Abstract
Most of our current understanding of the genetic predisposition to autoimmune disease can be traced to experiments performed in the decade from 1971 to 1981. Chella David was a key contributor to this research. Many of these early steps came from studies of experimental autoimmune thyroiditis. This model has been especially valuable because essentially the same disease can occur spontaneously in selected strains of animals or can be induced by deliberate immunization. From a genetic point of view, the disease has been investigated in three different species: mice, rats and chickens. The same antigen, thyroglobulin, initiates the disease in all three species. Among the main discoveries were the relationship of autoimmune disease to the major histocompatibility complex (MHC), the interplay of different subregions within the MHC in promoting or retarding development of disease, the differing roles of MHC class II and MHC I class genes in induction and effector phases, respectively, and the cumulative effect of non-MHC genes, each of which represents a small addition to overall susceptibility. Other experiments revealed that genetic differences in thyroglobulin allotypes influence susceptibility to thyroiditis. Thyroid glands differed in different strains in vulnerability to passive transfer of antibody. The first evidence of modulatory genes on the sex-related X chromosome emerged. All of these genetic findings were concurrently translated to the human disease, Hashimoto’s thyroiditis, where thyroglobulin is also the initiating antigen.
Keywords: thyroiditis, thyroglobulin, major histocompatibility complex, autoantibodies, cytotoxic T cells, X chromosome
PROLOGUE
One of the major advances in our understanding of autoimmune disease has been the identification of specific genetic traits that regulate the immune response and the susceptibility of a target organ to immune-mediated damage. Many of the most fundamental discoveries were made in the short time span from 1971 to 1981 in a number of laboratories investigating a variety of different diseases. In this essay I will illustrate some of these early steps based on our studies of genetic aspects of autoimmune thyroiditis in experimental animals (1). In these investigations, Chella David, the honoree in this special issue, played a key role.
INTRODUCTION
The possibility that genetic predisposition plays an important role in the initiation of autoimmune thyroiditis came to my attention when, as a medical student in the 1950’s, I had the priviledge of participating in the care of an 18 year old young lady with Hashimoto’s thyroiditis (Fig. 1). I was already acquainted with chronic thyroiditis from our ongoing studies aimed at demonstrating an autoimmune basis of the disease (2). I thought of chronic thyroiditis, however, as a disease that affects mainly women in their 40s and 50s. Its occurrence in a teenager suggested to me that there may be a heightened hereditary risk. When I inquired about a family history of thyroid disease, the results were quite striking. Past generations of both parents had several cases of Graves’ disease or Hashimoto’s thyroiditis. I inferred that to investigate the genetic control of autoimmune thyroiditis it might best be done in children who develop the disease precociously. The possibility to do that arose several years later and will be referred to briefly. Such human studies at best are capable of establishing an association and occasionally a genetic linkage, but are not likely to provide evidence of causation and mechanism. Studies designed to identify particular genes and determine their mode of action depended on studies of experimental models.
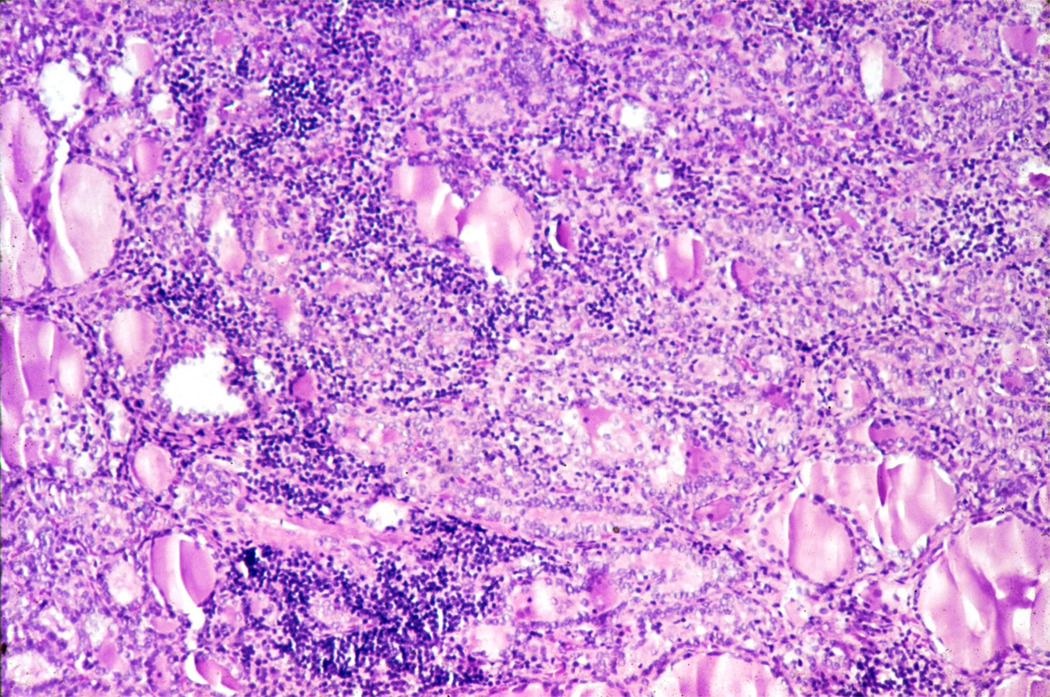
Figure 1.
Section of thyroid gland of patient DT (18 year old female) showing typical histologic findings of Hashimoto’s thyroiditis
H and E, original magnification × 100
Most of the early work on inducing thyroiditis was done in the rabbit. Further progress depended upon producing thyroiditis in an animal like the mouse where many genetically defined strains are available. It was not until 1971 that Frank Twarog and I accomplished the task of developing a consistent, reproducible model of experimental thyroiditis in the mouse replicating the pictures seen in humans (Fig. 2) (3). Critical factors were both the details of the adjuvant used with thyroglobulin to induce the disease and the strain of mouse employed.
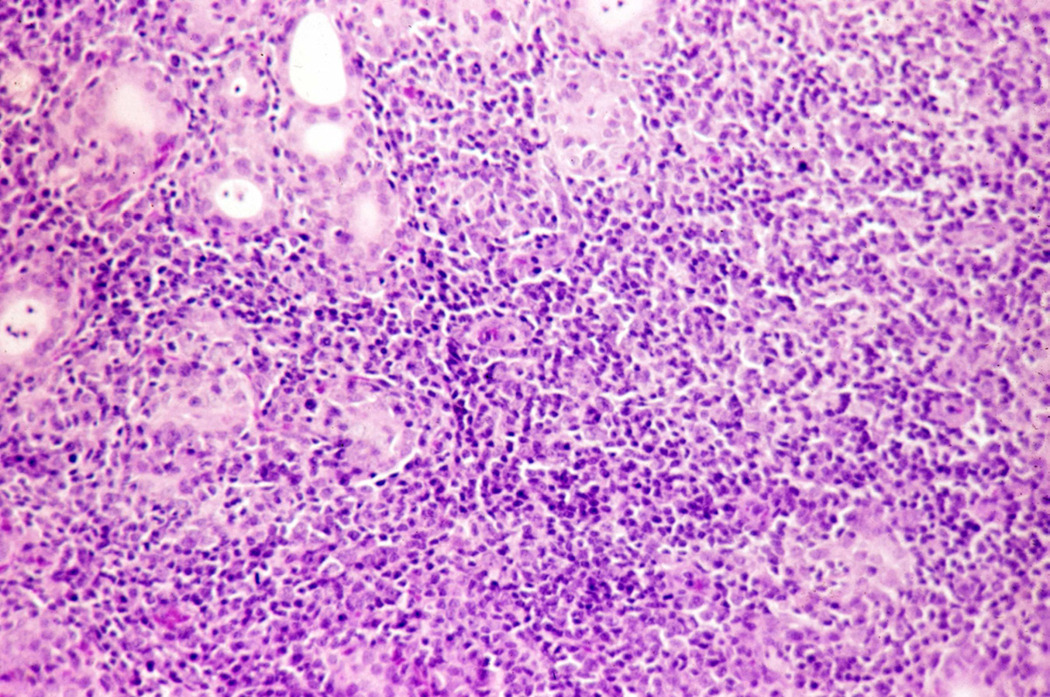
Figure 2.
Section of thyroid gland of CBA mouse immunized with murine thyroglobulin plus complete Freund adjuvant.
H and E, original magnification × 100
MAJOR HISTOCOMPATIBILITY COMPLEX IN THE MOUSE
Based on these findings we were able to undertake a large-scale study of the differing susceptibilities of inbred strains of mice to immunization with thyroglobulin measured in terms of autoantibody production as well as inflammatory lesions in the thyroid gland. These pains-taking studies were carried out by an extraordinarily talented and energetic postdoctoral fellow, Adrian Vladutiu. Briefly, we immunized 33 inbred strains with thyroglobulin and measured the production of thyroglobulin-specific autoantibodies and thyroiditis (4). Strains differed significantly in their response and, much to our surprise, the strength of the response was predictable on the basis of the murine major histocompatibility complex (MHC), H-2. Thus, mice bearing the H-2s or H-2k haplotype were all strong responders whereas H-2 b and H-2d strains were uniformly poor. These first findings were confirmed by testing congenic strains matched at H-2 on a variety of different background, non-H-2 genes. Clearly the H-2 region contain one or more genes that regulate the immune response to thyroglobulin.
This study established for the first time a relationship between the major histocompatibility region and susceptibility to any autoimmune disease. It was already well established that mice immunized with synthetic amino acid polymers mount different immune responses depending upon their H-2. The idea that response to a large multi-determinant molecule like thyroglobulin could be determined at H-2 seemed preposterous. We first reasoned that only a small number of the many potential epitopes on mouse thyroglobulin were recognized by the mouse. Only years later did we demonstrate that not all antigenic determinants are equally immunogenic; some are immunodominant while remain cryptic (5).
We had some initial concerns that the differential immune responses were due not to recognition of the thyroglobulin molecule, but rather susceptibility to the critical effect of adjuvant. That issue was settled definitively by later studies showing that exactly the same order of genetic susceptibilities was evident if one used other adjuvants such as lipopolysaccharide or poly adenine-uridine (poly AU) or even no adjuvant (6,7).
These findings were novel enough to warrant publication in Science where they elicited considerable attention. We quickly set about to define more precisely the locus within the H-2 region that was responsible for susceptibility to experimental thyroiditis. We, therefore, turned to Donald Shreffler of the University of Michigan, who was the leading expert on the fine structure of the murine MHC. Initial studies using two strains of mice (well characterized genetically by Chella David), BSVS and BRVR, showed that the response to thyroglobulin was mainly attributable to an immune response gene located near the K-end of the H-2 complex (8). These results wet our appetite to do more gene localization. At that time, however, Shreffler decided to leave the University of Michigan to go on sabbatical. He told us not to worry; that he had an extraordinarily talented postdoctoral fellow named Chella David who was willing to collaborate with us on MHC genetics. That was the beginning of a decade-long collaboration and friendship with Chella.
MAJOR HISTOCOMPATIBILITY COMPLEX IN THE CHICKEN
Before going on with more sophisticated genetic studies, we wanted to assure ourselves that the MHC association with autoimmune thyroiditis was a general phenomenon, not present only in mice deliberately immunized with thyroglobulin. Fortunately, we had available in our laboratory another model of autoimmune thyroiditis in the OS chicken (Fig. 3). Together with an avian geneticist, Larry Bacon, and an expert on the OS chicken, Joe Kite, we determined that the OS strain was heterogeneous with respect to their B blood groups which represents the major histocompatibility complex in birds. The allotypes could readily be determined by hemagglutination. In a second article in Science we were able to report that the thyroid pathology occurring spontaneously in OS chickens was associated with their B locus (9). We could relate susceptibility with particular alleles present in the OS strain and not in the original Cornell strain (CS) (10). With Larry Bacon and Randall Cole, the originator of the OS and CS strains, we proceeded to perform classical genetic studies in large numbers of hybrids between the OS and CS strains. At the F1 and F2 generations, we were able to detect subtle effects of the B complex on susceptibility. For example, female sex affected severity of thyroid disease, but only after seven weeks. We noted that certain sire families had more severe disease than others, showing that susceptibility to thyroiditis also depended on other, non MHC, genes (11).
Figure 3.
Obese strain (0S) chickens. Left - female (8 mos. old) with severe hypothyroidism due to thyroiditis. Right – male (1 year old) with mild disease
Knowledge of the B allele further allowed us to do selective matings and eventually develop three substrains of OS with differing degrees of thyroid pathology. Some B alleles actually reduced the autoimmune response. Further experiments using thymectomized chickens pointed to a distinct population of suppressor or regulatory T cells (12). One subline of OS birds that usually do not develop significant thyroiditis demonstrated significant thyroid pathology following thymectomy at hatching, showing clearly that increased suppressor function was sometimes responsible for lowered antibody response and thyroiditis. Finally, experiments showed that not only the immune system, but differences in the thyroid gland itself enhanced or diminished susceptibility to thyroid pathology.
The investigations of spontaneous thyroiditis in the OS chickens foreshadowed the types of problems and inconsistencies encountered in studies of genetic susceptibility in differing human populations. Using our three substrains of OS chickens, for example, we found that the influence of the B haplotype was marked in one, less marked but still significant in a second and barely detectable in the third substain. These differences were attributed to the overriding action of genes outside of the MHC (13).
MAJOR HISTOCOMPATIBILITY COMPLEX IN THE RAT
Having studied the genetics of thyroiditis in the mouse and the chicken, we wished to extend these findings to another rodent species, the rat. A sabbatical stay at Oxford University provided the opportunity of examining four well characterized inbred rat strains differing at the MHC (14). Strains HO and AU were both Ag-B5 and developed only minimal thyroid lesions following immunization with rat thyroglobulin. In contrast, AO rats (Ag-B2) and DA rats (Ag-B4) developed more severe thyroiditis. These MHC differences did not determine general autoimmune responsiveness, but were characteristic of thyroid autoimmunity. For example, susceptibility to experimental autoimmune encephalomyelitis among rats is also associated with the Ag-B locus but with entirely different MHC relationships.
Genetic studies of the rat provided another quite unexpected observation. Production of thyroid antibodies was much higher in female than male animals, but was not closely related to the Ag-B complex. Rather, the main factor controlling the response was associated with the X chromosome (15). Only relatively recently has the importance of genes on the X chromosome been implicated in the female sex bias in autoimmune disease among humans (16).
The research described in these sections raised the exciting possibility that autoimmune diseases in humans may all be associated with the MHC. In fact, as early as 1974 we were able to review the available literature and find a number of examples of possible associations (17). In retrospect, it is safe to say that essentially every human autoimmune disease seems to have some MHC bias, although it ranges from marked to minimal. With few possible exceptions, the associations are not usually close enough to be of clinical value but have proven to be extremely valuable for predictive studies, as will be described later. Ironically one of the diseases with the weakest HLA association is autoimmune thyroiditis.
Further Localization of H-2
As our initial studies of the murine MHC revealed, strains bearing the alleles H-2k and H-2s were highly susceptible to thyroiditis whereas strains carrying H-2b or H-2d were poor responders. while some strains, such as H-2a and H-2m, were intermediate. We took this as evidence that more than one locus within H-2 plays a role in the determination of immune response to thyroglobulin. In initial further investigations in collaboration with Tomazic and Shreffler (18), using a list of intra-H2 recombinants, we found that all mice bearing the K-end and I-A region derived from good responder H-2k strains showed moderate to high response to thyroglobulin. In contrast, mice that had K-end and I-A subregion from poor responders, H-2b or H-2d, had minimal or no thyroid inflammation and lower titers of thyroglobulin antibodies. Although these results confirmed our earlier paper using BSVS mice (8), strains were not available at that time to definitively separate the K and I-A loci. We could, however, discern evidence of influence by genes near the D-end of H-2 as well as by background, non-H-2 genes.
We returned to the problem a few years later in collaboration with Chella David together with my colleagues Yi-Chi M. Kong and Kirk Beisel, a postdoctoral fellow who was an alumnus of Chella’s laboratory. Using the intra-H-2 recombinants developed by Chella, we were finally able to pinpoint the gene regulating thyroglobulin response to the I-A subregion (19). These experiments enabled us to formally designate the major immune response gene within H-2 modulating thyroiditis as Irtg.
Our initial studies with Shreffler also hinted that there is additional control of the thyroglobulin response at the D-end of H-2. With the aid of Chella we reinvestigated this question in detail (20). When the Irtg gene is from a good responder H-2k or H-2s strain, cellular inflammation, but not antibody levels, was significantly reduced in mice with a d allele at the D-end. This was the first report that D-region (MHC class I) genes modify the effect of MHC class II immune response genes with respect to immune-induced pathology. The most likely explanation available was that MHC class I genes at the D-end (and in later experiments K-end) of the H-2 complex regulate production of cytotoxic T cells. These speculations were strengthened by our finding that MHC class I genes influence both the proliferation and cytotoxic effect of T cells on thyroid epithelial cell monolayers (21). A novel in vitro system allowed us to demonstrate MHC class I restriction since treatment of the target thyroid cells with antibodies to the K and D regions were both partially inhibitory to the cytotoxic response whereas both antibodies combined abrogated the entire response. The cytotoxic T cells were CD8+ but required CD4+ T cells for this generation, and were restricted in the action to syngeneic rather than allogeneic thyroid monolayers (21). These findings represented the first demonstration of MHC restriction in an autoimmune disease.
NON-H-2 GENES
Our studies on the genetics of thyroiditis in the mouse, the rat and the chicken all provided circumstantial evidence that genes outside of the MHC locus influence the autoimmune response to thyroglobulin. In collaboration with Chella David, we were able to compare a long list of mouse strains differing only at H-2 (22). Even strains that carried the low responder H-2 haplotype can develop relatively severe thyroid disease based on genes outside of the MHC. The exact number and location of these non-MHC genes could not be determined at that time, although “educated guesses” allowed us to predict that the genes were involved in the regulation of the immune response. Subsequent work in many laboratories, including our own, has clearly demonstrated that an “autoimmune diathesis” attributed to the chance accretion of many genetic alleles which combine to produce a heightened autoimmune response. In some instances, the combination of non-MHC genes may actually rival or exceed the influence of MHC genes themselves.
As a consequence of these experiments, we fully understood why susceptibility to an autoimmune disease represents a spectrum rather than a dichotomous function. Sometimes even strains that are considered “non-responders” on the basis of their MHC haplotype can respond if a potent combination of non-MHC immunoregulatory genes is present. Another prediction from the study was that some common immunoregulatory genes play a role in different autoimmune diseases, explaining to some degree the frequent co-occurrence of different autoimmune endocrinopathies in the same animal models or human populations (23). Notably, some of the non-MHC genes may even contribute to the lymphomas that are sometimes associated with autoimmune thyroid disease.
Genetic Control of T Cell Proliferation
Defining the complex genetic control of autoimmune thyroid disease represented a major advance in understanding these disorders, but the critical step in understanding the pathophysiology of thyroiditis depended upon determining the function of the major genes. With that goal in mind, we undertook experiments to define the cellular basis of MHC genetic control of immune responsiveness to murine thyroglobulin in mice (24). To this end, we performed cell transfer experiments from good to poor responder mice and vice versa. In some of the transfer experiments the recipients were thymectomized, or genetically athymic (“nude”) recepients were employed. The results showed clearly that transfer of T lymphocytes, but not B lymphocytes, from good to poor responder strains resulted in severe thyroiditis and high levels of antibody production. B cell transfers did not have that effect because there was no difference in the immune response whether B cells were obtained from genetically good responder or poor responder donors. If transfers were performed to mice that had an intact thymus, recipients developed only a moderate degree of thyroiditis and lowered antibody production. These results added to the growing body of evidence that there was a regulatory population of T cells produced in the thymus that were capable of suppressing the adoptively transferred disease. Very similar results were found using the BUF rat and OS chicken models of thyroiditis (25,26). The nature and role of a suppressor population of thymic derived T cells had a long and somewhat troubled history and are still a topic of vigorous discussion (27).
To continue investigations on the cellular basis of genetic susceptibility to thyroiditis we turned to in vitro proliferative response of T cells. We found initially that the proliferation to mouse thyroglobulin by lymphnode cells from immunized donors was much greater in good responder CBA (H-2k) than poor responder BALB/c (H-2d) mice (28). The proliferatory response was abrogated by treatment with T cell-specific antibody. Using the in vitro proliferative response we were able to collaborate with Chella David in correlating the response with genetically determined susceptibility (29). Control of T cell proliferation was mapped to the I-A region of H-2. Interestingly, this same genetic locus was important in T cell proliferation to foreign (bovine and porcine) thyroglobulins as well as murine thyroglobulin. The experiments further suggested that there were “suppressive” genes derived from the poor responder BALB/c mice located near the D locus.
Although these experiments all indicated that the major genetic control of T cell proliferation resided within the MHC haplotype, we wondered whether this proliferative control was antigen-dependent or non-specific. Using the rat model of experimental thyroiditis we found that the highly disease-susceptible CDF rat had significantly lower responses to the non-specific phytohemaglutinin than the poorly susceptible SHR rats (30). Cross-breeding experiments showed that the rat MHC did not segregate with the gene controlling mitogen responses. Thus, T cell proliferation was antigen-determined and not an intrinsic property of the T cell.
Target Organ Effects
The first hint that there may be genetic differences in the susceptibility to immune-mediated damage within the thyroid gland itself came from studies of the OS chicken. Detailed investigations were lead by Roy Sundick. Initially, we found that transfer of serum with high titers of thyroglobulin antibody from OS chickens with severe thyroiditis induced inflammatory lesions in the thyroid glands of OS chickens, but had no observable effect on CS chickens even bearing the same B haplotype (31). Absorption experiments showed that the effect was due to thyroglobulin-specific antibody. These findings meant that the OS thyroid gland is vulnerable to antibody-induced inflammatory disease and that the trait is not necessarily related to the MHC.
To pursue the idea that there are genetic differences in the vulnerability of the thyroid gland to experimental immunization, we turned to the mouse. Verna Tomazic and I found that purified thyroglobulin prepared from H-2k mice gave a greater immune response in both H-2k and H-2d mice than did H-2d thyroglobulin (32).
The allogeneic differences among thyroglobulins was more directly determined in comparing their immunogenicity in the absence of adjuvant. We compared the effect of repeated injections of allogeneic mouse thyroglobulin will syngeneic mouse thyroglobulin in good and poor responder mice. For the experiments in which no adjuvant was used, it was necessary to inject the mice 16 times over a 4 week period (33). Antibody production tended to be transient but did reach high levels in good responder mice and was accompanied by thyroid lesions. Lesions were observed in all of the mice given allogeneic thyroglobulin, but only half of those given the syngeneic preparation. Thus, in the absence of an overpowering adjuvant allogeneic mouse thyroglobulin is significantly more immunogenic than syngeneic mouse thyroglobulin.
Next we determined whether the antigenic differences resided in the thyroglobulin molecule itself or in some contaminant, and whether it was associated with H-2 (33). First we showed that the severity of thyroiditis was not enhanced by preimmunizing the mice with allogeneic lymphoid cells. Next we collaborated with Accavitti and Leon to prepare a panel of monoclonal antibodies to thyroglobulins from different mouse strains. Although most of the monoclonals reacted equally well with thyroglobulin from H-2k or H-2d mice, some were clearly able to distinguish these two thyroglobulin preparations. The results supported the possibility that allogeneic determinants enhanced the antigenicity of mouse thyroglobulin (33).
The basis of the differences among thyroglobulins of different mouse or chicken strains has never been fully resolved. An interesting possibility, however, arises from the observation that the thyroid cells of OS chickens take up more iodine than comparable epithelia from other birds (34). Perhaps this greater uptake of iodine is related to the metabolic differences in OS thyroid cells which was revealed by careful studies of growth rate and metabolism (35). Later studies by our group and by several others showed that the incorporation of iodine into the thyroglobulin molecules enhances its immunogenicity (36).
STUDIES IN HUMANS
Virtually all of the experimental work described in the previous sections of this article on mice, rats and chickens was carried out in the decade from 1971 – 1981. In parallel, we were able to perform some genetic studies on humans with autoimmune thyroid disease. We based the investigation on my earlier premise that genetic regulation might be more clearly expressed in children and juveniles than in adults. First, we predicted that disease would occur earlier in individuals with a strong family history. Second, there would be less opportunity for environmental factors to influence the outcome. Finally, in prebubesent children the sex difference obvious in adults might be inapparent.
I was fortunate in developing a collaboration with a very energetic pediatric endocrinologist, William Hoffman, and enlisting my colleague, Lynne Burek in an investigation of autoimmune thyroid disease in juveniles and their families. The details of these studies are beyond the scope of this article. However, Chella David played an important role. In the early 1980s he and G. S. Panayi invited us to contribute a chapter to a new book they were editing on immunogenetics. We decided this would be an appropriate place to summarize our genetic studies with special emphasis on the human investigations. In our laboratory this article is always referred to as the “Chella chapter” (37).
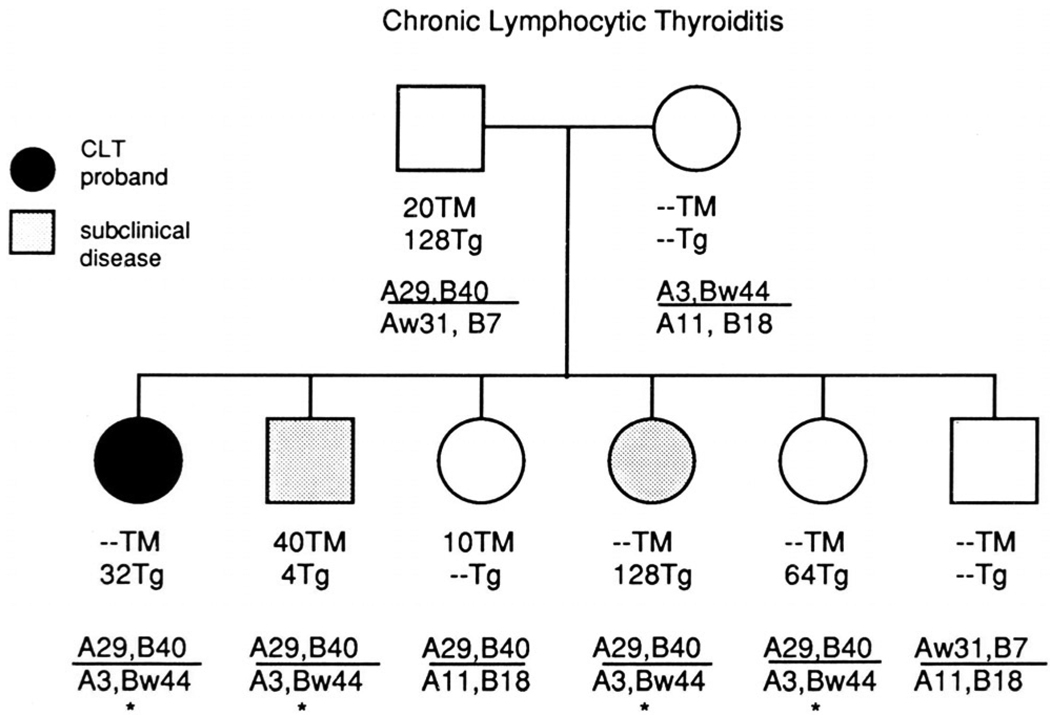
The studies were based on families of children seen by Dr. Hoffman in his pediatric endocrinology clinic. The probands had chronic lymphocytic thyroiditis or Graves’ disease. All patients were below the age of 18 and most of them below the age of 12. Our primary hypothesis was that the siblings of our patients would have a significantly elevated risk of thyroid disease. We found first that a clinically normal child in a family with a juvenile patient with thyroiditis or Graves’ disease had a striking increase in the frequency of thyroid autoantibodies directed to either thyroglobulin or thyroperoxidase. In a normal child of a family with a thyroid disease patient, the frequency of thyroid antibodies was highest if both parents themselves had antibodies, lower if one parent was antibody positive and even lower (but still very high) if neither parent was antibody positive (Fig. 4). We next looked at HLA haplotypes and found that if a clinically normal sibling shared both haplotypes with the proband there was an elevated frequency of thyroid autoantibodies and, even more strikingly, a 32% occurrence of biochemically detectable thyroid disease. Siblings who shared only one haplotype had a slightly lower frequency of autoantibodies and an 8% occurrence of subclinical thyroid disease. With the siblings sharing neither haplotype, more than half had antibodies but none had any evidence of functionally thyroid abnormality.
Figure 4.
Typical pedigree and HLA haplotypes of child (proband) with chronic lymphocytic thyroiditis (CLT), including siblings and parents. TM – titer of antibody to thyroperoxidase, Tg – titer of antibody to thyroglobulin. * - HLA haplotype shared with proband and father
Interestingly, although Graves’ disease was equally distributed in probands of European and African origin, Hashimoto’s disease occurred almost exclusively in children of European ancestry.
We found that the presence of both thyroid antibodies, to thyroglobulin or to thyroid peroxidase, correlated strongly to the presence of clinical thyroid disease, whereas one antibody was less of an indicator of current disease. However, unaffected members of families with either thyroid disease had a significantly greater frequency of thyroid autoantibodies than did normal controls. These data suggest that the presence of either thyroglobulin or thyroperoxidase antibodies may be an early predictive signal for the later development of disease. In subsequent studies we were able to follow up ten of the previously euthyroid siblings and found that the presence of the proband’s HLA-haplotype was predictive of subsequent thyroid disorder in six of them.
Although genome-wide association studies had not yet been invented, we did undertake to locate disease susceptibility genes by their association with 17 well-studied genetic loci. There were four significant associations, HLA, Km-1, ABO and Rh. In addition, the Duffy blood group was associated with Graves’ disease. Although it was much too early and the population examined too small to come to firm conclusions, the study clearly illustrated the potential value of combining a genetic approach with antibody studies as a pathway to earlier intervention or even institution of preventive measures for autoimmune disease.
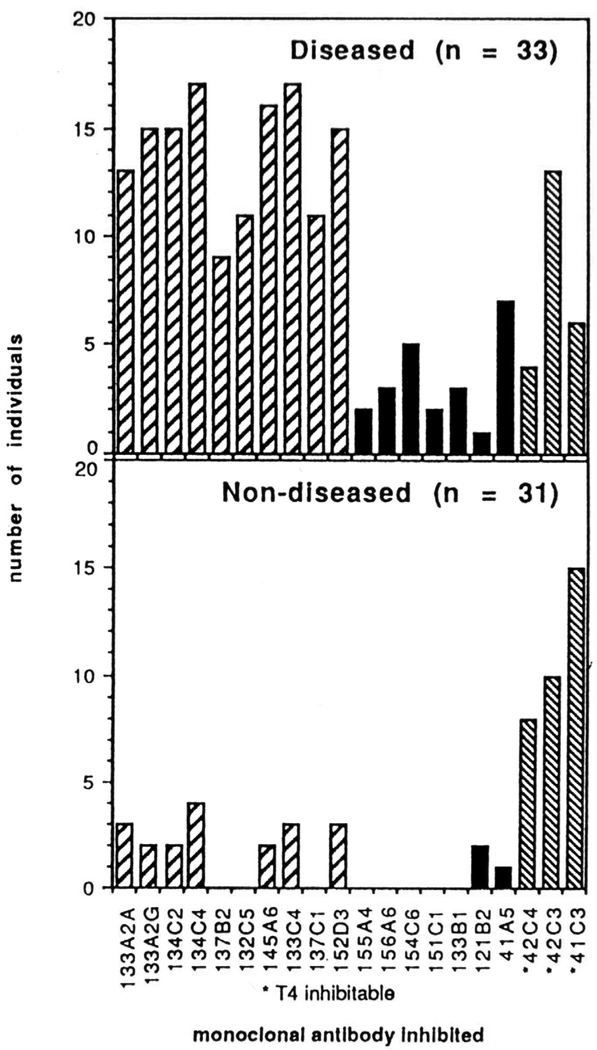
Complementing these genetic and serologic studies were our later investigations on the antigenic determinants of thyroglobulin itself (38). Many individuals with no evidence of thyroid disease have antibodies to thyroglobulin. We found that most of the antibodies are directed to a distinctive set of thyroglobulin epitopes; namely, conserved determinants that are highly cross-reactive and shared among different species. Patients with thyroid disease, on the other hand, produce an additional distinctive population of thyroglobulin-specific antibodies that react with the human-specific determinants (Fig. 5). Thus, the shift from antibodies to the conserved thyroglobulin epitopes to the species-specific ones can be an early signal that an individual is progressing from benign autoimmunity marked solely by the presence of autoantibodies to a pathogenic, inflammatory thyroiditis.
Figure 5.
Epitope mapping of human thyroglobulin using sera from a patient with chronic thyroiditis and normal controls. Monoclonal antibodies 41C3, 42C3 and 42C4 recognized conserved epitopes containing thyroxine (T4) whereas epitopes recognized by monoclonal 121B2, 133B1, 151C1, 154C6, 156A6 and 155A4 were primate – limited and recognized almost exclusively by patients. Remaining epitopes recognized by both groups.
CONCLUSION
Genetic studies of thyroiditis were particularly valuable because the primary antigen was well characterized in both humans and animals, and the disease could be compared in both experimental and spontaneous forms in animals. Investigations in rodents showed first that the MHC is involved in the initial immune response to thyroglobulin and that the MHC can serve as a determiner and predictor of susceptibility to thyroiditis. They also revealed that this gene complex encodes additional traits beyond the classical immune response genes associated with IA which modulate the immunopathology. In addition to MHC class II genes, the class I genes at the D-and K-ends limit one of the major effector mechanisms of thyroiditis, cytotoxic T cells. The synergy between MHC class I and MHC class II genes in inducing disease is arguably most clearly drawn in studies of experimental murine thyroiditis. Studies of thyroiditis in the rat, although more limited in number, have largely confirmed those in the mouse and have added the important finding that traits affecting susceptibility to thyroiditis are located on the X chromosome adding a plausible explanation for some of the sex-related biases of autoimmune disorders.
The investigations of spontaneously occurring thyroiditis in the OS chicken have been both confirmatory and complementary. They showed first of all the importance of the major histocompatibility locus in autoimmunity in the bird. They also demonstrated that the MHC control itself is complex and that MHC regulation is highly influenced by non-MHC genes. Finally, the chicken alerted us to the importance of the vulnerability of the target organ in determining susceptibility to thyroiditis.
Virtually all of the lessons learned during this remarkable decade have been translated to human disease. In a family with juvenile thyroid disease, HLA haplotype and the presence of multiple autoantibodies are highly predictive of the later onset of clinical disease. There would be no more fitting capstone to these early studies on the genetics of autoimmune thyroiditis than to open the pathway to practical means of diagnosing and preventing autoimmune disease before irreversible destruction occurs.
EPILOGUE
The investigations described in this article carried out largely in the decade of the 1970s were the beginning of many large-scale investigations of the genetics regulating resistance or susceptibility to other autoimmune diseases in many distinguished laboratories. Although I retain a lively interest in thyroiditis, the bulk of my personal research shifted since 1980 to studies of infection-induced autoimmune disease using myocarditis as an example. Here we used the tools of genetics to determine the steps involved in the progression from a viral cardiac inflammation to autoimmune myocarditis and then to dilated cardiomyopathy (39). Chella David has continued his landmark investigations on genetic susceptibility to autoimmune disease including thyroiditis in collaboration with Yi-Chi M. Kong, using mice in which they have replaced the murine MHC with human HLA. Their seminal discoveries, described elsewhere in this issue, have continued to shed fresh light on the genetics of autoimmune thyroid disease in humans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rose NR. The genetic basis of susceptibility to autoimmune disease. Chapter 1. In: Farid NR, editor. HLA in Endocrine and Metabolic Disorders. New York: Academic Press, Inc; 1981. pp. 1–10. [Google Scholar]
- 2.Witebsky E, Rose NR, Terplan K, Paine JR, Egan RW. Chronic thyroiditis and autoimmunization. J Am Medical Assn. 1957;164:1439–1447. doi: 10.1001/jama.1957.02980130015004. [DOI] [PubMed] [Google Scholar]
- 3.Rose NR, Twarog FJ, Crowle AJ. Murine thyroiditis: importance of adjuvant and mouse strain for the induction of thyroid lesions. J Immunol. 1971;106:698–704. [PubMed] [Google Scholar]
- 4.Vladutiu AO, Rose NR. Autoimmune murine thyroiditis: relation to histocompatibility (H-2) type. Science. 1971;174:1137–1139. doi: 10.1126/science.174.4014.1137. [DOI] [PubMed] [Google Scholar]
- 5.Bresler HS, Burek CL, Hoffman WH, Rose NR. Autoantigenic determinants on human thyroglobulin. II. Determinants recognized by autoantibodies from patients with chronic autoimmune thyroiditis compared to autoantibodies from healthy subjects. Clin Immunol Immunopathol. 1990;54:76–86. doi: 10.1016/0090-1229(90)90007-d. [DOI] [PubMed] [Google Scholar]
- 6.ElRehewy M, Kong YM, Giraldo AA, Rose NR. Syngeneic thyroglobulin is immunogenic in good responder mice. Eur J Immunol. 1981;11:146–151. doi: 10.1002/eji.1830110216. [DOI] [PubMed] [Google Scholar]
- 7.Esquivel PS, Rose NR, Kong Y-CM. Induction of autoimmunity in good and poor responder mice with mouse thyroglobulin and lipopolysaccharide. J Exp Med. 1977;145:1250–1263. doi: 10.1084/jem.145.5.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose NR, Vladutiu AO, David CS, Shreffler DC. Autoimmune murine thyroiditis. V. Genetic influence on the disease in BSVS and BRVR mice. Clin Exp Immunol. 1973;15:281–287. [PMC free article] [PubMed] [Google Scholar]
- 9.Bacon LD, Kite JH, Jr, Rose NR. Relation between the major histocompatibility (B) locus and autoimmune thyroiditis in obese chickens. Science. 1974;186:274–275. doi: 10.1126/science.186.4160.274. [DOI] [PubMed] [Google Scholar]
- 10.Bacon LD, Kite JH, Jr, Rose NR. Immunogenetic detection of B locus genotypes in chickens with autoimmune thyroiditis. Transplantation. 1973;16:591–598. doi: 10.1097/00007890-197312000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Bacon LD, Polley CR, Cole RK, Rose NR. Genetic influences on spontaneous autoimmune thyroiditis in (CS × OS)F2 chickens. Immunogenetics. 1981;12:339–349. doi: 10.1007/BF01561675. [DOI] [PubMed] [Google Scholar]
- 12.Rose NR, Bacon KD, Sundick RS. Genetic determinants of thyroiditis in the OS chicken. Transplant Rev. 1976;31:264–285. doi: 10.1111/j.1600-065x.1976.tb01457.x. [DOI] [PubMed] [Google Scholar]
- 13.Bacon LD, Rose NR. Influence of major histocompatibility haplotype on autoimmune disease varies in different inbred families of chickens. Proc Natl Acad Sci USA. 1979;76:1435–1437. doi: 10.1073/pnas.76.3.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose NR. Differing responses of inbred rat strains in experimental autoimmune thyrioditis. Cell Immunol. 1975;18:360–364. doi: 10.1016/0008-8749(75)90064-7. [DOI] [PubMed] [Google Scholar]
- 15.Lillehoj HS, Beisel K, Rose NR. Genetic factors controlling the susceptibility to experimental autoimmune thyroiditis in inbred rat strains. J Immunol. 1981;27:654–659. [PubMed] [Google Scholar]
- 16.Invernizzi P, Miozzo M, Selmi C, Persani L, Battezzati PM, Zuin M, et al. X chromosome monosomy: a common mechanism for autoimmune diseases. J Immunol. 2005;175:575–578. doi: 10.4049/jimmunol.175.1.575. [DOI] [PubMed] [Google Scholar]
- 17.Vladutiu AO, Rose NR. HL-A antigens: association with disease. Immunogenetics. 1974;1:305–308. [Google Scholar]
- 18.Tomazic V, Rose NR, Shreffler DC. Autoimmune murine thyroiditis. IV. localization of genetic control of the immune response. J Immunol. 1974;112:965–969. [PubMed] [Google Scholar]
- 19.Beisel KW, David CS, Giraldo AA, Kong Y-CM, Rose NR. Regulation of experimental autoimmune thyroiditis: mapping of susceptibility to the I-A subregion of the mouse H-2. Immunogenetics. 1982;15:427–430. doi: 10.1007/BF00364266. [DOI] [PubMed] [Google Scholar]
- 20.Kong Y-CM, David CS, Giraldo AA, Elrehewy M, Rose NR. Regulation of autoimmune response to mouse thyroglobulin: influence of H-2D-end genes. J Immunol. 1979;123:15–18. [PubMed] [Google Scholar]
- 21.Creemers P, Rose NR, Kong Y-CM. Experimental autoimmune thyroiditis: in vitro cytotoxic effects of T lymphocytes on thyroid monolayers. J Exp Med. 1983;157:559–571. doi: 10.1084/jem.157.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beisel KW, Kong YM, Babu KSJ, David CS, Rose NR. Regulation of experimental autoimmune thyroiditis: influence of non-H-2 genes. J Immunogenetics. 1982;9:257–265. doi: 10.1111/j.1744-313x.1982.tb00981.x. [DOI] [PubMed] [Google Scholar]
- 23.Beisel KW, Rose NR. Genetics of the autoimmune endocrinopathies: animal models. Chapter 2. In: Davies T, editor. Autoimmune Endocrine Disease. New York: John Wiley & Sons, Ltd; 1983. pp. 41–48. [Google Scholar]
- 24.Vladutiu AO, Rose NR. Cellular basis of the genetic control of immune responsiveness to murine thyroglobulin in mice. Cell Immunol. 1975;17:106–113. doi: 10.1016/s0008-8749(75)80010-4. [DOI] [PubMed] [Google Scholar]
- 25.Welch P, Rose NR, Kite JH., Jr Neonatal thymectomy increases spontaneous autoimmune thyroiditis. J Immunol. 1973;110:575–577. [PubMed] [Google Scholar]
- 26.Silverman DA, Rose NR. Neonatal thymectomy increases the incidence of spontaneous and methylcholanthrene-enhanced thyroiditis in rats. Science. 1974;184:162–163. doi: 10.1126/science.184.4133.162. [DOI] [PubMed] [Google Scholar]
- 27.Rose NR, Talor E. Antigen-specific immunoregulation and autoimmune thyroiditis. Ann N Y Acad Sci. 1991;636:306–320. doi: 10.1111/j.1749-6632.1991.tb33461.x. [DOI] [PubMed] [Google Scholar]
- 28.Okayasu IY, Kong Y-CM, David CS, Rose NR. In vitro T-lymphocyte proliferative response to mouse thyroglobulin in experimental autoimmune thyroiditis. Cell Immunol. 1981;6:32–39. doi: 10.1016/0008-8749(81)90351-8. [DOI] [PubMed] [Google Scholar]
- 29.Christadoss P, Chi MY, Kong Y-CM, Elrehewy M, Rose NR, David CS. Genetic control of T-lymphocyte proliferative autoimmune response to thyroglobulin in mice. Genetic Control of Autoimmune Disease. 1978:445–454. [Google Scholar]
- 30.Lillehoj HS, Rose NR. Relationship between genetic control of T-cell mitogen response and thyroiditis susceptibility in inbred rats. Cell Immunol. 1981;62:156–163. doi: 10.1016/0008-8749(81)90309-9. [DOI] [PubMed] [Google Scholar]
- 31.Jaroszewski J, Sundick RS, Rose NR. Effects of antiserum containing thyroglobulin antibody on the chicken thyroid gland. Clin Immunol Immunopathol. 1978;10:95–103. doi: 10.1016/0090-1229(78)90013-2. [DOI] [PubMed] [Google Scholar]
- 32.Tomazic V, Rose NR. Autoimmune murine thyroiditis. VIII. role of different thyroid antigens in the induction of experimental autoimmune thyroiditis. Immunology. 1976;30:63–68. [PMC free article] [PubMed] [Google Scholar]
- 33.Kong Y-CM, Rose NR, Elrehewy M, Michaels R, Giraldo AA, Accavitti MA, et al. Thyroid alloantigens in autoimmunity. Transplant Proc. 1980;12:129–134. [PubMed] [Google Scholar]
- 34.Wlodarski K, Sundick R, Rose NR. 131I--uptake by obese strain and Reaseheath Line R chicken thyroid and thymic epithelium cultured in vitro. Folia Biol (Krakow) 1979;27:85–89. [PubMed] [Google Scholar]
- 35.Truden JL, Sundick RS, Levine S, Rose NR. The decreased growth rate of obese strain chicken thyroid cells provides in vitro evidence for a primary target organ abnormality in chickens susceptible to autoimmune thyroiditis. Clin Immunol Immunopathol. 1983;29:294–305. doi: 10.1016/0090-1229(83)90031-4. [DOI] [PubMed] [Google Scholar]
- 36.Rose NR, Bonita R, Burek CL. Iodine: an environmental trigger of thyroiditis. Autoimmun Rev. 2002;1:97–103. doi: 10.1016/s1568-9972(01)00016-7. [DOI] [PubMed] [Google Scholar]
- 37.Burek CL, Rose NR, Najar GM, Gimelfarb A, Zmijewski CM, Polesky HF, et al. Autoimmune thyroid disease. Chapter 9. In: Panayi GS, David CS, editors. Immunogenetics. London: Butterworth & Co Ltd; 1984. pp. 207–233. [Google Scholar]
- 38.Vali M, Rose NR, Caturegli P. Thyroglobulin as autoantigen: structure-function relationships. Rev Endocr Metab Disorders. 2000;1:69–77. doi: 10.1023/a:1010016520778. [DOI] [PubMed] [Google Scholar]
- 39.Li HS, Ligons DL, Rose NR. Genetic complexity of autoimmune myocarditis. Autoimmunity Reviews. 2008;7:168–173. doi: 10.1016/j.autrev.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]