Summary
Background and objectives
Fibroblast growth factor 23 plays an important role in regulating phosphate and vitamin D homeostasis. Elevated levels of fibroblast growth factor 23 are independently associated with mortality in patients with CKD and ESRD. Whether fibroblast growth factor 23 levels are elevated and associated with adverse outcomes in patients with AKI has not been studied.
Design, setting, participants, & measurements
This study had 30 participants with AKI, which was defined as an increase in serum creatinine≥0.3 mg/dl or ≥50% from baseline, and 30 controls from the general hospital wards and intensive care units. Plasma levels of C-terminal fibroblast growth factor 23 and vitamin D metabolites were measured within 24 hours of AKI onset and 5 days later. The composite endpoint was death or need for renal replacement therapy.
Results
Enrollment fibroblast growth factor 23 levels were significantly higher among participants with AKI than controls (median [interquartile range]=1471 [224–2534] versus 263 [96–574] RU/ml, P=0.003). Enrollment fibroblast growth factor 23 correlated negatively with 25-hydroxyvitamin D (r=−0.43, P<0.001) and 1,25-dihydroxyvitamin D (r=−0.39, P=0.003) and positively with phosphate (r=0.32, P=0.02) and parathyroid hormone (r=0.37, P=0.005). Among participants with AKI, enrollment fibroblast growth factor 23 (but not other serum parameters) was significantly associated with the composite endpoint, even after adjusting for age and enrollment serum creatinine (11 events; adjusted odds ratio per 1 SD higher ln[fibroblast growth factor 23]=13.73, 95% confidence interval=1.75–107.50).
Conclusions
Among patients with AKI, fibroblast growth factor 23 levels are elevated and associated with greater risk of death or need for renal replacement therapy.
Introduction
Renal failure is almost universally accompanied by changes in mineral metabolism (1). Fibroblast growth factor 23 (FGF-23) is a potent phosphaturic hormone released by osteocytes that plays an important role in phosphate and vitamin D homeostasis (2,3). FGF-23 levels increase progressively as CKD worsens (4), which accounts for the progressive reduction of 1,25-dihydroxyvitamin D [1,25(OH)2D] levels in CKD (5). FGF-23 has gained widespread attention for its association with increased mortality among patients with CKD (6) and incident ESRD (7).
In contrast, little is known about FGF-23 levels among patients with AKI. We reported markedly elevated levels of FGF-23 in a patient with rhabdomyolysis-induced AKI accompanied by reduced levels of 1,25(OH)2D (8). A recent case series described elevated levels of FGF-23 among 12 participants with AKI; however, the study measured FGF-23 at only a single time point and did not measure vitamin D metabolites (9).
We tested the hypotheses that AKI is associated with elevated levels of FGF-23 and reduced levels of 1,25(OH)2D and that, among patients with AKI, FGF-23 is predictive of the composite clinical endpoint of in-hospital death or need for renal replacement therapy (RRT).
Materials and Methods
Study Design
We conducted a single-center, prospective pilot study among inpatients at New York Presbyterian Hospital—Columbia University Medical Center. All protocols for participant recruitment and specimen collection were approved by the Columbia University Medical Center Institutional Review Board.
Study Participants
Participants from general medical wards and intensive care units (ICUs) were recruited into two groups: AKI and control. AKI was defined in accordance with criteria established by the Acute Kidney Injury Network: an abrupt increase in serum creatinine≥0.3 mg/dl within 48 hours or a ≥50% increase in serum creatinine (10). Because of inconsistent reporting of urine volumes, particularly in non-ICU patients, we did not use oliguria as an AKI criterion, and we did not record detailed data on urine output. However, among participants diagnosed with AKI, the distinction between oliguric and nonoliguric AKI (defined as urine output<500 ml in 24 hours) was obtained in most cases (26 of 30) and recorded. Because our goal was to recruit participants with true renal injury, an additional criterion for AKI was the exclusion of prerenal azotemia, defined as resolution of increased serum creatinine within 24–48 hours of administration of intravenous fluid and/or discontinuation of diuretics. Control participants were selected to be closely representative of participants with AKI with regard to age, sex, race, and hospital location (i.e., general medical ward versus ICU), and they were required to have a stable serum creatinine<1.0 mg/dl.
Additional inclusion criteria for all participants included age≥18 and ≤70 years, capacity to give informed consent, and baseline estimated GFR≥60 ml/min per 1.73 m2 based on the Modification of Diet in Renal Disease equation and assessed within the preceding 3 months by electronic medical records. Patients greater than 70 years old were excluded to circumvent the issue of age-related decline in GFR.
Additional exclusion criteria for all participants included current or recent therapy with elemental vitamin D at doses≥800 IU/d or a history of parathyroid disease, metabolic bone disease, fat malabsorption, or duodenal resection as assessed by electronic medical records.
Study Procedures
Participants were identified as having AKI using a daily automated query of the hospital’s central data warehouse to rapidly identify inpatients with elevations in serum creatinine. After initial identification, additional review of chart records was performed to determine eligibility. Patients who met all criteria for the study and were agreeable to participating provided written informed consent. Patients who lacked capacity to provide written informed consent were not eligible for enrollment by surrogate consent. Accordingly, patients who were intubated, sedated, or suffered from altered mental status were excluded.
On enrollment in the study, 5 ml of blood was obtained in EDTA-containing vacutainers. Among those participants with AKI, enrollment collections were drawn, in most cases, within 24 hours after the clinical diagnosis was established. However, on rare occasions, enrollment collections were delayed up to 48 hours. Samples were centrifuged, aliquoted, and stored at −80°C. A second blood collection was obtained 5 days after the first collection from participants who remained hospitalized.
Laboratory Analyses
C-terminal FGF-23 was measured in duplicate using a standard ELISA kit (Immutopics, San Clemente, CA). 1,25(OH)2D (both D2 and D3) was measured using a standard radioimmunoassay kit (DiaSorin). Intra- and interassay coefficients of variation for FGF-23 were 1.4% and 2.4%, respectively. Intra- and interassay coefficients of variation for 1,25(OH)2D were 7%–11% and 12%–15%, respectively.
25-hydroxyvitamin D [25(OH)D; both D2 and D3] and its major metabolite 24R,25-dihydroxyvitamin D3 [24R,25(OH)2D3] were measured using ultra-performance liquid chromatography with tandem mass spectrometry, the details of which are provided in Supplemental Material. The ratio of 24R,25(OH)2D3 to 25(OH)D levels was used to standardize the results of the primary vitamin D metabolite to the total vitamin D stores.
Statistical Analyses
Statistical analysis was performed with SAS Version 9.2 (SAS Institute Inc., Cary, NC). Data are reported as median and interquartile range (IQR; 25–75 percentiles). Differences between groups were assessed by the Mann–Whitney U test. Differences within groups comparing enrollment and follow-up parameters were assessed by the Wilcoxon signed-rank test. Correlation between enrollment FGF-23 and other enrollment variables was analyzed using the Spearman’s rank correlation coefficient. Differences in rates of progression to the composite clinical endpoint of in-hospital death or need for RRT, according to tertiles of enrollment FGF-23 and serum creatinine levels, were assessed by Fisher’s exact test. Multivariable logistic regression models were used to compute adjusted odds ratios between enrollment FGF-23 (natural log-transformed given its skewed distribution and standardized to mean=0 and SD=1) and the composite clinical endpoint. The models were adjusted for age and serum creatinine at the time of enrollment. All comparisons are two-tailed, with P<0.05 considered significant.
Results
Baseline Characteristics
Thirty participants were enrolled into the AKI and control groups (n=60 total). Baseline characteristics are shown in Table 1. Participants in the two groups were similar with regard to age, sex, race, hospital location, baseline renal function, and comorbidities, with the exception of a greater number of participants with diabetes in the AKI group.
Table 1.
Baseline characteristics of the participants
| Variable | Control (n=30) | AKI (n=30) |
|---|---|---|
| Median age in years (IQR) | 56 (45–61) | 57 (50–64) |
| Female (%) | 12 (40) | 10 (36) |
| Race (%) | ||
| white | 13 (43) | 13 (43) |
| nonwhite | 17 (57) | 17 (57) |
| Comorbidities (%) | ||
| congestive heart failure | 9 (30) | 6 (20) |
| hypertension | 14 (47) | 14 (47) |
| liver disease | 9 (30) | 8 (27) |
| diabetes | 8 (27) | 17 (57) |
| Hospital location (%) | ||
| general medical ward | 17 (57) | 16 (53) |
| intensive care unit | 13 (43) | 14 (47) |
| Renal functiona | ||
| median serum creatinine (mg/dl; IQR) | 0.8 (0.7–0.9) | 0.9 (0.7–1.0) |
| median eGFR (ml/min per 1.73 m2; IQR) | 111 (92–126) | 103 (82–131) |
| Cause of AKI (%) | ||
| ischemic ATN | 14 (47) | |
| sepsis | 5 (17) | |
| nephrotoxin | 6 (20) | |
| HRS | 2 (7) | |
| AIN | 1 (3) | |
| rhabdomyolysis | 2 (6) |
IQR, interquartile range; eGFR, estimated GFR; ATN, acute tubular necrosis; HRS, hepatorenal syndrome; AIN, acute interstitial nephritis.
Represents renal function before study enrollment.
Enrollment Serum Parameters
Enrollment serum parameters are shown in Table 2. FGF-23 levels were significantly higher among participants with AKI compared with controls. Additionally, participants with AKI had lower levels of 1,25(OH)2D, 24R,25(OH)2D3, and calcium; higher levels of parathyroid hormone (PTH) and phosphate; and a trend toward lower levels of 25(OH)D. Despite lower absolute levels of 24R,25(OH)2D3 among participants with AKI, there was no significant difference in 24R,25(OH)2D3 when standardized to 25(OH)D levels (Table 2).
Table 2.
Enrollment serum parameters
| Reference Values | Control (n=30) | AKI (n=30) | P Value | |
|---|---|---|---|---|
| FGF-23 (RU/ml) | 7–71 | 263 (96–574) | 1471 (224–2534) | 0.003 |
| 25(OH)D (ng/ml) | 30–80 | 14 (8–21) | 8 (4–15) | 0.06 |
| 1,25(OH)2D (pg/ml) | 18–72 | 25 (15–35) | 17 (10–22) | 0.01 |
| 24R,25(OH)2D3 (ng/ml) | N/A | 1.5 (0.6–2.6) | 0.9 (0.3–1.5) | 0.01 |
| 24R,25(OH)2D3/25(OH)D | N/A | 0.09 (0.07–0.19) | 0.07 (0.04–0.11) | 0.10 |
| PTH (intact; pg/ml) | 8–51 | 40 (31–78) | 76 (51–182) | 0.004 |
| Calcium (mg/dl) | 8.7–10.0 | 8.7 (8.1–9.0) | 8.1 (7.5–8.6) | 0.004 |
| Phosphate (mg/dl) | 2.5–4.3 | 3.4 (2.9–4.0) | 4.7 (3.8–5.3) | <0.001 |
| Creatinine (mg/dl) | 0.5–1.0 | 0.7 (0.6–0.9) | 2.2 (1.8–2.7) | <0.001 |
Values represent median (interquartile ranges). FGF-23, fibroblast growth factor 23; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 24R,25(OH)2D3, 24R,25-dihydroxyvitamin D3; N/A, not applicable; PTH, parathyroid hormone.
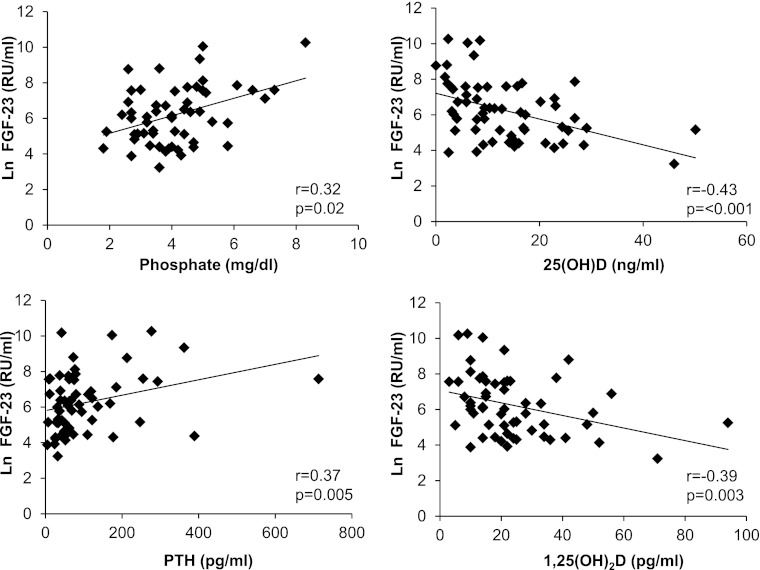
Correlations between enrollment FGF-23 (natural log-transformed) and other mineral metabolites in the overall sample are presented in Figure 1. Enrollment FGF-23 correlated positively with phosphate (P=0.02) and PTH levels (P=0.005) and negatively with 25(OH)D (P<0.001) and 1,25(OH)2D (P=0.003). It did not correlate with 24R,25(OH)2D3 (P=0.20) or calcium (P=0.50).
Figure 1.
Correlations between enrollment fibroblast growth factor 23 (FGF-23) and other serum parameters.
There were no significant differences in enrollment FGF-23 levels according to etiology of AKI. A trend toward higher levels of FGF-23 was observed among participants with oliguric compared with nonoliguric AKI; however, the differences did not reach significance (median [IQR]=2167 [1980–3116] and 1148 [151–3553] RU/ml, n=6 and n=20, respectively, P=0.15). The highest levels of enrollment FGF-23 tended to occur among participants with AKI in the ICU and were significantly higher than control participants in the ICU (1893 [352–6612] and 322 [170–668] RU/ml, n=14 and n=13, respectively, P=0.04). FGF-23 levels were lower among participants in the general wards but remained higher among those participants with AKI than controls (710 [186–2094] and 205 [74–562] RU/ml, n=16 and n=17, respectively, P=0.03).
Although enrollment FGF-23 levels were significantly lower among control participants compared with those participants with AKI, controls had higher FGF-23 levels than those levels previously reported in healthy, ambulatory volunteers (11) and even in many CKD patients (12). Among the control participants, those participants with FGF-23 levels above versus below the median were significantly more likely to have a history of congestive heart failure or left ventricular hypertrophy (LVH; 67% versus 13%, P=0.008). No other significant differences in comorbidities were observed between controls with high or low FGF-23 levels.
Day 5 Serum Parameters
Because of interim death or hospital discharge, fewer participants (16 controls and 19 AKI participants) were available for blood collections on day 5 after enrollment. By day 5, levels of 25(OH)D, 1,25(OH)2D, and 24R,25(OH)2D3 remained significantly lower among participants with AKI compared with controls (Table 3). FGF-23 levels declined significantly among participants with AKI (P<0.001), and therefore, levels were no longer significantly higher than in the control participants; levels in the control participants were unchanged over time.
Table 3.
Day 5 serum parameters
| Reference Values | Control (n=16) | AKI (n=19) | P Value | |
|---|---|---|---|---|
| FGF-23 (RU/ml) | 7–71 | 286 (127–454) | 459 (215–1478) | 0.17 |
| 25(OH)D (ng/ml) | 30–80 | 15 (8–18) | 6 (5–12) | 0.03 |
| 1,25(OH)2D (pg/ml) | 18–72 | 20 (16–28) | 13 (12–20) | 0.05 |
| 24R,25(OH)2D3 (ng/ml) | N/A | 1.7 (0.8–2.5) | 0.6 (0.3–1.1) | 0.04 |
| 24R,25(OH)2D3/25(OH)D | N/A | 0.11 (0.08–0.15) | 0.08 (0.04–0.11) | 0.22 |
| PTH (intact; pg/ml) | 8–51 | 48 (39–95) | 71 (46–108) | 0.56 |
| Calcium (mg/dl) | 8.7–10.0 | 8.9 (8.1–9.3) | 8.1 (7.5–8.7) | 0.07 |
| Phosphate (mg/dl) | 2.5–4.3 | 3.7 (3.3–4.1) | 3.4 (2.8–3.7) | 0.07 |
| Creatinine (mg/dl) | 0.5–1.0 | 0.8 (0.7–0.9) | 1.3 (1.1–2.4) | <0.001 |
Values represent median (interquartile ranges). FGF-23, fibroblast growth factor 23; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 24R,25(OH)2D3, 24R,25-dihydroxyvitamin D3; N/A, not applicable; PTH, parathyroid hormone.
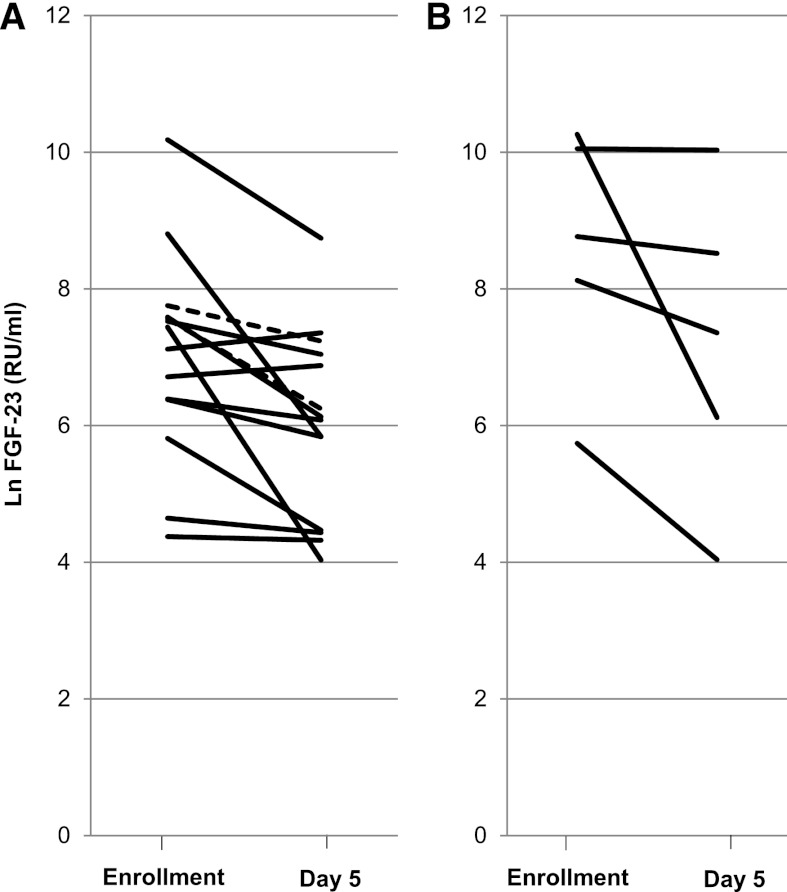
Among participants with AKI, no major differences were observed in the direction or magnitude of change in FGF-23 between those participants who did not require RRT (n=14) and those participants who did require RRT (n=5), which is shown in Figure 2. Among the five participants who required RRT, all showed a decline in FGF-23 over time; however, the magnitude of decline was variable, ranging from 20% to 80%. Finally, no significant associations were found between changes in FGF-23 and changes in serum phosphate, PTH, 25(OH)D, or 1,25(OH)2D.
Figure 2.
Line plot of FGF-23 trend over time among those participants with AKI. (A) Participants who did not require renal replacement therapy (RRT). (B) Participants who were initiated on RRT. In A, solid lines represent individuals with interval improvement in creatinine (n=12); dotted lines represent individuals with interval worsening of creatinine (n=2).
Additional changes in serum parameters over time included significant declines in serum creatinine (P<0.001) and phosphate (P<0.001) among participants with AKI. A significant decline is serum phosphate was also noted among the subgroup of participants with AKI who did not require RRT (4.7 [4.1–5.1] and 3.4 [3.0–3.4] mg/dl at enrollment and day 5, respectively, P=0.003). None of these participants developed hypophosphatemia at day 5, although many participants had values at the low end of the normal range.
FGF-23 as a Predictor of Outcomes in AKI
Among the 30 participants with AKI, 11 participants (5 participants from the general medical wards and 6 participants from the ICU) reached the composite clinical endpoint of death and/or need for RRT (2 participants required RRT and survived, 4 participants died without RRT, and 5 participants required RRT and died). Among the 7 participants who required RRT, the median (IQR) time of initiation was 0 (0–2.5) days after the enrollment blood collection. Among the 9 participants who died, the median (IQR) time to death was 22 (11–29) days. The cause of death was septic shock and multiorgan failure in all participants except a single participant who expired from cardiogenic shock. None of the controls reached the composite clinical endpoint; therefore, analyses between FGF-23 and outcomes were restricted to the AKI group.
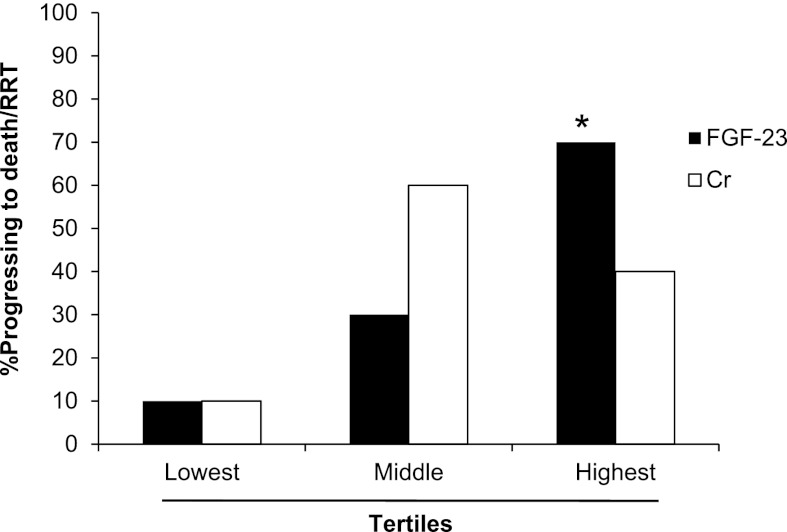
The association between tertiles of enrollment FGF-23 and creatinine with mortality/RRT in participants with AKI is show in Figure 3. Enrollment FGF-23 levels were significantly higher among those participants who died and/or required RRT compared with those participants who survived without RRT (3376 [1986–17318] versus 404 [136–1471] RU/ml, n=11 and 19, respectively, P<0.001). Elevated levels of FGF-23 were associated with a significantly higher risk of mortality or need for RRT in unadjusted analyses (odds ratio=7.48 per 1 SD of natural log-transformed FGF-23, 95% confidence interval=1.67–33.51). Adjustment for age and enrollment creatinine strengthened the association between FGF-23 and mortality/RRT (odds ratio=13.73 per 1 SD of natural log-transformed FGF-23, 95% confidence interval=1.75–107.50). Other enrollment serum parameters were not significantly associated with mortality/RRT among participants with AKI, including calcium, phosphate, albumin, creatinine, PTH, 25(OH)D, and 1,25(OH)2D. Participants with oliguric versus nonoliguric AKI had a nonsignificant trend to greater likelihood of mortality/RRT (66% and 30%, n=6 and n=20, respectively, P=0.16).
Figure 3.
Progression to death/RRT based on tertiles of enrollment FGF-23 and creatinine among participants with AKI. *P=0.02 likelihood of progression to death/RRT among those participants in the highest compared with lowest FGF-23 tertiles.
Discussion
We confirm that FGF-23 levels are elevated in AKI and provide the first evidence suggesting that elevated FGF-23 is independently associated with greater risk of in-hospital mortality and/or need for RRT. Additionally, we document that participants with AKI had significantly lower levels of enrollment 1,25(OH)2D and a trend toward lower levels of enrollment 25(OH)D compared with controls. This pilot study is the first to systematically evaluate the role of FGF-23 in AKI and simultaneously measure vitamin D metabolites, including 24R,25(OH)2D3.
Although FGF-23 correlated with phosphate and PTH, neither of these parameters nor any other serum parameter showed the strong dosage response relationship observed between increasing FGF-23 and adverse outcomes. A similar pattern of findings has been observed in patients with CKD and ESRD. FGF-23 levels are more predictive of kidney disease progression than serum phosphate among CKD patients (13), and they are more predictive of mortality than serum phosphate among incident hemodialysis patients (7). Cumulatively, these findings suggest that FGF-23 is a more sensitive biomarker for disordered mineral metabolism and adverse outcomes than serum phosphate.
We could not discern the precise mechanisms responsible for elevated FGF-23 among participants with AKI in this study. The contribution of changes in unmeasured variables, such as altered expression of Klotho, a coreceptor for FGF-23 (14), is unknown and requires additional study. Direct stimulation of FGF-23 by elevated serum levels of phosphate and PTH is a potential mechanism, especially because both mineral metabolites were positively correlated with FGF-23. However, no human studies have shown an increase in FGF-23 levels in response to increasing serum phosphate levels, despite the fact that they are often correlated. There is evidence that PTH directly stimulates FGF-23 production in both mice (15) and humans (16). Accordingly, FGF-23 elevation in the setting of AKI may reflect the transient secondary hyperparathyroidism that results from hypocalcemia. However, a recent study in uremic CKD patients suggests that FGF-23 may actually be the physiologic regulator of PTH secretion (17). Clearly, elucidating the complex interplay between FGF-23, PTH, and phosphate remains critical to our understanding of mineral metabolism, and it is an area of active investigation.
It is unclear whether elevated levels of FGF-23 contributed to the observed reduction in 1,25(OH)2D levels among participants with AKI like they do in CKD. FGF-23 is known to inhibit 25-hydroxyvitamin D-1α-hydroxylase (1α-hydroxylase) and stimulate 25-hydroxyvitamin D-24-hydroxylase, thereby simultaneously inhibiting the activation and enhancing the catabolism of 25(OH)D, respectively (5,18). In the overall sample, significant negative correlations were found between FGF-23 and both 25(OH)D and 1,25(OH)2D, supporting a possible link. To explore potential mechanisms underlying such a link, we measured 24R,25(OH)2D3, the major metabolite of 25(OH)D, and found that levels were not elevated in AKI, even after standardizing the results to the total vitamin D stores. Accordingly, enhanced catabolism of 25(OH)D does not seem to be the primary mechanism of reduced 1,25(OH)2D in AKI. Alternatively, it is possible that FGF-23 contributed to decreased synthesis of 1,25(OH)2D through its known inhibition of 1α-hydroxylase. Finally, reduced uptake of 25(OH)D because of severely reduced GFR or global nephron dysfunction itself may have contributed to diminished activity of renal 1α-hydroxylase.
An intriguing finding in this study was elevated levels of FGF-23 among hospitalized participants with normal renal function—a population in which FGF-23 has not been studied in detail. Elevation of FGF-23 in this setting may reflect a potential role as an acute-phase reactant. Alternatively, FGF-23 may be chronically elevated in certain patient populations. In particular, we observed dramatically higher levels of FGF-23 among controls with congestive heart failure or LVH. These observations are consistent with recent evidence that FGF-23 has a direct causal role in the pathogenesis of LVH (19). An alternative possibility that has yet to be investigated is that structural heart disease itself may signal FGF-23 release from bone.
We acknowledge several limitations of this study, including small sample size, single-center design, only two data points for each participant, and relatively short duration of follow-up (until hospital discharge). We did not include patients with prerenal azotemia and therefore, were unable to ascertain whether FGF-23 might reliably distinguish functional versus structural renal impairment. Exclusion of patients with prerenal azotemia also likely contributed to the high rates of RRT (23%) and death (30%) observed in the AKI group, which may not be representative of other AKI cohorts. We did not have access to data on urine output, which might have allowed earlier identification of AKI and would have been another traditional biomarker (other than serum creatinine) with which to compare the prognostic use of FGF-23. We did not measure circulating or soluble Klotho, which has recently been reported as an early AKI biomarker and renoprotective factor in rodents (20). Finally, although most enrollment laboratory collections in the AKI group were obtained within 24 hours after the clinical diagnosis was established, occasionally, the laboratory collections were delayed up to 48 hours, which may have altered the prognostic use of other serum parameters relative to FGF-23.
Additionally, surrogate informed consent was not approved for this study, which precluded enrollment of critically ill participants lacking decisional capacity because of sedation, intubation, or other acute processes affecting consciousness or cognition. However, given our observations that the highest levels of FGF-23 tended to occur among participants with the most severe AKI (and therefore, the most severe overall critical illness), it is likely that the above limitation would have, if anything, biased our results to the null. Future studies should aim to include the full spectrum of patients with and without AKI, including those patients who are most critically ill.
Severity of illness scores were not available for comparison between AKI and control participants in the ICU, which may have confounded the results by contributing to higher FGF-23 levels in the AKI group. However, participants in the two groups had very similar baseline characteristics, including demographics, comorbidities, and hospital location, and any differences between the two groups would be unlikely to account for the dramatically higher levels of FGF-23 observed in the AKI group.
Our findings do not unequivocally show whether levels of FGF-23 were elevated because of increased production or simply diminished clearance. However, at least two observations support increased production. First, FGF-23 levels were elevated even among ICU control participants with normal renal function; second, among peritoneal dialysis patients with residual renal function, renal and peritoneal dialysate clearance of FGF-23 is negligible (21). Whether convective forms of RRT such as continuous venovenous hemofiltration, which was used in this study, may result in greater clearance of FGF-23 is unknown and will require future studies.
Additional studies will be needed to further define the biologic role of FGF-23 in AKI and determine whether FGF-23 testing will provide diagnostic and prognostic use for clinical management of critically ill patients. Our data suggest that FGF-23 could have a role as a biomarker of adverse outcomes among patients with established AKI. Developing FGF-23 as a biomarker in AKI will require additional testing and validation in large AKI cohorts and comparisons of FGF-23 with other more established biomarkers of AKI. Irrespective of the performance of FGF-23 as a biomarker of risk prediction compared with other clinical variables, laboratory parameters, or novel biomarkers, our findings raise important questions about the potential pathophysiologic mechanisms that may underlie our observations of FGF-23 in critical illness and AKI. Whether elevated FGF-23 levels may be directly toxic to nontraditional target organs, such as the cardiovascular, skeletal, and endocrine systems, and thereby, contribute directly to adverse outcomes in AKI, which it seems to do in CKD, is an intriguing possibility that will require additional study.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Melanie Foley from Columbia University for invaluable assistance with the Institutional Review Board.
This work was supported by a generous donation from the Nortillo Foundation, Columbia University Clinical and Translational Science Award Grant UL1RR024156 from the National Center for Research Resources/National Institutes of Health, and National Institutes of Health Grants R01DK076116 (to M.W.) and R01DK081374 (to M.W.).
This work was presented as a poster abstract at the American Society of Nephrology’s Kidney Week, November 11, 2011, Philadelphia, Pennsylvania.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00550112/-/DCSupplemental.
References
- 1.Hruska KA, Teitelbaum SL: Renal osteodystrophy. N Engl J Med 333: 166–174, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Cai Q, Hodgson SF, Kao PC, Lennon VA, Klee GG, Zinsmiester AR, Kumar R: Brief report: Inhibition of renal phosphate transport by a tumor product in a patient with oncogenic osteomalacia. N Engl J Med 330: 1645–1649, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Berndt T, Kumar R: Phosphatonins and the regulation of phosphate homeostasis. Annu Rev Physiol 69: 341–359, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T: Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 78: 975–980, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leaf DE, Wolf M, Stern L: Elevated FGF-23 in a patient with rhabdomyolysis-induced acute kidney injury. Nephrol Dial Transplant 25: 1335–1337, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Hsu R, Hsu CY, Kordesch K, Nicasio E, Cortez A, McAlpine I, Brady S, Zhuo H, Kangelaris KN, Stein J, Calfee CS, Liu KD: FGF-23 and PTH levels in patients with acute kidney injury: A cross-sectional case series study. Ann Intensive Care 1: 21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laroche M, Boyer JF, Jahafar H, Allard J, Tack I: Normal FGF23 levels in adult idiopathic phosphate diabetes. Calcif Tissue Int 84: 112–117, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M: Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P, MMKD Study Group : Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Kawata T, Imanishi Y, Kobayashi K, Miki T, Arnold A, Inaba M, Nishizawa Y: Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol 18: 2683–2688, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Wesseling-Perry K, Harkins GC, Wang HJ, Elashoff R, Gales B, Horwitz MJ, Stewart AF, Jüppner H, Salusky IB: The calcemic response to continuous parathyroid hormone (PTH)(1-34) infusion in end-stage kidney disease varies according to bone turnover: A potential role for PTH(7-84). J Clin Endocrinol Metab 95: 2772–2780, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komaba H, Goto S, Fujii H, Hamada Y, Kobayashi A, Shibuya K, Tominaga Y, Otsuki N, Nibu K, Nakagawa K, Tsugawa N, Okano T, Kitazawa R, Fukagawa M, Kita T: Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int 77: 232–238, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Inoue Y, Segawa H, Kaneko I, Yamanaka S, Kusano K, Kawakami E, Furutani J, Ito M, Kuwahata M, Saito H, Fukushima N, Kato S, Kanayama HO, Miyamoto K: Role of the vitamin D receptor in FGF23 action on phosphate metabolism. Biochem J 390: 325–331, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu MC, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OW: Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 78: 1240–1251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isakova T, Xie H, Barchi-Chung A, Vargas G, Sowden N, Houston J, Wahl P, Lundquist A, Epstein M, Smith K, Contreras G, Ortega L, Lenz O, Briones P, Egbert P, Ikizler TA, Jueppner H, Wolf M: Fibroblast growth factor 23 in patients undergoing peritoneal dialysis. Clin J Am Soc Nephrol 6: 2688–2695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.