Summary
Background and objectives
Intradialytic hypertension may be caused by an impaired endothelial cell response to hemodialysis. Carvedilol has been shown to improve endothelial cell function in vivo and to block endothelin-1 release in vitro. This study hypothesized that carvedilol would improve endothelial cell function and reduce the occurrence of intradialytic hypertension.
Design, setting, participants, & measurements
A prospective 12-week pilot study of carvedilol titrated to 50 mg twice daily was performed among 25 hemodialysis participants with intradialytic hypertension. Each patient served as his or her own control. Paired tests were used to analyze changes in BP and endothelial cell function—assessed by flow-mediated vasodilation, endothelial progenitor cells (aldehyde dehydrogenase bright activity and CD34+CD133+), asymmetric dimethylarginine, and endothelin-1—from baseline to study end.
Results
Flow-mediated vasodilation was significantly improved with carvedilol (from 1.03% to 1.40%, P=0.02). There was no significant change in endothelial progenitor cells, endothelin-1, or asymmetric dimethylarginine. Although prehemodialysis systolic BP was unchanged (144–146 mmHg, P=0.5), posthemodialysis systolic BP, 44-hour ambulatory systolic BP, and the frequency of intradialytic hypertension decreased with carvedilol (159–142 mmHg, P<0.001; 155–148 mmHg, P=0.05; and 77% [4.6 of 6] to 28% [1.7 of 6], P<0.001, respectively).
Conclusions
Among hemodialysis participants with intradialytic hypertension, targeting endothelial cell dysfunction with carvedilol was associated with modest improvements in endothelial function, improved intradialytic and interdialytic BP, and reduced frequency of intradialytic hypertension. Randomized controlled trials are required to confirm these findings.
Introduction
Intradialytic hypertension is a common complication of hemodialysis that is under-recognized and its significance is underappreciated (1). Our work has shown that intradialytic hypertension is independently associated with an increased risk of hospitalization and all-cause mortality (2–4). Our laboratory and others have shown that intradialytic hypertension is associated with severe impairments in endothelial cell (EC) function (5–7). However, it remains unknown if pharmacologic interventions targeting EC dysfunction can improve intradialytic hypertension.
Prior uncontrolled investigations have suggested that aggressive volume reduction may improve intradialytic increases in BP (8), but this approach is not effective in all individuals (1). Others have suggested that renin-angiotensin-aldosterone system (RAAS) inhibition may improve intradialytic hypertension (9), but studies with conflicting results fail to consistently support this hypothesis (6). Recent investigations have identified imbalances in EC-derived mediators of vascular function, including intradialytic increases in plasma endothelin-1 (ET-1) relative to nitric oxide among those with intradialytic hypertension (6,7). We have identified in vivo impairments in EC function among participants with intradialytic hypertension compared with controls, as measured by lower endothelial progenitor cells (EPCs) and impaired flow-mediated vasodilation (FMD) (5). Considering that EC dysfunction seems to be one of the primary mechanisms responsible for intradialytic hypertension and is independently predictive of adverse cardiovascular outcomes (10–13), we propose that targeting EC dysfunction may represent a novel therapeutic target.
Carvedilol has been shown to block ET-1 release in vitro and to improve EC function as measured by improved FMD in patients with diabetes and hypertension (14,15). Thus, among a cohort of prevalent hemodialysis patients with intradialytic hypertension, we performed a pilot study testing the hypothesis that carvedilol would be associated with improved EC function as measured by improved FMD and increased EPCs. We further hypothesized that carvedilol would reduce the occurrence of intradialytic hypertension.
Materials and Methods
Patient Eligibility and Enrollment
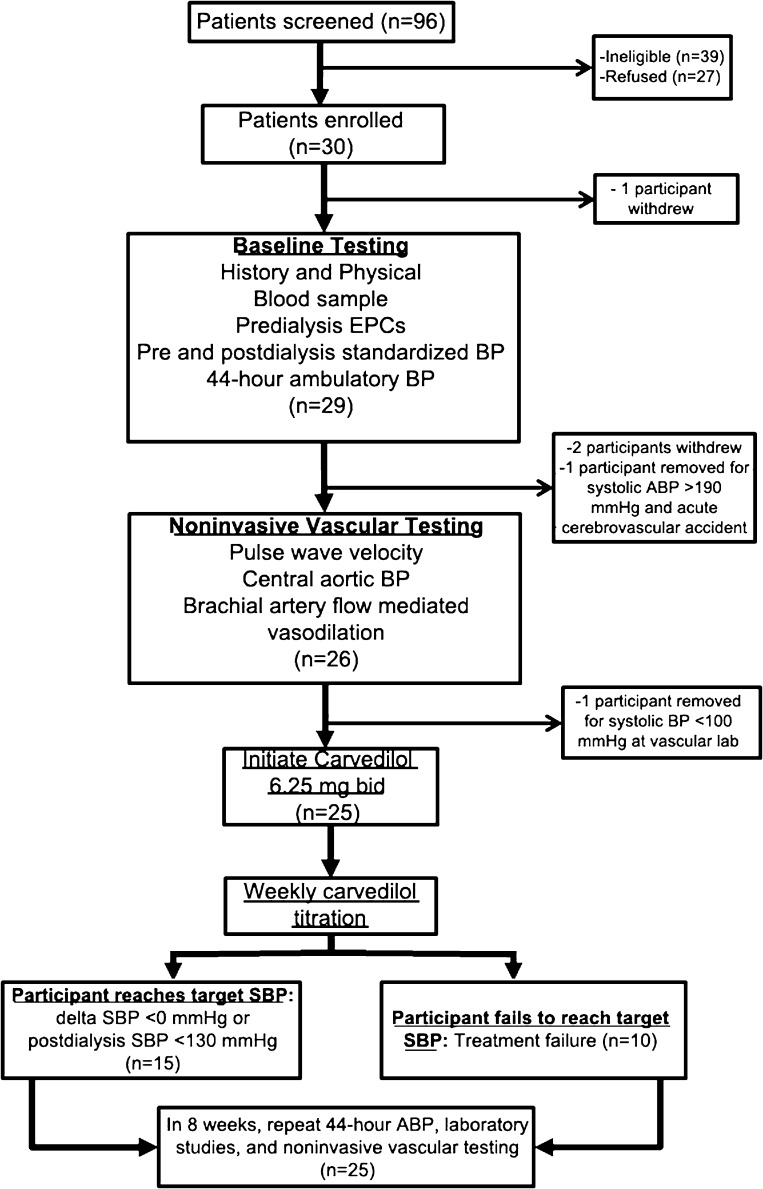
We prospectively enrolled hemodialysis patients with intradialytic hypertension into an open-label intervention study that sought to determine if carvedilol could improve EC function and intradialytic hypertension (Figure 1). Each patient served as his or her own control. Potential participants were screened using consecutive sampling from three hemodialysis facilities affiliated with the University of Texas Southwestern Medical Center in Dallas, Texas. We screened 96 patients for inclusion, 39 of which were ineligible, 27 refused participation, 30 enrolled, and 25 completed the study. Inclusion criteria were as follows: being on hemodialysis >30 days, age 18–80 years, ability to provide consent, patient deemed at their target dry weight, hypertension (predialysis systolic BP >140 mmHg or postdialysis systolic BP >130 mmHg), and the presence of intradialytic hypertension (defined as an increase in systolic BP from pre- to postdialysis ≥10 mmHg) occurring ≥4 of 6 consecutive hemodialysis sessions (2). Exclusion criteria were as follows: active neoplasm or wounds, BP unable to be measured by routine methods, planned kidney transplant or planned move, intolerance or contraindication to β or α blockers, active therapy with carvedilol, inability to safely administer nitroglycerin, life expectancy <6 months, or pregnancy.
Figure 1.
Study flow chart of participants with intradialytic hypertension in the Mechanisms and Treatment of Intradialytic Hypertension Study. EPC, endothelial progenitor cells; ABP, ambulatory BP; SBP, systolic BP.
Study Procedures
After providing informed consent, participants underwent a history and physical examination with a detailed review of their medical history and medications. To confirm that the screening BP pattern was consistent, up to 6 months of hemodialysis unit BP readings were reviewed. At baseline and monthly, we recorded dialysis unit laboratory results and concurrent dialysis prescription. At baseline and at least weekly, standardized BP readings (an average of three readings after 5 minutes of rest using an Omron HEM-907 monitor) were obtained by trained personnel before and after dialysis. After a mid-week hemodialysis session, blood was drawn before and after dialysis (see below) and participants underwent 44-hour ambulatory BP monitoring (Spacelabs 90207). The morning after the next mid-week hemodialysis session, participants were seen in our vascular laboratory for noninvasive vascular testing (see below).
Blood Sampling.
Blood was drawn from the arterial port immediately after cannulation of the vascular access. Blood was processed and sent locally for measurement of complete lipid panel, high-sensitivity C-reactive protein, albumin, hemoglobin, and sodium. A 10-ml sample of whole blood was shipped overnight in EDTA-containing vacutainers for EPC analyses (16). Plasma (taken before and after dialysis) was frozen at −80°C for measurement of ET-1 and asymmetric dimethylarginine (ADMA). At the study conclusion, ET-1 was quantitatively measured by commercially available ELISA kits (R&D Systems, Minneapolis, MN), with a detection limit of 0.02 pg/ml and intra- and interassay coefficients of variation of 2.6% and 4.6%, respectively. ADMA was measured by commercially available ELISA kits (Immundiagnostik AG, Germany), with a detection limit of 0.05 µmol/L and intra- and interassay coefficients of variation of 2.7% and 3.3%, respectively. ET-1 and ADMA were run in duplicate.
Measurement of EPCs.
The EPC number was assayed using flow cytometry based on cell surface expression of CD34+CD133+ aldehyde dehydrogenase bright (ALDHbr) activity (16,17). Briefly, PBMCs were isolated using density gradient centrifugation and were washed, and relative level of EPCs in the mononuclear cell population was determined. ALDHbr cells were identified using Aldecount as previously described (18). EPCs were also identified based on cell surface expression after incubation with CD133-PE (Miltenyi Biotec) and CD34-FITC (Becton Dickinson). After incubation and washing, cells were sorted by a Becton Dickinson FACS Caliber machine, analyzed using FlowJo software, and the percentage of EPCs in the mononuclear cell population was determined.
Vascular Laboratory Testing.
Brachial Artery Vasodilation.
Testing of brachial artery FMD and nitroglycerin-mediated vasodilation was per standard guidelines and per our previously published protocol (5,19). Briefly, the brachial artery diameter was measured at baseline and after 5 minutes of brachial artery occlusion. FMD was expressed as a percentage change in brachial artery diameter (from baseline) and was normalized for peak shear stress (20,21). After 10 minutes of rest, endothelium-independent vasodilation was determined by the maximal vasodilation after sublingual administration of nitroglycerin (0.4 mg).
Carotid-Femoral Pulse Wave Velocity.
Arterial tonometry and simultaneous electrocardiography was obtained from the brachial, radial, femoral, and carotid arteries using a pulse transducer device (Cardiovascular Engineering Inc, Waltham, MA). All data were digitized during the primary acquisition and analyzed in a blinded fashion. Waveforms from the carotid artery were used to measure central aortic BP. Pulse wave velocity (PWV) was measured as distance/time using the foot-to-foot method (22).
Study Follow-Up.
After baseline testing, all participants were instructed to stop β blocker and α blocker antihypertensive medications and were initiated on 6.25 mg twice daily of carvedilol without a washout period. Participants were seen three times per week and carvedilol was titrated weekly over 4 weeks to a maximum dosage of 50 mg twice daily until cessation of intradialytic hypertension (Δ systolic BP <0 during ≥2 consecutive hemodialysis sessions) or postdialysis systolic BP <130 mmHg ≥2 consecutive hemodialysis sessions (Figure 1). Throughout the study, standardized BP measurements taken before and after dialysis were used to target antihypertensive treatment. Frequency of intradialytic hypotension (systolic BP <90 during dialysis), symptomatic hypotension (intradialysis systolic BP <90 mmHg plus symptoms), and intradialytic bradycardia (heart rate of <50 during hemodialysis) was collected throughout the study. If a patient exhibited hypotension or bradycardia in the interdialytic period, the study drug was cut in half for 1 week; if there were no further complications, carvedilol was titrated the following week to the maximally tolerated dose. Any adverse events were recorded and reported to the primary investigator and the institutional review board. Participants who were hospitalized during the study were allowed to complete the study unless comorbid conditions warranted otherwise. Participants’ dialysis prescription remained unchanged throughout the study period. At the end of the 4-week titration period, participants were maintained on the highest tolerated dose of carvedilol and underwent repeat testing after 8 weeks on the maintenance dose.
Endpoints
The primary endpoints were changes in EC function measured in vivo by changes in FMD and by EPCs. Secondary endpoints included changes in plasma ET-1, ADMA, ambulatory BP, PWV, and frequency of intradialytic hypertension.
Power Calculations
On the basis of our previously published data (18), we determined that we needed 25 participants to provide 86% power to demonstrate a 22% increase in FMD, 85% power to detect a 40% increase in ALDHbr cells, and 90% power to detect a 60% increase in CD34+CD133+ cells from before therapy to after therapy with a correlation of 0.6 and a two-sided α of 0.05.
Statistical Analyses
Changes in EC function (FMD and EPCs) from baseline to the study end were determined by paired t tests or Wilcoxon matched-pairs signed rank tests. Paired tests were also used to determine changes in BP, laboratory variables, PWV, and frequency of intradialytic hypertension. All analyses were performed with SAS 9.2 software (SAS Institute, Cary, NC). Exploratory analyses were performed comparing changes in markers of EC function, arterial stiffness, and BP between participants with resolution of intradialytic hypertension (Δ systolic BP <0 mmHg based on 2-week BP average at the study end) and those with persistent intradialytic increases in BP (Δ systolic BP >0 mmHg). This study was approved by the institutional review board at the University of Texas Southwestern and is registered with ClinicalTrials.gov (NCT00827775). All procedures were in accordance with the Declaration of Helsinki.
Results
Baseline Characteristics
Study participants had an average age of 53.9 years, and 80% were male, 36% were black, and 64% were Hispanic (Table 1). Comorbid conditions were prevalent, and 88% of participants had diabetes and 32% had cardiovascular disease. Antihypertensive utilization was high among participants, and 64% were taking either an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker or both and 68% were taking a β-blocker before enrollment.
Table 1.
Baseline characteristics of 25 participants with intradialytic hypertension enrolled into the Mechanisms and Treatment of Intradialytic Hypertension studya
| Characteristic | Intradialytic Hypertension (n=25) |
|---|---|
| Age (yr) | 53.9 (±11.1) |
| Male sex | 20 (80) |
| Black race versus other | 9 (36) |
| Hispanic ethnicity | 16 (64) |
| Current tobacco use | 5 (20) |
| Dialysis duration <1 yr | 9 (36) |
| Comorbid conditions | |
| diabetes mellitus | 22 (88) |
| cardiovascular diseaseb | 8 (32) |
| congestive heart disease | 4 (16) |
| hypertension | 25 (100) |
| autoimmune disorder | 2 (8) |
| atrial flutter or fibrillation | 0 (0) |
| Antihypertensive medications | |
| ether ACE-I or ARB or both | 16 (64) |
| β blocker (including labetalol) | 17 (68) |
| calcium channel blocker | 15 (60) |
| clonidine | 8 (32) |
| hydralazine | 6 (24) |
| nitroglycerin or isosorbide | 0 (0) |
| Other medications | |
| aspirin | 10 (40) |
| cinacalcet HCL | 6 (24) |
| erythropoiesis-stimulating agents | 22 (88) |
| intravenous iron | 22 (88) |
| vitamin D analogs | 21 (84) |
| calcium-containing phosphorus binder | 14 (56) |
| noncalcium-containing phosphorus binder | 11 (44) |
| cholesterol lowering agents | 12 (48) |
| clopidogrel | 2 (8) |
| phosphodiesterase type 5 inhibitors | 2 (8) |
Values are reported as mean ± SD or n (%). ACE-I angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Compared with patients who refused participation (n=27), participants were slightly younger (53.9 versus 56.6 years) and more likely to be male (80% versus 44%).
Either coronary artery disease, cerebrovascular disease, or peripheral vascular disease.
Changes in EC Function, Endothelin-1, and Arterial Stiffness
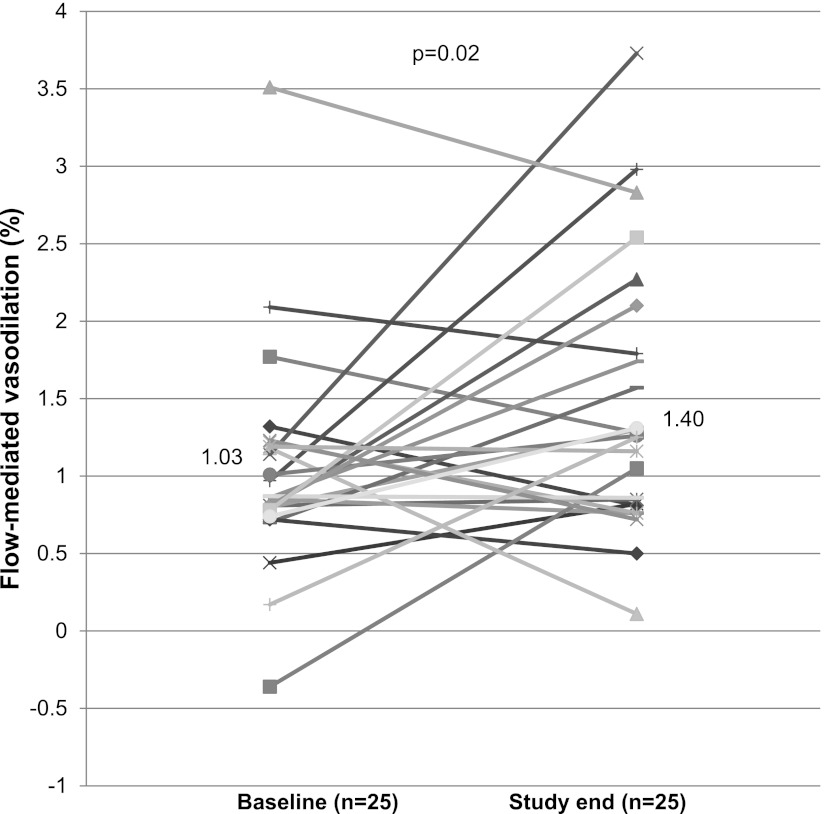
At baseline, participants had impaired (endothelial-dependent) flow-mediated vasodilation that significantly improved after 8 weeks of maximally tolerated carvedilol (from 1.03%±0.70% to 1.40%±0.84, P=0.02) (Figure 2 and Table 2). As expected, nitroglycerin-mediated (endothelial-independent) vasodilation was unchanged with carvedilol therapy. Peripheral blood markers of potential EPCs, including ALDHbr and CD34+CD133+ cells, were low at baseline and did not change significantly during the study (Table 2). There was no significant change in either prehemodialysis or posthemodialysis plasma ET-1 levels with carvedilol. However, the intradialytic rise in plasma ET-1, while significant at baseline (from 4.97±2.01 prehemodialysis to 5.98±2.94 posthemodialysis, P=0.05), was abolished with carvedilol (from 5.57±2.25 to 5.64±2.91, P=0.8). However, the change in the change in intradialytic ET-1, while improved, did not reach statistical significance (P=0.1). There was no significant change in either plasma ADMA levels or PWV with carvedilol.
Figure 2.
Change in individual brachial artery flow-mediated vasodilation from baseline to study. Mean flow-mediated vasodilation at baseline was 1.03, which improved to 1.40 by study end (P=0.02). Each marker and line represent an individual participant in the study.
Table 2.
Changes in endothelial-dependent flow-mediated vasodilation, endothelial-independent brachial artery vasodilation, endothelial progenitor cells and pulse wave velocity among 25 participants with intradialytic hypertension before and after 8 weeks on maximally tolerated dose of carvedilol
| Baseline (n=25) | Study End (n=25) | Change from Baseline to Study End | P Value | |
|---|---|---|---|---|
| Endothelial-dependent FMD (%)a | 1.03 (±0.70) | 1.40 (±0.84) | 0.47 (±0.93) | 0.02 |
| Endothelial-independent nitroglycerin-mediated vasodilation (%) | 6.65 (±4.59) | 7.30 (±6.80) | 0.71 (±5.0) | 0.5 |
| Endothelial progenitor cell markers | ||||
| ALDHbr (% of mononuclear cells) | 0.034 (0.017, 0.080) | 0.027 (0.013, 0.048) | −0.004 (−0.03, 0.014) | 0.4 |
| CD34+CD133+ (% of mononuclear cells) | 0.033 (0.016, 0.051) | 0.029 (0.019, 0.049) | −0.001 (−0.028, 0.012) | 0.9 |
| PWV (m/s) | 11.8 (±3.1) | 11.6 (±3.2) | −0.45 (±2.04) | 0.3 |
| ET-1 (pg/ml) | ||||
| prehemodialysis | 4.97 (±2.01) | 5.57 (±2.25) | 0.20 (±1.81) | 0.6 |
| posthemodialysis | 5.98 (±2.94) | 5.64 (±2.91) | −0.80 (±2.78) | 0.2 |
| Δ hemodialysis (posthemodialysis–prehemodialysis) | 1.01 (±2.32)a | 0.07 (±2.02)b | −1.00 (±3.74) | 0.1 |
| ADMA (µmol/L) | 0.68 (±0.18) | 0.65 (±0.17) | −0.04 (±0.14) | 0.2 |
Values are reported as mean ± SD or median and 25th to 75th interquartile range. Vasodilation units are the percentage of dilation and endothelial progenitor cells are the percentage of mononuclear cells. P values determined from paired t tests or nonparametric signed rank test. FMD was normalized for peak shear rate and calculated as follows: (FMD/peak shear rate) × 100 (20). Two PWV measurements were unable to be performed for technical reasons, two patients had inadequate samples for ET-1, and four patients had inadequate samples for ADMA. FMD, flow-mediated vasodilation; PWV, pulse wave velocity; ALDHbr, aldehyde dehydrogenase activity; ET-1, endothelin-1; ADMA, asymmetric dimethylarginine.
P=0.03 for change in ET-1 from prehemodialysis to posthemodialysis at baseline.
P=0.9 for change in ET-1 from prehemodialysis to posthemodialysis at study end.
Changes in BP
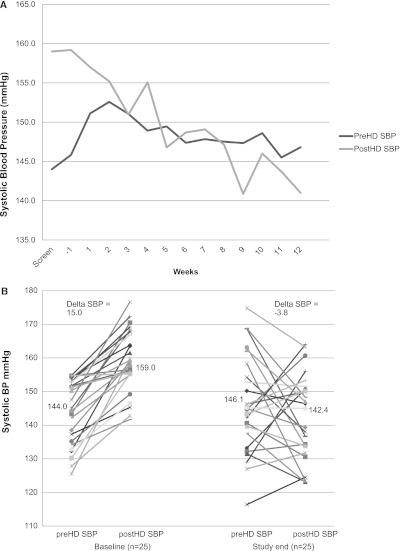
At baseline, 77% (4.6 of 6) of hemodialysis sessions were complicated by intradialytic hypertension that improved to 28% (1.7 of 6) at study end (P<0.001.) Screening dialysis unit BP and subsequent weekly average standardized BP measurements are shown in Figure 3A. Individual 2-week average intradialytic changes in systolic BP from baseline and study end are shown in Figure 3B and Table 3. As illustrated, 15 participants had resolution of intradialytic hypertension and 10 participants continued to exhibit an increase in systolic BP from predialysis to postdialysis. Overall, although predialysis systolic BP was unchanged, postdialysis systolic BP significantly decreased. Diastolic BP was not significantly changed during the study. The 2-week average Δ systolic BP (posthemodialysis–prehemodialysis) at baseline was +15±9.1 mmHg and declined to −3.8±16.1 mmHg at study end (P<0.001). Systolic and diastolic 44-hour ambulatory BP was also improved with carvedilol.
Figure 3.
Changes in systolic BP during the Mechanisms and Treatment of Intradialytic Hypertension (MATCH) Study. (A) Two-week screening hemodialysis unit systolic BP and subsequent weekly average standardized predialysis and postdialysis systolic BP during the course of the study. (B) Change in individual 2-week average predialysis and postdialysis systolic BP from baseline to study end. Mean predialysis systolic BP was unchanged from baseline (144–146.1 mmHg, P=0.5), whereas mean postdialysis systolic BP was significantly reduced (from 159 to 142.4 mmHg, P<0.001). PreHD SBP, prehemodialysis systolic BP; postHD SBP, posthemodialysis systolic BP.
Table 3.
BP measurements before and after 8 weeks of maximally tolerated carvedilol among 25 participants with intradialytic hypertension
| Baseline (n=25) | Study End (n=25) | Mean Change from Baseline to Study end | P Value | |
|---|---|---|---|---|
| 2-wk frequency of intradialytic hypertension (%) | 77% (4.6 of 6 sessions) | 28% (1.7 of 6 sessions) | 49% (2.9 of 6 sessions) | <0.001 |
| 2-wk average BP (mmHg)a | ||||
| predialysis systolic | 144.0 (±9.7) | 146.1 (±14.4) | 2.2 (±16.9) | 0.5 |
| postdialysis systolic | 159.0 (±9.3) | 142.4 (±12.7) | −16.7 (±16.7) | <0.001 |
| predialysis diastolic | 77.5 (±9.2) | 75.9 (±8.9) | −1.6 (±9.6) | 0.4 |
| postdialysis diastolic | 80.9 (±7.6) | 72.5 (±23.9) | −8.4 (±22.8) | 0.08 |
| Δ systolic (postdialysis–predialysis) | +15.0 (±9.1) | −3.8 (±16.1) | −18.8 (±16.5) | <0.001 |
| Ambulatory BP (mmHg) | ||||
| systolic (44-hr) | 155.4 (±14.2) | 147.7 (±16.2) | −7.5 (±16.8) | 0.04 |
| daytime systolic | 155.7 (±14.9) | 146.9 (±15.9) | −8.2 (±18.5) | 0.04 |
| nighttime systolic | 155.6 (±16.4) | 149.8 (±19.7) | −4.1 (±18.2) | 0.3 |
| diastolic (44-hr) | 82.4 (±10.8) | 77.7 (±9.7) | −4.2 (±7.7) | 0.01 |
| daytime diastolic | 83.2 (±11.8) | 77.6 (±9.7) | −4.7 (±8.8) | 0.02 |
| nighttime diastolic | 80.9 (±10.6) | 77.0 (±11.1) | −2.8 (±7.3) | 0.09 |
| Central aortic BP (mmHg) | ||||
| systolic | 153.7 (±30.8) | 147.3 (±34.8) | −6.4 (±25.7) | 0.2 |
| diastolic | 76.1 (±11.5) | 75.6 (±12.4) | −0.5 (±10.2) | 0.8 |
Values are reported as mean ± SD or n (%). P values determined from paired t tests
Values are 2-week averages of BP from the six hemodialysis sessions before intervention and the six hemodialysis sessions at study end.
Trends in Differences in EC Function, Arterial Stiffness, and BP among Participants with and without Resolution of Intradialytic Hypertension
Among the 15 participants with resolution of intradialytic hypertension, there was a significant increase in FMD from baseline to study end and either no change in EPCs or modest increases (Table 4). Although ET-1 levels overall were not significantly different, those with resolution of intradialytic hypertension exhibited an overall decline in ET-1 from before hemodialysis to after hemodialysis at study end. Furthermore, plasma ADMA levels declined among those with resolution of intradialytic hypertension. Among those with persistent increases in systolic BP during hemodialysis, FMD nonsignificantly increased and both EPC markers declined during the study. Finally, the intradialytic rise in ET-1 persisted throughout the study and ADMA did not change among participants who continued to exhibit increases in BP during hemodialysis. There was no difference in dry weight or dry weight management between those that improved and those who had persistent intradialytic hypertension.
Table 4.
Changes in endothelial cell function, arterial stiffness, and BP among those with and without resolution of intradialytic hypertension
| Baseline | Study End | Mean Change from Baseline to Study End | P Value | |
|---|---|---|---|---|
| Resolution of intradialytic hypertension (n=15) | ||||
| endothelial-dependent FMD (%) | 1.00 (±0.78) | 1.37 (±0.91) | 0.55 (±0.96) | 0.05 |
| endothelial progenitor cell markers | ||||
| ALDHbr (% of mononuclear cells) | 0.026 (0.013, 0.093) | 0.027 (0.012, 0.051) | 0.001 (−0.048, 0.016) | 0.6 |
| CD34+CD133+ (% of mononuclear cells) | 0.033 (0.016, 0.076) | 0.042 (0.016, 0.049) | 0.001 (−0.014, 0.012) | 0.9 |
| ET-1 (pg/ml) | ||||
| prehemodialysis | 4.76 (±1.52) | 5.20 (±1.67) | 0.36 (±2.02) | 0.5 |
| posthemodialysis | 5.79 (±2.94) | 4.87 (±2.34) | −1.02 (±3.40) | 0.3 |
| Δ hemodialysis | 1.04 (±2.83) | −0.34 (±1.90) | −1.38 (±4.45) | 0.3 |
| asymmetric dimethylarginine (µmol/L) | 0.70 (±0.19) | 0.63 (±0.19) | −0.08 (±0.15) | 0.07 |
| pulse wave velocity (m/s) | 12.4 (±3.5) | 11.8 (±3.0) | −0.6 (±2.2) | 0.3 |
| ambulatory BP (mmHg) | ||||
| systolic | 159.3 (±13.8) | 152.4 (±15.4) | −6.9 (±20.0) | 0.2 |
| diastolic | 84.1 (±9.8) | 78.4 (±7.7) | −5.0 (±9.6) | 0.07 |
| target dry weight | 81.5 (±21.7) | 81.3 (±22.9) | −0.1 (±3.9) | 0.9 |
| Persistent intradialytic hypertension (n=10) | ||||
| endothelial-dependent FMD (%) | 1.06 (±0.58) | 1.43 (±0.77) | 0.37 (±0.94) | 0.2 |
| endothelial progenitor cell markers | ||||
| ALDHbr (% of mononuclear cells) | 0.045 (0.031, 0.058) | 0.031 (0.016, 0.045) | −0.006 (−0.029, −0.001) | 0.5 |
| CD34+CD133+ (% of mononuclear cells) | 0.035 (0.019, 0.05) | 0.025 (0.019, 0.049) | −0.006 (−0.034, −0.022) | 0.8 |
| ET-1 (pg/ml) | ||||
| prehemodialysis | 5.26 (±2.57) | 6.19 (±3.06) | 0.09 (±1.46) | 0.8 |
| posthemodialysis | 6.22 (±3.09) | 6.96 (±3.50) | 0.42 (±1.28) | 0.4 |
| Δ hemodialysis | 0.96 (±1.57) | 0.77 (±2.17) | −0.33 (±2.23) | 0.7 |
| ADMA (µmol/L) | 0.65 (±0.15) | 0.70 (±0.14) | 0.03 (±0.10) | 0.4 |
| PWV (m/s) | 10.7 (±2.20) | 11.3 (±3.55) | 0.1 (±1.9) | 0.8 |
| ambulatory BP (mmHg) | ||||
| systolic | 149.5 (±13.3) | 141.1 (±15.6) | −8.4 (±11.7) | 0.05 |
| diastolic | 79.8 (±12.3) | 76.7 (±12.4) | −3.1 (±4.0) | 0.04 |
| target dry weight | 82.1 (±18.2) | 81.9 (±18.3) | −0.2 (±0.9) | 0.5 |
Values are reported as mean ± SD or medians and 25th to 75th interquartile ranges. Vasodilation units are the percentage of dilation and endothelial progenitor cells are the percentage of mononuclear cells. P values determined from paired t tests or nonparametric signed rank. FMD was normalized for peak shear rate and calculated as (FMD/peak shear rate) × 100 (21). Two PWV measurements were unable to be performed for technical reasons, two patients had inadequate samples for ET-1, and four patients had inadequate samples for ADMA. FMD, flow-mediated vasodilation; ALDHbr, aldehyde dehydrogenase activity; ET-1, endothelin-1; ADMA, asymmetric dimethylarginine; PWV, pulse wave velocity.
Changes in Laboratory Parameters and Weight
There were no significant changes in serum albumin, high-sensitivity C-reactive protein, hemoglobin, serum sodium, or cholesterol levels throughout the study period (Table 5). Target dry weight and intradialytic weight loss remained unchanged throughout the study (Table 5).
Table 5.
Laboratory variables and weight parameters at baseline and after 8 weeks of maximally tolerated dose of carvedilol among 25 participants with intradialytic hypertension
| Baseline (n=25) | Study End (n=25) | Mean Change from Baseline to Study End | P Value | |
|---|---|---|---|---|
| Albumin (g/dl) | 3.8 (±0.55) | 3.8 (±0.35) | −0.01 (±0.30) | 0.8 |
| High-sensitivity C-reactive protein (mg/L) | 5.2 (1.5, 11.6) | 3.3 (1.5, 8.6) | −0.1 (−7.5, 0.7) | 0.6 |
| Hemoglobin (g/dl) | 12.3 (±1.1) | 12.2 (±1.0) | −0.16 (±1.5) | 0.6 |
| Serum sodium (mEq/L) | 135.8 (±3.7) | 136.1 (±3.5) | −0.2 (±2.6) | 0.7 |
| Total cholesterol (mg/dl) | 133.8 (±23.1) | 131.1 (±25.8) | −2.8 (±16.2) | 0.4 |
| LDL cholesterol (mg/dl) | 65.6 (±23.4) | 62.4 (±24.0) | −3.2 (±17.2) | 0.4 |
| HDL cholesterol (mg/dl) | 43.5 (±14.0) | 44.4 (±13.1) | 0.9 (±9.1) | 0.6 |
| Target dry weight, 2-wk average (kg) | 81.7 (±20.0) | 81.6 (±20.8) | −0.2 (±3.1) | 0.8 |
| Intradialytic weight loss, 2-wk average (kg) | 2.8 (±1.0) | 3.0 (±1.2) | −0.2 (±1.0) | 0.3 |
Values are reported as mean ± SD or medians and 25th to 75th interquartile ranges. P value determined from paired t tests or nonparametric signed rank tests.
Study and Drug Tolerability
Eighteen participants tolerated 50 mg twice daily of carvedilol, two tolerated 37.5 mg twice daily, one tolerated 25 mg twice daily, and two tolerated 12.5 mg twice daily. During the course of the study, 0.1% (1 of 920) of sessions were complicated by transient intradialytic bradycardia, 9.0% (83 of 920) were complicated by an intradialytic drop in systolic BP <90 mmHg, and 2.4% (22 of 920) were complicated by symptomatic intradialytic hypotension. In addition, one participant was hospitalized briefly with gastroparesis exacerbation, one participant was hospitalized for an access revision, one participant was hospitalized with pneumonia, and two participants were hospitalized for management of fluid removal due to large interdialytic weight gain. There were no deaths during the study.
Discussion
The principal finding in this study is that carvedilol administered at the maximum dose tolerated (not exceeding 50 mg twice daily) is associated with significant improvements in vivo in EC function in hemodialysis patients. Our study also identified carvedilol to be associated with a reduction in intradialytic BP, with lower interdialytic ambulatory BP, and with a reduced frequency of intradialytic hypertension. Thus, this study supports evidence from our laboratory and others that EC dysfunction may play a primary role in intradialytic hypertension and introduces new evidence that targeted pharmacologic therapy is associated with improvements in both EC function and intradialytic hypertension. Considering that we previously showed that intradialytic hypertension is associated with adverse clinical outcomes (2–4), carvedilol may represent a simple therapeutic option for these high-risk patients. However, this must be tested in randomized controlled trials.
In our study, we observed a 47% improvement in flow-mediated vasodilation after 8 weeks of maximally tolerated carvedilol among participants with intradialytic hypertension. Prior studies in nondialysis patients with diabetes have shown that carvedilol improves FMD in vivo (15). Our study extends these findings and is the first to show the association between carvedilol with improvements in EC function in hemodialysis patients. Although we identified improvements in vivo in EC function, we failed to identify significant improvements in circulating markers of potential EPC. It is plausible that although carvedilol improved in vivo EC function as measured by improved FMD, the study follow-up was not long enough to identify bone marrow regeneration of reparative EPCs (23,24).
This study provides additional evidence for the relationship between EC dysfunction and intradialytic hypertension. Recent studies into the pathogenesis of intradialytic hypertension identified intradialytic imbalances in ET-1 relative to nitric oxide (6,7). In our recent case-control study, we identified lower predialysis EPCs and decreased FMD among patients with intradialytic hypertension compared with control hemodialysis patients (5). Thus, EC dysfunction is not only a prevalent finding in patients with intradialytic hypertension, but may also be a mechanism for the increases in BP during hemodialysis. Carvedilol has previously been shown to suppress ET-1 release in vitro in cultured human ECs (14). In this study, carvedilol was associated with improved FMD but was not significantly associated with improvements in ET-1. Compared with patients with persistent intradialytic increases in systolic BP, those with resolution of intradialytic hypertension exhibited improvements in markers of EC function, although this study was not powered to identify significant treatment effects within subgroups. Taken together, these findings support the hypothesis that EC dysfunction may be a key mechanism responsible for intradialytic hypertension.
In this study, carvedilol was associated with significant improvements in the frequency of intradialytic hypertension (from 77% to 28%). Prior small uncontrolled studies have suggested that either dry weight reduction or captopril may improve intradialytic hypertension (8,9). We did not lower dry weight because these patients had previously failed lowering of dry weight as a therapeutic option. In addition, we did not select RAAS inhibitors as a study drug because recent studies failed to show evidence of intradialytic RAAS activation among patients with intradialytic hypertension (6). On the basis of pathophysiologic evidence supporting EC dysfunction as contributing to intradialytic hypertension, carvedilol was selected based on evidence that carvedilol (but not other β blockers) improves EC function (14,15). Our study showed that carvedilol was associated with both improved EC function and a reduced frequency of intradialytic hypertension. However, it is possible that intensification of other antihypertensive agents could have resulted in similar improvements in postdialysis BP. Thus, randomized studies are required to confirm these findings.
Carvedilol was also associated with improvements in interdialytic ambulatory BP. In an uncontrolled study of eight hemodialysis patients, supervised administration of atenolol postdialysis was associated with a 17 mmHg decline in systolic ambulatory BP (25). In our study, the use of carvedilol was associated with a 7.7 mmHg additional reduction in systolic ambulatory BP. This occurred in the context of 68% of patients already taking a β blocker, thus the BP lowering was additive to what the patient exhibited with other β blockers. Although carvedilol lowered ambulatory BP, it is unknown if this improvement partially explains the observed improvement in FMD. In a study among 37 hypertensive non-ESRD patients with diabetes, carvedilol, but not metoprolol, improved FMD despite similar changes in BP (15). Although we found no significant differences in ambulatory BP among those who did and did not exhibit resolution of intradialytic hypertension, it remains to be determined if the observed improvements in EC function were unique to carvedilol or partially attributable to the overall improvements in BP.
Although our study identified novel associations between carvedilol and improvements in EC function and intradialytic hypertension, this study is not without limitations. This study did not include a placebo arm. We chose to use each patient as his or her own control given the known interindividual variability in FMD. A placebo controlled trial would require 175 patients per arm to be adequately powered to identify the predicted 22% differences in FMD. Given our study design, we cannot exclude the possibility that identified improvements were due to chance or changes in extracellular volume. Although undetected improvements in extracellular volume status could explain the observed reduction in ambulatory BP, the natural time course of EC dysfunction is that it worsens, not improves. In this pilot study, we sought first to probe whether carvedilol was associated with changes in EC function and to explore whether targeting EC dysfunction could improve BP patterns. This study was necessary to generate hypotheses and to provide data for planning for a larger trial. We acknowledge that there is an increased risk for a type II error with a small sample size and that there is an increased risk of a type I error with multiple testing. Thus, a randomized study design will be required to confirm these findings.
In summary, our study identified that carvedilol up to 50 mg twice daily is associated with modest improvements in EC function in vivo measured by increased FMD. We also found that carvedilol was associated with improved interdialytic BP and reduced frequency of intradialytic hypertension. Considering that we previously identified an association of intradialytic hypertension with increased morbidity and mortality as well as with impaired EC function (2–5,26), cause-targeted agents, such as carvedilol, may represent a simple therapeutic option in this high-risk patient population. However, randomized controlled trials are required to confirm these findings.
Disclosures
None.
Acknowledgments
We thank our study coordinators, Yvonne Gordon (deceased) and Gloria Williams, for their work recruiting and enrolling patients, as well as assisting the DaVita staff and personnel. We also thank our vascular laboratory technician, Zhongyun Wang, for performing FMD and PWV as well as our laboratory technician, Jessica Lucas, for performing ELISA.
This research was supported by the University of Texas Southwestern O’Brien Kidney Research Core (National Institutes of Health [NIH] Grant P30DK079328), a NIH University of Texas Southwestern Clinical Translational Science Award (NIH UL1RR024982), and NIH Grants K23 HL092297 (J.K.I.), F32DK085965 (P.V.B.), and 5K24DK002818 (R.T.).
This work was presented in part as an oral communication at the 26th Annual Meeting of the American Society of Hypertension, May 22, 2011, in New York, New York.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Inrig JK: Intradialytic hypertension: A less-recognized cardiovascular complication of hemodialysis. Am J Kidney Dis 55: 580–589, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inrig JK, Oddone EZ, Hasselblad V, Gillespie B, Patel UD, Reddan D, Toto R, Himmelfarb J, Winchester JF, Stivelman J, Lindsay RM, Szczech LA: Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int 71: 454–461, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inrig JK, Patel UD, Toto RD, Reddan DN, Himmelfarb J, Lindsay RM, Stivelman J, Winchester JF, Szczech LA: Decreased pulse pressure during hemodialysis is associated with improved 6-month outcomes. Kidney Int 76: 1098–1107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inrig JK, Patel UD, Toto RD, Szczech LA: Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: A secondary analysis of the Dialysis Morbidity and Mortality Wave 2 Study. Am J Kidney Dis 54: 881–890, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inrig JK, Van Buren P, Kim CM, Vongpatanasin W, Povsic TJ, Toto RD: Intradialytic hypertension and its association with endothelial cell dysfunction. Clin J Am Soc Nephrol 6: 2016–2024, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou KJ, Lee PT, Chen CL, Chiou CW, Hsu CY, Chung HM, Liu CP, Fang HC: Physiological changes during hemodialysis in patients with intradialysis hypertension. Kidney Int 69: 1833–1838, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Raj DS, Vincent B, Simpson K, Sato E, Jones KL, Welbourne TC, Levi M, Shah V, Blandon P, Zager P, Robbins RA: Hemodynamic changes during hemodialysis: Role of nitric oxide and endothelin. Kidney Int 61: 697–704, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Cirit M, Akçiçek F, Terzioğlu E, Soydaş C, Ok E, Ozbaşli CF, Başçi A, Mees EJ: ‘Paradoxical’ rise in blood pressure during ultrafiltration in dialysis patients. Nephrol Dial Transplant 10: 1417–1420, 1995 [PubMed] [Google Scholar]
- 9.Bazzato G, Coli U, Landini S, Lucatello S, Fracasso A, Morachiello P, Righetto F, Scanferla F: Prevention of intra- and postdialytic hypertensive crises by captopril. Contrib Nephrol 41: 292–298, 1984 [DOI] [PubMed] [Google Scholar]
- 10.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G: Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353: 999–1007, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T: Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348: 593–600, 2003 [DOI] [PubMed] [Google Scholar]
- 12.London GM, Pannier B, Agharazii M, Guerin AP, Verbeke FH, Marchais SJ: Forearm reactive hyperemia and mortality in end-stage renal disease. Kidney Int 65: 700–704, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA: Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol 41: 1769–1775, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Saijonmaa O, Metsärinne K, Fyhrquist F: Carvedilol and its metabolites suppress endothelin-1 production in human endothelial cell culture. Blood Press 6: 24–28, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Bank AJ, Kelly AS, Thelen AM, Kaiser DR, Gonzalez-Campoy JM: Effects of carvedilol versus metoprolol on endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Hypertens 20: 777–783, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Povsic TJ, Adams SD, Zavodni KL, Kelly F, Melton LG, Rao SV, Najjar SS, Harrington RA, Peterson ED: Aldehyde dehydrogenase activity allows reliable EPC enumeration in stored peripheral blood samples. J Thromb Thrombolysis 28: 259–265, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Povsic TJ, Zavodni KL, Vainorius E, Kherani JF, Goldschmidt-Clermont PJ, Peterson ED: Common endothelial progenitor cell assays identify discrete endothelial progenitor cell populations. Am Heart J 157: 335–344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Povsic TJ, Zavodni KL, Kelly FL, Zhu S, Goldschmidt-Clermont PJ, Dong C, Peterson ED: Circulating progenitor cells can be reliably identified on the basis of aldehyde dehydrogenase activity. J Am Coll Cardiol 50: 2243–2248, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R, International Brachial Artery Reactivity Task Force : Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Pyke KE, Tschakovsky ME: The relationship between shear stress and flow-mediated dilatation: Implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris RA, Nishiyama SK, Wray DW, Richardson RS: Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahous SA, Stephan A, Barakat W, Blacher J, Asmar R, Safar ME: Aortic pulse wave velocity in renal transplant patients. Kidney Int 66: 1486–1492, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Park CS, Kim W, Woo JS, Ha SJ, Kang WY, Hwang SH, Park YW, Kim YS, Ahn YK, Jeong MH, Kim W: Green tea consumption improves endothelial function but not circulating endothelial progenitor cells in patients with chronic renal failure. Int J Cardiol 145: 261–262, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Yoshida O, Kondo T, Kureishi-Bando Y, Sugiura T, Maeda K, Okumura K, Murohara T: Pitavastatin, an HMG-CoA reductase inhibitor, ameliorates endothelial function in chronic smokers. Circ J 74: 195–202, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Agarwal R: Supervised atenolol therapy in the management of hemodialysis hypertension. Kidney Int 55: 1528–1535, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Van Buren PN, Kim C, Toto R, Inrig JK: Intradialytic hypertension and the association with interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol 6: 1684–1691, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]