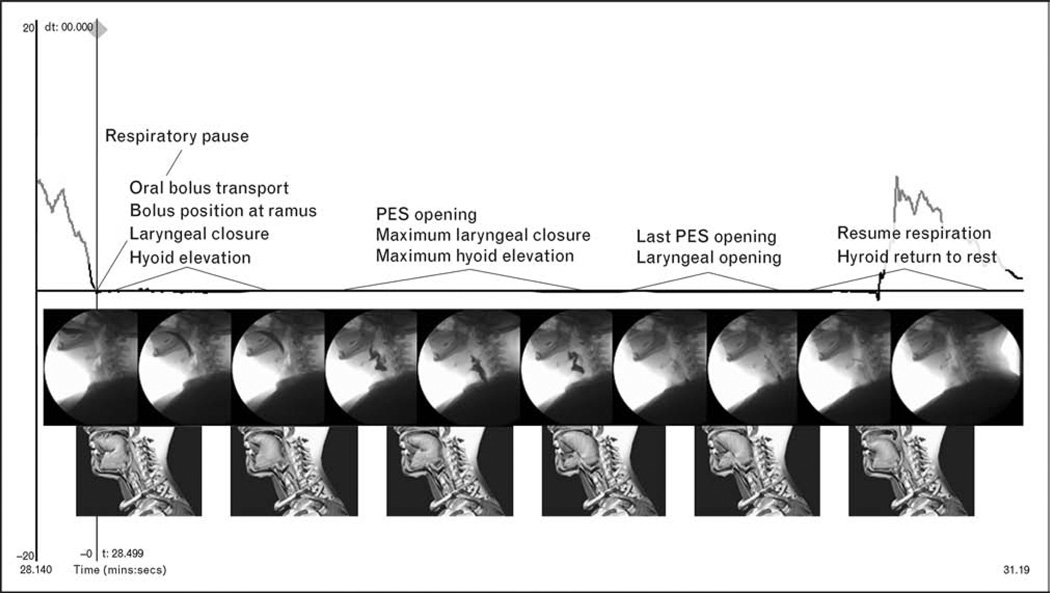
A growing body of literature suggests the existence of a stable coordinative relationship between respiration and oropharyngeal swallowing in healthy adults [3–7]. The article by Martin-Harris et al. [3] represents the first large scale study of breathing and swallowing behavior during liquid swallows throughout the adult lifespan. A unique feature of this work was the identification of functional units describing temporal relationships of swallowing physiology and breathing behavior. Solid food tends to differ from liquids and viscous materials in terms of the regularity of respiration, but the cycle of breathing in which the swallow typically occurs is during the expiratory phase of respiration between middle and lower lung volumes [8]. Initiating swallowing in the expiratory phase at mid-to-low lung volumes poses significant physiologic advantages for hyolaryngeal anterior–superior movement, airway closure, and pharyngo-esophageal segment (PES) opening [9]. There is an obligate pause in respiration that occurs to accommodate swallowing. This pause has historically been termed ‘apnea’ in the majority of studies that have investigated respiratory–swallowing relationships. However, given the implications of disease associated with the term ‘apnea’, the naturally occurring halt in breathing associated with swallowing will be hereafter termed respiratory pause (D.H. McFarland, personal communication, 19 July 2007). This duration of the respiratory pause averages between 1.0 and 1.5 s during liquid swallows in most healthy adults, but there is variability in the timing, depending on the swallowing task and bolus viscosity [3–7]. Onset of the respiratory pause has been associated with a seemingly protective, momentary medialization of the true vocal folds that is followed by complete vocal fold adduction at some point during anterior–superior hyolaryngeal motion [10,11]. In many individuals, respiration resumes during descent of the larynx at the later stages of swallow and is marked by the brief exhalation that occurs during this time [3,4,10,11]. An animated functional model has been derived from simultaneous videofluoroscopy and airflow recordings that temporally link the critical biomechanical components of swallowing with the respiratory phases surrounding swallowing and the respiratory pause that accommodates swallowing [3] (Fig. 1). This model enables clinicians to recognize aberrations from the stable patterning and coupling of breathing and swallowing events during liquid swallows obtained in a videofluoroscopic evaluation. Variations in the stable pattern are clinical indicators of risk for aspiration.
Figure 1.
The animated model derived from simultaneous videofluorographic and respiratory recordings that illustrates the temporal relationships between respiratory pause onset and offset with functional groupings of oropharyngeal swallowing events
PES, pharyngo-esophageal segment. Adapted from [3].
Emerging data are pointing to disruptions in this stable coupling between respiration and swallowing under certain physiologic conditions such as development and aging, presence of neurologic disease and cancers of the head and neck, swallowing task, and compensatory postures [12,13•,14,15••–18••]. These swallowing conditions have been associated with swallows being initiated in and followed by an inspiration versus the typical pattern of expiration surrounding swallowing activity. Although our understanding of the relationship between swallowing disorders alone and between respiratory disorders alone on patient outcome are increasingly clear, understanding of the role of disorders that cross between the swallowing and respiratory systems, that is, breathing and swallowing coordination and patterning, is unknown. Evidence supports that liquid swallows initiated during the inspiration and followed by inspiration place patients at an airway protective disadvantage. Liquids flow from the mouth and into the pharynx at rapid velocities. Inspiration, therefore, might result in airway entry of hesitated material prior to initiating a swallow or residue in the pharynx following a swallow in patients with dysphagia. A better understanding of the nature and consequences of reversal or modification in the stable pattern and the benefits of relearning a potentially protective pattern may be a hallmark in the prevention of aspiration.
Development and aging
Fragile patients on the extreme ends of the age continuum fall victim to swallowing disorders, aspiration, and aspiration pneumonia. In contrast to findings from studies of healthy adults, less regularity and stability in the respiratory– swallowing pattern with equal distribution of expiratory and inspiratory phases of respiration surrounding swallowing have been reported in infants [19]. It has been suggested that the inspiratory activity surrounding a swallow in infants may be related to collapse of the pharyngeal airway and becomes a source of obstructive apnea that is associated with sudden infant death syndrome.
Contrary to this early work that investigated respiratory– swallowing coordination in infants, however, the experiments by Nixon et al. [17••] revealed a potentially protective role between the act of swallowing and episodes of apnea. The investigators studied medically stable, preterm infants at term using combined recordings of nasopharyngeal manometry, respiratory inductance plethysmography, and polysomnography. Unlike many previous studies, not all respiratory pauses were included in the Nixon et al. analysis. Rather, the criteria for registering an apneic episode were clearly specified as greater than normal periods of pauses that accommodate swallowing, and included only those pauses that were greater than 3 s. Consistent with the specified details that defined apneas, arousals were also operationally defined and were indicated by changes in the EEG (electroencephalography), submental EMG (electromyography), and heart rate signals and by movement artifacts on the respiratory channels and pulse waveforms. Using these criteria, swallows occurred more frequently during periods of apnea when compared to control periods, were more frequently associated with mixed and obstructive apneas rather than central apneas, and tended to occur after apnea had begun. These data refute the notion that swallowing is causally linked to apnea, rather the movements associated with swallowing may be a coordinated, protective response to apnea that serve to open an obstructed airway. The investigators discussed the need to extend these experiments to clinically susceptible populations and note that common treatments such as nCPAP (nasal continuous positive airway pressure) have been shown to inhibit swallowing frequency in newborn lambs. If this inhibition of swallowing activity were replicated in human infants, it may be that the intended respiratory treatment renders the infant at risk for poor airway clearance and airway obstruction.
Of particular relevance to the discussion of respiratory–swallowing stability, this study found a highly stable coupling between breathing and swallowing during nonnutritive swallows in healthy infants. Swallows occurred in the end-expiratory phase of the respiratory cycle during sleep and wakefulness and mirrored data obtained in healthy adults by the investigative team. These findings differ from the work by Kelly et al. [16••] who studied respiratory–swallowing coordination during nutritive swallows in 10 healthy term infants throughout the first year of life. Similar to the Nixon et al. study and typical adult pattern, swallowing resulted in a respiratory pause that occurred during the mid-expiratory phase of the respiratory cycle during the first 48 h of life. There was, however, a rapid shift to swallows occurring in the inspiratory phase of the respiratory cycle in the ages of 9–12 months. This shift in respiratory–swallow phase patterning occurred without negative nutritional or airway protective consequences. The infants’ respiratory–swallowing pattern resumed the adultlike pattern by 12 months of age. These data suggest that changes in respiratory–swallowing pattern are common, occur at predictable periods in maturation, and do not signal respiratory or nutritional compromise in healthy term infants. The data also speak to the possibility that the physiologic load associated with feeding may impart instability in respiratory–swallowing coordination at critical periods during the maturation process when compared to nonnutritive swallowing.
Like the alterations in respiratory–swallow coupling that occur with maturation, evidence also supports modifications to the stable respiratory–swallow coupling during advanced aging. A few studies have shown a greater occurrence of liquid swallows initiated and followed by the inspiratory phase of respiration [3,20,21]. These pattern changes have not been associated with swallowing impairment or aspiration; however, they may have negative airway protective and bolus clearance implications in patients with already compromised swallowing function secondary to diseases and conditions that occur during old age such as, stroke, chronic obstructive pulmonary disease, and head and neck cancer. In addition to alteration in respiratory–swallow phase patterning, the respiratory pause to accommodate swallowing has been shown to nearly double in duration during liquid swallowing in older individuals [3]. The functional significance of the prolonged respiratory pause remains uncertain particularly if overlaid with conditions known to cause dysphagia.
Neurologic disease
A recent study lends clarification to respiratory–swallowing relationships in young to mid-aged adults with neurological conditions [18••]. The authors approached a clinically challenging question that sought to address the effects of mechanical ventilation on perceived swallowing effort and respiratory–swallowing coordination. Study participants included 29 patients with a variety of neuromuscular disorders and chronic respiratory failure and 10 healthy controls. Respiratory–swallowing phase relationships were examined via submental EMG and inductive respiratory plethysmography during random presentation of three volumes of water swallows (5, 10, and 15 ml). The tracheostomized patients (n = 19) who were capable of spontaneous breathing (n = 11) were studied during mechanical ventilation and during spontaneous breathing. The results showed that nearly 100%of swallows produced by the controls were followed by the expiratory phase of the respiratory cycle. In contrast, only 50% of the swallows produced by the patients were followed by expiration. Interestingly, the number of swallows per bolus and swallowing time correlated to maximal inspiratory pressures (MIPs) but not to maximal expiratory pressures (MEP). The presence of the tracheostomy tube significantly affected the number of swallows per bolus and the duration of swallowing when compared to the control group. Further, mechanical ventilation was consistently associated with shorter swallowing times per bolus, fewer swallows per bolus, and significantly lower Borg scale scores (i.e., index of perceived effort). Finally, patients who could not swallow the largest bolus during spontaneous breathing were able to accomplish the task during mechanical ventilation. Although there was a primary weakness of this study that included failure to identify the physiologic nature and severity of the swallowing disorder, particularly in view of a varied neurological population, the findings that relate respiratory muscle function and mechanical ventilation to swallowing ability and respiratory–swallow phasing are compelling and may have important clinical implications if replicated in future studies. Intuitively, it would seem that constraining respiratory phase associated with swallowing by a ventilator would serve to disrupt swallowing coordination and efficiency. However, the swallowing parameters of timing and perceived effort actually improved. The authors gave several plausible and intriguing explanations for their results: swallowing likely increased the work of breathing and demands of the respiratory musculature, whereas these demands were decreased by mechanical ventilation allowing the upper airway muscles to dedicate their efforts to swallowing; and spontaneous breathing may result in increases in patient anxiety, hypercapnia, and concentration on breathing with poorer swallowing performance. The authors also stated that positive subglottic pressures generated by mechanical ventilation may improve swallowing ability by promoting airway clearance of oropharyngeal residual and aspirate, as postulated in earlier studies. Though this study must be extended to like populations of patients with quantified levels and types of swallowing impairment, the implications of these current findings are interesting and unlike current practice. Speech-language pathologists and physicians often opt to withhold swallowing therapy and oral intake when tracheostomized patients are being weaned from the ventilator. The results of this study, though very preliminary, speak to the potential functional, physiologic advantages of feeding patients with tracheostomy tubes during mechanical ventilation.
A final interesting finding from this study was that MIP was associated with swallowing ability. Expiratory muscle strength training is under investigation for a potential role in the rehabilitation and recovery of swallowing function in healthy individuals [22•]. The results of this work suggest that the role of inspiratory muscle strength training for improving swallowing function warrants exploration.
Compensatory strategies
Compensatory postures are often applied as temporary measures with dysphagic patients because evidence has shown their effectiveness in facilitating airway protection and bolus flow in carefully selected patients [2].What is not clear, however, is whether these postural alterations somehow interfere with the normal respiratory–swallowing pattern, a potentially critical factor in airway protection. An experiment by McFarland et al. [23] set out to investigate the potential alterations that may occur with postural alteration to the upper airway. Their study showed that the segment of the expiratory phase during which swallowing occurs can be influenced by changes in the whole body posture in adult humans. It was revealed that when human adults attempted to swallow with their hands and knees planted on the ground (i.e., ’on all fours’), the swallow occurred during the early part of the expiratory phase of the respiratory cycle. In contrast, swallowing occurred late in the expiratory phase when participants were standing upright. A more recent study by Ayuse and colleagues [12] carried this concept further and studied the effect of two postural modifications that are commonly used as temporary compensatory strategies during dysphagia rehabilitation – chin tuck and partial recline. Combined use of submental EMG and manometry revealed that a 60° recline from vertical position with a 60° chin tuck posture resulted in increased durations of respiratory pause during swallowing and increased total swallowing duration when compared to a neutral, upright positioning. Although the Ayuse study suffers from lack of measurement or description of the physiologic swallowing mechanism, the findings demonstrate in a very preliminary way that some compensatory techniques may impose physiologic loads that somehow alter the otherwise tight neural coupling between respiration and swallowing.
The coupling of respiratory–swallowing phase patterning also seems to change according to the swallowing task. Differences in the onset and duration of the respiratory pause to accommodate swallowing and respiratory– swallow phase pattern have been shown between cued syringe swallows and cup drinking tasks. Studies that employ prompted, liquid swallows administered to healthy individuals from a syringe demonstrate highly replicable phase patterning, pause onsets and durations [10,11]. Spontaneous small volume, liquid swallows during cup drinking have been shown to occur during the expiratory phase of the respiratory cycle; however, there is a great deal of variability in the onset of the respiratory pause. Although the respiratory pause consistently began prior to or coincident with the onset of hyoid movement, it often began earlier during loading of the liquid into the mouth or during oral bolus transport [3].Amore recent study by Dozier et al. [14] showed a significant increase in the occurrence of inspiration surrounding spontaneous, sequential swallowing of larger volumes when compared to small volume liquid swallows. This finding, together with their finding of laryngeal vestibular opening between swallows in a sequence, led the investigators to surmise that sequential swallows of liquid from a cup may predispose patients with swallowing disorders to greater risk for aspiration when compared to a small volume, single swallowing task. These findings are consistent with current clinical practice of monitoring bolus volume in order to optimize temporal coordination of swallowing. This practice may also optimize respiratory–swallowing phase patterning (i.e., swallowing occurring during and followed by expiration) that further prevents risk for aspiration.
Similar to the compensatory alterations made to bolus volume and task, modifications in the texture of swallowed materials are also routinely made by speech-language pathologists because these variables have shown an immediate influence on swallowing behavior observed during videofluoroscopic or endoscopic imaging. The majority of studies that have explored breathing and swallowing relationships have employed liquid swallows. A few studies have examined the integration of respiration, mastication, and swallowing behavior. McFarland et al. [23] found that mastication could have a profound effect on the respiratory rhythm in some individuals, even to the extent of producing a long period of apnea. Like the McFarland et al. study, Matsuo et al. [24••] also reported abrupt changes in breathing pattern during eating. The chewed bolus aggregated in the valleculae as breathing was maintained. It would appear from these findings that food swallows impart additional coordinative demands on these cross-system functions of patients with dysphagia, particularly in those who present with delayed initiation of airway closure. Further studies of natural eating and drinking behavior will be essential to further understanding the neural mechanisms that mediate peripheral inputs on central respiratory–swallow coupling during various eating and drinking tasks. If these mechanisms were better understood, clinicians could identify and optimize peripheral inputs to effect a ‘normalization’ or stabilization of aberrant respiratory–swallowing patterns.
Conclusion
The functional significance of cross-system neural control and respiratory swallowing coordination is far from understood. Future studies are warranted to test the effects of respiratory–swallowing instability on the swallowing safety and health outcomes of like groups of patients. Preliminary studies have demonstrated the occurrence of alterations in the otherwise stable respiratory–swallowing coupling during maturation, in the presence of neurological disease and cancers of the head and neck, and in certain swallowing tasks. These alterations in respiratory–swallow phase patterns have also been associated with increases in the presence of aspiration and swallowing impairment. Studies are warranted and underway that will test whether or not retraining stable coupling is feasible and test the effect of respiratory–swallowing phase training alone or in combination with traditional swallowing treatments on the recovery of swallowing disorders.
Acknowledgements
The author’s work was supported by grants NIDCD R03 DC04864, 5 K23 DC005764, R24 DC008647-01, and the Mark and Evelyn Trammell Trust.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 293).
- 1.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 2.Logemann JA. Rehabilitation of oropharyngeal swallowing disorders. Acta Otorhinolaryngol Belg. 1994;48:207–215. [PubMed] [Google Scholar]
- 3.Martin-Harris B, Brodsky MB, Michel Y, et al. Breathing and swallowing dynamics across the adult lifespan. Arch Otolaryngol Head Neck Surg. 2005;131:762–770. doi: 10.1001/archotol.131.9.762. [DOI] [PubMed] [Google Scholar]
- 4.Martin-Harris B, Brodsky MB, Price CC, et al. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: single liquid swallows. J Appl Physiol. 2003;94:1735–1743. doi: 10.1152/japplphysiol.00806.2002. [DOI] [PubMed] [Google Scholar]
- 5.Paydarfar D, Gilbert RJ, Poppel CS, Nassab PF. Respiratory phase resetting and airflow changes induced by swallowing in humans. J Physiol (Lond) 1995;483(Pt 1):273–288. doi: 10.1113/jphysiol.1995.sp020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perlman AL, He X, Barkmeier J, Van Leer E. Bolus location associated with videofluoroscopic and respirodeglutometric events. J Speech Lang Hear Res. 2005;48:21–33. doi: 10.1044/1092-4388(2005/003). [DOI] [PubMed] [Google Scholar]
- 7.Hiss SG, Strauss M, Treole K, et al. Effects of age, gender, bolus volume, bolus viscosity, and gustation on swallowing apnea onset relative to lingual bolus propulsion onset in normal adults. J Speech Lang Hear Res. 2004;47:572–583. doi: 10.1044/1092-4388(2004/044). [DOI] [PubMed] [Google Scholar]
- 8.McFarland DH, Lund JP. Modification of mastication and respiration during swallowing in the adult human. J Neurophysiol. 1995;74:1509–1517. doi: 10.1152/jn.1995.74.4.1509. [DOI] [PubMed] [Google Scholar]
- 9.Charbonneau I, Lund JP, McFarland DH. Persistence of respiratory–swallowing coordination after laryngectomy. J Speech Lang Hear Res. 2005;48:34–44. doi: 10.1044/1092-4388(2005/004). [DOI] [PubMed] [Google Scholar]
- 10.Martin BJ, Logemann JA, Shaker R, Dodds WJ. Coordination between respiration and swallowing: respiratory phase relationships and temporal integration. J Appl Physiol. 1994;76:714–723. doi: 10.1152/jappl.1994.76.2.714. [DOI] [PubMed] [Google Scholar]
- 11.Martin BJW. The influence of deglutition on respiration [doctoral dissertation] Evanston, Illinois: Northwestern University; 1991. [Google Scholar]
- 12.Ayuse T, Ayuse T, Ishitobi S, et al. Effect of reclining and chin-tuck position on the coordination between respiration and swallowing. J Oral Rehabil. 2006;33:402–408. doi: 10.1111/j.1365-2842.2005.01586.x. [DOI] [PubMed] [Google Scholar]
- 13. Butler SG, Stuart A, Pressman H, et al. Preliminary investigation of swallowing apnea duration and swallow/respiratory phase relationships in individuals with cerebral vascular accident. Dysphagia. 2007;22:215–224. doi: 10.1007/s00455-007-9077-4. This current study supports early work that found respiratory–swallowing phase relationship differences and respiratory pause prolongations with associated aspiration in cerebrovascular accident (CVA) patients versus normal controls.
- 14.Dozier TS, Brodsky MB, Michel Y, et al. Coordination of swallowing and respiration in normal sequential cup swallows. Laryngoscope. 2006;116:1489–1493. doi: 10.1097/01.mlg.0000227724.61801.b4. [DOI] [PubMed] [Google Scholar]
- 15. Dozier TS, Martin-Harris B, Brodsky MB, et al. Aberrant breathing and swallowing patterns in patients treated for oropharyngeal cancer. 14th Annual Dysphagia Research Society; Scottsdale, Arizona. 2006. The above is the first study documenting a reversal from dominant to nondominant respiratory phase patterning with associated aspiration in patients treated for oropharyngeal cancers.
- 16. Kelly BN, Huckabee M-L, Jones RD, Frampton CMA. The first year of human life: coordinating respiration and nutritive swallowing. Dysphagia. 2007;22:37–43. doi: 10.1007/s00455-006-9038-3. The above is the first reported longitudinal study showing maturation of respiratory–swallowing phase relationships within the first year of life.
- 17. Nixon GM, Charbonneau I, Kermack AS, et al. Respiratory–swallowing interactions during sleep in premature infants at term. Respir Physiol Neurobiol. 2008;160:76–82. doi: 10.1016/j.resp.2007.08.010. The above study was carefully controlled and outcome measures were meticulously defined. The findings suggest that swallowing is associated with arousal and may serve a protective role in preventing deleterious effects of obstructive sleep apnea in term infants.
- 18. Terzi N, Orlikowski D, Aegerter P, et al. Breathing-swallowing interaction in neuromuscular patients: a physiological evaluation. Am J Respir Crit Care Med. 2007;175:269–276. doi: 10.1164/rccm.200608-1067OC. The above is the first known study that has investigated respiratory–swallowing phase patterning in mechanically ventilated patients and that showed a potential functional advantage of mechanical ventilation during swallowing. Further, this investigation is the first study linking swallowing problems to inspiratory muscle function.
- 19.Wilson SL, Thach BT, Brouillette RT, Abu-Osba YK. Coordination of breathing and swallowing in human infants. J Appl Physiol. 1981;50:851–858. doi: 10.1152/jappl.1981.50.4.851. [DOI] [PubMed] [Google Scholar]
- 20.Hirst LJ, Ford GA, Gibson GJ, Wilson JA. Swallow-induced alterations in breathing in normal older people. Dysphagia. 2002;17:152–161. doi: 10.1007/s00455-001-0115-3. [DOI] [PubMed] [Google Scholar]
- 21.Hiss SG, Treole K, Stuart A. Effects of age, gender, bolus volume, and trial on swallowing apnea duration and swallow/respiratory phase relationships of normal adults. Dysphagia. 2001;16:128–135. doi: 10.1007/s004550011001. [DOI] [PubMed] [Google Scholar]
- 22. Wheeler KM, Chiara T, Sapienza CM. Surface electromyographic activity of the submental muscles during swallow and expiratory pressure threshold training tasks. Dysphagia. 2007;22:108–116. doi: 10.1007/s00455-006-9061-4. The above study describes the first investigation of expiratory muscle strength training on some biomechanic components of swallowing function.
- 23.McFarland DH, Lund JP, Gagner M. Effects of posture on the coordination of respiration and swallowing. J Neurophysiol. 1994;72:2431–2437. doi: 10.1152/jn.1994.72.5.2431. [DOI] [PubMed] [Google Scholar]
- 24. Matsuo K, Hiiemae KM, Gonzalez-Fernandez M, Palmer JB. Respiration during feeding on solid food: alterations in breathing during mastication, pharyngeal bolus aggregation and swallowing. J Appl Physiol. 2008;104:674–681. doi: 10.1152/japplphysiol.00527.2007. The importance of this work is the observation that the airway remains open and breathing continues during chewing and bolus aggregation in the valleculae. These data indicate that chewing of solid foods requires fine temporal coordination between respiration, mastication, pharyngeal swallowing initiation, and airway closure.