Summary
Background and objectives
Peritonitis is a major infectious complication in peritoneal dialysis patients, and intraperitoneal antibiotic administration is preferred to ensure maximal antibiotic concentrations at the site of infection. This study aimed to describe the plasma and infection site pharmacokinetics of intraperitoneal gentamicin in patients with peritonitis.
Design, setting, participants, & measurements
This prospective pharmacokinetic study of intraperitoneal gentamicin was conducted in peritoneal dialysis patients presenting to hospital with clinically defined signs and symptoms of peritonitis. Twenty-four patients were administered a 0.6-mg/kg dose of intraperitoneal gentamicin, which was allowed to dwell for 6 hours. Serial blood and dialysate samples were collected for 24 hours after the first dose. Gentamicin concentrations in plasma and dialysate were measured using a validated assay.
Results
The median percentage of the dose absorbed into the systemic circulation was 76% (interquartile range=69%–82%) and significantly different between patients with low average, high average, and high peritoneal membrane transporter status (P=0.03). The calculated pharmacokinetic parameters were plasma terminal elimination half-life of 24.7 (20.4–29.9) hours, terminal volume of distribution of 0.30 (0.20–0.36) L/kg, observed peak plasma concentration of 3.1 (2.4–3.4) mg/L, and observed trough plasma concentration of 1.9 (1.4–2.2) mg/L. The peak gentamicin concentration in dialysate was at least eight times the minimum inhibitory concentration of the likely pathogens.
Conclusions
The high systemic absorption of gentamicin in patients with peritonitis and prolonged plasma elimination half-life may lead to drug accumulation in the systemic circulation, increasing the risk of toxicity.
Introduction
Peritonitis is a major infectious complication in peritoneal dialysis (PD) patients and remains one of the main reasons for the transfer of many patients from PD to hemodialysis, accounting for about 42% of all transfers (1,2). Antibiotic therapy for the treatment of peritonitis may be administered by the intravenous, oral, or intraperitonal (ip) route. The ip route is preferred, because it ensures maximal antibiotic concentrations in the peritoneum and cells lining the peritoneal cavity (3).
As much as 65%–100% of an antibiotic dose administered ip can be absorbed into the systemic circulation (4). The work by Somani et al. (4) describes what has been termed the unidirectional transport of antibiotics where there is rapid distribution of the drug from the peritoneal space into the systemic circulation compared with slow distribution of drug into dialysate after intravenous administration (4). This has been shown for gentamicin (4) and tobramycin (5), and it is best explained by the small volume of the peritoneal cavity compared with the larger systemic volume of distribution of aminoglycosides. Peritonitis alters the permeability of the peritoneal membrane in PD patients, leading to increased systemic absorption of drugs across the peritoneal membrane compared with volunteer PD patients without peritonitis (6). Furthermore, poor systemic clearance in PD patients leads to drug accumulation in the systemic circulation, increasing the potential for toxicity of renally eliminated antibiotics such as gentamicin.
Gentamicin is an aminoglycoside antibiotic, and it is indicated for empirical Gram-negative cover in PD-related peritonitis (7). Aminoglycosides display concentration-dependent activity, with maximal bacterial killing occurring at high peak drug concentrations (8). A peak to minimum inhibitory concentration (Cmax:MIC) ratio of 8–10 for aminoglycosides has been associated with maximal antibiotic efficacy (9). Aminoglycosides also display a prolonged postantibiotic effect (PAE), where bacterial growth continues to be inhibited even when drug concentrations fall below the MIC of the bacteria (10). These pharmacodynamic characteristics suggest that dosing regimens that achieve high peak concentrations are optimal and that prolonged antibiotic exposure is not advantageous.
Many studies have examined the effectiveness and outcomes of ip aminoglycoside therapy in PD-related peritonitis (11–16); however, only a few small studies have reported the pharmacokinetics of ip gentamicin (4,6,17–20). Most of these pharmacokinetic studies were conducted in volunteer PD patients without peritonitis (4,17,19), and therefore, they do not account for the altered pharmacokinetics in patients with peritonitis. It follows that pharmacokinetic data that support the current dosing recommendations for gentamicin may not be optimal.
This study aims to describe the infection site and plasma pharmacokinetics of ip gentamicin in PD patients with peritonitis. We also examined the effect of peritoneal membrane transporter status of PD patients on the systemic absorption of ip gentamicin.
Materials and Methods
Patient Population
We have previously published a protocol paper on this study (21). This prospective pharmacokinetic study involved 24 PD patients (both anuric and nonanuric) and was a convenience sample. Patients who presented with clinical signs and symptoms of peritonitis (abdominal pain, nausea, vomiting, diarrhea, fever, or cloudy dialysate) and were prescribed empirical ip antibiotic therapy by the treating clinician were included in the study. The clinical diagnosis of peritonitis was confirmed by PD effluent cell count, differential, and culture. Patients above the age of 18 years who have been receiving continuous ambulatory PD or automated PD (APD) for at least 1 month were eligible for the study. On hospital admission, APD patients were switched to continuous ambulatory PD during their acute phase of peritonitis. All patients received four exchanges per day with dialysate volumes of 2 L per exchange. PD solutions used were either Dianeal (1.5% or 2.5% glucose; Baxter Healthcare, Deerfield, IL) or Stay Safe (1.5% or 2.3% glucose; Fresenius Medical Care, Bad Homburg, Germany). For some patients, Extraneal (7.5% icodextrin; Baxter Healthcare, Deerfield, IL) was used for a long dwell; however, none of these patients had ip gentamicin added to their Extraneal dialysis fluid. The study included patients with different peritoneal membrane transporter statuses (low average, high average, and high transporters). Patients who received a course of ip antibiotics in the preceding 2 weeks were not eligible for the study.
This study was performed in a 986-bed tertiary referral hospital. Ethics approval was obtained from the Royal Brisbane and Women’s Hospital Human Research and Ethics Committees (HREC/08/QRBW/8) and the Medical Research Ethics Committee of the University of Queensland (2009000673). Written informed consent was obtained from all participants. The trial was also registered on the Australian New Zealand Clinical Trial Registry (ANZCTRN 12609000446268).
Study Protocol
Drug dosing was as prescribed by clinicians based on the local hospital Peritonitis Treatment Protocol that reflects the International Society for Peritoneal Dialysis Peritonitis Treatment Recommendations (3). A gentamicin dose of 0.6 mg/kg was added to a 2-L bag of dialysate, and the bag was shaken to ensure adequate mixing of drug in dialysate. The antibiotic containing dialysate was infused into the peritoneal cavity and allowed to dwell for 6 hours. Serial blood samples were collected in lithium heparin-coated blood collection tubes from an indwelling peripheral intravenous cannula after the instillation of the ip antibiotic. Blood samples (5 ml each) were collected at 1, 3, 6, 7, and 24 hours after the ip antibiotic dose. Dialysate samples (10 ml each) were collected at 3 and 6 hours and the end of the three subsequent dialysis exchanges up to 24 hours after the first ip antibiotic dose. Samples collected were stored on ice; the blood samples were then centrifuged at 3,000 rpm, and the plasma aliquots and dialysate samples were all stored at −80°C until assayed. A 24-hour urine collection was also performed at the start of the study to determine patient’s residual renal function. Urinary creatinine clearance over 24 hours corrected for body surface area was calculated according to the equation
 |
Clinical and demographic data were also collected for each patient, including their peritoneal membrane transporter status as classified by the standardized peritoneal equilibration test (22).
Assay Method
Gentamicin concentrations in plasma and peritoneal dialysate were determined using a validated liquid chromatography tandem mass spectrometry assay method on an API 2000 Mass Spectrometer (Applied Biosystems). The stationary phase was a Phenomenex Luna C18 column (5 µm, 50×2 mm), and the mobile phase was 50% methanol/50% 0.11 M trifluoroacetic acid. Tobramycin was used as the internal standard. Gentamicin standards and quality controls (QCs) were prepared in matrices of plasma and peritoneal dialysate.
For plasma samples, 200 µl plasma was diluted with the internal standard and deproteinated with 30% trichloroacetic acid before 20 µl supernatant was injected. For peritoneal dialysate samples, 500 µl peritoneal dialysate was diluted with 0.1% formic acid and internal standard. A sample cleanup process was carried out, which involved loading the diluted sample onto an Oasis MCX solid-phase extraction cartridge. A wash step using 0.1% formic acid (10 ml) was undertaken for each sample before gentamicin was eluted from the solid-phase extraction cartridge using a 50% methanol/50% ammonium hydroxide solution. The eluent was dried and reconstituted with 50% methanol/50% formic acid (0.1%), and 10 µL reconstituted solution was then injected.
Linearity was validated from 0.25 to 125 mg/L (plasma) and from 0.5 to 50 mg/L (peritoneal dialysate). Accuracy and precision were determined from n=6 replicates of QCs at high, medium, and low concentrations. For both plasma and peritoneal dialysate, intraday precision was within 10%, and intraday accuracy was within 15% at all QC levels. Interday precision ranged between 6% and 18% at the different QC levels, whereas interday accuracy was within 10% at all QC levels for both plasma and peritoneal dialysate.
Pharmacokinetic Analysis
A noncompartmental pharmacokinetic approach was used to analyze the data. The equations and methods used to calculate the various pharmacokinetic parameters are outlined in Table 1.
Table 1.
Summary of equations for pharmacokinetic parameters
| Pharmacokinetic Parameter | Definition | Equation or Method of Calculation |
|---|---|---|
| F | Fraction of ip dose absorbed | F = (Total dose − Amount of drug remaining in dialysate at the end of antibiotic dwell)/Total dose |
| kel | Plasma elimination rate constant | Negative slope of the log-transformed concentration versus time data obtained from plasma samples between 6 and 24 hours |
| t1/2, el | Plasma elimination half-life | t1/2, el=ln2/kel |
| kpc 0–6 | Rate constant for distribution of drug from the peritoneal cavity between 0 and 6 hours | Negative slope of the log-transformed concentration versus time data obtained from peritoneal dialysate samples between 0 and 6 hours |
| t1/2, pc 0–6 | Distribution half-life in the peritoneal cavity between 0 and 6 hours | t1/2, pc 0–6=ln2/kpc 0–6 |
| t1/2, eq | Equilibration half-life | t1/2, eq=ln2/(kel+kpc 0–6) |
| CLtotal | Total clearance from the systemic circulation | CLtotal = (F × Dose)/AUCplasma 0–∞ |
| AUCplasma 0–∞ | Area under the plasma concentration time curve between time 0 and infinity | Linear trapezoidal rule between 0 and 24 hours; then, extrapolation from 24 hours to infinity was calculated assuming log-linear decline |
| AUC0–24 | Area under the concentration time curve between 0 and 24 hours | Linear trapezoidal rule between 0 and 24 hours |
| Vd | Volume of distribution | Vd=CLplasma 0–24/kel |
| CLplasma 0–24 | Plasma clearance between 0 and 24 hours | CLplasma 0-24 = (F × Dose)/AUCplasma 0–24 |
| CLperitoneal 6–24 | Peritoneal clearance from 6 to 24 hours |  |
| CLnondial | Nondialysate clearance | CLnondial=(CLplasma 0–24)−(CLperitoneal 6–24) |
ip, intraperitoneal.
Statistical Analyses
Continuous variables are expressed as median (interquartile range). Statistically significant differences in pharmacokinetic parameters between the anuric and nonanuric patients were calculated using the Wilxocon Mann–Whitney test. Specific pharmacokinetic parameters were transformed to normal distribution, and the differences between peritoneal membrane types were tested using linear regression analysis. P value<0.05 was considered statistically significant. Statistical analysis was conducted using Stata Statistical Software: Release 10 (Stata Corporation, College Station, TX).
Results
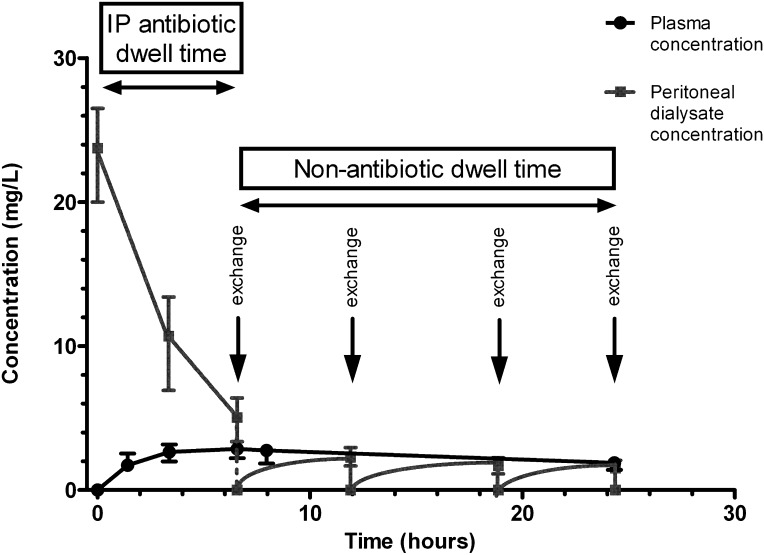
A total of 24 PD patients with peritonitis were enrolled. A summary of the clinical and demographic data are presented in Table 2. Figure 1 presents the concentration–time profile of gentamicin in plasma and peritoneal dialysate after ip administration of gentamicin.
Table 2.
Clinical and demographic data
| Parameter | Anuric (n=12) | Nonanuric (n=12) | Total (n=24) |
|---|---|---|---|
| Males | 6 (50%) | 6 (50%) | 12 (50%) |
| Age (years) | 75 (64–82) | 52 (37–68) | 68 (46–78) |
| Weight (kg) | 79.0 (70.3–88.0) | 77.5 (65.5–88.0) | 78.0 (68.8–88.0) |
| Intraperitoneal gentamicin dose (mg) | 47.5 (41.0–55.0) | 47.5 (40.0–52.0) | 47.5 (40.0–52.0) |
| Intraperitoneal gentamicin dose (mg/kg) | 0.60 (0.59–0.60) | 0.60 (0.57–0.62) | 0.60 (0.58–0.61) |
| Urine output (ml/24 h) | 0 (0–42) | 533 (177–827) | 173 (19–625) |
| Peritoneal membrane type | |||
| low average | 3 (25%) | 3 (25%) | 6 (25%) |
| high average | 5 (42%) | 6 (50%) | 11 (46%) |
| high | 4 (33%) | 3 (25%) | 7 (29%) |
| Duration of intraperitoneal gentamicin therapy (days) | 3.5 (2–11) | 14 (4.5–21) | 5 (3–21) |
All values are reported as n (%) or median (interquartile range).
Figure 1.
Gentamicin plasma and peritoneal dialysate concentrations in peritoneal dialysis patients with peritonitis. The figure shows the concentration–time profile of gentamicin in plasma and peritoneal dialysate of 24 peritoneal dialysis patients with peritonitis after the first dose of intraperitoneal gentamicin (0.6 mg/kg) was added to a 2-L bag of peritoneal dialysis fluid and allowed to dwell in the peritoneal cavity for 6 hours. Patients received four peritoneal dialysis fluid exchanges in a 24-hour period, with one of the dwells being the intraperitonal antibiotic dwell. During the antibiotic dwell, a decrease of gentamicin concentration in peritoneal dialysate was observed together with an increase in plasma concentration of gentamicin, which indicated systemic absorption of intraperitoneal gentamicin. During the nonantibiotic dwell time, slow elimination of gentamicin from plasma was observed, indicating a prolonged plasma elimination half-life in this patient population.
The pharmacokinetic parameters observed and calculated from gentamicin concentrations in plasma and peritoneal dialysate are summarized in Table 3 and also reported according to residual renal function status (anuric versus nonanuric). Table 4 summarizes significant differences in pharmacokinetic parameters between patients with different peritoneal membrane transporter status.
Table 3.
Summary of pharmacokinetic parameters grouped according to residual renal function
| Parameter | Total | Anuric | Nonanuric | P Valuea |
|---|---|---|---|---|
| F | 0.76 (0.69–0.82) | 0.76 (0.70–0.82) | 0.78 (0.69–0.84) | 0.98 |
| Cmax, plasma (mg/L) | 3.12 (2.44–3.36) | 2.86 (2.22–3.20) | 3.15 (2.54–3.53) | 0.43 |
| Cmin, plasma (mg/L) | 1.89 (1.42–2.15) | 1.88 (1.46–2.15) | 1.97 (1.38–2.19) | 0.88 |
| AUCplasma 0–24 (mg⋅h/L) | 60.45 (37.78–65.66) | 58.14 (39.92–65.83) | 60.45 (26.59–65.66) | 0.56 |
| Cmax, peritoneal (mg/L) | 23.75 (20–26) | 23.75 (20.5–27.5) | 23.75 (20–26) | 0.91 |
| Cmin, peritoneal (mg/L) | 1.52 (1.25–2.00) | 1.38 (1.15–1.58) | 1.82 (1.49–2.11) | 0.10 |
| AUCperitoneal 0–24 (mg⋅h/L) | 121.92 (108.77–145.16) | 121.44 (108.58–148.86) | 125.74 (108.77–145.16) | 1.00 |
| kel×10(h−1) | 0.30 (0.20–0.30) | 0.25 (0.20–0.30) | 0.30 (0.30–0.40) | 0.01 |
| t1/2, el (h) | 24.71 (20.37–29.89) | 28.78 (24.54–30.77) | 21.10 (18.90–27.14) | 0.03 |
| kpc 0–6 (h−1) | 0.23 (0.19–0.26) | 0.22 (0.19–0.26) | 0.24 (0.19–0.27) | 0.62 |
| t1/2, pc 0–6 (h) | 2.98 (2.69–3.59) | 3.19 (2.69–3.59) | 2.88 (2.60–3.60) | 0.62 |
| t1/2, eq (h) | 2.89 (2.38–3.13) | 2.87 (2.48–3.04) | 3.03 (2.38–3.79) | 0.77 |
| Vd (L/kg) | 0.30 (0.20–0.36) | 0.31 (0.26–0.42) | 0.22 (0.17–0.32) | 0.20 |
| CLtotal (L/h) | 0.25 (0.22–0.27) | 0.25 (0.23–0.31) | 0.24 (0.19–0.26) | 0.44 |
| CLplasma 0–24 (L/h) | 0.54 (0.46–0.64) | 0.58 (0.50–0.66) | 0.43 (0.37–0.58) | 0.12 |
| CLperitoneal 6–24 (L/h) | 0.26 (0.25–0.28) | 0.25 (0.24–0.28) | 0.27 (0.26–0.28) | 0.18 |
| CLnondial (L/h) | 0.31 (0.14–0.39) | 0.33 (0.26–0.39) | 0.14 (0.14–0.31) | 0.14 |
All values are reported as median (interquartile range). F, fraction of intraperitoneal dose absorbed; Cmax, plasma, peak plasma concentration; Cmin, plasma, trough plasma concentration at 24 hours; AUCplasma 0–24, area under the plasma concentration time curve between 0 and 24 hours; Cmax, peritoneal, peak concentration in peritoneal dialysate during the intraperitoneal antibiotic dwell; Cmin, peritoneal, trough concentration in peritoneal dialysate at 24 hours; AUCperitoneal 0–24, area under the peritoneal dialysate concentration time curve between 0 and 24 hours; kel, plasma elimination rate constant; t1/2, el, plasma elimination half-life; kpc 0–6, rate constant for distribution of drug from the peritoneal cavity between 0 and 6 hours; t1/2, pc 0–6, distribution half-life in the peritoneal cavity between 0 and 6 hours; t1/2, eq, equilibration half-life; Vd, volume of distribution; CLtotal, total clearance from the systemic circulation; CLplasma 0–24, plasma clearance between 0 and 24 hours; CLperitoneal 6–24, peritoneal clearance from 6 to 24 hours; CLnondial, nondialysate clearance.
Wilcoxon Mann–Whitney test.
Table 4.
Selected pharmacokinetic parameters grouped according to peritoneal membrane transporter status
| Parameter | Low Average | High Average | High | P Valuea | r2 |
|---|---|---|---|---|---|
| F | 0.64 | 0.78 | 0.76 | 0.03 | 0.30 |
| t1/2, eq (hours) | 5.60 | 2.38 | 2.94 | 0.04 | 0.48 |
All values are reported as median. F, fraction of intraperitoneal dose absorbed; t1/2, eq, equilibration half-life.
Linear regression analysis after transformation of data to normal distribution.
The proportion of drug absorbed (bioavailability; F) differed in patients based on the membrane transporter status (P=0.03, r2=0.30), with greater bioavailability observed among high average and high transporters than low average transporters. The equilibration half-life (t1/2, eq) also differed between patients based on the membrane transporter status (P=0.04, r2=0.48), with shorter equilibration time among patients with high average and high transporter status compared with low average transporters. The difference in mean plasma elimination half-life (t1/2, el) between anuric and nonanuric patients (28.7±6.3 versus 21.9±4.6 hours) was also found to be statistically significant (P=0.03), with nonanuric patients having residual creatinine clearance ranging from 1 to 11 ml/min per m2.
Discussion
This study is the largest pharmacokinetic study to date of ip gentamicin in PD patients with peritonitis. Our results show that a significant proportion of a gentamicin dose administered intraperitoneally is absorbed into the systemic circulation. The mean systemic absorption of ip gentamicin (73±16%) in our study conducted in patients with peritonitis was much higher than the absorption that has been reported in two other previous studies conducted in PD patients without peritonitis (49±15% and 56±11%) (17,19). It has been postulated that peritonitis alters the permeability of the peritoneal membrane, meaning that an inflamed peritoneal membrane during the acute phase of peritonitis allows increased absorption of drugs from the peritoneal cavity into the systemic circulation (23). Our data support previous smaller pharmacokinetic studies with gentamicin and tobramycin that reported statistically significant higher mass transfer coefficients for ip aminoglycosides in patients with active peritonitis infection compared with control patients without peritonitis (6,24).
This study also described a difference in the fraction of ip dose absorbed between patients grouped according to their peritoneal membrane transport status. Different peritoneal membrane types explain about 30% of the variability observed in the proportion of an ip gentamicin dose absorbed among PD patients with peritonitis. This finding has not been previously shown for gentamicin; however, previous data for ip cephazolin in APD patients showed a trend between peritoneal clearance and transport status as described by 4-hour peritoneal equilibration test values for dialysate to plasma creatinine (r=0.834, P<0.10, n=6) (25). Our results highlight that peritoneal membrane transporter status is one of the factors that can influence the proportion of an ip gentamicin dose absorbed into the systemic circulation. Furthermore, a statistically significant difference was also observed in the equilibration half-life based on membrane transporter status (P=0.04, r2=0.48). Our results show that almost one-half of the variability in the time that it takes to achieve 50% equilibration of gentamicin between plasma and peritoneal dialysate is explained by the different peritoneal membrane transporter status (i.e., low average, high average, or high transporters).
After gentamicin is absorbed into the systemic circulation from the peritoneum, it is eliminated from the body predominantly by glomerular filtration but also through PD. The plasma elimination half-life of gentamicin in healthy volunteers is approximately 2 hours; however, in end-stage kidney disease patients who are anuric and not on dialysis, the plasma elimination half-life of gentamicin is reported to be between 50 and 70 hours (26). In our anuric patients (defined as patients with urine output<100 ml/d), our observed mean half-life of 28.7 hours was shorter than the mean values of 36 hours reported in two previous studies that were conducted in anuric volunteer PD patients without peritonitis (17,19). The shorter half-life that we observed is most likely explained by the increased membrane permeability during peritonitis and therefore, increased clearance of gentamicin from the systemic circulation into the peritoneal cavity in our patients with infected and inflamed membranes compared with PD patients without peritonitis. Our study included both anuric and nonanuric PD patients with peritonitis. As expected, we found that anuric patients had a longer half-life compared with nonanuric patients (28.7±6.3 versus 21.9±4.6 hours, P=0.03). These findings support the importance of residual renal function in the elimination of gentamicin from the systemic circulation.
Maintenance dosing is dependent on the clearance of the drug; however, residual renal function should not be used to determine the initial dosing of ip gentamicin. Basic pharmacokinetic principles dictate that the initial dosing of a drug should be based on volume of distribution and not clearance. We raise this important point, because the current International Society for Peritoneal Dialysis Peritonitis Treatment Recommendations (7) have a general recommendation that nonanuric patients should receive a 25% higher ip antibiotic dose for renally eliminated drugs. This dosing is not used in our hospital, and all patients in our study received 0.6 mg/kg ip gentamicin. When administered the same ip gentamicin dose, we observed no difference in mean peak plasma concentration between anuric and nonanuric patients on the first day of ip antibiotic therapy. Furthermore, all patients, regardless of their residual renal function, achieved adequate peak drug concentrations in the peritoneal cavity, the main site of infection. Based on this information and from a pharmacokinetic and pharmacodynamic perspective, we are of the opinion that residual renal function should not be used empirically as a determining factor in prescribing a higher initial dose of ip gentamicin to nonanuric patients.
After initial dosing of the drug, plasma gentamicin concentration is often monitored in clinical practice and used as a guide to determine if and when redosing should occur. The main concern for clinicians is the risk of nephrotoxicity and ototoxicity with accumulation of gentamicin in the systemic circulation after subsequent repeated dosing. Trough plasma gentamicin concentrations of <2 mg/L have generally been targeted based on evidence from early studies showing increased nephrotoxicity with trough levels greater than 2 and 4 mg/L (27,28). Ototoxicity, however, has been associated with peak plasma concentrations above 12 mg/L (29). In our study, a median trough plasma concentration of 1.9 mg/L was observed at 24 hours after the first ip gentamicin dose. A previous pharmacokinetic study by Tosukhowong et al. (18) reported mean trough concentrations of 1.1 and 2.2 mg/L on days 1 and 5, respectively, of ip gentamicin therapy, suggesting that drug accumulation occurs with repeated dosing. Monitoring of plasma gentamicin concentrations is still necessary to minimize the potential for toxicity.
Although minimizing the drug concentration in plasma is important to reduce antibiotic toxicity, drug concentration in the peritoneal cavity, the main site of infection, is the major factor in determining antibiotic efficacy. Recent papers have described how aminoglycosides can be best used to optimize efficacy by concentration-dependent killing (8,30). The present International Society for Peritoneal Dialysis Peritonitis Treatment Recommendations (7) do not strictly reflect these principles, and therefore, there may be scope to optimize the use of aminoglycosides. For aminoglycosides, the Cmax:MIC and the area under the curve to MIC ratio (AUC:MIC) are the pharmacokinetic/pharmacodynamic indices that correlate with antibacterial efficacy (8). A review of data from animal in vivo and human clinical studies suggests that a Cmax:MIC of 8–10 and AUC:MIC of 80–100 are the general pharmacokinetic/pharmacodynamic targets for aminoglycosides (9). In vitro studies also support that a Cmax:MIC of greater than eight is also important in preventing the emergence of resistant subpopulations of bacteria (31).
The duration of the PAE of antibiotics can vary for different bacteria and is also dependent on the different antibiotic exposure concentrations, with longer duration of the PAE observed after exposure to higher concentrations. The PAE duration for aminoglycosides against Gram-negative bacilli has been reported to be up to 7.5 hours in in vivo animal infection models (10). A study by Low et al. (19) on the pharmacokinetics of once-daily ip gentamicin raised some concerns that nonanuric patients would be exposed to prolonged periods of time with subtherapeutic gentamicin concentrations in plasma and dialysate. Rather than use the duration of time that concentrations remain above the MIC as the pharmacodynamic target to define ip gentamicin efficacy, we would argue that a Cmax:MIC of 8–10 in peritoneal dialysate should be the appropriate target based on an understanding of the pharmacodynamic properties of the drug. With the current recommended ip gentamicin dose (0.6 mg/kg) administered once-daily and assuming an MIC90 of 2 mg/L for most commonly encountered susceptible Gram-negative organisms, our peritoneal dialysate concentration data showed peak gentamicin concentrations of greater than 16–20 mg/L (i.e., 8–10×MIC) for at least the first 30–60 minutes of the ip antibiotic dwell time (when at least 40 mg gentamicin was added to a 2-L bag of dialysate). By 3 hours, approximately 50% of the ip dose had been absorbed into the systemic circulation. At the end of the recommended 6-hour antibiotic dwell time, approximately three-quarters of the total ip dose had been absorbed. Therefore, if a shorter dwell time was used for ip gentamicin instead of the recommended 6 hours, it would still result in the same Cmax:MIC ratio in peritoneal dialysate but lower systemic absorption into the systemic circulation and decreased potential for toxicities.
Although the Cmax:MIC ratio in the peritoneal cavity would not change with a shorter dwell time, the AUC:MIC ratio in the peritoneal cavity would decrease with shorter antibiotic exposure. Given that the rate of bacterial killing is primarily correlated with Cmax:MIC, achieving this pharmacokinetic/pharmacodynamic target should be considered an essential part of ip gentamicin dosing. The AUC:MIC has also been correlated with efficacy, albeit to a lesser extent; however, the long ip gentamicin dwell time required to achieve this exposure target is intrinsically linked with higher systemic absorption and increased risk of toxicity. Therefore, it is reasonable to ensure the Cmax:MIC target is achieved, even at the expense of decreased AUC:MIC with a shorter antibiotic dwell time. Furthermore, in current clinical practice where a long 6-hour dwell time is recommended, ip gentamicin doses are often withheld by day 2 or 3 of therapy, because plasma gentamicin concentrations are deemed high (>2 mg/L). This often results in prolonged periods of up to 48 hours or more that the ip gentamicin dose is withheld, and therefore, there is no exposure during this time to a high peak concentration of gentamicin in the peritoneal cavity, which is required for maximal bacterial killing. This approach of using a shorter ip gentamicin dwell time would minimize systemic absorption and result in lower trough plasma concentrations; thus, it would enable more consistent daily dosing (with less doses being withheld), which would result in more effective bacterial killing and potentially shorter courses of therapy. The safety and efficacy of a shorter ip gentamicin dwell time will need to be studied in clinical practice. Merely reducing the current ip gentamicin dose instead of reducing the ip antibiotic dwell time would not be the appropriate solution for this class of antibiotic considering its concentration-dependent pharmacodynamic effect.
In our view, the primary limitation of this study is that we were unable to follow up with patients on subsequent days to determine whether pharmacokinetics of ip gentamicin changed with resolution of infection and inflammation. Unfortunately, local practice results in patients being discharged from the hospital frequently within 24–72 hours on home ip antibiotic therapy. Because intravenous access is required for pharmacokinetic sampling, ongoing collection was not possible after decannulation. Other limitations include the absence of actual MIC for the causative organism in individual patients. In our discussion, we used an assumed MIC90 of 2 mg/L for most susceptible Gram-negative organisms based on data from the European Committee on Antimicrobial Susceptibility Testing (http://www.eucast.org/clinical_breakpoints/). Furthermore, antibiotic pharmacodynamics in PD fluid are not very well understood, particularly the interaction between the antibiotic and bacteria in the presence of PD fluid. There is some in vitro data to suggest that, for certain antibiotics, antibiotic susceptibility against certain organisms is diminished in PD fluid, which is reflected by higher MICs when measured in PD fluid compared with standard broth (32). However, the significance of this finding is poorly understood, because there have been no studies describing the relationship between the concentration in the PD fluid and clinical outcomes in this patient population.
Although the observed peak and trough plasma concentrations were not in the toxic range after the first dose of ip gentamicin, the potential systemic toxicity should not be underestimated in PD patients with peritonitis. The prolonged plasma elimination half-life in this patient population as well as the high systemic absorption (particularly during the acute phase of peritonitis) and the long ip antibiotic dwell time may lead to drug accumulation in the systemic circulation. However, as the peritonitis episode resolves, systemic absorption of ip antibiotics is also likely to decrease with the reduced inflammation of the peritoneal membrane. Taking into consideration the concentration-dependent pharmacodynamics of aminoglycosides, clinical studies comparing short and long ip gentamicin dwell times are required before we can reconsider the current dosing recommendations and guidelines for ip gentamicin in PD patients with peritonitis.
Disclosures
None.
Acknowledgments
The authors thank the medical and nursing staff at the Department of Renal Medicine at the Royal Brisbane and Women’s Hospital for their help in completing this study. Particular thanks to the renal consultants (Dr. Vincent D’Intini, Dr. Adrian Kark, Dr. Sharad Ratanjee, and Dr. George John), the nurse unit manager of the renal ward (Ilse Berquier), and all nursing staff on the renal ward and peritoneal dialysis nurses from the Home Independent Dialysis Centre for their valuable assistance in recruiting patients and ensuring that this study was a success.
Funding for this project was received from the Society of Hospital Pharmacists of Australia. We acknowledge funding of the Burns, Trauma and Critical Care Research Centre by National Health and Medical Research Council of Australia Project Grant 519702. J.M.V. is funded by a University of Queensland Research Scholarship. J.A.R. is funded by a fellowship from the Australian National Health and Medical Research Council of Australia (Australian-Based Health Professional Research Fellowship 569917).
Part of this manuscript was presented as an abstract (A-1098) at the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, September 17–20, 2011, Chicago, Illinois.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Brown F, Liu WJ, Kotsanas D, Korman TM, Atkins RC: A quarter of a century of adult peritoneal dialysis-related peritonitis at an Australian medical center. Perit Dial Int 27: 565–574, 2007 [PubMed] [Google Scholar]
- 2.Davenport A: Peritonitis remains the major clinical complication of peritoneal dialysis: The London, UK, peritonitis audit 2002-2003. Perit Dial Int 29: 297–302, 2009 [PubMed] [Google Scholar]
- 3.Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, Kuijper EJ, Li PK, Lye WC, Mujais S, Paterson DL, Fontan MP, Ramos A, Schaefer F, Uttley L, ISPD Ad Hoc Advisory Committee : Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 25: 107–131, 2005 [PubMed] [Google Scholar]
- 4.Somani P, Shapiro RS, Stockard H, Higgins JT: Unidirectional absorption of gentamicin from the peritoneum during continuous ambulatory peritoneal dialysis. Clin Pharmacol Ther 32: 113–121, 1982 [DOI] [PubMed] [Google Scholar]
- 5.Bunke CM, Aronoff GR, Brier ME, Sloan RS, Luft FC: Tobramycin kinetics during continuous ambulatory peritoneal dialysis. Clin Pharmacol Ther 34: 110–116, 1983 [DOI] [PubMed] [Google Scholar]
- 6.de Paepe M, Lameire N, Belpaire F, Bogaert M: Peritoneal pharmacokinetics of gentamicin in man. Clin Nephrol 19: 107–109, 1983 [PubMed] [Google Scholar]
- 7.Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, Johnson DW, Kuijper EJ, Lye WC, Salzer W, Schaefer F, Struijk DG, International Society for Peritoneal Dialysis : Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 30: 393–423, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Craig WA: Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin Infect Dis 26: 1–10, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Turnidge J: Pharmacodynamics and dosing of aminoglycosides. Infect Dis Clin North Am 17: 503–528, v, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Vogelman B, Gudmundsson S, Turnidge J, Leggett J, Craig WA: In vivo postantibiotic effect in a thigh infection in neutropenic mice. J Infect Dis 157: 287–298, 1988 [DOI] [PubMed] [Google Scholar]
- 11.Kent JR, Almond MK, International Society for Peritoneal Dialysis : A survey of CAPD peritonitis management and outcomes in North and South Thames NHS regions (U.K.): Support for the ISPD guidelines. Perit Dial Int 20: 301–305, 2000 [PubMed] [Google Scholar]
- 12.Blunden M, Zeitlin D, Ashman N, Fan SL: Single UK centre experience on the treatment of PD peritonitis—antibiotic levels and outcomes. Nephrol Dial Transplant 22: 1714–1719, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Lye WC, van der Straaten JC, Leong SO, Sivaraman P, Tan SH, Tan CC, Lee EJ: Once-daily intraperitoneal gentamicin is effective therapy for gram-negative CAPD peritonitis. Perit Dial Int 19: 357–360, 1999 [PubMed] [Google Scholar]
- 14.Bailie GR, Haqqie SS, Eisele G, Gorman T, Low CL: Effectiveness of once-weekly vancomycin and once-daily gentamicin, intraperitoneally, for CAPD peritonitis. Perit Dial Int 15: 269–271, 1995 [PubMed] [Google Scholar]
- 15.Weber J, Staerz E, Mettang T, Machleidt C, Kuhlmann U: Treatment of peritonitis in continuous ambulatory peritoneal dialysis (CAPD) with intraperitoneal cefazolin and gentamicin. Perit Dial Int 9: 191–195, 1989 [PubMed] [Google Scholar]
- 16.Lye WC, Wong PL, van der Straaten JC, Leong SO, Lee EJ: A prospective randomized comparison of single versus multidose gentamicin in the treatment of CAPD peritonitis. Adv Perit Dial 11: 179–181, 1995 [PubMed] [Google Scholar]
- 17.Pancorbo S, Comty C: Pharmacokinetics of gentamicin in patients undergoing continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother 19: 605–607, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tosukhowong T, Eiam-Ong S, Thamutok K, Wittayalertpanya S, Na Ayudhya DP: Pharmacokinetics of intraperitoneal cefazolin and gentamicin in empiric therapy of peritonitis in continuous ambulatory peritoneal dialysis patients. Perit Dial Int 21: 587–594, 2001 [PubMed] [Google Scholar]
- 19.Low CL, Bailie GR, Evans A, Eisele G, Venezia RA: Pharmacokinetics of once-daily IP gentamicin in CAPD patients. Perit Dial Int 16: 379–384, 1996 [PubMed] [Google Scholar]
- 20.Mars RL, Moles K, Pope K, Hargrove P: Use of bolus intraperitoneal aminoglycosides for treating peritonitis in end-stage renal disease patients receiving continuous ambulatory peritoneal dialysis and continuous cycling peritoneal dialysis. Adv Perit Dial 16: 280–284, 2000 [PubMed] [Google Scholar]
- 21.Ranganathan D, Varghese JM, Fassett RG, Lipman J, D’Intini V, Healy H, Roberts JA: Optimising intraperitoneal gentamicin dosing in peritoneal dialysis patients with peritonitis (GIPD) study. BMC Nephrol 10: 42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Twardowski ZJ, Nolph KO, Khanna R, Prowant BF.Ryan LP, Moore HL, Nielsen MP: Peritoneal equilibration test. Perit Dial Bull 7: 138–148, 1987 [Google Scholar]
- 23.Wideröe TE, Smeby LC, Dahl K, Jörstad S: Definitions of differences and changes in peritoneal membrane transport properties. Kidney Int Suppl 24: S107–S113, 1988 [PubMed] [Google Scholar]
- 24.Rubin J: Tobramycin absorption from the peritoneal cavity. Perit Dial Int 10: 295–297, 1990 [PubMed] [Google Scholar]
- 25.Elwell RJ, Bailie GR, Manley HJ: Correlation of intraperitoneal antibiotic pharmacokinetics and peritoneal membrane transport characteristics. Perit Dial Int 20: 694–698, 2000 [PubMed] [Google Scholar]
- 26.Wilson TW, Mahon WA, Inaba T, Johnson GE, Kadar D: Elimination of tritiated gentamicin in normal human subjects and in patients with severely impaired renal function. Clin Pharmacol Ther 14: 815–822, 1973 [DOI] [PubMed] [Google Scholar]
- 27.Dahlgren JG, Anderson ET, Hewitt WL: Gentamicin blood levels: A guide to nephrotoxicity. Antimicrob Agents Chemother 8: 58–62, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman EL, Van Gelder J, Holmes R, Hull AR, Sanford JP: Prospective comparative study of variable dosage and variable frequency regimens for administration of gentamicin. Antimicrob Agents Chemother 8: 434–438, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson GG, Arcieri G: Ototoxicity of gentamicin in man: A survey and controlled analysis of clinical experience in the United States. J Infect Dis 124[Suppl]: S130–S137, 1971 [DOI] [PubMed] [Google Scholar]
- 30.Craig WA: Does the dose matter? Clin Infect Dis 33[Suppl 3]: S233–S237, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Blaser J, Stone BB, Groner MC, Zinner SH: Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother 31: 1054–1060, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zelenitsky S, Franczuk C, Fine A, Ariano R, Harding G: Antibiotic tolerance of peritoneal bacterial isolates in dialysis fluids. J Antimicrob Chemother 49: 863–866, 2002 [DOI] [PubMed] [Google Scholar]