Abstract
Rationale
The regenerative potential of the heart is insufficient to fully restore functioning myocardium after injury, motivating the quest for a cell-based replacement strategy. Bone marrow derived mesenchymal stem cells (MSC) have the capacity for cardiac repair that appears to exceed their capacity for differentiation into cardiac myocytes.
Objective
Here we test the hypothesis that bone marrow derived MSCs stimulate the proliferation and differentiation of endogenous cardiac stem cells (CSCs) as part of their regenerative repertoire.
Methods And Results
Female Yorkshire pigs (n=31) underwent experimental myocardial Infarction (MI); and 3 days later received transendocardial injections of allogeneic male bone marrow-derived MSCs, MSC concentrated conditioned medium (CCM), or placebo (Plasmalyte). A no-injection control group was also studied. MSCs engrafted and differentiated into cardiomyocytes and vascular structures. In addition, endogenous c-kit+ CSCs increased 20-fold in MSC treated animals vs. controls (p<0.001), there was a 6-fold increase in GATA-4+ CSCs in MSC vs. control (p<0.001), and mitotic myocytes increased 4-fold. Porcine endomyocardial biopsies were harvested and plated as organotypic cultures in the presence or absence of MSC feeder layers. In vitro, MSCs stimulated c-kit+ CSCs proliferation into enriched populations of adult cardioblasts that expressed Nkx2-5 and troponin I.
Conclusions
MSCs stimulate host CSCs, a new mechanism of action underlying successful cell-based therapeutics.
Keywords: Myocardial Infarction, Mesenchymal Stem Cells, Cardiac Stem Cells, Myocardial Regeneration
Introduction
The quest to restore damaged organs is one of the major challenges in medicine1;2. Recently, it has been demonstrated both experimentally3 and in patients4;5 that the heart has the capacity to replace cardiac myocytes throughout life, but that this response is inadequate to compensate for major injuries such as myocardial infarction. These recent observations coupled with the description of endogenous cardiac stem cells6-9 have raised enthusiasm for tapping this intrinsic property therapeutically. Another avenue of cardiac cell-based therapy has utilized diverse adult cell sources such as fat, bone marrow, umbilical cord blood, and adipose tissue10;11. Some of these cell types, particularly mesenchymal stem cells, have properties that could allow them to stimulate endogenous cardiac repair in a regulated manner 12;13.
Bone marrow-derived MSCs regulate hematopoietic and other stem cell niches 14-16 while maintaining substantial multilineage differentiation capacity 17. Notably, recent studies document that interactions with MSCs are essential to the stimulation of in vitro proliferation and differentiation of other progenitor cell populations, in a process requiring direct cell-cell contacts16;18-20. Currently, in experimental models, MSCs exert major functional recovery in the injured heart 12;21-24, through incompletely understood mechanisms 25-27. Here we hypothesized that MSCs stimulate cardiac repair through cell-autonomous effects that stimulate host myocardial precursor cells to amplify and differentiate into cardiomyocytes 6;22;28. To address this prediction, we injected GFP-labeled, male porcine MSCs into the infarct and border zone in female pigs 3 days following myocardial infarction (MI); another group received injection of concentrated conditioned medium (CCM), so as to test whether secreted factors alone would be sufficient to stimulate host cardiac repair.
Materials and Methods
This study was reviewed and approved by the University of Miami Institutional Animal Care and Use Committee and complies with all Federal and State guidelines concerning the use of animals in research and teaching as defined by The Guide For the Care and Use of Laboratory Animals (NIH Pub. No. 80-23, revised 1985).
For this study, 31 healthy female Yorkshire swine weighing 25-35 kg underwent experimental myocardial infarction (MI) followed by reperfusion [Online. Figure 1]. The study was conducted in 2 phases. In the first phase, three groups were studied: Yorkshire pigs received transendocardial injections (TEI) (Stiletto, Boston Scientific, Natick, Massachusetts) of 75×106 GFP labeled MSCs (n=8), Placebo (n=8) or no injection (n=3) three days following the MI. Animals were sacrificed at 24h (n=2 placebo and n=2 MSCs treated), 72h (n=3 placebo and n=3 MSCs treated) and 2 weeks (n=3 MSCs treated n=3 placebo and n=3 control) after transplantation in order to study the fate of the allogeneic cells.
In the second phase, two groups were studied: The Yorkshire pigs were randomized to receive TEI of 100×106 cells of male GFP-labeled MSCs or their 10x concentrated condition medium 3 days after MI and followed by MRI analyses (Siemens Symphony, Erlangen, Germany) at multiple time points (baseline, 2 days post MI, 4days, 2 weeks and 8 weeks post-injection)in order to assess the amount of functional recovery. The animals were sacrificed at 2 weeks (n=3 CCM and n=3 MSCs treated) and 8 weeks (n=3 CCM and n=3 MSCs treated) after injections.
GFP transduction of MSCs
Passage 1 (P1) MSCs were plated in a T25cm2 flask and transduced with lentiviral green fluorescence protein (GFP). At approximately 50% confluency the media was removed and replaced with 5ml of transduction media consisting of alpha MEM plus 20% FCS plus 8 ug/ml polybrene and 10 ul of lenti viral vector LV-173GFP (Lentigen, Gaithersburg, MD). The culture flask was incubated for 72 hours total, with fresh transduction media being added every 24h. The next day the media was removed and alpha MEM plus 20%FCS added to the culture. The flask was further incubated until confluent.
Cultures were expanded with each passage of the GFP+ MSC until sufficient numbers of MSC were obtained. The cells were then frozen in liquid nitrogen until needed. Prior to injection, the cells were thawed rapidly and washed to remove dimethylsulfoxide (DMSO), then resuspended in PBS plus 1% human serum albumin (HSA) to the required cell dose.
Transendocardial Injections of MSCs
Delivery of the cells at the sites of myocardial injury was performed as previously described22. Briefly, left ventriculograms from 2 different angiographic projections [left (-30°) and right (+30°) anterior oblique] were used to manually map the endocardial contours of the LVs in both projections. The IZ and BZ were then delineated on the contours, and a total of 15 injections were performed in each animal, with each injection containing 0.5 ml of the injectate. Each injection was fluoroscopically guided to distribute cells evenly throughout the entire infarct and border zones.
Histology
For microscopic evaluation, the regions of interest were selected from each of the transverse ventricular sections based on CMRI and gross pathology findings: (i). One sample from the middle of the scarred, infarcted tissue. (ii) One sample containing the left border of the infarct along with non-scarred tissue; one sample containing the right border of the infarct along with non-scarred tissue (these two samples were defined as the border zones macroscopically). Microscopically, within these samples, border zones were defined as the areas that were 1-1 ½ high power fields distant from scarred zones. (iii) One sample from the posterior non-infarcted LV wall. Confocal analysis was performed as previously described.
Statistical Analysis
All the values are presented as means ± SEM. All analyses were performed by using the SPSS for Windows version 15.0 (SPSS Inc., Chicago, IL). Differences between groups following immunohistological evaluation were compared by using One Way ANOVA. Differences between groups in ejection fraction and infarct size based on cMRI were calculated by using two-way repeated measures ANOVA. The Tukey's test was used for the post-hoc analysis. A level of P≤0.05 was considered statistically significant.
A detailed Materials and Methods section describing all procedures and protocols is provided in the online supplement.
Results
MSC engraftment reduces infarct size
As shown by serial cardiac magnetic resonance imaging (cMRI), MI led to a reduced ejection fraction (EF) [27.9±1.1% and 25.8±3.1% for MSC and CCM groups respectively, p=NS, p<0.001 vs. baseline] and scar tissue that comprised ~25% of the left ventricle (24.9±2.4% and 24.4±3.2% of the left ventricles of the MSC and CCM groups, respectively, p=NS) [Table 1 and figure 1].
Table 1.
Myocardial Infarct phenotype before and after treatment (values determined by cMRI)
| Parameter | Groups | Baseline | Post-MI | Week 2 Post-Inj | Week 8 Post-Inj | p-value |
|---|---|---|---|---|---|---|
| Infarct Size (%LV mass) | CCM | 0 | 24.4±3.2 | 23.5±1.7 | 26.3±1.5 | |
| MSCs | 0 | 24.9±2.4 | 14.5±3.1 | 10.9±5.1 | p=0.002*† | |
| Infarct Volume (ml) | CCM | 0 | 13.4±1.3 | 11.9±1.0 | 13.4±1.0 | p=0.009* |
| MSCs | 0 | 11.7±1.7 | 7.7±1.8† | 7.3±3.5† | p≤0.001† | |
| LV mass (g) | CCM | 51.2±3.3 | 51.2±3.3 | 51.7±5.0 | 51.5±6.0 | |
| MSCs | 45.9±3.6 | 45.9±3.6 | 51.2±4.2 | 62.1±5.4 | p=NS | |
| Circumferential Extent of MI (%LV) | CCM | 0 | 41.5±5.0 | 44.8±2.4 | 38.04±4.3 | |
| MSCs | 0 | 38.8±4.0 | 27.8±6.5 | 27.6±12.1 | p=NS | |
| End-Diastolic Volume (ml) | CCM | 33.2±3.4 | 38.8±4.0 | 43.0±4.8 | 40.5±1.2 | |
| MSCs | 32.2±2.4 | 34.2±3.8 | 41.0±4.7 | 46.5±10.7 | p=NS | |
| End-Systolic Volume (ml) | CCM | 20.3±2.3 | 28.9±3.6 | 30.8±4.1 | 28.2±0.9 | |
| MSCs | 20.1±1.6 | 24.5±2.6 | 26.7±5.1 | 31.4±11.3 | p=NS | |
| Stroke Volume (ml) | CCM | 12.9±1.3 | 9.8±1.1 | 12.2±0.8 | 12.2±0.3 | p= 0.003† |
| MSCs | 12.1±1.0 | 9.7±1.3 | 14.3±1.4□ | 15.1±1.1† | p=0.005□ | |
| Heart Rate | CCM | 86.2±6.8 | 82.2±3.0 | 69.1±1.5 | 81.1±19.5 | |
| MSCs | 102.5±9.0 | 85.7±4.3 | 81.0±5.5 | 84.1±17.3 | p=NS | |
| Ejection Fraction | CCM | 39.1±1.6 | 25.8±3.1 | 29.1±1.4 | 30.2±0.4 | p=0.042□ |
| MSCs | 37.8±1.6 | 27.9±1.1 | 37.0±5.0□ | 36.4±9.4† | p=0.026† |
indicates p values significant between groups
indicate p values significant within group
indicate p values significant within group
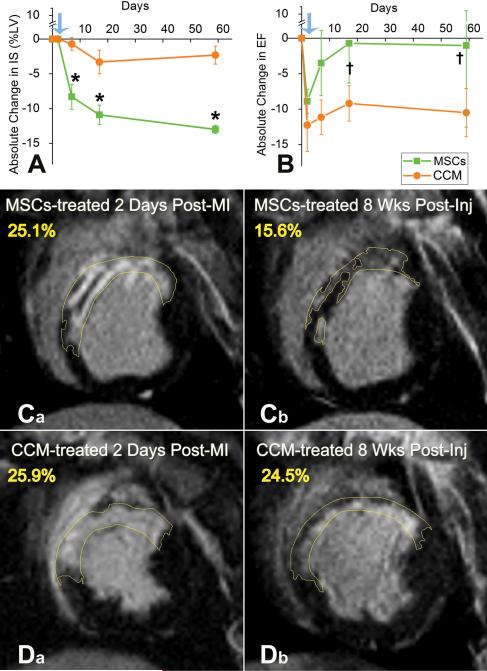
Figure 1. Impact of MSC and CCM on infarct size and global LV function by cardiac MRI.
(A,B) Cardiac MRI documents that targeted TEI of MSCs but not CCM causes infarcted porcine hearts to improve significantly (*p=0.002), by achieving ~50% reduction in IS (A) and restoring EF (B) towards normal (†p=0.042 and †p=0.026 within MSCs group at 2 and 8 weeks respectively). (C,D) Representative delayed contrast hyper-enhanced images of MSCs (C a,b) and CCM (D a,b) treated animals before and 8 weeks after injections. Infarct size (yellow tracings) is reduced by MSC but not CCM administration; values in yellow correspond to scar size (%LV). Blue arrows indicate the day before TEI. Mean values± SEM (n=6 each at baseline, 4-days and 2 weeks, n=3 each at 8 weeks).
Consistent with previous observations21-24, as early as 4 days following TEI of MSCs, the absolute size of the myocardial infarct29 was reduced by 8.3±1.8% (% LV mass) in the animals treated with MSCs, while remaining unchanged in the CCM group (P=0.018; Figure 1). Eight weeks following MI, myocardial scar was reduced to 10.9±5.1% of the LV in the MSC-treated animals, while CCM caused a mild reduction in the absolute IS by 2.3±1.3% (p=0.002 between groups) [Figure 1A]. In addition, by 2 weeks the MSC group had a significant improvement in EF compared to post-MI (p<0.05), which persisted through the 8-week follow-up period [Figure 1B].
Localization of injected MSCs in infarcted hearts
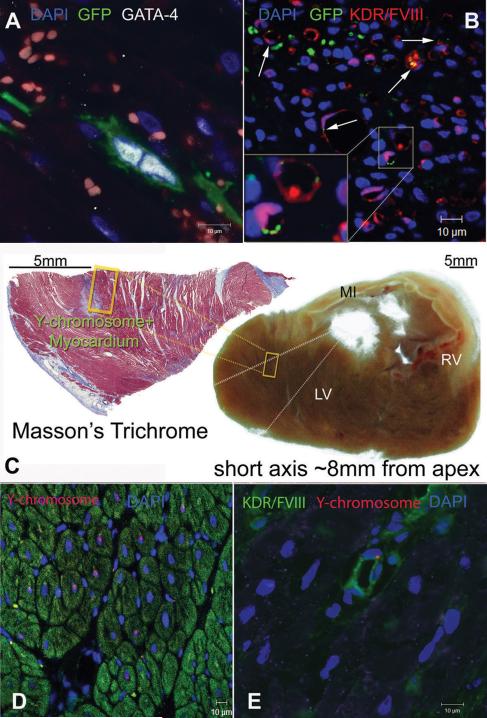
Engraftment and lineage tracing of transplanted MSCs were assessed with confocal immunofluorescence. As determined by both GFP and Y-chromosome tags, MSCs occupied the infarcted (IZ) and border (BZ) zones but not the healthy, remote areas (NIZ) of the infarcted myocardium. Interestingly, while MSCs lacked markers of cardiovascular lineage in vitro [figure 2A-C], evidence for their cardiac commitment could be documented within 24 hours [figure 3A, Online Figures IV, VI, VIII, XI], and by 2 weeks MSCs had differentiated into new, mature cardiomyocytes and vascular structures [figure 3C-E, Online video I, Online Figures II, V, X]. The number of MSCs committed to cardiomyocytic lineage was quantified based on the expression of GFP+ and/or Y-chromosome+ tags co-localized with the cardiomyocyte specific-markers GATA-4 and α-sarcomeric actinin. Commitment of MSCs peaked at 3-days post-implantation (640±240 cells/cm3 GFP+/GATA-4+ cells/cm3 at 24h vs. 1980±360 GFP+/GATA-4+ cells/cm3 at 72h) [Online figure IV] and the extent of myocardial chimerism remained constant in the regenerated hearts [figure 4A]. The number of MSCs committed to vascular lineage was quantified based on the expression of GFP+ and/or Y-chromosome+ tags co-localized with the endothelial cell specific-markers KDR and Factor-VIII related antigen. Importantly, differentiation of MSCs into vascular phenotype did not occur during the first 24h, but by 72h 79±23 GFP+ cells/cm3 exhibited commitment to vascular lineage [Figure 3B, Online Figures IV, VII]. Two weeks later, coronary vascular chimerism was still present within the myocardium [Figure 3E].
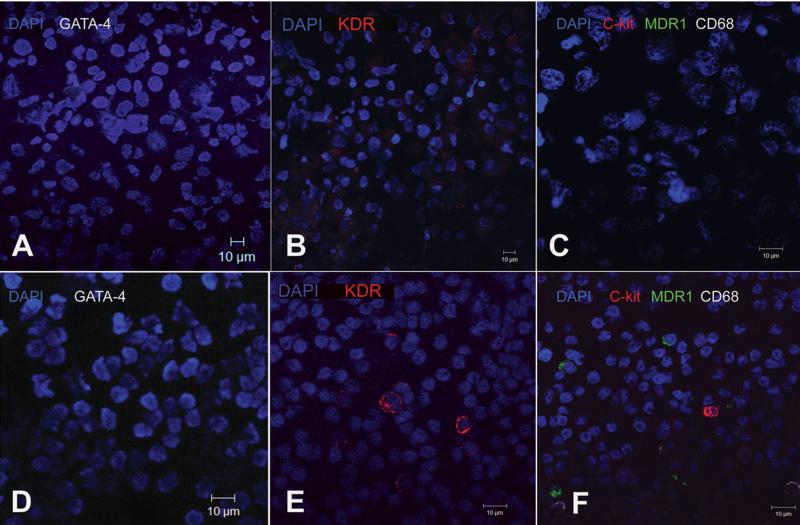
Figure 2. Immunophenotypic characteristics of porcine MSCs before transplantation.
(A-C), Immunocytochemical staining of porcine MSCs illustrating native phenotype; all cells are negative for markers such as GATA-4 (A), KDR (B), CD68, MDR1 and c-kit (C). (D-F) Porcine Peripheral Blood Mononuclear Cells (PBMCs) used as a control cell type, for evaluating the aforementioned markers. PBMCs are negative for GATA-4 (D) but contain positive fractions for KDR (E), c-kit, CD68 and MDR1 (F).
Figure 3. MSCs differentiate into new cardiac myocytes and vessels.
(A) A GFP+ MSC committed into cardiomyocytic lineage and undergoing symmetric division 72h after transplantation, as indicated by nuclear co-localization with GATA-4. (B) Differentiation of MSCs into endothelial lineages as indicated by co-localization of GFP with KDR and Factor-VIII related antigen (arrows). (C) Masson's trichrome stained tissue section showing the context of a Y-chromosome containing region with respect to the infarct, 2 weeks after MSCs therapy. MSCs differentiated into new myocardial tissue at the border line of a previously infarcted region. The section is located ~8mm from the apex and ~30mm from base of the LV. (D) Chimeric myocardium as indicated by the Y-chromosome containing myocytes in the cross-section of panel (C). (E) New coronary vessel formation 2 weeks after transplantation, illustrated by the Y-chromosome containing endothelial cell. [MI, myocardial infarct; LV, left ventricle; RV, right ventricle].
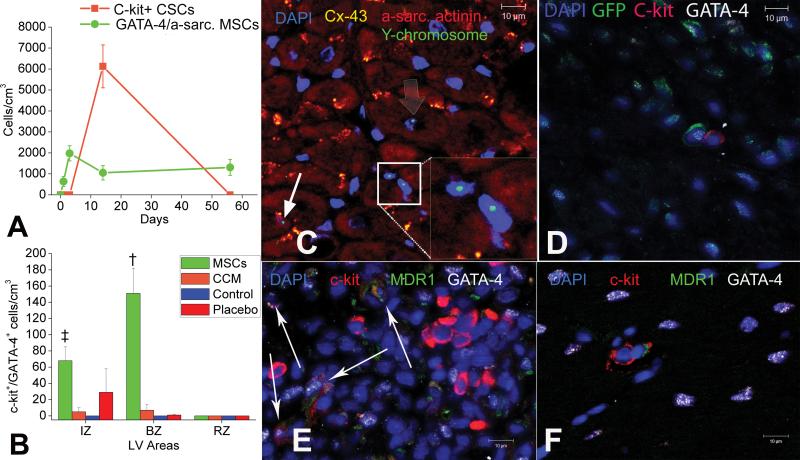
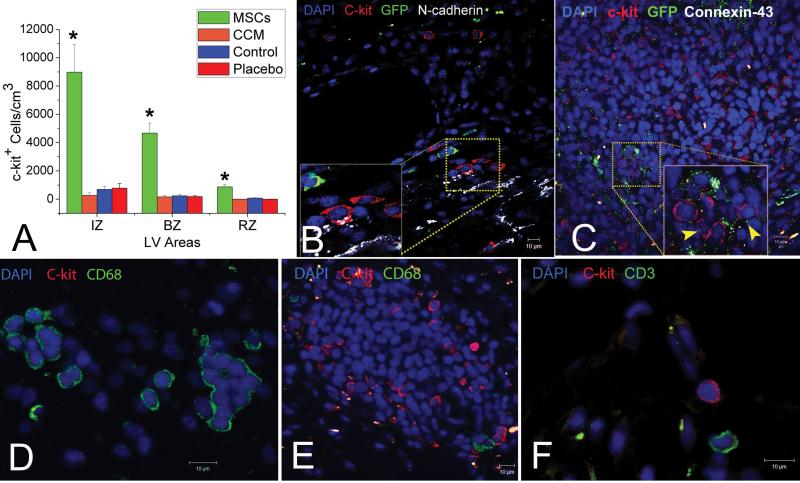
Figure 4. MSCs stimulate endogenous CSCs.
(A) Graph depicting the contribution of cardiomyocyte precursors following exogenous administration of MSCs (green line) and endogenous CSCs (orange line), during cardiac repair after MI. MSC differentiation occurs rapidly after delivery. At 2-weeks, MSCs activate endogenous expansion c-kit+ CSCs (orange line). (B) Two weeks following TEI, the number of C-kit+ cells co-expressing GATA-4 is greater in MSCs vs. non-MSCs treated hearts. The cardiac precursors are preferentially located in the IZ and BZ of the MI, indicating an active process of endogenous regeneration (‡p=0.019 and †p<0.0001) (C,D) The 2-week old chimeric myocardium contains mature cardiomyocytes (open arrow), immature MSCs (arrowheads, inset) and cardiac precursors of MSCs origin (arrow), coupled to host myocardium by connexin-43 gap junctions; Interestingly, endogenous c-kit+ CSCs are found in close proximity to MSCs (D). (E) Cluster of c-kit+ CSCs in an MSCs-treated heart; numerous CSCs are committed to cardiac lineage documented by GATA-4 and MDR-1 co-expression (arrows). (F) Few, isolated c-kit+ cells were found in non-MSC treated animals.
MSCs engraftment induces C-kit+ CSCs recruitment
Next, we tested the hypothesis that MSCs, in addition to direct tissue replacement could also contribute to myocardial repair by supporting endogenous CSCs to regenerate myocardium. Interestingly, two weeks after MSC injection, the MSC-treated hearts exhibited chimeric clusters containing both immature MSCs of exogenous origin and endogenous CSCs [figures 4, 5, Online. Figure III A-C], defined by the expression of the stem cell factor-receptor, c-kit 6;8. These clusters were mainly localized within the IZ and BZ but not the NIZ or the non-MSC treated hearts; important cell-cell interactions between the MSCs and endogenous CSCs could be documented [figures 4, 5, Online. Fig. III A-C, Online Fig XII]. C-kit+ cells lacked GFP confirming that they did not arise from the MSCs [figure 4D,G,H and figure 5]. Quantification of c-kit+ cells in infarct hearts illustrated sporadic distribution during the first 3 days after transplantation similar in MSC and CCM groups. At 2 weeks following TEI, endogenous c-kit+ CSCs increased 20-fold in MSC but not CCM treated animals [Figure 4A, figure 5]. In addition, CSCs formed potential connexin-43 mediated gap junctions and N-cadherin mechanical connections with other c-kit+ cells, adult cardiomyocytes, and with GFP+ MSCs [figures 4, 5, online Fig XII], and formed structures that resemble cardiac stem cell niches6-9. Further immunohistochemical characterization demonstrated that a number of CSCs co-expressed MDR1 and GATA-4, indicating a cardiac lineage commitment [figure 4E]. The number of cardiac committed CSCs increased 2-fold in the IZ and 15-fold in the BZ of MSC-treated animals compared to animals receiving either CCM, non-cellular vehicle alone, and untreated controls [figure 4B]. There were no differences between groups in the non-infarcted zones, indicating the presence of an active endogenous repair mechanism targeting the damaged zones of the treated hearts [figure 4B]. Finally, by 8 weeks the level of endogenous CSCs decreased to baseline post-MI levels, and were detected as scattered, isolated cells with no differences observed between treatment groups [figure 4A].
Figure 5. MSCs stimulate amplification of endogenous c-kit+ CSCs 2 weeks after injection.
(A) Recruitment of c-kit+ CSCs in the MSCs-treated vs. non-treated hearts and distribution of the c-kit cells within the different zones. (*p≤0.001) (B) Endogenous c-kit+ CSCs develop putative mechanical connections with the infarcted myocardium as indicated by co-localization with N-cadherin (arrows). (C), A large cluster of c-kit+ CSCs connected to each other and to adjacent GFP+ MSCs by connexin-43. (D-F), Representative figures illustrating the non-inflammatory/mast cell phenotype of the c-kit+ CSCs. While clusters of CD68pos/c-kitneg cells could be detected in the non MSCs-treated hearts (C), the MSCs-treated hearts were rich in ckitpos/CD-68neg and CD3neg clusters of CSCs (D) Mean values ±SEM (n=6 MSCs, 3 placebo, 3 CCM and 3 Control).
MSCs stimulate cardiomyocyte cell cycling
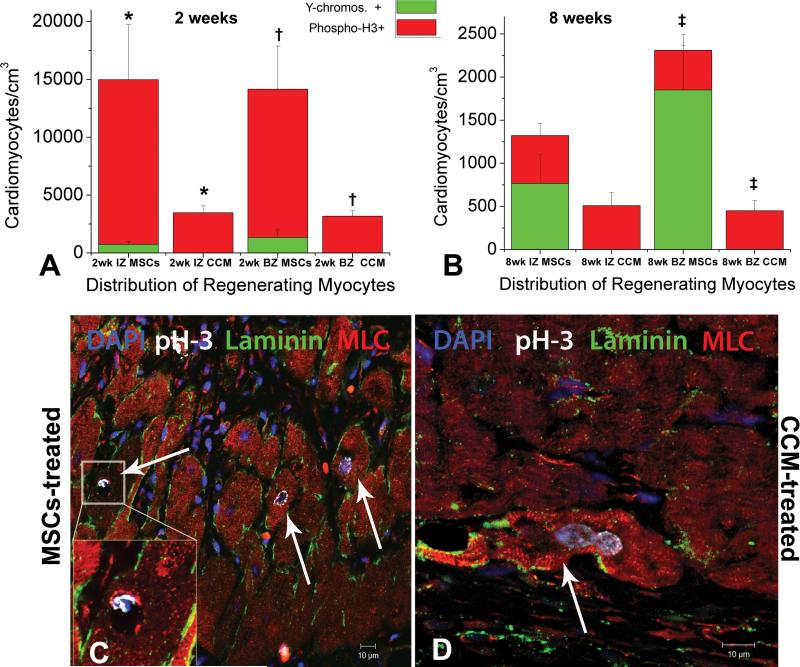
In addition to activating c-kit+ cardiac precursors to enter cardiomycytic lineages, MSCs also stimulated cardiomyocyte replication [figure 6, Online. Figure IX]. Host cardiomyocyte turnover peaked at 2 weeks after therapy as indicated by the expression of the mitotic marker of serine 10-phosphorylated Histone-H3 (phospho-H3), and was 4-fold greater in the IZ and BZ of the MSCs treated hearts compared to CCM group [figure 6A]. The number of mitotic myocytes in the MSCs-group was significantly higher in both the IZ (14,220±4750 vs 3,480±590 cardiomyocytes/cm3, p=0.03) and BZ (12,810±3720 vs 3,190±470 cardiomyocytes/cm3, p=0.005) compared to the CCM-treated animals [figure 6A]. Co-localization of GFP and/or Y-chromosome tags with phospho-H3+ cardiomyocytes could be rarely documented, indicating that the majority of amplifying cardiomyocytes detected were of host origin (data not shown). The levels of cycling myocytes in the MSCs-treated group decreased by 2 months, and no differences could be observed between groups [figure 6B]. However, the total number of newly formed cardiomyocytes of both donor and host origin was still greater in the BZ of the MSCs treated animals compared to the CCM-treated animals (2,310±640 vs. 450±120 total new cardiomyocytes/cm3 in the BZ of MSCs and CCM-treated respectively, p=0.02), illustrating that direct tissue replacement by differentiated MSCs contributed significantly to the regeneration of the BZ areas [figure 6B].
Figure 6. MSCs stimulate endogenous cardiomyocyte cell cycling.
A,B Quantification of newly formed myocytes of both host (red bargraph, phospho-H3+) and donor (green bargraph, Y-chromosome+) origin, 2 and 8 weeks post-injection respectively. MSCs stimulated host cardiomyocytes to amplify during the first 2 weeks following TEIs. The new CMs were mainly distributed at the IZ and BZ of the treated hearts, indicating active regeneration of injured myocardium. By 8 weeks endogenous cycling CMs levels had returned to normal values. Correlation between the extent of cardiomyocytes that arose from differentiated MSCs vs. amplifying host cardiomyocytes, depicted a substantially higher contribution of the latter to new cardiac muscle formation indicating the important actions of MSCs on enhancing endogenous therapeutic potentials C,D Mitotic features in endogenous cardiomyocytes from the BZ of an MSCs-treated and CCM-treated heart respectively. Mean values ±SEM (n=3 hearts, each). (* and †, indicate p<0.05 between groups; □ indicates p=0.05 between groups).
MSCs stimulate proliferation and myocardial commitment of c-kit+ CSCs in vitro
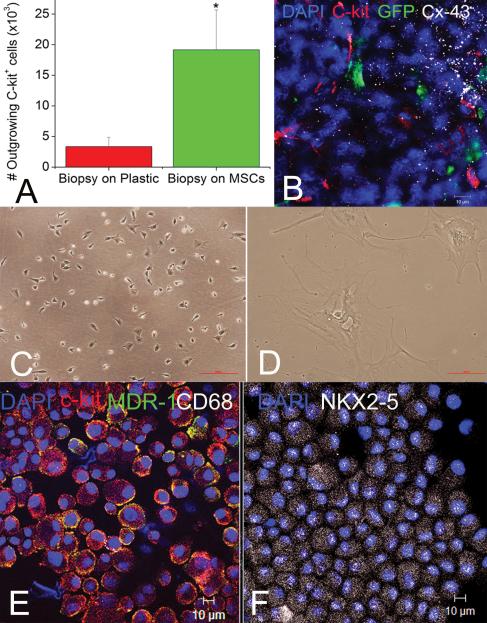
We next performed in vitro experiments to study the function and origin of endogenous CSCs in relation to MSCs [figure 7, Online Figure XIII]. Fresh (n=12) or cryopreserved (n=26) endomyocardial biopsies from porcine hearts were cultured for one week with or without MSCs as feeder layers [figure 7B, C]. In additional control experiments, MSCs were cultured under the same conditions without a myocardial biopsy. After 3 days, myocardial biopsies became infiltrated by MSCs and adhered to the MSC monolayers [Online figure XIII, B]. In contrast, biopsies cultured without MSCs remained in suspension [Online figure XIII, A]. Within one week organotypic co-cultures became confluent, and purification by repeated immune panning30 illustrated that the number of GFP-negative, c-kit+ cells egressing from the biopsy were 6-fold greater compared to biopsies cultured alone [figure 7]. These cells were CD68neg, small, semi-adherent and self-renewing [figure 7, Online Figure XIII]. As expected, c-kit+ cells could not be harvested from MSC-control cultures [figure 2]. A mean of 19,159±6,506 vs. 3,347±1,519 c-kit+ cells were purified from biopsies cultured with and without MSCs respectively (p=0.003). Similar to our in-situ findings, immunocytochemical analysis documented the development of connexin-43 mediated cell-cell interactions between GFP+ and c-kit+ cells [figure 7B]. In contrast, c-kit+ cells purified from biopsies cultured without MSCs had a large, antigen-presenting cell-morphology that did not proliferate [figure 7D].
Figure 7. Development of cardiac stem cell niches ex-vivo.
(A) MSCs stimulate outgrowth of c-kit+ CSCs from endomyocardial biopsies. (B), Immunostaining of the primary cell cultures documents interactions between GFP+ MSCs (green) and c-kit+ cells (red) as indicated by co-localization with connexin-43 (white); these clusters resemble cardiac stem cell niches. (C, D) C-kit+ cells outgrowing after a week from the biopsy-alone are large, quiescent cells with a macrophages morphology (D); The fraction of the c-kit+ cells that were produced from the co-cultures was 3.4±0.9% of the total cell number in co-culture vs. 5.0±2.2% of the cells without co-culture. However, there were 10-fold more cells with co-culture than without, yielding many more c-kit+ cells (1.01 × 106 cells/co-culture vs. 0.9 × 105 cells/biopsy alone panned 1 week after plating the organotypic cultures in each group). In comparison, co-culture with MSCs egress small, semi-adherent CSCs that renew their population constantly (C). (E, F) Immunocytological stainings of c-kit+ CSCs purified and expanded from the organotypic co-cultures with MSCs, illustrate the high percentage of c-kit+ cells in MDR1 (E, yellow) and NKX2-5 (F, white), while lacking the mast-cell surface epitope CD68 (E, white). Mean values ±SEM (n=19 each).
In comparison to previous studies 6-9;31;32, purification of c-kit+ CSCs from single biopsy samples was accelerated by co-culture with MSCs, and facilitated an outgrowth of highly myocardiocytic CSCs; greater than 90% of the cells expressed the cardiac transcription factors Nkx2.5 and GATA-4 and the ATP-binding cassette transporter MDR1 while lacking expression of the VEGF-receptor, KDR [figure 7, Online fig. 3D-G, Online Figure XIII].
Porcine CSCs differentiate into myocytes by co-culturing with neonatal cardiomyocytes
To test the capacity of the CSCs to differentiate into myocytes we performed co-cultures with neonatal rat cardiomyocytes (NRCMs). Porcine CSCs were seeded on transwell membranes, and placed on top of NRCM monolayers. After 3-4 days in co-culture, CSCs illustrated phenotypical characteristics of mature cardiomyocytes, evidenced by a striated cytoskeleton expressing cardiac troponin-I [Online video II]. Importantly, differentiation of CSCs into cardiomyocytes in a transwell culture system which prevents the development of direct cell contact with NRCMs, excludes cell-fusion and demonstrates a cardiomyocytic phenotype of this adult cardiac precursor cell type.
Discussion
Here we demonstrate that bone marrow derived MSCs, when injected into the post myocardial infarct porcine heart, facilitate substantial cardiac recovery involving host cell-based repair as well as MSC engraftment and differentiation. Differentiation of MSCs occurs acutely after transplantation, while MSCs stimulate endogenous cardiomyocyte turnover in two likely related ways; first, by stimulating endogenous c-kit+ CSCs, and second by enhancing cardiomyocyte cell cycling. Together these phenomena represent the spectrum of changes necessary for the amplification and differentiation of new adult cardiomyocytes. The interaction between cardiac derived c-kit cells and bone marrow derived MSCs could be replicated in-vitro, where the co-culturing amplified c-kit cell expansion and differentiation.
Efficacy of MSCs
Several previous papers from our group21-24;33 and that of others34-36 demonstrate in large animal models major degrees of infarct size reduction and functional recovery with MSC cell therapy. We have previously documented reappearance of myocardial tissue in the border zones of infarction including a rim of tissue on the endocardial surface21 that is associated with improved tissue perfusion24 and recovery of regional function21;23. Despite the reproducible demonstration of major cardiac recovery with MSC therapy, the underlying mechanism of action has been a challenge to demonstrate given the rarity of differentiated MSC-derived myocytes in the post infarction heart.
In the current study we demonstrate a multifold mechanism of action for this effect that includes MSC engraftment and trilineage differentiation. Importantly, MSCs interact with host CPCs, promoting their recruitment and/or expansion and differentiation. In addition, there is evidence of extensive myocyte mitosis which very likely represents the terminal stage of cycling of CPC derived myocytes5. It is also possible that this observation could represent cell cycling of mature myocytes as has been suggested to occur in the zebrafish37;38. The engraftment of MSCs appears to be necessary as tissue recovery is not achieved with the injection of MSC conditioned media.
Direct vs. Indirect tissue replacement following MSCs-based cardiac repair
The current findings argue against a solely paracrine mechanism for MSC stimulated cardiac repair and strongly support that transplanted MSCs engraft and respond directly to cues of injury in infarcted myocardium. By using dual labeling with GFP and sex mismatch strategies to trace allograft fate, we document that transplanted MSCs exhibit features of differentiated cardiomyocytes suggesting direct replacement of myocardial tissue by chimeric CMs. Without excluding the possibility of cell fusion between exogenous and endogenous cells, our data indicate that MSCs generate new CMs and that this process occurs during the first 24 hours following transplantation. However, even though the newly produced chimeric CMs last for at least 2 months and contribute significantly to the total number of new CMs regenerating the injured heart, their number remains unlikely to account in total for the extensive degree of cardiac recovery documented by cMRI. Accordingly we demonstrate alternative mechanisms for cell autonomous, MSC mediated cardiac repair - MSCs stimulated a series of secondary endogenous responses that caused substantial amounts of adult CMs and, more importantly, immature CSCs to proliferate and replenish the damaged regions with new CMs of host origin.
Our findings permit an estimate of the degree of myocardial tissue regenerated by varying mechanisms. Using rates of appearance of various cell types as a percentage of total new myocytes within the infarct border zones, we estimate that MSCs could contribute up to ~8% of the newly formed cardiomyocytes39;40. Chimeric cardiomyocytes require less than 2 weeks to reach a mature appearance and their presence at 2 months argues that they are not rejected. However, a greater extent of cardiac repair originates endogenously. We document the concerted appearance of c-kit+ CSCs and phospho-H3+ mitotic CMs5;37 (likely representing transient amplifying myocytes originating from CSCs) that peaked 2 weeks after therapy. Together, CSCs and mitotic CMs are estimated to contribute at least 45% of the new myocardial mass. Interestingly, the abundance of both CSCs and transient amplifying myocytes decline in parallel by 8 weeks further suggesting a linkage between the appearance of CSCs and transient amplifying cells5;41. This phenomenon strongly supports the notion that repeated application of cell therapy may lead to further declines in scar tissue within injured myocardium.
None of the above effects occurred when CCM was used as treatment following MI, highlighting that even though paracrine signaling can be a major contributor to cardiac regeneration, the degrees of repair that occur with cell injections cannot be achieved with single applications of factors secreted from MSCs. Indeed while this may simply be an issue of pharmacokinetics as specific paracrine and authocrine pathways are shown to stimulate endogenous tissue repair, MSC delivery clearly provides an enormous advantage in that a single delivery provides a long-standing and sustained biologic effect.
These findings offer a broader perspective on the biology of regeneration and should be viewed in the context of several other major advancements. First, substantial efforts are underway to genetically manipulate MSCs to enhance their survival and/or to use them as delivery vectors42; indeed our findings showing cell-cell coupling of MSCs support this approach. Second, successful cardiac repair in our model was based upon interaction of administered MSCs with host cells, and in this regard there are other attractive candidates for host cells such as recently described endogenous multipotent circulating progenitors 43. As mentioned above, a major hypothesis underlying regenerative effects of MSCs has been termed the “paracrine hypothesis” in which a number of paracrine44 and autocrine factors such as nucleostemin45 drive regeneration of injured hearts. Our findings are not at odds with this hypothesis, and likely MSCs orchestrate a broad array of reparative effects. As a corollary to the paracrine hypothesis, MSCs release immunomodulatory cytokines as part of their secretome 46,47 that could enhance long term graft tolerance following heart transplantation. Finally, it is highly likely that MSCs will have therapeutic potential in a broad array of cardiac disorders in addition to ischemic heart disease.
MSCs support expansion of the local CSCs pool and enhance their cardiomyocytic potentials
Two key questions that are addressed here warrant mention. First, is whether the clusters of c-kit+ CSCs present in the MSCs-treated hearts represent cells mobilized from extra-cardiac tissues such as the bone marrow and circulating blood, or whether they originated from the heart. Secondly, we addressed the differentiation capacity of these endogenous cells. To address the potential cardiac origin of the c-kit cells, we co-cultured heart biopsies on MSC lawns demonstrating that the MSCs-feeder layers stimulated the expansion of c-kit+ CSCs. The CSCs that resulted from MSC co-culture were more than 90% positive for Nkx2-5, a phenotype previously found only in the developing fetal hearts. In addition, the adult CSCs exhibit significantly enhanced cardiomyocytic potentials compared to CSCs that exist in the adult myocardium, and suggest that bone marrow MSCs may provide significant information to link cardiac development to repair. Thus, endogenous CSCs clusters detected in situ following MSCs implantation seem unlikely to originate from distant tissues such as the bone marrow, since their isolation from single heart biopsies suggests they stem from the cardiac pool. While our results do not exclude the possibility of mobilization from distant sources43 (indeed the circulating multipotent cells could represent a master reservoir of tissue specific precursors), these results confirm the presence of precursor reservoirs within the heart itself, and provide evidence that therapeutic CSCs can be harnessed from endomyocardial biopsies. With regard to differentiation, both in vivo and ex vivo studies document lineage commitment and potential differentiation of c-kit cells.
Timing and mode of delivery
It is not firmly established whether selecting different routes, timing and doses on delivering the MSCs would have a greater or less impact on prevention of remodeling following MI. Comparing our previous21-24;33 and current laboratory findings , we believe that hastening 24;36 or delaying22;33;34 cell therapy may not have as a robust an effect as to the targeted delivery of MSCs 3 days post -MI21;23. Importantly, we have found that late treatment following full infarct healing, with the goal of reversing infarct remodeling can produce substantial recovery. In this setting22;33 the degree of infarct size reduction, although substantial, is not as great as that observed with treatment in the early post infarction period.
Certain technical features of our study warrant mention – first, we employed a transendocardial injection in a reperfused MI model. Most investigators have employed intravenous or intracoronary routes to deliver cells in infarcted heart tissue48 assuming that in this way important paracrine actions of the infused cells could still be harnessed without risking unnecessary myocardial damage. However, our study provides novel insights to delivering cell-based therapies and, besides providing further evidence that TEIs can be a safe and efficient method to deliver MSCs21;23, suggests that TEIs delivery to the infarct border zone may provide an optimal substrate for cell engraftment and interaction with host cells. Indeed, it is attractive to speculate that delivery of boluses of MSCs may provide an ideal mechanism to foster reconstitution of cardiac stem cell niches6;12.
Study Limitations
With regard to the adequacy of the conditioned media generated from porcine MSCs cultures, we used the technique described by Maitra et al49 with minor modifications to concentrate the media ten-fold. We acknowledge that we cannot fully rule out the importance of secreted factors contained within the conditioned media. Moreover, it is well described that several factors secreted by MSCs are shown to recruit CSCs50. The fact that our CCM produced little to no reparative effects could be due to a pharmacological effect, and that single dosing could not produce the sustained effects observed with MSCs. Work is ongoing to define the factors produced by MSCs that stimulate endogenous repair in cell non-autonomous manners.
Conclusion
Together these results offer insights into the mechanism of action of a cell-based therapy, whereby significant cardiac repair occurs in the absence of a degree of engraftment and differentiation sufficient to account for the degree of cardiac functional recovery. Moreover these findings require a broadening of our therapeutic perspective solely away from cells with maximum differentiation capacity to those that have a spectrum of activities that include cell-cell interactions. As such cell-cell interactions may offer therapeutic synergies not fully embodied in a single cell preparation. Thus, we suggest that MSCs may replenish or restore cardiac stem cell niches lost or injured during myocardial infarction.
Supplementary Material
Novelty and Significance.
What is known?
The adult mammalian heart can no longer be viewed as a post-mitotic organ, as it has reservoirs of stem cells and is capable of generating substantial numbers of myocytes that enter mitosis.
Despite these endogenous regenerative mechanisms, cardiac injury due to ischemic and other mechanisms remains the leading cause of morbidity and mortality, which has prompted the quest to harness cell-based therapies.
One of the most promising sources for adult cell therapy is the mesenchymal stem cell found in bone marrow and other sources.
What New Information Does This Article Contribute?
MSCs can directly regenerate new myocardium when injected into infarcted hearts, by differentiating into cardiomyocytes and coronary vessels.
The bulk of regenerative process is mediated by interactions between MSCs and endogenous CPCs which cause the latter to amplify and differentiate into cardiomyocytes of host origin.
MSCs can be utilized ex-vivo to enhance the production of CPCs from single endomyocardial biopsies into therapeutic quantities.
Summary.
Emerging clinical and pre-clinical studies demonstrate the therapeutic capacity of MSCs following acute and chronic ischemic cardiac injury. Despite these promising results, whether MSCs engraft and differentiate into myocytyes remains controversial, prompting a paracrine hypothesis that MSCs exert therapeutic effects through secreted factors. In this study we illustrate that MSCs reduce infarct size by regenerating only a maximum of ~8% of newly formed myocardium. The majority of new tissue formed originates from host mechanisms that require interactions with the MSCs. We show for the first time that MSCs enhance CPC proliferation and differentiation into transient amplifying cells and mature CMs. Therefore, without excluding paracrine modes of action, our findings show that the effects of MSCs are cell-autonomous. The structures formed by MSCs and CPCs resemble stem cell niches. Together these findings have important biological and therapeutic implications that may pave the way for designing future studies that tap the heart's own regenerative potential. Successful cell based therapy must be viewed in a broader context – cell differentiation and replacement is not the only imperative; interaction with host elements with the capability of generating new cells is a crucial component of successful tissue regeneration.
Acknowledgements
We thank Dr. George McNamara from Diabetes Research Institute's Analytical Imaging Core Facility for his confocal imaging support.
Sources of Funding
This work was supported by National Heart, Lung, and Blood Institute grants U54-HL081028 (Specialized Center for Cell Based Therapy), R01-HL084275, and P20 HL101443. Dr. Hare is also supported by RO1's AG025017, HL065455, and HL094849.
Non-standard Abbreviations and Acronyms
- LV
Left Ventricle
- MI
Myocardial Infarction
- EF
Ejection Fraction
- SV
Stroke Volume
- EDV
End-diastolic volume of LV
- ESV
End-systolic volume of LV
- HR
Heart rate under ventilation
- MI EXT
Circumferential extent of MI
- IZ
Infarcted myocardial zone
- BZ
Borderline zone
- NIZ
Non-infarcted, remote myocardium
- MSCs
Male Porcine, Allogeneic, Bone Marrow-Derived Mesenchymal Stem Cells
- CSCs
Native Porcine c-kit+ Cardiac Stem Cells
- CMs
Cardiomyocytes
- TEI
Transendocardial Injections
- GFP
Green Fluorescence Protein
- CCM
Concentrated Conditioned Medium from the MSCs cultures
- cMRI
Cardiac Magnetic Resonance Imaging
- phospho-H3
Serine-10 phosphorylated Histone-H3
- MLC
cardiac myosin light chain
- IS
Infarct size
- BM
Bone marrow
- MNCs
Mononuclear cells
- FCS
fetal calf serum
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 2.Boyle AJ, Schulman SP, Hare JM, Oettgen P. Is stem cell therapy ready for patients? Stem Cell Therapy for Cardiac Repair. Ready for the Next Step. Circulation. 2006;114:339–352. doi: 10.1161/CIRCULATIONAHA.105.590653. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kajstura J, Urbanek K, Perl S, Hosoda T, Zheng H, Ogorek B, Ferreira-Martins J, Goichberg P, Rondon C, D'Amario D, Rota M, Del MF, Orlic D, Tisdale J, Leri A, Anversa P. Cardiomyogenesis in the Adult Human Heart. Circ Res. 2010 doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De AA, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 10.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 11.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Werner N, Haase J, Neuzner J, Germing A, Mark B, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 12.Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S21–S26. doi: 10.1038/ncpcardio0770. [DOI] [PubMed] [Google Scholar]
- 13.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr., Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miura Y, Gao Z, Miura M, Seo BM, Sonoyama W, Chen W, Gronthos S, Zhang L, Shi S. Mesenchymal stem cell-organized bone marrow elements: an alternative hematopoietic progenitor resource. Stem Cells. 2006;24:2428–2436. doi: 10.1634/stemcells.2006-0089. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Chen S, Yuan J, Yang Y, Li J, Ma J, Wu X, Freund M, Pollok K, Hanenberg H, Goebel WS, Yang FC. Mesenchymal stem/progenitor cells promote the reconstitution of exogenous hematopoietic stem cells in Fancg-/- mice in vivo. Blood. 2009;113:2342–2351. doi: 10.1182/blood-2008-07-168138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 18.Robinson SN, Ng J, Niu T, Yang H, McMannis JD, Karandish S, Kaur I, Fu P, Del AM, Messinger R, Flagge F, de LM, Decker W, Xing D, Champlin R, Shpall EJ. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37:359–366. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stappenbeck TS, Miyoshi H. The Role of Stromal Stem Cells in Tissue Regeneration and Wound Repair. Science. 2009;324:1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 20.Alakel N, Jing D, Muller K, Bornhauser M, Ehninger G, Ordemann R. Direct contact with mesenchymal stromal cells affects migratory behavior and gene expression profile of CD133(+) hematopoietic stem cells during ex vivo expansion. Exp Hematol. 2009 doi: 10.1016/j.exphem.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Amado LC, Saliaris AP, Schuleri KH, St JM, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amado LC, Schuleri KH, Saliaris AP, Boyle AJ, Helm R, Oskouei B, Centola M, Eneboe V, Young R, Lima JA, Lardo AC, Heldman AW, Hare JM. Multimodality noninvasive imaging demonstrates in vivo cardiac regeneration after mesenchymal stem cell therapy. J Am Coll Cardiol. 2006;48:2116–2124. doi: 10.1016/j.jacc.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 24.Schuleri KH, Amado LC, Boyle AJ, Centola M, Saliaris AP, Gutman MR, Hatzistergos KE, Oskouei BN, Zimmet JM, Young RG, Heldman AW, Lardo AC, Hare JM. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am J Physiol Heart Circ Physiol. 2008;294:H2002–H2011. doi: 10.1152/ajpheart.00762.2007. [DOI] [PubMed] [Google Scholar]
- 25.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 26.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 27.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbanek K, Cesselli D, Rota M, Nascimbene A, De AA, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heiberg E, Engblom H, Engvall J, Hedstrom E, Ugander M, Arheden H. Semi-automatic quantification of myocardial infarction from delayed contrast enhanced magnetic resonance imaging. Scand Cardiovasc J. 2005;39:267–275. doi: 10.1080/14017430500340543. [DOI] [PubMed] [Google Scholar]
- 30.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 31.Steele A, Jones OY, Gok F, Marikar Y, Steele P, Chamizo W, Scott M, Boucek RJ., Jr Stem-like cells traffic from heart ex vivo, expand in vitro, and can be transplanted in vivo. J Heart Lung Transplant. 2005;24:1930–1939. doi: 10.1016/j.healun.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Smits AM, van VP, Metz CH, Korfage T, Sluijter JP, Doevendans PA, Goumans MJ. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: an in vitro model for studying human cardiac physiology and pathophysiology. Nat Protoc. 2009;4:232–243. doi: 10.1038/nprot.2008.229. [DOI] [PubMed] [Google Scholar]
- 33.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 35.Lim SY, Kim YS, Ahn Y, Jeong MH, Hong MH, Joo SY, Nam KI, Cho JG, Kang PM, Park JC. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res. 2006;70:530–542. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 37.Jopling C, Sleep E, Raya M, Marti M, Raya A, Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 40.Hosoda T, D'Amario D, Cabral-Da-Silva MC, Zheng H, Padin-Iruegas ME, Ogorek B, Ferreira-Martins J, Yasuzawa-Amano S, Amano K, Ide-Iwata N, Cheng W, Rota M, Urbanek K, Kajstura J, Anversa P, Leri A. Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci U S A. 2009;106:17169–17174. doi: 10.1073/pnas.0903089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–1463. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 42.Penn MS, Mangi AA. Genetic enhancement of stem cell engraftment, survival, and efficacy. Circ Res. 2008;102:1471–1482. doi: 10.1161/CIRCRESAHA.108.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cesselli D, Beltrami AP, Rigo S, Bergamin N, D'Aurizio F, Verardo R, Piazza S, Klaric E, Fanin R, Toffoletto B, Marzinotto S, Mariuzzi L, Finato N, Pandolfi M, Leri A, Schneider C, Beltrami CA, Anversa P. Multipotent progenitor cells are present in human peripheral blood. Circ Res. 2009;104:1225–1234. doi: 10.1161/CIRCRESAHA.109.195859. [DOI] [PubMed] [Google Scholar]
- 44.Rota M, Padin-Iruegas ME, Misao Y, De AA, Maestroni S, Ferreira-Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siddiqi S, Gude N, Hosoda T, Muraski J, Rubio M, Emmanuel G, Fransioli J, Vitale S, Parolin C, D'Amario D, Schaefer E, Kajstura J, Leri A, Anversa P, Sussman MA. Myocardial induction of nucleostemin in response to postnatal growth and pathological challenge. Circ Res. 2008;103:89–97. doi: 10.1161/CIRCRESAHA.107.169334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D'Alessandro DA, Kajstura J, Hosoda T, Gatti A, Bello R, Mosna F, Bardelli S, Zheng H, D'Amario D, Padin-Iruegas ME, Carvalho AB, Rota M, Zembala MO, Stern D, Rimoldi O, Urbanek K, Michler RE, Leri A, Anversa P. Progenitor cells from the explanted heart generate immunocompatible myocardium within the transplanted donor heart. Circ Res. 2009;105:1128–1140. doi: 10.1161/CIRCRESAHA.109.207266. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Le BK, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 48.Heldman AW, Hare JM. Cell therapy for myocardial infarction: Special delivery. J Mol Cell Cardiol. 2008;44:473–476. doi: 10.1016/j.yjmcc.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, Koc ON. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 50.Nakanishi C, Yamagishi M, Yamahara K, Hagino I, Mori H, Sawa Y, Yagihara T, Kitamura S, Nagaya N. Activation of cardiac progenitor cells through paracrine effects of mesenchymal stem cells. Biochem Biophys Res Commun. 2008;374:11–16. doi: 10.1016/j.bbrc.2008.06.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.