Summary
Background and objectives
Inflammation is closely associated with cardiovascular disease, the leading cause of mortality in patients with CKD. Serum decoy receptor 3 (DcR3) is a member of the TNF receptor superfamily. CKD patients have higher levels of DcR3 than the general population, but whether DcR3 predicts mortality in CKD patients on hemodialysis has not been explored.
Design, setting, participants, & measurements
DcR3 levels were measured in 316 prevalent hemodialysis patients who were followed up from November 1, 2004, to June 30, 2009, for cardiovascular and all-cause mortality.
Results
The baseline DcR3 concentration showed a strong positive correlation with inflammatory markers including high-sensitivity C-reactive protein, IL-6, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1). During a follow-up period of 54 months, 90 patients died (34 cardiovascular deaths). Kaplan–Meier survival analysis showed higher cardiovascular and all-cause mortality in patients with higher DcR3 levels. The hazard ratios (95% confidence intervals) of the highest versus lowest tertiles of DcR3 were 2.8 (1.1–7.3; P for trend=0.04) for cardiovascular mortality and 2.1 (1.1–3.7; P for trend=0.02) for all-cause mortality, respectively. Based on the minimal increase in the area under the receiver operating characteristic curve from 0.79 to 0.80, the addition of DcR3 to established risk factors including VCAM-1, albumin, and IL-6 does not improve the prediction of mortality.
Conclusions
Higher DcR3 levels strongly correlate with inflammation and independently predict cardiovascular and all-cause mortality in CKD patients on hemodialysis.
Introduction
Cardiovascular disease (CVD) is the leading cause of both morbidity and mortality in patients with CKD (1). Persistent inflammation in CKD plays a pathogenic role in CVD (2). Although several studies have examined the association between cytokine levels and clinical outcomes in CKD, few have reported whether such associations suggest a pathogenic role distinct from that of other mediators (3). Decoy receptor 3 (DcR3), a member of the TNF receptor superfamily (4), is an antiapoptotic soluble receptor considered to play an important role in immune modulation. DcR3 may participate in immune suppression (5–8). Alternatively, DcR3 may have proinflammatory functions. An excessive inflammatory response to various forms of endothelial injury to an artery is characteristic of the atherosclerotic process. Atherosclerosis involves various inflammatory mediators including adhesion molecules, chemokines, and cytokines (9). Recently, an association between DcR3 and CVD was proposed on the basis of its ability to elicit the secretion of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and IL-8 from endothelial cells (10). Adhesion of circulating monocytes to endothelial cells and subsequent trans-endothelial migration to sites of inflammation are key steps in the initiation and aggravation of atherosclerotic lesions (11).
CKD patients have higher DcR3 expression levels than the general population (12), but whether DcR3 associates with the increased cardiovascular mortality observed in CKD patients has not been explored. On the basis of this information, we conducted a longitudinal analysis to test the hypothesis that elevated DcR3 levels are a predictor for cardiovascular and all-cause mortality in CKD patients on hemodialysis.
Materials and Methods
Study Design
This prospective cohort study was carried out at four dialysis centers in the Taipei metropolitan area. Study participants were recruited from November 1 to December 31, 2004. Initially, all patients (n=402) undergoing hemodialysis were screened, and 350 patients aged >20 years who had been on hemodialysis for >6 months were included. Exclusion criteria were dialysis for <12 h/wk (n=4); inadequacy of dialysis defined as Kt/V urea <1.2 (n=5); and conditions of malignancy (n=3), infectious disease, or sepsis (n=5), or hepatobiliary disease (n=17). Finally, 316 clinically stable patients (150 men and 166 women; mean age of 59 years) were followed up until June 30, 2009. All of the patients were subjected to a standard bicarbonate dialysis session. Hemodialysis was performed three times weekly using single-use dialyzers with a membrane surface area of 1.6−1.7 m2. The median duration of hemodialysis before study entry was 71 months (interquartile range, 34−120). The study protocol was approved by the institutional review board of each affiliated hospital. Informed consent was obtained from all participants, and our study complies with the Declaration of Helsinki.
In all patients, a thorough medical history was taken at the time of study enrollment. Presence of CVD was defined as a medical history and clinical findings of congestive heart failure and coronary artery, cerebrovascular, and/or peripheral vascular disease. No major modifications were made in dialysis treatments or schedules during the follow-up period. The primary outcome measures were cardiovascular and all-cause mortality from the time of inclusion in the study. Cardiovascular mortality included fatal myocardial infarction, stroke, congestive heart failure, arrhythmia, complicated peripheral vascular disease, and sudden death. The overall mortality category comprised death due to cardiovascular events, infection, sepsis, malignancy, gastrointestinal bleeding, chronic obstructive lung disease, or cachexia. Each medical chart was reviewed and a trained physician who was blinded to the DcR3 levels independently assigned the cause of death on the basis of all of the available clinical information.
Laboratory Measurements
All blood samples were drawn from patients who had fasted overnight at the start of a mid-week dialysis session, and heparin was then administered. Plasma and serum were separated and kept frozen at –70°C when not analyzed immediately. Serum levels of DcR3 (Biovendor, Modrice, Czech Republic), ICAM-1 and VCAM-1 (BioSource, Camarillo, CA), and IL-6 (R&D Systems, Minneapolis, MN) were measured using commercially available ELISA kits according to the manufacturer’s instructions. Intra-assay and interassay coefficients of variance for DcR3 were 4.8% and 4.3% at 0.50 ng/ml and 1.5% and 1.6% at 3.50 ng/ml, respectively. Intra-assay and inter-assay coefficients of variance for other concentrations were <5%. Albumin, urea, creatinine, calcium, phosphate, iron, and total iron-binding capacity in serum were determined using commercial kits by a Hitachi 7600 autoanalyzer (Roche Modular; Hitachi Ltd, Tokyo, Japan). Transferrin saturation was calculated as the serum iron concentration/total iron-binding capacity × 100. Serum ferritin and intact parathyroid hormone levels were determined with a RIA (Incstar, Stillwater, MN). Serum high-sensitivity C-reactive protein (hs-CRP) levels were measured using an immunoturbidimetric assay and rate nephelometry (IMMAGE; Beckman Coulter, Galway, Ireland). The adequacy of dialysis was estimated by measuring mid-week urea clearance (Kt/V urea) using the standard method (13). BP was measured and recorded by an automated sphygmomanometer. Predialysis BP was measured in the nonaccess arm after a 5-minute rest while the patient was seated with both feet on the floor before placement of a dialysis needle.
Statistical Analyses
All variables were expressed as percentages for categorical data and as means ± SD or medians and interquartile ranges for continuous data with or without a normal distribution, respectively. Potential differences among the three patient groups for each baseline serum DcR3 tertile were assessed by ANOVA, the Kruskal–Wallis test, or the chi-squared test, as appropriate. A Pearson correlation analysis was used to assess the associations of DcR3 with malnutrition-inflammation parameters. Multivariate regression analyses were used to assess independent predictors of serum DcR3 levels. To assess the predictive accuracy of the DcR3 for mortality, we used the time-dependent receiver operating characteristic (ROC) curve for censored data and the area under the ROC curve (AUC) as the criterion (14). An AUC of 0.5 indicates no predictive ability, whereas a value of 1 represents perfect predictive ability. Using the C statistic with stepwise addition of VCAM-1, albumin, IL-6, and DcR3 to traditional cardiovascular risk factors, the incremental change in AUC was assessed for mortality prediction at 48 months of the study.
The Kaplan–Meier method was used to describe survival curves. During the follow-up, 14 patients received a kidney transplant, 4 were shifted to peritoneal dialysis, and 33 were transferred to other dialysis units. Censoring occurred at the time of kidney transplantation, peritoneal dialysis, and withdrawal from the study, or on June 30, 2009. Cox proportional hazards regression analysis was used to examine the association of baseline variables with cardiovascular and all-cause mortality. The univariate and multivariate Cox regression analyses are presented as hazard ratios (HRs) and 95% confidence intervals (95% CIs). Adjustments for age and sex were initially performed to calculate adjusted HRs. The multivariate regression analysis was further adjusted for age and sex, and covariates served as potential confounders of the association between DcR3 concentration and cardiovascular or all-cause death. The potential confounding factors were smoking status, diabetes, prior CVD, body mass index, total cholesterol, systolic BP, dialysis vintage, urea Kt/V, hemoglobin, serum albumin, IL-6, and VCAM-1. Furthermore, the significance of linear trends across the DcR3 tertiles was tested by assigning each patient the median of the tertile and modeling this value as a continuous variable. The Akaike Information Criterion (AIC) (15) was used for calculating the prediction gain by DcR3 when this receptor was added to a multivariate Cox regression model adjusted for traditional cardiovascular risk factors. The prediction gain was then calculated by adding VCAM-1, albumin, hs-CRP, or IL-6 into the same regression model, respectively. P<0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences (version 16.0; SPSS Inc, Chicago, IL).
Results
Baseline Characteristics of Patients
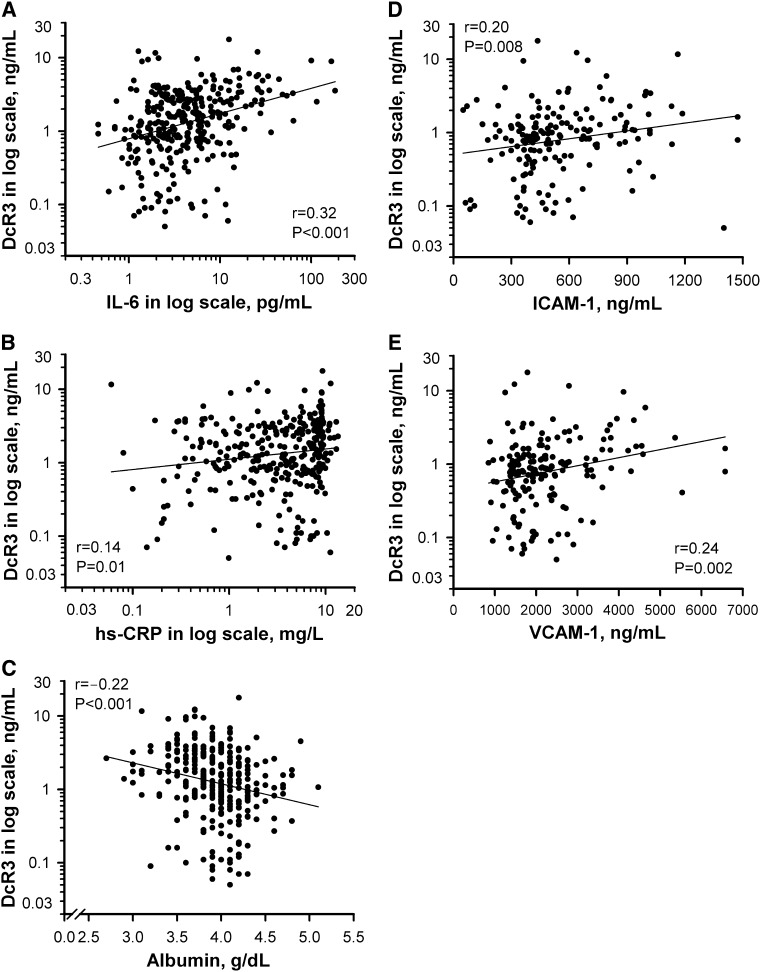
Clinical characteristics of the 316 hemodialysis patients, stratified according to the serum DcR3 tertiles, are summarized in Table 1. There were 104 patients (33%) with diabetes mellitus, and 66 patients (21%) had a clinical history of CVD. Patients with higher DcR3 levels were more likely to have a previous history of CVD. In addition, plasma concentrations of hs-CRP, IL-6, ICAM-1, and VCAM-1 were significantly higher in patients with serum DcR3 levels in the highest tertile. In the univariate analysis (Figure 1), DcR3 levels were positively correlated with IL-6 (P<0.001) and hs-CRP (P=0.01) levels and negatively correlated with albumin levels (P<0.001). Furthermore, DcR3 levels were positively associated with ICAM-1 (P=0.008) and VCAM-1 (P=0.002) levels. No correlation was found between DcR3 levels and traditional risk factors, including age, sex, hypertension, dyslipidemia, smoking status, and diabetes. The use of renin-angiotensin blockers or statins did not have an effect on DcR3 levels. We performed a multivariate regression analysis of contributing factors to explain DcR3 levels. Serum albumin (P=0.009), IL-6 (P=0.001), VCAM-1 (P=0.01), and history of CVD (P=0.04) all were significantly associated with DcR3 levels (Table 2).
Table 1.
Patients’ characteristics according to tertiles of DcR3 levels
| Characteristic | Serum DcR3 Tertiles | P Value | ||
|---|---|---|---|---|
| 0.05−0.92 ng/ml (n=105) | 0.93−2.41 ng/ml (n=105) | 2.45−17.78 ng/ml (n=106) | ||
| Age (yr) | 58±12 | 59±14 | 61±13 | 0.06a |
| Male sex | 48 (45.7) | 52 (49.5) | 50 (47.2) | 0.86b |
| Smoking history | 35 (33.3) | 32 (30.5) | 35 (33.0) | 0.89b |
| Hypertension | 47 (44.8) | 46 (43.8) | 52 (49.1) | 0.72b |
| Diabetes mellitus | 33 (31.4) | 36 (34.3) | 35 (33.0) | 0.91b |
| Previous CVD | 15 (14.3) | 21 (20.0) | 30 (28.6) | 0.04b |
| Hemodialysis vintage (mo) | 72 (37–131) | 76 (36–120) | 61 (26–118) | 0.34c |
| Body mass index (kg/m2) | 22.1±3.4 | 22.3±3.1 | 22.1±4.2 | 0.87a |
| Systolic BP (mmHg) | 134±26 | 134±22 | 138±24 | 0.40a |
| Diastolic BP (mmHg) | 75±15 | 75±10 | 76±11 | 0.73a |
| RAS blockade | 27 (25.7) | 30 (28.6) | 29 (27.3) | 0.90b |
| Total no. of antihypertensives | 3 (0–4) | 3 (0–3) | 4 (0–5) | 0.67c |
| Statin | 16 (15.2) | 15 (14.3) | 19 (17.9) | 0.75b |
| Laboratory parameters | ||||
| Kt/V urea | 2.10±0.48 | 2.05±0.41 | 2.01±0.74 | 0.58a |
| albumin (g/dl) | 4.01±0.31 | 3.95±0.41 | 3.79±0.35 | <0.001a |
| hs-CRP (mg/L) | 3.75 (1.30–6.39) | 3.86 (1.52–8.24) | 5.70 (2.23–8.81) | 0.01c |
| IL-6 (pg/ml) | 2.79 (1.66–5.53) | 4.09 (2.20–4.09) | 5.36 (2.52–14.4) | <0.001c |
| ICAM-1 (ng/ml) | 431 (333–599) | 511 (385–738) | 668 (437–818) | 0.02c |
| VCAM-1 (ng/ml) | 1903 (1491–2391) | 2139 (1585–2798) | 2550 (1672–3771) | 0.04c |
| plasma glucose (mg/dl) | 113±28 | 112±29 | 110±29 | 0.90a |
| total cholesterol (mg/dl) | 193±48 | 192±45 | 192±47 | 0.90a |
| triglyceride (mg/dl) | 190 (182–248) | 188 (181–260) | 189 (184–237) | 0.88c |
| calcium (mg/dl) | 9.7±0.7 | 9.6±0.9 | 9.6±0.8 | 0.62a |
| phosphate (mg/dl) | 5.1±1.3 | 5.2±1.5 | 4.9±1.3 | 0.21a |
| intact PTH (pg/ml) | 254 (109–421) | 217 (94–530) | 202 (65–468) | 0.81c |
| hemoglobin (g/dl) | 10.6±1.5 | 10.6±1.6 | 10.3±1.6 | 0.30a |
| dose of epoetin (U/kg per week) | 71 (30–99) | 68 (33–94) | 71 (20–95) | 0.67c |
| ferritin (μg/L) | 288 (160–452) | 322 (182–492) | 346 (196–585) | 0.29c |
| transferrin saturation (%) | 25±12 | 25±11 | 26±14 | 0.68a |
All variables were expressed as n (%) for categorical data and as means ± SD or medians and interquartile ranges for continuous data with or without a normal distribution, respectively. Previous CVD category consisted of coronary artery disease, cerebrovascular disease, and peripheral arterial disease. DcR3, decoy receptor 3; CVD, cardiovascular disease; RAS, renin-angiotensin system; hs-CRP, high-sensitivity C-reactive protein; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; PTH, parathyroid hormone.
Statistical analysis by ANOVA.
Statistical analysis by the Pearson chi-squared test.
Statistical analysis by the Kruskal–Wallis test.
Figure 1.
DcR3 correlates with inflammatory markers. Univariate analysis of the correlation of DcR3 with (A) IL-6, (B) hs-CRP, (C) serum albumin, (D) ICAM-1, and (E) VCAM-1. Logarithmic transformation of DcR3, IL-6, and hs-CRP was used to normalize the distributions for univariate analyses. DcR3, decoy receptor 3; hs-CRP, high-sensitivity C-reactive protein; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1.
Table 2.
Multivariate regression models for the prediction of DcR3
| Parameter | Difference in DcR3 | SEM | P Value |
|---|---|---|---|
| Age (yr) | −0.004 | 0.013 | 0.75 |
| Sex (male) | 0.014 | 0.058 | 0.81 |
| Diabetes mellitus (presence) | 0.008 | 0.073 | 0.42 |
| Previous CVD (presence) | 0.165 | 0.061 | 0.04 |
| HD vintage (mo) | −0.009 | 0.018 | 0.69 |
| Kt/V urea | −0.036 | 0.021 | 0.28 |
| log IL-6 (pg/ml) | 0.248 | 0.071 | 0.26 |
| log ICAM-1 (ng/ml) | 0.134 | 0.072 | 0.07 |
| log VCAM-1 (ng/ml) | 0.202 | 0.096 | 0.01 |
| Albumin (g/dl) | −0.229 | 0.087 | 0.009 |
| Body mass index (kg/m2) | −0.016 | 0.009 | 0.52 |
The adjusted r2 of the model was 0.294. Logarithmic transformation of DcR3, IL-6, hs-CRP, ICAM-1 and VCAM-1 was used to normalize the distributions for multivariate analysis. DcR3, decoy receptor 3; CVD, cardiovascular disease; HD, hemodialysis; hs-CRP, high-sensitivity C-reactive protein; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1.
Association of DcR3 Levels with Outcome
During a median follow-up period of 54 months (interquartile range, 27–107), 33 patients who transferred to other dialysis units were followed using questionnaire forms completed by the attending physicians at the units. At the end of the follow-up period, 208 patients were confirmed to be alive on hemodialysis treatment, and 90 patients had died while being treated, 34 (37.7%) of which were deaths due to CVD-related causes.
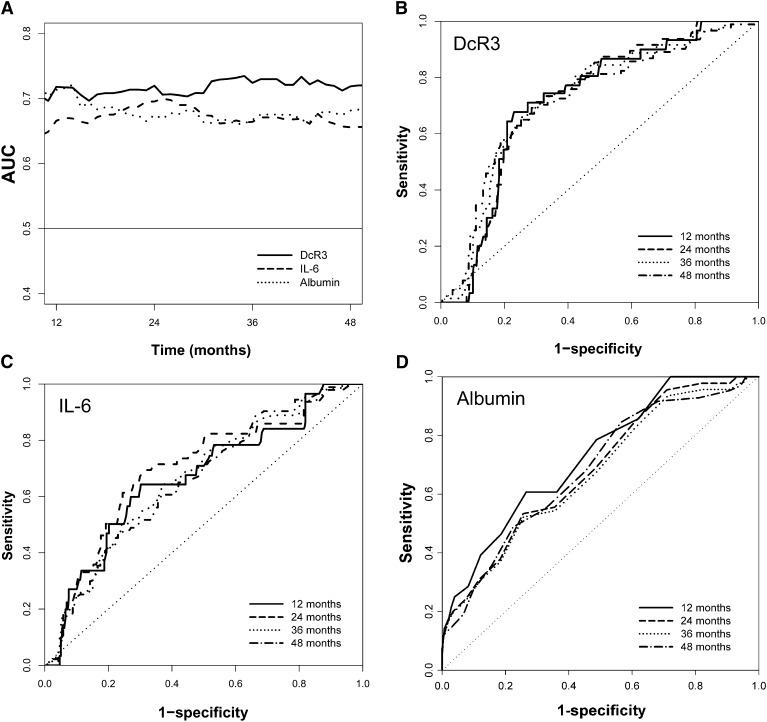
The AUCs of DcR3, IL-6, and albumin assessed by time-dependent ROC curve analysis showed consistently perfect predictive performance over the follow-up duration (Figure 2). The AUCs by stepwise addition of VCAM-1, albumin, IL-6, and DcR3 were all statistically significant for predicting mortality at 48 months (Table 3). However, the increase in the AUC from 0.79 to 0.80 was not substantial when DcR3 was added, indicating no additional predictive ability of DcR3 to the established risk factors.
Figure 2.
Predictive accuracy of DcR3, IL-6, and albumin estimated by using time-dependent ROC curve analysis. A shows the time-dependent AUCs for all of the markers and B (DcR3), C (IL-6), and D (albumin) show the ROC curves at different time periods. The AUCs at 36 months for DcR3, IL-6, and albumin were 0.74, 0.66 (P<0.01 versus DcR3), and 0.66 (P<0.01 versus DcR3), respectively. The AUCs at 48 month for DcR3, IL-6, and albumin were 0.73, 0.65 (P<0.01 versus DcR3), and 0.68 (P<0.05 versus DcR3), respectively. AUC, area under curve; DcR3, decoy receptor 3; ROC, receiver operating characteristic.
Table 3.
Prediction model of pertinent factors for mortality using AUC
| Variable | AUC |
|---|---|
| Traditional cardiovascular risk factors | 0.75 (0.70−0.80) |
| VCAM-1 | 0.76 (0.71−0.80) |
| VCAM-1 + albumin | 0.78 (0.74−0.83) |
| VCAM-1 + albumin + IL-6 | 0.79 (0.75−0.84) |
| VCAM-1 + albumin + IL-6 + DcR3 | 0.80 (0.75−0.84) |
Risk prediction was assessed by the C statistic. Each variable was stepwise added to assess the incremental change in AUC for predicting mortality at 48 months. Traditional cardiovascular risk factors included age, sex, smoking status, diabetes, total cholesterol, and systolic BP in the model. AUC, area under the ROC curve; VCAM-1, vascular cell adhesion molecule-1; DcR3, decoy receptor 3.
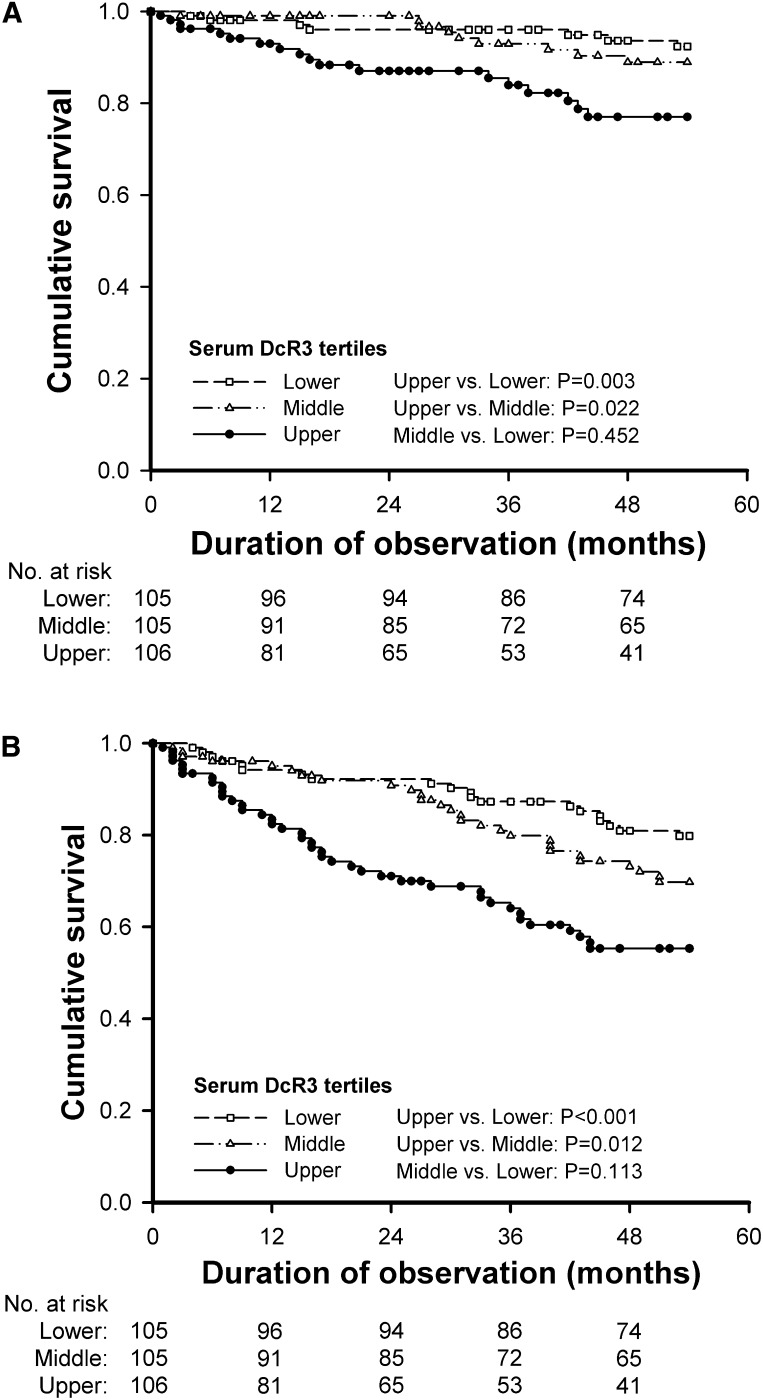
Kaplan–Meier survival curves corroborated the finding that higher DcR3 levels are associated with a lower probability of survival in hemodialysis patients (Figure 3). Patients were categorized according to tertiles of baseline serum DcR3 levels, and differences in the survival rates between patients in the upper versus middle tertiles and the upper versus lower tertiles were statistically significant. The association between DcR3 and cardiovascular or all-cause mortality was studied through univariate and multivariate Cox analysis (Table 4). A comparison of the upper versus lower tertiles of DcR3 levels showed that the crude HR was 3.6 for cardiovascular mortality (95% CI, 1.5–8.6; P for trend=0.002) and 2.9 for all-cause mortality (95% CI, 1.7–4.9; P for trend <0.001). The predictive value of DcR3 on cardiovascular mortality (HR, 2.8; 95% CI, 1.1–7.3; P for trend=0.04) and all-cause mortality (HR, 2.1; 95% CI, 1.1–3.7; P for trend=0.02) persisted after adjustment for various potential confounders. To further elucidate the contribution of elevated DcR3 levels, we performed Cox analyses with the log DcR3 level as a continuous variable. These analyses confirmed an increased risk of cardiovascular mortality (HR, 1.4; 95% CI, 1.1–2.1; P<0.05) and all-cause mortality (HR, 1.3; 95% CI, 1.1–1.7; P=0.04) per SD increase in log DcR3 concentration, respectively, after multivariate adjustment. Using the AIC for pertinent biomarkers (Table 5), DcR3 was independently associated with the outcomes but the prediction gain was minimal when DcR3 was added in the Cox regression model.
Figure 3.
Kaplan–Meier analysis curves in hemodialysis patients at risk for cardiovascular and all-cause mortality. All patients were stratified by the tertiles of baseline serum decoy receptor 3 (DcR3) to assess the unadjusted risks for (A) cardiovascular mortality and (B) all-cause mortality.
Table 4.
Multivariate Cox proportional hazards analysis for relative risk of cardiovascular and overall mortality calculated for DcR3 tertiles and for a 1-SD unit change in log DcR3 levels in a follow-up of 54 months
| Cardiovascular Mortality | All-Cause Mortality | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | Hazard Ratio (95% Confidence Interval) | |||||
| Crude | Age- and Sex-Adjusted | Multivariate Adjustment | Crude | Age- and Sex-Adjusted | Multivariate Adjustment | |
| DcR3 by tertiles | ||||||
| lower tertile | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| middle tertile | 1.4 (0.5−3.9) | 1.3 (0.4−3.6) | 1.3 (0.5−3.8) | 1.6 (0.9−2.8) | 1.5 (0.8−2.6) | 1.4 (0.9−2.5) |
| upper tertile | 3.6 (1.5−8.6) | 3.0 (1.2−7.2) | 2.8 (1.1−7.3) | 2.9 (1.7−4.9) | 2.5 (1.4−4.2) | 2.1 (1.1−3.7) |
| P for trend | 0.002 | 0.007 | 0.04 | <0.001 | 0.001 | 0.02 |
| Log DcR3 | ||||||
| 1-SD unit increase | 1.7 (1.2−2.6) | 1.6 (1.2−2.4) | 1.4 (1.1−2.1) | 1.7 (1.3−2.1) | 1.6 (1.2−2.0) | 1.3 (1.1−1.7) |
| P value | 0.007 | 0.03 | <0.05 | <0.001 | 0.001 | 0.04 |
Hazard ratios and 95% confidence intervals were derived from Cox regression analysis with DcR3 taken into account as a time-dependent covariate. The multivariate model included variables for age, sex, smoking status, diabetes, prior cardiovascular disease, body mass index, total cholesterol, systolic BP, hemodialysis duration, urea Kt/V, serum albumin, hemoglobin, IL-6, and vascular cell adhesion molecule-1. DcR3, decoy receptor 3.
Table 5.
Prediction gain by DcR3 over a model of traditional cardiovascular risk factors
| Multivariate Cox Hazards Model | HR (95% CI) | P Value | AICa | ΔAICb |
|---|---|---|---|---|
| Cardiovascular mortality | ||||
| cardiovascular risk factors | 335.5 | 0.0 | ||
| + VCAM-1 (1 SD unit) | 1.8 (0.7−4.4) | 0.15 | 346.7 | 11.2 |
| + albumin (1 g/L) | 0.9 (0.6−1.3) | 0.48 | 345.0 | 9.5 |
| + hs-CRP (1 SD unit) | 1.6 (0.9−2.5) | 0.05 | 341.4 | 5.9 |
| + IL-6 (1 SD unit) | 1.7 (1.1−2.7) | 0.01 | 339.3 | 3.8 |
| + DcR3 (1 SD unit) | 1.5 (1.1−2.4) | 0.03 | 341.5 | 6.0 |
| All-cause mortality | ||||
| cardiovascular risk factors | 872.5 | 0.0 | ||
| + VCAM-1 (1 SD unit) | 1.7 (1.0−3.1) | 0.03 | 889.6 | 17.1 |
| + albumin (1 g/dl) | 0.7 (0.5−0.9) | 0.01 | 882.7 | 10.2 |
| + hs-CRP (1 SD unit) | 1.3 (0.9−1.7) | 0.05 | 892.3 | 19.8 |
| + IL-6 (1 SD unit) | 1.6 (1.2−2.1) | 0.01 | 885.4 | 12.9 |
| + DcR3 (1 SD unit) | 1.5 (1.2−2.0) | <0.01 | 884.0 | 11.5 |
DcR3, decoy receptor 3; HR, hazard ratio; 95% CI, 95% confidence interval; AIC, Akaike Information Criterion; VCAM-1, vascular cell adhesion molecule-1; hs-CRP, high-sensitive C-reactive protein.
AIC (15) for a given model is a function of its maximized log-likelihood and the number of estimable parameters (K): AIC = −2 (maximized log-likelihood) + 2K. In the baseline Cox proportional hazards model, there were six traditional cardiovascular risk factors, including age, sex, smoking status, diabetes, total cholesterol, and systolic BP. VCAM-1, albumin, hs-CRP, IL-6, or DcR3 was added subsequently in order to assess the independent predictive contribution by DcR3.
ΔAIC is the difference between the AIC of baseline model and the AIC of VCAM-1, albumin, hs-CRP, IL-6, or DcR3, respectively. Therefore, the lower the ΔAIC, the higher prediction gain of pertinent biomarker.
Discussion
This study has elucidated DcR3 as a prognostic factor for hemodialysis patients. Serum levels of DcR3 are strongly and positively associated with inflammation and independently predict long-term cardiovascular and all-cause mortality.
DcR3 is a soluble receptor lacking a transmembrane domain that is capable of neutralizing the biologic effects of the following three members of the TNF superfamily: Fas ligand (FasL) (4), LIGHT (16), and TNF-like molecule 1A (17). Because FasL, LIGHT, and TNF-like molecule 1A play critical roles in apoptosis and inflammatory responses of immune cells, DcR3 can be defined as an immune suppressor on the basis of its neutralizing effects. In contrast, DcR3 also acts as an effector molecule that modulates proinflammatory responses via “nondecoy” activities (18). DcR3 induces NF-κB–mediated expression of ICAM-1, VCAM-1, and IL-8 by monocytes such that their binding to endothelial cells is enhanced (10). DcR3 may play a pathogenic role in the development of CVD in patients with CKD because increased expression of cell adhesion molecules and their ligands mediate the recruitment of inflammatory cells from the circulation and their trans-endothelial migration, both of which are essential processes in atherogenesis (11). This notion was supported by a strong positive association between the expression of DcR3 and adhesion molecules (ICAM-1 and VCAM-1) (Figure 1, D and E) in our study.
Chronic inflammation, as evidenced by the presence of proinflammatory cytokines, may cause malnutrition and atherosclerosis in the dialysis population. Cumulative evidence has indicated that IL-6 plays a key role in the pathogenesis of inflammation and atherosclerosis in dialysis patients (19). Therefore, it has been proposed that IL-6 is the best biomarker for stratifying risk in dialysis patients due to its strong link to clinical outcomes (20). In our study, DcR3 outperforms IL-6 in the prediction of long-term mortality of hemodialysis patients. Fong recently found that DcR3 expression is markedly upregulated by IL-6 via the JAK-STAT signaling pathway (21). Our data showed that serum DcR3 is highly correlated with IL-6 levels (Figure 1A), which may mean that DcR3 is also involved in the inflammatory cascades that accompany CKD. It is postulated that overexpression of DcR3 might be one missing link in explaining why IL-6 contributes to the development of CVD in patients with CKD (22). Furthermore, in this study, the predictive value of DcR3 for cardiovascular mortality persisted after adjustment for IL-6 and other proinflammatory cytokines, leading us to hypothesize that DcR3 might also associate with atherogenesis and increased mortality by yet uncharacterized non-proinflammatory mechanisms.
The origin of DcR3 in CKD patients is unknown, although studies have suggested that endothelium and bacterial antigen-stimulated immune cells can secrete DcR3 protein (23). In accordance with previous reports, our study confirms that serum concentrations of DcR3 are elevated in patients with CKD compared with those in healthy individuals (12). The increased DcR3 levels could be a result of increased DcR3 expression over the course of renal disease or decreased removal of the protein due to renal dysfunction. Increased DcR3 expression may have a protective role in preventing renal tissue damage because of its neutralizing effects on FasL-mediated apoptosis (8,24). On the other hand, DcR3 causes endothelial dysfunction by inducing expression of adhesion molecules and formation of cell aggregates (10,25). Taken together, these data suggest that increased DcR3 levels in CKD might have both a protective role at the renal level and a harmful role in the arterial wall.
DcR3 is almost undetectable in nonpathologic conditions. Recent studies have demonstrated that DcR3 expression is upregulated in inflammatory diseases, and serum DcR3 levels correlate with disease progression in these illnesses. Han et al. showed that serum DcR3 could serve as an additional parameter for diagnosing SLE and that DcR3 secreted from cells of hematopoietic origin was pathogenic for SLE in mice (26). In a recent study, Chen et al. demonstrated that high levels of circulating DcR3 correlate with the development of multiple organ failure and can independently predict 28-day mortality in patients with adult respiratory distress syndrome (27). CKD is an inflammatory process. Our study corroborates the findings that serum DcR3 is also a valuable marker for predicting the outcome of CKD patients on hemodialysis.
Some limitations of this study should be acknowledged. We acknowledge that the pathophysiology underlying various CVD outcomes may differ. However, we combined CVD events because we lacked sufficient power to examine specific events. Due to few cardiovascular events, the statistical power and the scope for multivariate adjustment in the Cox regression models were somewhat limited. Another limitation is the lack of data on fibroblast growth factor 23. Given that this has been strongly associated with mortality in hemodialysis patients (28), it would be important to know whether DcR3 adds predictive ability after including fibroblast growth factor 23. Kaplan–Meier curves are unadjusted and it is unclear if DcR3 is predictive for incident dialysis patients. We also cannot exclude the possibility of residual confounding. Finally, an observational study design cannot definitively examine whether biomarkers are causally related to clinical CVD. High DcR3 levels may act as a causal risk factor for atherosclerosis or may represent a compensatory response to atherosclerosis. Although it ultimately cannot be ruled out that high levels of DcR3 are entirely an epiphenomenon of inflammatory processes associated with CKD, a number of experimental studies suggest an active role of DcR3 in vascular pathology (10,25). DcR3 antagonists might provide new avenues for CVD therapy in CKD patients on hemodialysis. Recently, the crystal structures of the unliganded DcR3 ectodomain were reported, providing a mechanistic basis for the rational manipulation of specificity and affinity of DcR3 and its ligands (29).
In conclusion, DcR3 levels are elevated and have close associations with inflammation in hemodialysis patients. Serum DcR3 levels correlate with a clinical history of CVD and are an independent predictor of mortality. The association of DcR3 with mortality in hemodialysis patients may be explained, at least in part, by its proinflammatory effects. Further studies are needed to clarify the mechanism by which higher DcR3 concentrations are associated with CVD and whether therapeutic interventions to lower DcR3 concentrations are of clinical benefits in CKD patients on hemodialysis.
Disclosures
None.
Acknowledgments
We are deeply indebted to Ms. P.C. Lee for her expert secretarial assistance and graphic design.
This study was supported by grants from the National Science Council (NSC 93-2314-B010-032, NSC 94-2314-B010-033, and NSC 96-2628-B-010-001-MY3), Taipei Veterans General Hospital (V95S5-003, V96S5-004, and V97S5-004), National Yang-Ming University Hospital (RD2011-025), Bureau of Health Promotion, Department of Health (DOH98-HP-1110), and Ministry of Education’s aim for the Top University Plan.
Part of this work was presented in abstract form at the annual meeting of the American Society of Nephrology, November 16–21, 2010, Denver, Colorado.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Carrero JJ, Stenvinkel P: Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: A hypothesis proposal. Clin J Am Soc Nephrol 4[Suppl 1]: S49–S55, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K: Inflammatory marker mania in chronic kidney disease: Pentraxins at the crossroad of universal soldiers of inflammation. Clin J Am Soc Nephrol 2: 872–875, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW, Baldwin DT, Godowski PJ, Wood WI, Gurney AL, Hillan KJ, Cohen RL, Goddard AD, Botstein D, Ashkenazi A: Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 396: 699–703, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Salcedo TW, Wan X, Ullrich S, Hu B, Gregorio T, Feng P, Qi S, Chen H, Cho YH, Li Y, Moore PA, Wu J: Modulation of T-cell responses to alloantigens by TR6/DcR3. J Clin Invest 107: 1459–1468, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi G, Wu Y, Zhang J, Wu J: Death decoy receptor TR6/DcR3 inhibits T cell chemotaxis in vitro and in vivo. J Immunol 171: 3407–3414, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Chang YC, Hsu TL, Lin HH, Chio CC, Chiu AW, Chen NJ, Lin CH, Hsieh SL: Modulation of macrophage differentiation and activation by decoy receptor 3. J Leukoc Biol 75: 486–494, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Ka SM, Sytwu HK, Chang DM, Hsieh SL, Tsai PY, Chen A: Decoy receptor 3 ameliorates an autoimmune crescentic glomerulonephritis model in mice. J Am Soc Nephrol 18: 2473–2485, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Theroux P: Pathophysiology of coronary artery disease. Circulation 111: 3481–3488, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Yang CR, Hsieh SL, Ho FM, Lin WW: Decoy receptor 3 increases monocyte adhesion to endothelial cells via NF-kappa B-dependent up-regulation of intercellular adhesion molecule-1, VCAM-1, and IL-8 expression. J Immunol 174: 1647–1656, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Blankenberg S, Barbaux S, Tiret L: Adhesion molecules and atherosclerosis. Atherosclerosis 170: 191–203, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Zhang L, Kim S: Quantification and detection of DcR3, a decoy receptor in TNFR family. J Immunol Methods 285: 63–70, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Daugirdas JT: Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J Am Soc Nephrol 4: 1205–1213, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Heagerty PJ, Zheng Y: Survival model predictive accuracy and ROC curves. Biometrics 61: 92–105, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Posada D, Buckley TR: Model selection and model averaging in phylogenetics: Advantages of akaike information criterion and bayesian approaches over likelihood ratio tests. Syst Biol 53: 793–808, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Yu KY, Kwon B, Ni J, Zhai Y, Ebner R, Kwon BS: A newly identified member of tumor necrosis factor receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J Biol Chem 274: 13733–13736, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Migone TS, Zhang J, Luo X, Zhuang L, Chen C, Hu B, Hong JS, Perry JW, Chen SF, Zhou JX, Cho YH, Ullrich S, Kanakaraj P, Carrell J, Boyd E, Olsen HS, Hu G, Pukac L, Liu D, Ni J, Kim S, Gentz R, Feng P, Moore PA, Ruben SM, Wei P: TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity 16: 479–492, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Lin WW, Hsieh SL: Decoy receptor 3: A pleiotropic immunomodulator and biomarker for inflammatory diseases, autoimmune diseases and cancer. Biochem Pharmacol 81: 838–847, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Stenvinkel P, Barany P, Heimburger O, Pecoits-Filho R, Lindholm B: Mortality, malnutrition and atherosclerosis in ESRD: What is the role of interleukin-6? Kidney Int Suppl 80: 103–108, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Tripepi G, Mallamaci F, Zoccali C: Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: Searching for the best risk marker by multivariate modeling. J Am Soc Nephrol 16[Suppl 1]: S83–S88, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Fong PF: Elucidation of Signaling Pathways Involving in Interleukin-6 (IL-6) Induced Decoy Receptor 3 (DcR3) Expression in a Colorectal Cancer Cell Line [Master’s Thesis]. Taipei, National Yang-Ming University, 2009 [Google Scholar]
- 22.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V: Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis 148: 209–214, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Kim S, McAuliffe WJ, Zaritskaya LS, Moore PA, Zhang L, Nardelli B: Selective induction of tumor necrosis receptor factor 6/decoy receptor 3 release by bacterial antigens in human monocytes and myeloid dendritic cells. Infect Immun 72: 89–93, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortiz A, Lorz C, Justo P, Catalán MP, Egido J: Contribution of apoptotic cell death to renal injury. J Cell Mol Med 5: 18–32, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tateishi K, Miura Y, Hayashi S, Takahashi M, Kurosaka M: DcR3 protects THP-1 macrophages from apoptosis by increasing integrin α4. Biochem Biophys Res Commun 389: 593–598, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Han B, Bojalil R, Amezcua-Guerra LM, Springall R, Valderrama-Carvajal H, Wu J, Luo H: DcR3 as a diagnostic parameter and risk factor for systemic lupus erythematosus. Int Immunol 20: 1067–1075, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Chen CY, Yang KY, Chen MY, Chen HY, Lin MT, Lee YC, Perng RP, Hsieh SL, Yang PC, Chou TY: Decoy receptor 3 levels in peripheral blood predict outcomes of acute respiratory distress syndrome. Am J Respir Crit Care Med 180: 751–760, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhan C, Patskovsky Y, Yan Q, Li Z, Ramagopal U, Cheng H, Brenowitz M, Hui X, Nathenson SG, Almo SC: Decoy strategies: The structure of TL1A:DcR3 complex. Structure 19: 162–171, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]