Summary
Background and objectives
Within the last few years, anti–human leukocyte antigen detection assays have significantly improved. This study asked, using the Luminex single-antigen assay, whether an allograft nephrectomy allowed donor-specific alloantibodies to appear that were not previously detected in the serum when the failed kidney was still in place.
Design, setting, participants, & measurements
After losing the kidney allograft and stopping immunosuppressive therapy, the proportions of donor-specific alloantibodies and nondonor-specific alloantibodies were compared in patients who had (n=48; group I) and had not (n=21; group II) undergone an allograft nephrectomy. Allograft nephrectomies were performed at 150 days after kidney allograft loss, and the time between allograft nephrectomy and last follow-up was 538±347 days.
Results
At kidney allograft loss, donor-specific alloantibodies were detected in three group II patients (14.2%) and six group I patients (12.5%). At last follow-up, donor-specific alloantibodies were detected in 11 patients (52.4%) without and 39 patients (81%) with an allograft nephrectomy (P=0.02). Anti–human leukocyte antigen class I donor-specific alloantibodies were positive in 23.8% of group II and 77% of group I patients (P<0.001); anti–human leukocyte antigen class II donor-specific alloantibodies were positive in 42.8% of group II and 62.5% of group I patients. Independent predictive factors for developing donor-specific alloantibodies after losing kidney allograft and stopping immunosuppressants were number of anti–human leukocyte antigen A/B mismatches at transplantation (zero versus one or more) and allograft nephrectomy.
Conclusions
The development of donor-specific alloantibodies was significantly greater in patients with a failed kidney who had undergone an allograft nephrectomy compared with those patients who had not undergone allograft nephrectomy.
Introduction
Although improved immunosuppressive therapies have increased kidney allograft survival within the last decade (1), the number of patients with a failed kidney transplant that requires retransplantation has also grown (2). The majority of patients undergoing kidney retransplantation are sensitized against the human leukocyte antigen (HLA) system. The risk of antibody-mediated rejection (AMR) is increased in these high-risk immunologic patients (3). Donor-specific anti-HLA antibodies (DSAs) are responsible for the acute and chronic AMR that reduces long-term kidney allograft survival (4). For this reason, anti-HLA antibodies are characterized before a first transplantation or retransplantation to determine donor-mismatch acceptability.
In patients undergoing kidney retransplantation, it has been suggested that allograft nephrectomy of the failed kidney may promote anti-HLA antibodies (5–7); these antibodies may be attached to the kidney allograft and/or have a very low titer that cannot be detected by standard immunologic assays. Indeed, DSAs have been previously detected in the eluates of allograft nephrectomies obtained from patients with DSAs not detected in the serum (8). However, within the last few years, anti-HLA detection assays have significantly improved.
The Luminex single-antigen assay is much more sensitive than previous assays at detecting DSAs (9,10). Hence, we wondered whether an allograft nephrectomy allowed DSAs to appear that were not previously detected in the serum when the failed kidney was still in place. The aim of our study was to compare the incidence of DSAs using the Luminex single-antigen assay in patients with a failed kidney allograft after ceasing immunosuppressive therapy and who had or had not undergone an allograft nephrectomy. We also assessed the incidence of DSAs in patients who had a systematic or clinically indicated allograft nephrectomy.
Materials and Methods
Between February of 2007 and November of 2010, 133 kidney allograft failures, defined as a permanent return to dialysis, occurred in our department. However, only patients for whom a kidney retransplantation was planned were included in this study (n=95). After kidney allograft loss and reinitiation of dialysis, all immunosuppressive drugs, except for steroids (if any), were stopped. Steroids were then stopped at 6 months after initiation of dialysis. Between February of 2007 and July of 2008, no kidney allograft nephrectomies were conducted unless clinically indicated (i.e., acute rejection after ceasing immunosuppressants). Between July of 2008 and November of 2010, systematic allograft nephrectomies were performed before patients, who gave written informed consent, were registered on the waiting list for a kidney retransplantation. Hence, 21 patients did not undergo an allograft nephrectomy (group II), and 74 patients underwent an allograft nephrectomy. Of the latter group, 26 patients underwent an allograft nephrectomy within 3 months of the initial transplantation, mainly because of early vascular thrombosis, and therefore, they were excluded from this study; 48 patients (group I) underwent an allograft nephrectomy either systematically (n=17) or as clinically indicated (n=31).
Anti-HLA antibodies were assessed in both groups at graft loss and 3- to 6-month intervals until the last follow-up of patients on a waiting list. Group I patients had additional analyses: before an allograft nephrectomy, on day 5, and at months 3 and 9 after an allograft nephrectomy.
Immunologic Analysis
Luminex assays determined the specificity of HLA class I and II IgG antibodies in the recipients’ sera (centrifuged at 10,000 × g for 10 minutes) using Labscreen single Ag HLA class I and class II detection tests (One Lambda, Canoga Park, CA) according to the manufacturer’s instructions. The presence and specificity of antibodies were then detected using a Labscan 100, and the mean fluorescence (baseline value) for each sample in each bead was evaluated. The baseline value was calculated as follows: (raw sample mean fluorescence intensity [MFI]−raw negative serum control MFI)−(negative bead raw MFI with sample−negative bead raw MFI with negative serum control). A baseline value of >500 was considered positive. Immunodominant DSA was defined as the DSA with the highest MFI.
HLA Matchmaker Analysis
We used the HLA Matchmaker program version 2.1 (www.hlamatchmaker.net) to determine donor–recipient compatibility at the structural level in class I HLA as described (11,12).
Pathologic Analysis of the Explanted Allograft
Forty-two of forty-eight explanted kidney allografts were analyzed by light microscopy and scored according to 2009 Banff criteria (13). C4d staining was also performed.
Statistical Analyses
Reported values represent the means (±SD) or medians (ranges). Proportions were compared using the Fisher exact test. Quantitative variables were compared using the Mann–Whitney nonparametric or t test. The predictive factors for developing DSA after graft failure were determined by univariate and multivariate regression analyses. Factors associated by univariate analyses (at a significance of P<0.10) with the detection of DSA after graft failure were selected for multivariate analyses. A P value<0.05 was considered statistically significant.
Results
The patients’ characteristics are presented in Table 1.
Table 1.
Comparisons between patients who had or had not undergone an allograft nephrectomy
| Parameters | Patients with an Allograft Nephrectomy (Group I; n=48) | Patients with a Clinically Indicated Allograft Nephrectomy (n=31) | Patients with a Systematic Allograft Nephrectomy (n=17) | Patients without an Allograft Nephrectomy: (Group II; n=21) |
|---|---|---|---|---|
| Recipient age at transplantation (years) | 38.6±16 | 40.3±17 | 35.6±14 | 34.6±11 |
| Recipient sex: male (%) | 35 (72.9) | 22 (71) | 13 (76) | 19 (90) |
| Donor age (years) | 39.6±18.7 | 43.4±18 | 36±17 | 40.3±15 |
| Type of donor: deceased (%) | 47 (98) | 30 (97) | 17 (100) | 21 (100) |
| Number of previous transplantations | 1.27±0.57 | 1.22±0.42 | 1.35±0.79 | 1.38±0.59 |
| Nephrectomy for previous kidney transplantation (%) | 9/11 (81.8) | 7/7 (100) | 2/4 (50) | 4/7 (57.1) |
| Number of HLA A/B/DR/DQ mismatches | 3.98±1.83 | 4.03±2.02 | 3.88±1.45 | 3.5±1.8 |
| Number of HLA A/B mismatches | 2.45±1.05 | 2.38±1.11 | 2.59±0.94 | 2.1±1.27 |
| Number of HLA DR/DQ mismatches | 1.48±1.24 | 1.64±1.35 | 1.17±0.95 | 1.57±0.87 |
| Patients with DSAs at transplantation | 0 | 0 | 0 | 0 |
| Induction therapy: yes (%) | 39 (81.3) | 25 (81) | 14 (82) | 18 (85.7) |
| Polyclonal ab/anti–IL-2R induction therapy | 24/15a | 15/10 | 9/5 | 17/1a |
| Rituximab induction therapy: yes (%) | 1 (2) | 1 (3) | 0 | 0 |
| Initial immunosuppression after transplantation: CNIs (%) | 47 (97.8) | 30 (97) | 17 (100) | 20 (95) |
| Cyclosporine A/tacrolimus (%) | 31 (66)/16 (34) | 22 (70)/8 (30) | 9 (53)/8 (47) | 19 (89.4)/2 (10.6) |
| mTOR inhibitors (%) | 4 (8.9) | 3 (9.5) | 1 (6) | 1 (5) |
| MPA (%) | 32 (66.7) | 21 (67.8) | 11 (60) | 17 (83) |
| steroids (%) | 48 (100) | 31 (100) | 17 (100) | 21 (100) |
| Immunosuppression at graft loss | 38 (79.1) | |||
| CNIs (%) | 10 (26.3)/28 (73.7) | 25 (80.6) | 13 (76.5) | 16 (75) |
| cyclosporine A/tacrolimus (%) | 4 (9) | 6 (24)/19 (76) | 4 (31)/9 (69) | 9 (53)/7 (47) |
| mTOR inhibitors (%) | 36 (75) | 1 (3) | 3 (17) | 2 (9) |
| MPA (%) | 43 (89.6) | 23 (77.4) | 13 (82) | 17 (81) |
| steroids (%) | 30 (93.5) | 15 (88) | 21 (100) | |
| Rejection episodes during the transplant period (%) | 27 (56) | 18 (58) | 9 (53) | 12 (55) |
| Number of rejection episodes per patient | 1 (0–4) | 1 (0–4) | 1 (0–2) | 1 (0–2) |
| Steroid-sensitive rejection episodes (%) | 16 (33.5) | 13 (42) | 3 (18) | 10 (48) |
| Number of steroid-sensitive episodes per patient | 0 (0–2) | 0 (0–2) | 0 (0–1) | 0 (0–2) |
| Steroid-resistant rejection episodes (%) | 15 (31) | 9 (29) | 6 (41) | 4 (20) |
| Humoral rejection episodes (%) | 6 (12.5) | 4 (12.9) | 2 (11.7) | 3 (14.2) |
| Rituximab use during transplant period: yes (%) | 11 (23) | 8 (26) | 3 (18) | 6 (28.5) |
| Number of Rituximab injections/patientb | 2 (2–5) | 3 (2–5) | 2 | 2 |
| Time between last Rituximab and graft failure (days) | 410 (20–1740) | 330 (120–870) | 596 (20–1740) | 405 (60–2160) |
| Time between last Rituximab therapy and nephrectomy (days) | 581 (95–1800) | 498 (180–1050) | 800 (31–1800) | — |
| Plasma exchange during transplant period: yes (%) | 7 (14.6) | 6 (19) | 1 (6) | 6 (28.5) |
| Number of plasma exchanges during the transplant period | 9 (5–14)a | 9 (5–14) | 12 | 6c |
| Time between transplantation and graft failure (days) | 1768 (81–7785) | 1340 (81–7351) | 3330 (80–7785) | 4099 (493–10013) |
| Time between graft failure and graft nephrectomy (days) | 150 (100–3390) | 169 (100–3390) | 143 (110–1119) | — |
| Time between graft failure and last follow-up (days) | 680 (111–3939) | 696 (111–3939) | 673 (137–1436) | 836 (33–3018) |
| Number of patients with DSAs at graft failure (%) | 6 (12.5) | 4 (12.9) | 2 (11.7) | 3 (14.2) |
| Number of patients with DSAs at graft nephrectomy (%) | 17 (35.4) | 10 (32.2) | 7 (41.2) | — |
| Number of patients with DSAs at last follow-up (%) | 39 (81)a | 25 (80.6) | 14 (82.3) | 11 (52.4)c |
| Time between nephrectomy and de novo DSA (days) | 5 (5–1097) | 5 (5–1097) | 5 (5–270) | — |
HLA, human leukocyte antigen; DSA, donor-specific antibodies; CNIs, calcineurin inhibitors; mTOR, mammalian target of rapamycin; MPA, mycophenolic acid.
The Rituximab dose of each injection was 375 mg/m2.
P<0.05.
Emergence of DSAs after Graft Loss in Patients Who Did or Did Not Have an Allograft Nephrectomy
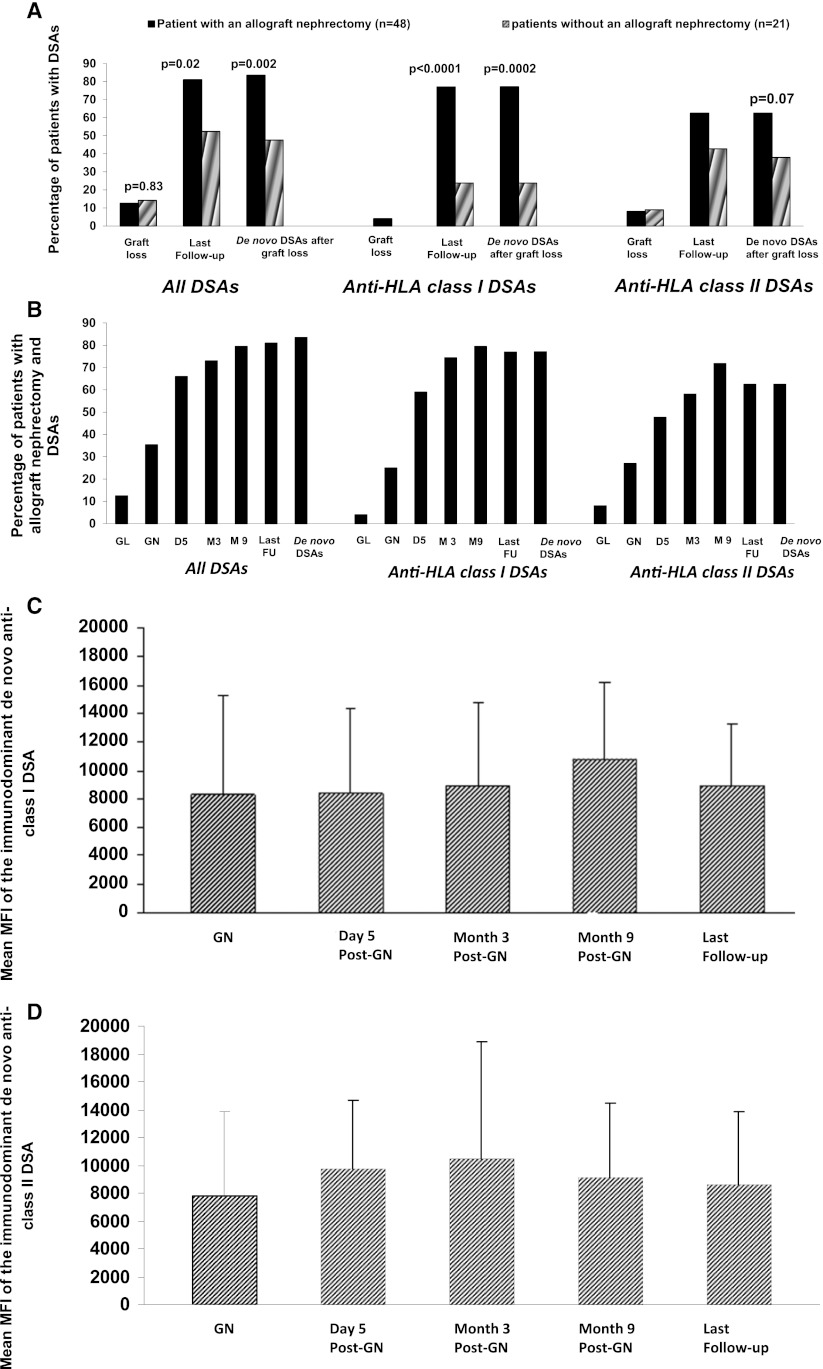
At graft loss, DSAs were detected in three patients (14.2%) from group II and six patients (12.5%) from group I. At last follow-up, DSAs were detected in 11 patients (52.4%) without an allograft nephrectomy and 39 patients (81%) with an allograft nephrectomy (P=0.02). Anti-HLA class I DSAs were found to be positive in 23.8% of patients from group II and 77% of patients from group I (P<0.001), and anti-HLA class II DSAs were found to be positive in 42.8% of patients from group II and 62.5% for patients from group I.
De novo DSAs after graft loss were seen in 10 patients (47.6%) from group II and 40 patients (83.3%) from group I (P=0.002). In one patient from each group, de novo DSA, which occurred after graft loss, disappeared during follow-up and was not detected at the last follow-up. De novo anti-HLA class I DSAs occurred in 23.8% of patients from group II and 77.1% of patients from group I (P=0.001). De novo anti-HLA class II DSAs occurred in 38% of patients from group II and 62.5% of patients from group I (P=0.06) (Figure 1A).
Figure 1.
The proportion of patient with donor-specific antibodies (DSAs) increases after an allograft nephrectomy. (A) Proportions of patients with DSAs, anti–human leukocyte antigen (anti-HLA) class I DSAs, and anti-HLA class II DSAs who did or did not have an allograft nephrectomy. (B) Kinetics of DSAs after an allograft nephrectomy. (C) Mean fluorescence intensity of de novo immunodominant anti-HLA class I DSAs. (D) Mean fluorescence intensity of de novo immunodominant anti-HLA class II DSAs. FU, follow-up; GL, graft loss; GN, graft nephrectomy.
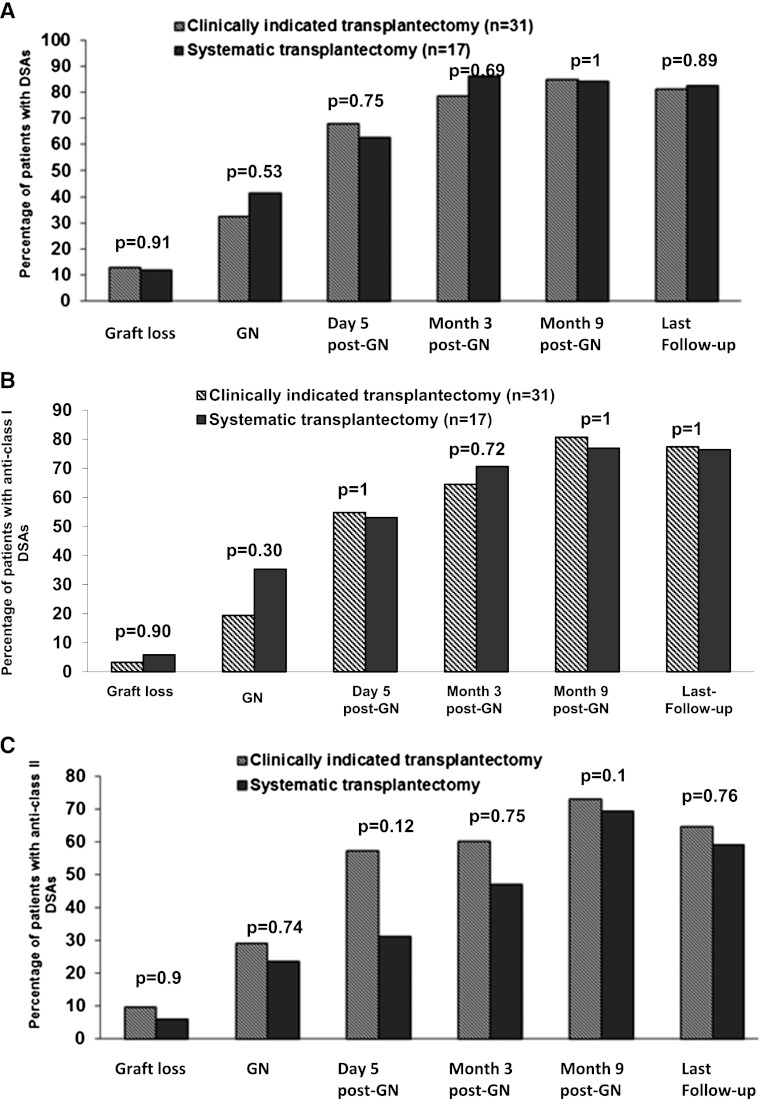
Emergence of DSAs in Patients Who Underwent a Systematic or Clinically Indicated Allograft Nephrectomy
The patients’ characteristics and their induction therapies, initial immunosuppressive therapies, immunosuppressive therapies at graft loss, acute rejection rates, and numbers, types, and treatments for acute rejection episodes (if any) did not differ significantly between patients who had undergone a systematic or clinically indicated allograft nephrectomy (data not shown). With respect to DSAs, the proportions of patients that had at least one DSA were similar in both groups at graft loss, the time of an allograft nephrectomy, and last follow-up. The incidence of anti-HLA classes I and II antibodies was similar in the two groups (Figure 2).
Figure 2.
Proportion of patients with donor specific antibodies (DSAs) after a systematic or clinically indicated allograft nephrectomy. (A) Proportion of patients with anti–human leukocyte antigen (anti-HLA) class I + II DSAs regarding the indication of allograft nephrectomy. (B) Proportion of anti-HLA class I DSAs. (C) Proportion of anti-HLA class II DSAs. GN, graft nephrectomy.
Kinetics of the Appearance of DSAs in Patients after an Allograft Nephrectomy
The kinetic appearance of DSAs after graft loss is illustrated in Figure 1B. At graft loss, six patients (12.5%) had at least one DSA. At the time of allograft nephrectomy (i.e., 150 [100–3390] days after graft loss and cessation of immunosuppressive therapy), 35.4% of patients had developed at least one DSA. As early as 5 days after an allograft nephrectomy, this percentage increased to 66% and thereafter, 81.4% and 84.6% at months 3 and 9 postallograft nephrectomy, respectively. At last follow-up (i.e., at 538±347 days after allograft nephrectomy), 81% of patients had at least one DSA. The incidence of DSAs after allograft nephrectomy was 73%, and 59% of patients who had a DSA before an allograft nephrectomy later developed another DSA. The incidence of DSAs after graft loss was 83.3%. In one patient, a de novo DSA had disappeared at last follow-up. Interestingly, the incidence of DSAs after allograft nephrectomy did not differ between patients who did or did not receive a blood transfusion after an allograft nephrectomy: 71.4% versus 71.8%.
The MFI of immunodominant de novo anticlass I and/or anticlass II DSAs remained stable from day 5 after an allograft nephrectomy until the last follow-up (Figure 1, C and D).
Predictive Factors for the Occurrence of De Novo DSA after Graft Loss
All collected variables were analyzed. The statistically significant results from the univariate and multivariate analyses are presented in Table 2. The independent predictive factors for the development of DSAs after graft loss and cessation of immunosuppressants were the number of anti-HLA A/B mismatches (e.g., zero versus one or more mismatches) at transplantation and having undergone an allograft nephrectomy.
Table 2.
Predictive factors for the development of donor-specific antibodies after graft loss
| Variables | De Novo DSA after Graft Loss (n=50) | No DSA after Graft Loss (n=19) | P Value |
|---|---|---|---|
| Univariate analysis | |||
| anti-HLA A/B/DR/DQ MM (zero versus one or more MM) | 4.3±1.63 | 2.84±1.95 | 0.002 |
| anti-HLA A/B MM (zero versus one or more MM) | 2.64±0.96 | 1.64±1.12 | <0.001 |
| transplantectomy (yes/no) | 40/10 | 8/11 | 0.004 |
| anti–IL-2R induction therapy (yes/no) | 15/35 | 1/18 | 0.05 |
| Multivariate analysis | OR | 95% CI | |
| anti-HLA A/B MM (zero versus one or more MM) | 2.32 | 1.32–4.08 | 0.004 |
| transplantectomy (yes/no) | 5.56 | 1.56–4.08 | 0.008 |
DSA, donor-specific antibodies; HLA, human leukocyte antigen; MM, mismatches; CI, confidence interval.
Pathologic Analysis of Explanted Kidney Allografts
All elementary histologic lesions were scored according to the Banff 2009 classification; they did not differ significantly between patients with or without DSAs (Table 3). However, total interstitial fibrosis score and positive C4d staining were significantly higher in patients who had developed DSAs. No difference was observed between patients who had undergone a systematic or clinically indicated allograft nephrectomy.
Table 3.
Histologic analyses of allograft nephrectomies
| Patients with DSAs at Last Follow-Up (n=34) | Patients without DSAs at Last Follow-Up (n=8) | P Value | Patients with an Allograft Nephrectomy for Clinical Symptoms (n=17) | Patients with a Systematic Allograft Nephrectomy (n=17) | P Value | |
|---|---|---|---|---|---|---|
| t | 0 (0–3) | 0 (0–2) | 0.4 | 0 (0–3) | 0 (0–3) | 0.60 |
| i | 0 (0–3) | 0 (0–1) | 0.7 | 0 (0–3) | 0 (0–1) | 0.10 |
| g | 0 (0–3) | 0 (0–3) | 0.9 | 0 (0–3) | 0 (0–3) | 0.25 |
| v | 0 (0–3) | 0 (0–3) | 0.7 | 1 (0–3) | 0 (0–3) | 0.08 |
| ah | 3 (0–3) | 3 (3–3) | 0.1 | 3 (0–3) | 3 (2–3) | 0.06 |
| cg | 0 (0–3) | 0 (0–3) | 0.5 | 0 (0–3) | 0 (0–3) | 0.60 |
| ci | 3 (1–3) | 3 (0–3) | 0.5 | 1 (0–3) | 3 (0–3) | 0.20 |
| ct | 3 (1–3) | 3 (1–3) | 0.6 | 3 (1–3) | 3 (1–3) | 0.20 |
| cv | 3 (0–3) | 3 (0–3) | 0.4 | 3 (0–3) | 3 (0–3) | 0.90 |
| mm | 2 (0–3) | 1 (0–3) | 0.9 | 1 (0–3) | 2 (0–3) | 0.80 |
| ti | 3 (0–3) | 0 (0–2) | 0.002 | 2 (0–3) | 2 (0–3) | 0.95 |
| PTc | 0 (0–3) | 0 (0–3) | 0.8 | 0 (0–3) | 0 (0–3) | 0.74 |
| Positive C4d staining | 19 (55.9%) | 1 (12.5%) | 0.05 | 10 (59%) | 9 (53%) | 0.99 |
DSA, donor-specific antibodies; t, tubulitis; i, mononuclear cell interstitial inflammation; g, glomerulitis; v, intimal arteritis; ah, arteriolar hyaline thickening; cg, allograft glomerulopathy; ci, interstitial fibrosis; ct, tubular atrophy; cv, vascular fibrous intimal thickening; mm, mesangial matrix increase, ti, total interstitial inflammation; PTc, peritubular capillaritis.
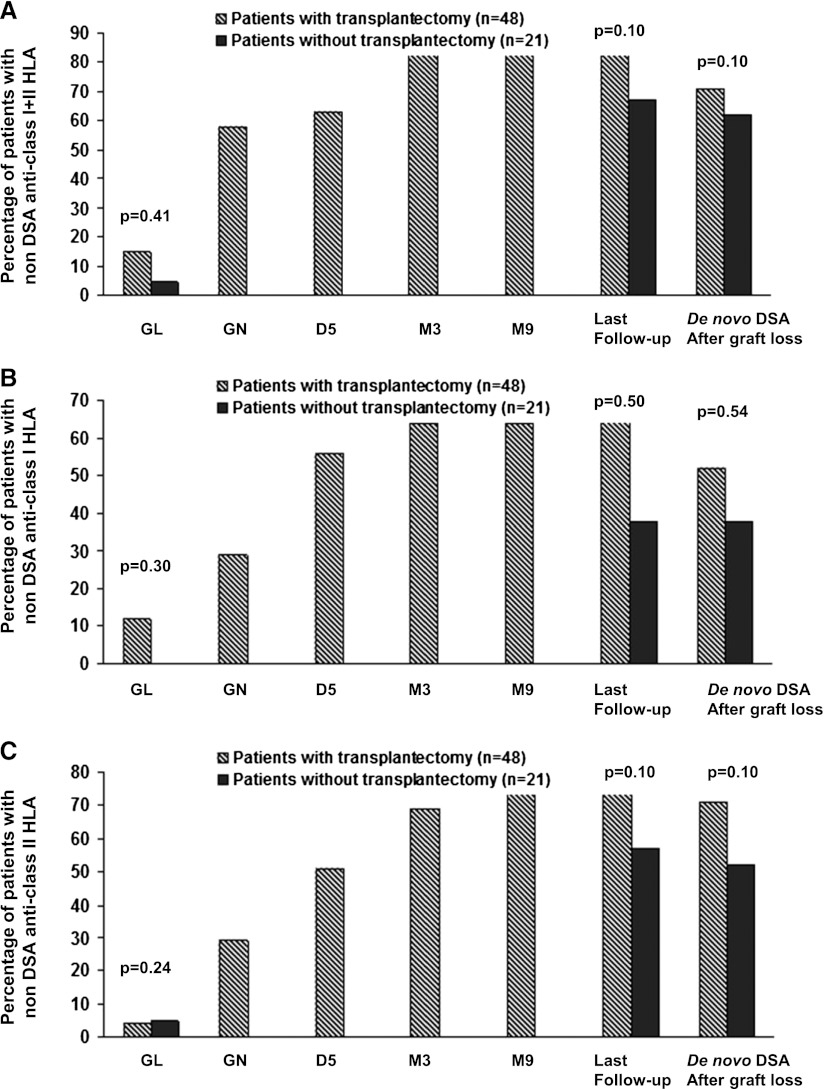
Emergence of Non-DSA Antibodies after Graft Loss in Patients Who Did or Did Not Have an Allograft Nephrectomy
At graft loss, there was no significant difference in the presence of non-DSA antibodies in patients with (seven patients; 15%) or without (one patient; 5%) an allograft nephrectomy (Figure 3). After graft loss, the proportion of patients that had non-DSA antibodies increased in patients with and without an allograft nephrectomy; at last follow-up, no significant difference was observed between these two groups (i.e., 41 patients in group I [85%] and 14 patients in group II [67%]).
Figure 3.
The proportion of patient with non-DSA anti-HLA antibodies is not significantly different in both groups. (A) Proportions of patients with no donor-specific antibodies (DSAs) anti–human leukocyte antigens (anti-HLAs) antibodies either with or without allograft nephrectomy. (B) Proportions of patients with no DSAs anti-HLA class I antibodies with or without allograft nephrectomy. (C) Proportions of patients with no DSAs anti-HLA class II antibodies with or without allograft nephrectomy. GL, graft loss; GN, graft nephrectomy.
HLA Matchmaker analysis showed that the proportion of de novo non-DSA anti-HLA antibodies that reacted to the donors’ epitopes was 88.9%.
Complications from Transplantectomies
Thirty percent of patients experienced a complication after an allograft nephrectomy. The complication rate did not differ significantly between patients who had a systematic or clinically indicated allograft nephrectomy (Table 4).
Table 4.
Allograft nephrectomy-related complications
| All Patients (n=48) | Systematic Transplantectomy (n=17) | Clinically Indicated Transplantectomy (n=31) | P Value: Systematic Versus Clinically Indicated Transplantectomy | |
|---|---|---|---|---|
| Duration of hospitalization (days) | 6.5 (3–36) | 6 (3–31) | 7 (3–36) | 0.91 |
| Overall complications (%) | 13 (30); 7 (15) | 5 (30); 3 (18) | 8 (30); 4 (13) | 1 |
| Infectious complications (%) | 4 (8) | 2 (11) | 2 (6) | 0.71 |
| pulmonary infection (%) | 1 (2) | 1 (6) | 0 | |
| bacteremia (%) | 1 (2) | 0 | 1 (3) | |
| kidney allograft site infection (%) | 1 (2) | 0 | 1 (3) | |
| cytomegalovirus reactivation (%) | ||||
| Surgical complications (%) | 9 (19) | 3 (18) | 6 (19)a | 1 |
| hematoma | 7 (15) | 3 (18) | 4 (13) | |
| cecal perforation (%) | 1 (2) | 0 | 1 (3) | |
| wound dehiscence (%) | 1 (2) | 0 | 1 (3) | |
| Blood transfusion (%) | 14 (30) | 6 (35) | 8 (26) | 1 |
| Death (%) | 0 | 0 | 0 | 1 |
One patient required additional surgery to drain a hematoma.
Discussion
The harmful impact of AMR on kidney allograft survival (3,4,14) has prompted transplant physicians to test recipients’ sera for anti-HLA antibodies using the very sensitive Luminex single-antigen assay to determine donor-acceptable mismatches. In patients considered for retransplantation, several previous studies tested for anti-HLA antibodies using lymphocytotoxicity or ELISA techniques; they suggested that removal of the failed graft might allow the appearance of previously undetected DSAs in serum (5,15,16). In the present study, we assessed the use of the Luminex single-antigen assay to determine the incidence of DSAs after ceasing immunosuppression in patients who had a failed kidney allograft with or without a subsequent allograft nephrectomy and were waiting for retransplantation.
Our findings from this study are fivefold. (1) The appearance of DSAs was significantly greater in patients who had undergone an allograft nephrectomy after ceasing immunosuppressive therapy compared with those patients who had not undergone allograft nephrectomy. (2) De novo DSAs were detected in the sera as soon as 5 days after an allograft nephrectomy. (3) Donor-specific HLA class I antibodies appeared more frequently than donor-specific HLA class II antibodies after an allograft nephrectomy. (4) No significant difference in the incidence of DSAs was observed between patients who underwent a clinically indicated allograft nephrectomy and patients who underwent a systematic allograft nephrectomy. (5) The only predictive factors for the appearance of DSAs after kidney allograft failure were the number of HLA class I mismatches and having an allograft nephrectomy.
The work by Billen et al. (7) used the Luminex assay to test for anti-HLA antibodies and DSAs in 53 patients who had an allograft nephrectomy after a first kidney allograft failure. All patients were DSA-negative at transplantation; 16% of patients showed DSAs before the allograft nephrectomy, whereas DSAs appeared in 84% after an allograft nephrectomy.
More recently, the work by Marrari and Duquesnoy (6) tested for DSAs using the Luminex SA assay in 65 patients who had undergone an allograft nephrectomy at >1 year post-transplantation. Sera were tested at 35 (1–306) days before and 44 (14–337) days after transplantectomy. In patients with HLA class I mismatches, the incidences of DSAs before and after allograft nephrectomy were 64% and 87%, respectively (P=0.003). In patients with HLA-DRB1 mismatches, the incidence of DSAs was increased from 57% before to 86% after an allograft nephrectomy (P=0.001). Surprisingly, in both studies, it was not mentioned whether patients were receiving immunosuppressive drugs before the allograft nephrectomy and if they were not, what the delay was between immunosuppressant cessation and allograft nephrectomy.
Other studies have shown that the percentages of classes I and II panel-reactive antibodies were significantly increased after an allograft nephrectomy (5,16,17). In the present study, we found that de novo DSAs appeared in 47.6% of patients without allograft nephrectomy when immunosuppressive therapy was stopped. In contrast, in patients who had an allograft nephrectomy, the incidence of de novo DSAs increased from 35.4% after cessation of immunosuppressive therapy and before a transplantectomy to 83.3% (P=0.002) at the last follow-up after an allograft nephrectomy. Both donor-specific anticlasses I and II antibodies were detected after an allograft nephrectomy. However, after graft loss, the incidence of de novo DSA anti-HLA class I antibodies was significantly higher in patients who had an allograft nephrectomy compared with patients who had not had an allograft nephrectomy (77.1% versus 23.8%, P=0.001). The incidence of de novo DSA anti-HLA class II antibodies tended to be higher in patients who had an allograft nephrectomy (62.5%) compared with patients who had not had an allograft nephrectomy (38%; P=0.07). Non-DSA anti-HLA antibodies, which have a negative impact on allograft survival (18), significantly increased after cessation of immunosuppressive therapy. However, the proportion of patients with non-DSAs antibodies did not differ between patients with or without a nephrectomy. Interestingly, 89% of non-DSA antibodies reacted with the donors’ epitopes.
Allograft nephrectomy and the number of anti-HLA class I mismatches were the only two independent predictive factors for de novo DSAs after graft loss and cessation of immunosuppressive therapy. In patients who had an allograft nephrectomy, the work by Billen et al. (7) found that donor age was a predictive factor for de novo DSAs, whereas the work by Knight et al. (17) found that both the number of rejection episodes and having a <10-month interval between graft failure and allograft nephrectomy were both significantly associated with greater percentages of panel-reactive antibodies and mean DSA levels.
How an allograft nephrectomy allows the appearance of DSAs is not fully known. It has been suggested that DSAs are absorbed by the kidney allograft and that removal of the kidney allows DSAs to appear in the blood circulation (15,19). In contrast, it has been also suggested that an allograft nephrectomy may cause damage that results in danger signals that stimulate the immune system, leading to the appearance of DSAs (20–22). The results of our study support the first hypothesis, suggesting that DSAs are absorbed by the kidney. Indeed, DSAs appeared as soon as 5 days after allograft nephrectomy, suggesting that antibodies were already preformed. Furthermore, although MFI is not quantitative and may have significant interassay variability, the MFI of immunodominant antibodies remained stable or was decreased during follow-up, whereas if DSAs had appeared because of injury caused by the nephrectomy, the MFI would have increased during follow-up.
The improved ability to detect DSAs after an allograft nephrectomy makes it more difficult to find a suitable kidney with acceptable HLA mismatches for retransplantation. Nevertheless, if no allograft nephrectomy is performed, the acceptance of a kidney allograft when an HLA antigen against a DSA is present in the failed kidney allograft but not in the sera may cause an AMR after retransplantation, leading to reduced kidney allograft survival. Because of the shortage of transplant organs, it may be reasonable to propose the use of a kidney when there are no DSAs in the sera after an allograft nephrectomy. Although the time taken to find a more suitable kidney is prolonged, this proposal may be recommended rather than performing a kidney retransplantation without being sure of the absence of preformed DSAs in the failed kidney that are not detected in the sera.
The removal of failed kidney allografts may reduce chronic inflammation and be responsible for erythropoietin resistance (23). The work by Ayus et al. (24) showed that allograft nephrectomy was associated with improved patient survival and interestingly, a higher retransplantation rate compared with patients without an allograft nephrectomy. The work by Johnston et al. (25) found that, when graft failure occurred at >12 months post-transplant, allograft nephrectomy was associated with a decreased risk of death. However, in the study by Johnston et al. (25), allograft nephrectomy significantly increased the risk of repeat transplant failure. The work by Schleicher et al. (16) showed that graft nephrectomy had a negative impact on graft function and survival after retransplantation, whereas the work by Ahmad et al. (26) found that nephrectomy of a failed allograft did not significantly influence the survival of the subsequent kidney transplant. However, it is not possible to draw any conclusions from these three studies, because DSAs were not assessed routinely before or after transplantation using a sensitive assay.
A mortality rate of between 0.7% and 7% has been reported after graft nephrectomy (24–29). In the present study, no patients died during or immediately after an allograft nephrectomy. However, the complication rate was relatively high at 30%. Hence, an allograft nephrectomy should be proposed with caution.
There are several limitations to the present study. It is a nonrandomized study that included a relatively small number of patients. We did not look for anti-HLA C and anti-HLA DP in our patients. However, the difference in de novo DSAs between patients with and without an allograft nephrectomy was statistically significant and may have been increased if these two HLA subtypes had been assessed. We also did not assess inflammatory markers before and after a nephrectomy. However, this assessment was not the purpose of our study.
In conclusion, the incidence of DSAs is significantly greater in patients with a failed kidney who have undergone an allograft nephrectomy compared with those patients who have not undergone an allograft nephrectomy. Prospective studies are required to assess the mechanism(s) responsible for the appearance of DSAs after allograft nephrectomy and the impact of allograft nephrectomy on patient and graft survival rates and acute rejection rates after retransplantation.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Sequestration and Suppression of Anti-HLA Antibodies by a Failed Kidney Allograft,” on pages 1209–1210.
References
- 1.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJP, Stablein D: Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 342: 605–612, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK: Long-term survival in renal transplant recipients with graft function. Kidney Int 57: 307–313, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, Gautreau C, Charron D, Glotz D, Suberbielle-Boissel C: Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol 21: 1398–1406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terasaki PI: Humoral theory of transplantation. Am J Transplant 3: 665–673, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Khakhar AK, Shahinian VB, House AA, Muirhead N, Hollomby DJ, Leckie SH, McAlister VC, Chin JL, Jevnikar AM, Luke PP: The impact of allograft nephrectomy on percent panel reactive antibody and clinical outcome. Transplant Proc 35: 862–863, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Marrari M, Duquesnoy RJ: Detection of donor-specific HLA antibodies before and after removal of a rejected kidney transplant. Transpl Immunol 22: 105–109, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Billen EV, Christiaans MH, Lee J, van den Berg-Loonen EM: Donor-directed HLA antibodies before and after transplantectomy detected by the luminex single antigen assay. Transplantation 87: 563–569, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Heinemann FM, Roth I, Rebmann V, Arnold ML, Spriewald BM, Grosse-Wilde H: Characterization of anti-HLA antibodies eluted from explanted renal allografts. Clin Transpl 2006: 371–378, 2006 [PubMed] [Google Scholar]
- 9.Tait BD, Hudson F, Brewin G, Cantwell L, Holdsworth R: Solid phase HLA antibody detection technology—challenges in interpretation. Tissue Antigens 76: 87–95, 2010 [DOI] [PubMed] [Google Scholar]
- 10.El-Awar N, Lee J, Terasaki PI: HLA antibody identification with single antigen beads compared to conventional methods. Hum Immunol 66: 989–997, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Adeyi OA, Girnita AL, Howe J, Marrari M, Awadalla Y, Askar M, Martell J, Zeevi A, Shapiro R, Nalesnik M, Randhawa P, Demetris AJ, Duquesnoy RJ: Serum analysis after transplant nephrectomy reveals restricted antibody specificity patterns against structurally defined HLA class I mismatches. Transpl Immunol 14: 53–62, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Del Bello A, Congy N, Sallusto F, Cardeau-Desangles I, Fort M, Esposito L, Guitard J, Cointault O, Lavayssiere L, Nogier MB, Game X, Blancher A, Rostaing L, Kamar N: Anti-human leukocyte antigen immunization after early allograft nephrectomy. Transplantation 93: 936–941, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Baldwin WM, 3rd, Bracamonte ER, Broecker V, Cosio F, Demetris AJ, Drachenberg C, Einecke G, Gloor J, Glotz D, Kraus E, Legendre C, Liapis H, Mannon RB, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Rodriguez ER, Seron D, Seshan S, Suthanthiran M, Wasowska BA, Zachary A, Zeevi A: Banff ’09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 10: 464–471, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Rowshani AT, Bemelman FJ, Lardy NM, Ten Berge IJ: Humoral immunity in renal transplantation: Clinical significance and therapeutic approach. Clin Transplant 22: 689–699, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Lenaers J, Christiaans M, van Heurn E, van Hooff H, van den Berg-Loonen E: Frequent but late donor-directed antibody formation after kidney transplantectomy within one month after grafting. Transplantation 81: 614–619, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Schleicher C, Wolters H, Kebschull L, Anthoni C, Suwelack B, Senninger N, Palmes D: Impact of failed allograft nephrectomy on initial function and graft survival after kidney retransplantation. Transpl Int 24: 284–291, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Knight MG, Tiong HY, Li J, Pidwell D, Goldfarb D: Transplant nephrectomy after allograft failure is associated with allosensitization. Urology 78: 314–318, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Lachmann N, Terasaki PI, Budde K, Liefeldt L, Kahl A, Reinke P, Pratschke J, Rudolph B, Schmidt D, Salama A, Schönemann C: Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation 87: 1505–1513, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Zou Y, Heinemann FM, Grosse-Wilde H, Sireci G, Wang Z, Lavingia B, Stastny P: Detection of anti-MICA antibodies in patients awaiting kidney transplantation, during the post-transplant course, and in eluates from rejected kidney allografts by Luminex flow cytometry. Hum Immunol 67: 230–237, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Tullius SG, Nieminen M, Bechstein WO, Jonas S, Steinmüller T, Pratschke J, Zeilinger K, Graser E, Volk HD, Neuhaus P: Chronically rejected rat kidney allografts induce donor-specific tolerance. Transplantation 64: 158–161, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Tullius SG, Nieminen M, Bechstein WO, Jonas S, Steinmüller T, Pratschke J, Zeilinger K, Graser E, Volk HD, Neuhaus P: A second native renal allograft of donor origin in a model of chronic rejection demonstrates improved long-term function. Transplant Proc 29: 3029, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Lair D, Coupel S, Giral M, Hourmant M, Karam G, Usal C, Bignon JD, Brouard S, Soulillou JP: The effect of a first kidney transplant on a subsequent transplant outcome: An experimental and clinical study. Kidney Int 67: 2368–2375, 2005 [DOI] [PubMed] [Google Scholar]
- 23.López-Gómez JM, Pérez-Flores I, Jofré R, Carretero D, Rodríguez-Benitez P, Villaverde M, Pérez-García R, Nassar GM, Niembro E, Ayus JC: Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erythropoietin resistance. J Am Soc Nephrol 15: 2494–2501, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Ayus JC, Achinger SG, Lee S, Sayegh MH, Go AS: Transplant nephrectomy improves survival following a failed renal allograft. J Am Soc Nephrol 21: 374–380, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston O, Rose C, Landsberg D, Gourlay WA, Gill JS: Nephrectomy after transplant failure: Current practice and outcomes. Am J Transplant 7: 1961–1967, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Ahmad N, Ahmed K, Mamode N: Does nephrectomy of failed allograft influence graft survival after re-transplantation? Nephrol Dial Transplant 24: 639–642, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Mazzucchi E, Nahas WC, Antonopoulos IM, Piovesan AC, Ianhez LE, Arap S: Surgical complications of graft nephrectomy in the modern transplant era. J Urol 170: 734–737, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Secin FP, Rovegno AR, del Rosario Brunet M, Marrugat RE, Dávalos Michel M, Fernandez H: Cumulative incidence, indications, morbidity and mortality of transplant nephrectomy and the most appropriate time for graft removal: Only nonfunctioning transplants that cause intractable complications should be excised. J Urol 169: 1242–1246, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Voesten HG, Slooff MJ, Hooykaas JA, Tegzess AM, Kootstra G: Safe removal of failed transplanted kidneys. Br J Surg 69: 480–481, 1982 [DOI] [PubMed] [Google Scholar]