Background: Aggregated biotherapeutics have the potential to induce an immune response.
Results: Aggregates can enhance innate and adaptive immune responses of PBMC.
Conclusion: The response depends on aggregate type, immunogenicity of the monomer, donor immune status, and high particle numbers in the in vitro assay.
Significance: This is the first study showing the impact of aggregate characteristics on the potential immune response of PBMC.
Keywords: Aggregation, Antibodies, Biomarkers, Cytokine Induction, Innate Immunity, PBMC, Biotherapeutics, Immunogenicity, Particle Numbers, Secondary Structure
Abstract
Aggregation of biotherapeutics has the potential to induce an immunogenic response. Here, we show that aggregated therapeutic antibodies, previously generated and determined to contain a variety of attributes (Joubert, M. K., Luo, Q., Nashed-Samuel, Y., Wypych, J., and Narhi, L. O. (2011) J. Biol. Chem. 286, 25118–25133), can enhance the in vitro innate immune response of a population of naive human peripheral blood mononuclear cells. This response depended on the aggregate type, inherent immunogenicity of the monomer, and donor responsiveness, and required a high number of particles, well above that detected in marketed drug products, at least in this in vitro system. We propose a cytokine signature as a potential biomarker of the in vitro peripheral blood mononuclear cell response to aggregates. The cytokines include IL-1β, IL-6, IL-10, MCP-1, MIP-1α, MIP-1β, MMP-2, and TNF-α. IL-6 and IL-10 might have an immunosuppressive effect on the long term immune response. Aggregates made by stirring induced the highest response compared with aggregates made by other methods. Particle size in the 2–10 μm range and the retention of some folded structure were associated with an increased response. The mechanism of aggregate activation at the innate phase was found to occur through specific cell surface receptors (the toll-like receptors TLR-2 and TLR-4, FcγRs, and the complement system). The innate signal was shown to progress to an adaptive T-cell response characterized by T-cell proliferation and secretion of T-cell cytokines. Investigating the ability of aggregates to induce cytokine signatures as biomarkers of immune responses is essential for determining their risk of immunogenicity.
Introduction
Fully human protein-based therapeutics have the potential to be immunogenic when administered to human subjects. One important factor that might enhance potential immunogenicity is aggregation (1–4). The number of monoclonal antibody (IgG)-based investigational drug candidates undergoing clinical trials, or in development, has increased exponentially over the last few years making them the fastest growing class of protein therapeutics (5–7). They are typically administered at high doses, increasing the probability for aggregate formation, and often subcutaneously, which increases the risk for immunogenicity (1, 8, 9). Establishing biomarkers for immunogenicity due to aggregate characteristics is necessary for ensuring the safety and efficacy of biopharmaceutical drug products (5, 10).
Protein therapeutics can potentially induce an early innate immune response through cells that defend the host from infection in an immediate and nonspecific manner that is not associated with long term memory. An innate response can lead to a late stage T-cell mediated adaptive response, if a relevant danger signal is presented by an antigen presenting cell (APC)3 to a CD4+ T-cell (11, 12). This can result in the breaking of tolerance and induction of both T-cell dependent and B-cell mediated anti-drug antibody (ADA) responses. The development of ADA may in turn impact the safety and efficacy of the drug product (13–16). Several product- and patient-related factors are believed to contribute to the potential innate response and subsequent development of immunogenicity to protein therapeutics.
In vitro and in vivo assays using IFN-α (17–20), recombinant growth hormone (21, 22), and hemophilia factor VIII (23, 24), among other proteins, have shown that some protein aggregates can elicit an immune response. Growth hormone absorbed onto glass or alum particles and metal-catalyzed aggregates of IFN-α were shown to break tolerance in mice (18, 19, 21), whereas heat-aggregated factor VIII was found to be less immunogenic than the monomeric protein (23, 24). Predictive in vitro assays that can evaluate the impact of distinct types of IgG aggregates on cells of the immune system are essential for determining the risk associated with aggregates of therapeutic proteins, including monoclonal antibodies. These assays can be utilized at the early development phase of the molecule to investigate the impact of critical quality attributes, hence enabling quality by design.
Protein aggregates can occur during any stage of manufacturing and/or administration, with different stress treatments generating different populations of aggregates (25). These protein aggregates have widely varying properties such as particle size distribution and counts, particle morphology, chemical modifications, reversibility, percent aggregation, conformation, and particle surface hydrophobicity depending on the method of generation (26, 27). Small particulate size is one of the few characteristics that have been linked to enhancing the immune response (28). For instance, smaller sized adjuvant aggregates (3 μm) were preferentially internalized by dendritic cell phagocytosis over larger sizes (≥10 μm) (29), and smaller protein-coated beads (0.4 μm) yielded the highest T-cell responses as compared with other sizes (30). Protein aggregates in the 0.1–10 μm range are suspected of being the most immunogenic (1); however, it remains to be established if the aggregates found in therapeutic products in this size range can be linked to the induction of an ADA-mediated immune response.
The attributes of aggregates that may contribute to their ability to induce an immune response include the formation of neoepitopes, the immunomodulatory properties of the product, the exposure of post-translational modifications, the number of protein molecules that are linked to form the aggregate (i.e. monomers, dimers, and trimers to oligomers), and the concentration and size of such particulates (14, 17, 31, 32). Regulatory agencies are concerned about the safety impact of protein aggregates (3, 33, 34), as rigidly organized protein assemblies in the micron size range have been shown to be immunogenic (30, 35, 36). Protein aggregates that retain some of their folded structure may also trigger this response by mimicking rigidly organized protein arrays or by increasing the dose of antigen internalized. Unlike rigid arrays, protein aggregates, however, appear to be irregular and amorphous and thus are unlikely to display repeating antigenic motifs. The aggregates studied here were previously shown to be at least partially reversible upon dilution (26), indicating that they may pose less of a concern in a physiological environment. Host factors such as immune competence, route of administration, dose, and frequency can also play a role in propagation of the immune response.
The aim of this work was to use an in vitro cell-based model to study the potential impact of aggregated protein therapeutics on the immune system in vivo. Because the amount of protein aggregates in actual product is too low to allow for isolation and utilization in these types of studies, the aggregate had to be created by accelerated stress conditions. Previously, we generated aggregated forms of antibody-based therapeutics by a variety of different accelerated stress conditions and then classified them based on the traits identified (26, 27). These aggregates represent the type of particles that could be generated during various stages of the manufacturing process and therefore could potentially be present at very low amounts in formulated drug products. As these highly aggregated solutions could not be evaluated in the clinic due to safety concerns, we developed a clinically relevant in vitro human peripheral blood mononuclear cell (PBMC) assay to help rank the potential immunogenic risk of therapeutic aggregates. This assay helped avoid challenges from animal models (37, 38), incorporated the diversity of the human population, and mimicked the administration of drug and subsequent potential immune cell activation (39). These highly aggregated solutions represent a worst case scenario and should let us detect any potential impact of such formulations in a physiologically relevant system. In the results reported here, we have explored the ability of these different aggregate types to enhance potential biomarkers of the innate and adaptive responses using our in vitro cell-based assay, multiplex cytokine assessment, statistical analysis, biophysical characterization, inhibition experiments, and ex vivo T-cell assays. This study is the first to our knowledge that shows the effect of aggregate characteristics, including morphology, size, concentration, and degree of folded structure on the potential to induce an in vitro immune response in a population of human PBMC.
EXPERIMENTAL PROCEDURES
Aggregate Preparation
Purified human IgG2 monoclonal antibodies, mAb1 and mAb2, were supplied by Amgen as high concentration solutions. Human IgG1 monoclonal antibody (mAb3) is commercially available as a highly purified solution used therapeutically. Endotoxin levels were measured and found to be within the limit acceptable for these assays. Rates of incidence of immunogenicity in human studies are 44.7% for mAb1 and 0.1% for mAb3. Although mAb2 has not been tested in the clinic, current in silico methods that determine potential risk of protein sequence-associated immunogenicity as measured by HLA class II agretope content suggest that mAb2 is likely to have a higher level of clinical immunogenicity when compared with mAb1 (data not shown). mAb1 and mAb2 were diluted to 1 mg/ml in 10 mm acetate, pH 5.0, and mAb3 was diluted to 1 mg/ml in water (according to the manufacturer's instructions). The diluted samples before stress treatment were used to monitor the response to the monomeric form of the mAb (labeled monomer).
Aggregates were generated to simulate those that occur during the manufacturing, shipping, and administration of biotherapeutics, as described previously (26). For stirring stress, 2 ml of the protein sample was stirred with a 6 × 6-mm Teflon stirrer bar at ∼700 rpm, creating a vortex, in a 5-ml glass vial capped and placed vertically on a magnetic stir plate over 20 h (labeled stir-20h) or 3 days (labeled stir-3d). For syringe stress, the protein samples were manually pumped 50 times through a disposable 18-gauge × 1.5-inch needle (VWR Scientific) attached to a 3-ml disposable polyethylene syringe containing silicone oil (Fisher) (labeled syringe-so+). Thermal stress was used to assess the effect of aggregate of the unfolded antibody. To achieve this, the protein solution was diluted to 1 mg/ml in 10 mm acetate, pH 8.5, followed by incubation in a 65 °C bath for 1 h (labeled 65C/pH 8.5).
PBMC Preparation
Human PBMC were prepared as described in the supplemental material.
PBMC Stimulation Assay
PBMC from 22 donors were plated at 3 × 106 cells/ml in a total volume of 200 μl in a 96-well culture plate and acclimatized overnight in a 5% CO2 incubator at 37 °C. Acclimatized PBMC were challenged with the monomeric and aggregated solutions at 40 μg/ml (optimized in a dose titration curve, data not shown). Negative controls testing a stress-treated buffer (treated in the same manner as the aggregated samples) or medium-treated cells only (referred to as the background response), as well as a lipopolysaccharide (LPS)-positive control were also included. Of the 22 donors, 7 that were tested for a response to mAb2 were from different individuals than those tested for responses to mAb1 or mAb3. To assess the innate immune response, the challenged cells were incubated in a 5% CO2 incubator at 37 °C for an optimized time of 20 h (defined in an initial time course assay, data not shown). Cell derived supernatants were then frozen and stored at −70 °C for multiplexed cytokine assessment.
Multiplex Cytokine Analysis
Multiplex cytokine analysis was performed on culture supernatants using Luminex both in-house and by Myriad Rules-based Medicine (Myriad RBM, Austin, TX) as described in supplemental material. For data analysis, the stimulation index (SI) was calculated by dividing the amount of cytokine detected (pg/ml) in the treated sample by the relevant control sample. For the innate phase, a response was considered to be positive if the SI was two times greater than the relevant control (SI ≥2.0). The percentage of donors (% donors) that responded was calculated by taking the total number of donors that had an SI ≥2.0 as a percentage of the total number of donors tested.
Statistical Analysis
The objectives of the statistical analyses were first to determine whether the monomeric mAbs increased the cytokine response, as compared with the background, and second to probe the statistical significance of the increased cytokine response in the presence of the aggregated mAbs versus the cytokine response to their corresponding monomeric forms. A mixed effect model was used for this evaluation. The model assumed correlated responses from the same donor and independent responses from different donors. The response was either log transformed or rank transformed before the statistical analysis to ensure the validity of the statistical model. When evaluating the response to the monomeric mAb relative to background, the model included compound (mAb1, mAb2, and mAb3), condition (monomeric and background), and the two-way interaction between compound and condition as fixed effects, calculated for each cytokine. The response to each monomeric mAb was compared with the background response, and a one-sided p value was reported with the expectation that an increased response should be observed in the presence of the monomeric mAbs. When evaluating the response to aggregated mAbs relative to the response to the monomeric form, the model included compound (mAb1, mAb2, and mAb3), condition (different types of aggregates, monomeric and background), and the two-way interaction between compound and condition as fixed effects, calculated for each cytokine. The response to different types of mAb aggregates was compared with the response to the monomeric mAb or the background response, and the one-sided p values were reported with the expectation that an increased response should be observed in PBMC treated with aggregated mAbs. p values less than 0.048 were considered as statistically significant. Borderline p values between 0.048 and 0.050 were considered not significant based on known biology.
Protein-coated Microspheres
Polystyrene microspheres 5 μm in diameter (Bangs Laboratories) were coated with mAb1 by direct absorption. 3 mg/ml polystyrene microspheres were incubated with 80 μg/ml mAb1 in 10 mm acetate, pH 5.0, for ∼10 min at room temperature (mAb1-microspheres). Acclimatized PBMC from eight donors were challenged with mAb1-microspheres, monomeric mAb1, and mAb1-aggregated samples (stir-20h, stir-3d, 65C/pH 8.5, syringe-so+) at a mAb1 protein concentration of 40 μg/ml for 20 h. Uncoated microspheres, stress-treated buffer, medium only, and LPS were also tested as controls.
To determine the amount of mAb1 bound to the microspheres, the mAb1-microspheres were washed four times with 10 mm acetate, pH 5.0, in a total volume of 10 ml to remove any free mAb1, until protein could no longer be detected in the wash supernatant. The concentration of mAb1 on the microspheres was assessed by incubating the mAb1-microspheres in a reducing SDS buffer heated at 95 °C for 5 min to release all bound protein. Protein levels were quantitated by integration of peaks from rCE-LIF against a standard curve. Enough protein to form approximately a monolayer of antibodies on the surface of the microspheres was detected (data not shown).
To test mAb1-microspheres free of excess mAb1, mAb1-microspheres were generated by incubating the microspheres with a 10-fold excess of mAb1 for 2 h at room temperature with gentle mixing and then continued incubation overnight at 4 °C. The mAb1-microspheres were then washed, and the concentration was measured as above. Protein absorbed onto the surface was found to be stable after washing and for up to 1 month of storage (data not shown). PBMC from two of the eight donors were incubated with the washed mAb1-microspheres at 40, 20, 10, 1, 0.1, and 0.01 μg/ml mAb1 for 20 h. Supernatants were frozen and stored at −70 °C for multiplexed cytokine assessment.
Biophysical Characterization
Aggregated solutions were assessed for particle number and changes in secondary structure as described in our previous report (26), and in the supplemental material.
Cell Receptor Inhibition
To block individual FcγRs and TLRs, acclimatized PBMC from four donors were incubated with 10 μg/ml F(ab′)2 anti-FcγR1 (clone 10.1), anti-F(ab′)2 FcγRII (clone 7.3), or F(ab′)2 anti-FcγRIII (clone 3G8)(Ancell), or 20 μg/ml anti-TLR-2 or anti-TLR-4 (eBioscience) in a 5% CO2 incubator at 37 °C for 1 h (40). Isotype controls for the blocking antibodies from both the IgG1 and IgG2 subtypes were also tested. Monomeric and stir-20h samples were added to the blocked cells at 40 μg/ml and incubated in a 5% CO2 incubator at 37 °C for 20 h. The response to medium only and LPS was also monitored as controls.
Here, opsonization refers to the process by which the surface of an aggregate is coated by fragments of complement component 3 (C3) for ingestion and destruction by a phagocyte through relevant complement receptors. To prepare opsonized aggregates, trypsin-treated C3 was first prepared by adding 30 μl of 1 mg/ml complement C3 from human serum (Sigma) to 30 μl of 0.05% trypsin/EDTA (Invitrogen). The reaction sat at room temperature for 45 s and was stopped by adding 60 μl of growth media. Aggregates were then opsonized by adding 1 μl of trypsin-treated complement C3 to 100 μl of stir-20h samples (1 mg/ml) and incubating in a 37 °C bath for 1 h. Opsonization with heat-inactivated C3 was achieved by incubating the trypsin-treated C3 at 56 °C for 45 min before adding to the stir-20h samples. As a control, the monomeric mAbs were treated in an identical manner as the stir-20h samples. Acclimatized PBMC from the same four donors were challenged with opsonized aggregates at 40 μg/ml and incubated in a 5% CO2 incubator at 37 °C for 20 h. Supernatants from both experiments were assessed by multiplexed cytokine assessment as described. The % release was calculated by dividing the amount of cytokines detected when treatment with inhibitory antibodies or heat inactivation was performed by the amount detected in the absence of this treatment. Donors that showed at least a 20% reduction (below both the original values and the relevant isotype control) in monocyte-derived cytokines were considered inhibited.
CD4+ T-cell Assays
Ex vivo T-cell assays were performed to assess CD4+ T-cell responses by Antitope (Antitope Ltd., Babraham Institute, Cambridge, UK) (41–43). PBMC were isolated from 50 healthy donors from the UK National Blood Transfusion Service (Addenbrooke's Hospital, Cambridge, UK). PBMC were isolated using Lymphoprep (Axis-shield, Dundee, UK) density centrifugation, and CD8+ T-cells were depleted using CD8+ RosetteSep (StemCell Technologies Inc, London, UK). Donors were characterized by identifying HLA-DR haplotypes and selected to represent the world population. PBMC were added at a concentration of 4–6 × 106 cells/ml to the 24-well proliferation and enzyme-linked immunosorbent spot (ELISPOT) plates and then challenged with 40 μg/ml of the mAb2 monomeric or stir-20h samples.
Cultures for T-cell proliferation assays were incubated for a total of 8 days at 37 °C with 5% CO2. On days 5–8, the cells in each well were gently resuspended, transferred to a round bottomed 96-well plate, and then pulsed with 0.75 μCi of [3H]thymidine (PerkinElmer Life Sciences), and incubated for 18 h. Cultures were harvested onto filter mats (PerkinElmer Life Sciences) using a TomTec Mach III harvester (Hamden). Counts/min were determined by scintillation counting using a Wallac Microbeta TriLux (PerkinElmer Life Sciences) in ParaLux, low background counting. For each donor, responses to reproducibility controls, keyhole limpet hemocyanin (Pierce), peptides derived from influenza A and Epstein-Barr viruses, and medium only were monitored, and inter-assay variability was <10% (data not shown). Supernatants from 35 donors from day 7 were assessed by multiplex cytokine analysis (as described above) for the following 13 cytokines: GM-CSF, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, and TNF-α.
IL-2 ELISPOT plates were incubated for 8 days before developing according to the manufacturer's instructions (R&D Systems). Negative controls testing medium only or no cells as well as a mitogen-positive control were also included. Dried plates were scanned on an Immunoscan Analyzer, and spots per well were determined using Immunoscan version 4 software. For data analysis, the SI was calculated as the counts/min, spots per well, or picograms/ml for donors treated with aggregated mAbs divided by the background response. An SI ≥1.9 was considered positive, based on a statistically derived cut point for the population tested for an adaptive response.
RESULTS
In Vitro PBMC Assay for Innate Immune Reactivity
Before assessing the ability of the different mAb aggregates to elicit an immune response, an in vitro PBMC assay was used to evaluate the innate immune response in a diverse donor population. The responses of human PBMC from 22 naive healthy donors to two IgG2 monoclonal antibodies (mAb1 and mAb2) and one IgG1 monoclonal antibody (mAb3) were tested (Fig. 1). The commercial grade preparations of the antibody therapeutics, mAb1, mAb2, and mAb3, contain very low amounts of protein aggregates as formulated solutions (26). These solutions prior to aggregation were therefore labeled as monomer in the text and figures. mAb1, mAb2, and mAb3 were selected as they have varied rates of clinical or sequence-driven predicted immunogenicity. Rates of incidence of immunogenicity in human studies are 44.7% for mAb1 and 0.1% for mAb3. mAb2 has not been tested in the clinic; however, the predicted immunogenicity of mAb2 as determined by in silico methods suggests that mAb2 may have a higher rate of immunogenicity than mAb3 (data not shown).
FIGURE 1.
Representative cytokine profiles of a population of PBMC in response to monomeric or aggregated antibodies. A, cytokine expression profile of PBMC from one representative responding donor treated with either monomeric or aggregated mAb1. Multiplex cytokine analysis was used to detect the amount (pg/ml) of 47 cytokines released as an innate response. Vertical black lines represent cytokines that were found to increase in the presence of the aggregated mAbs above the monomeric mAb or background (SI ≥2.0) for this donor. B, heat map illustration of statistically significant increases in cytokine release from PBMC (15–22 donors) at the innate phase (20 h) treated with monomeric mAb1, mAb2, or mAb3. A subset of the 47 cytokines tested is shown, where the remaining cytokines were not impacted. p values generated by a mixed effect statistical model were used to indicate statistically significant differences between donors treated with each monomeric mAb and the background response. Cytokine abbreviations are as follows: A1AT, α1-antitrypsin; A2M, α2-macroglobulin; B2M, β2-microglobulin; BDNF, brain-derived neurotrophic factor; C3, complement 3; CRP, C reactive protein; GM-CSF, granulocyte-macrophage colony-stimulating factor; Hp, haptoglobin; ICAM-1, intercellular adhesion molecule-1; IFN-γ, interferon-γ; IL-1α, -1β, -1ra, -2, -3, -4, -5, -6, -7, -8, -10, -12p40, -12p70, -15, -17, -18, -23, interleukin-1α, -1β, -1 receptor antagonist, -2, -3, -4, -5, -6, -7, -8, -10, -12p40, -12p70, -15, -17, -18, and -23; MCP-1, monocyte chemotactic protein-1; MIP-1α, -1β, macrophage inflammatory protein-1α, -1β; MMP-2, -3, -9, matrix metalloproteinase-2, -3, -9; SCF, stem cell factor; TIMP-1, tissue inhibitor of metalloproteinase-1; TNF-α, -β, -RII, tumor necrosis factor-α, -β, -receptor II; VCAM-1, vascular cell adhesion molecule-1; VDBP, vitamin D-binding protein; VEGF, vascular endothelial growth factor; vWF, von Willebrand factor.
We first tested monomeric mAb1, mAb2, and mAb3 in the in vitro PBMC assay to determine the base-line response to each monomeric mAb prior to aggregation. The secretion of 47 different cytokines from a broad panel was assessed at the innate phase (20 h post-stimulation) by multiplex cytokine analysis. The 22 donor population showed a wide range of responses, which included high responders as well as nonresponders that only responded to the positive control. Fig. 1A displays a cytokine profile of PBMC generated in response to mAb1 from one representative responding donor. The highest increase in the cytokines induced were those associated with monocytes/macrophages. PBMC treated with growth media were used as a base-line for donor activation in all cases (background). A mixed effect statistical model was used to compare the response of PBMC treated with the three different molecules and the background response to identify cytokines that showed statistically significant increases. Fig. 1B shows a heat map of a subset of 31 of the total 47 cytokines that were tested, where the remaining 16 cytokines were not affected. mAb1 was found to have the greatest number of increased cytokines with statistically significant one-sided p values, as compared with mAb2 and mAb3, which is consistent with the high rate of immunogenicity associated with this molecule. The ability of the in vitro PBMC assay to identify the most immunogenic molecule at the innate phase demonstrates that this assay can be a useful tool for screening molecules for immunogenic potential. However, further studies must be conducted to determine whether there is a correlation between the in vitro PBMC assay at the innate phase and ADA produced by immunogenic therapeutics in vivo.
Aggregation of Biotherapeutics Promotes the Innate Immune Response
To generate aggregates that might be encountered during the manufacturing, storage, and delivery of biopharmaceuticals, mAb1, mAb2, and mAb3 were subjected to a variety of accelerated stress conditions. These stress methods are meant to represent conditions for changes that an antibody might go through during manufacturing or following clinical administration of the drug and to produce substantial amounts of aggregate so that they can be tested in the in vitro assays. Different types of stresses were used, including the following: stirring for 20 h (labeled stir-20h) or 3 days (labeled stir-3d), heating and raising the pH (labeled 65C/pH 8.5), and manually pumping though a syringe in the presence of silicone oil (labeled syringe-so+). As described in our previous report (26), all stressed samples contained substantial amounts of aggregated species. Commercial grade antibody solutions in the absence of stress (labeled as monomer in the table and figures) contained very low levels of protein aggregates and were used for comparison.
To determine whether the innate immune response to monomeric mAbs could be enhanced upon aggregation, the aggregated solutions were tested in the in vitro PBMC assay. PBMC from 22 donors were challenged with mAb1, mAb2, and mAb3 monomeric and aggregated samples, and the secretion of 47 cytokines was assessed at the innate phase (20 h) by multiplex cytokine analysis. Fig. 1A shows the variability of expression levels for different cytokines as well as cytokines that are enhanced in the presence of aggregates for this particular donor (Fig. 1A, vertical black lines). The following three types of calculations were performed on the cytokine profile data to evaluate activation of PBMC (Fig. 2). 1) The SI was calculated for each cytokine by dividing the amount of cytokine detected in the presence of aggregates by the amount detected in samples treated with the relevant monomeric mAb (Fig. 2A, colored bars). An SI ≥2.0 was considered positive. 2) The percentage of donors that responded (% donors) was calculated as the percentage of donors that had an SI ≥2.0 above the relevant monomeric mAb (Fig. 2A, gray bars). 3) One-sided p values were generated by applying a mixed effect statistical model to identify cytokines that responded to aggregated mAbs with statistically significant increases over the relevant monomeric mAb or background (Fig. 2B). Calculated values were placed in a unique population bar graph to assist with the simultaneous visualization of several data sets including the following: the SI of individual donors, the average SI of responding donors, and the percentage of donors that responded. The effect of aggregates can be interpreted by looking at both the response of the individual (shown in Fig. 2A, by the magnitude and percentage of responding donors) and the average response of the population (shown in Fig. 2B, by the statistical significance of the response across all donors).
FIGURE 2.
Aggregation of biotherapeutics enhances the innate response of PBMC. PBMC from 22 donors were challenged with either monomeric or aggregated mAbs and tested for the release of signature cytokines by multiplex cytokine analysis at the innate phase (20 h). A, average SI of positive donors (SI ≥2.0, colored bars) and percentage of donors that responded (% donors, gray bars) to the aggregated mAb above the monomeric mAb is shown for mAb1, mAb2, and mAb3. Black dots depict responding individuals and highlight the distribution of responses across the population tested. These population bar graphs highlight the response of individuals to capture all events. Different aggregate types are depicted horizontally as follows: stir-20h (red), stir-3d (orange), 65C/pH 8.5 (green), and syringe-so+ (blue). B, heat maps of statistically significant increases in the cytokine signature above the monomeric mAb (top panel) or background (bottom panel) are shown for mAb1, mAb2, and mAb3. These panels reflect the average response of the population. p values generated by a mixed effect statistical model were used to indicate statistically significant differences between donors treated with the aggregated mAbs and the same donors treated with either the monomeric mAb or the background response.
All aggregate types tested (stir-20h, stir-3d, 65C/pH 8.5, and syringe-so+) induced an increase in cytokine release compared with the monomeric form of the molecule, with the extent of the increase dependent on the aggregate type, demonstrating that aggregation of biotherapeutics can enhance the innate immune signal to varying degrees (Fig. 2). A combination of eight cytokines was identified as a potential biomarker of PBMC in response to aggregates based on the overall SI of the response, the % donors that responded, and the statistical significance of the response when it occurred (Fig. 2 and Table 1). Table 1 summarizes the average change in these parameters for each cytokine in the signature in the presence of stir-20h aggregates across all three mAbs. The cytokine signature included the following: IL-1β, IL-6, IL-10, MCP-1, MIP-1α, MIP-1β, MMP-2, and TNF-α. The response also appeared to depend on molecule and aggregate type. For instance, aggregates of mAb1 and mAb2 induced a greater overall response than aggregates of mAb3, consistent with the immunogenicity ranking of the monomeric molecules. However, in the case of mAb1, the strong monomeric signal decreased the apparent strength of response to aggregated mAb1 (Fig. 2B, mAb1 only). Of all the aggregate types tested, the stir-20h aggregates induced the highest response by all analyses employed (SI, % donors and statistical significance) (Fig. 2 and Table 1). It is important to note that several of the cytokines in the signature, including IL-6 (44) and IL-10 (45, 46), have been shown to mediate anti-inflammatory and immunosuppressive effects that may counteract the formation of long term ADA and immunogenicity.
TABLE 1.
Cytokine signature of PBMC as a potential biomarker of the response to aggregates
| Cytokine | Average SI (above monomer)a | Average responding donorsb | Concentration rangec | p valued |
|---|---|---|---|---|
| % | pg/ml | |||
| IL-1β | 12 ± 10 | 23 | 1–190 | *** |
| IL-6 | 14 ± 14 | 23 | 5–12,800 | *** |
| IL-10 | 25 ± 27 | 18 | 1–410 | *** |
| MCP-1 | 16 ± 37 | 47 | 360–52,700 | *** |
| MIP-1α | 31 ± 32 | 24 | 110–18,600 | *** |
| MIP-1β | 15 ± 17 | 29 | 320–82,000 | *** |
| MMP-2 | 5 ± 2 | 29 | 2,830–68,500 | *** |
| TNF-α | 38 ± 51 | 26 | 4–960 | *** |
a Average SI of cytokine secretion for responding donors of a 22-donor set was calculated for mAb1, mAb2, and mAb3 stir-20h samples above the relevant monomeric mAb. The standard deviation represents the variability of the population.
b The average percentage of a 22-donor set that responded to mAb1, mAb2, or mAb3 stir-20h samples.
c Concentration range extends over the minimum and maximum concentration of cytokines detected for responding donors.
d Representative p values for mAb2 are shown. p values generated by a mixed effect statistical model were used to indicate statistically significance differences between 22 donors treated with either the mAb2 monomer or stir-20h samples. Statistically significant increases are denoted with three asterisks (p value < 0.001).
High Particle Numbers Are Required for Aggregate Activation of the Innate Response
We next tested the effect of an aggregate of defined size and concentration by absorbing protein onto microspheres, which could also potentially mimic protein coated onto foreign particles of similar size and shape. Protein-coated microspheres were generated by coating polystyrene microspheres 5 μm in diameter with mAb1 (labeled mAb1-microspheres). PBMC from eight donors were tested for their response at the innate phase (20 h) to mAb1-microspheres and mAb1 stir-20h, stir-3d, 65C/pH 8.5, and syringe-so+ aggregated samples (Fig. 3). The highest percentage of donors was found to respond above the monomeric mAb1 to the mAb1-microspheres (100%), followed by the other aggregate types (14–41%) (Fig. 3, A and B). The activation appeared to require the presence of protein as the uncoated microspheres caused a response above the monomeric mAb1 in only 13% of donors (Fig. 3A). Most of the individual cytokines in the signature also showed a higher response to mAb1-microspheres than any other aggregate type both by the average SI of responding donors and the % donors that responded (Fig. 3, C and D). All eight cytokines in the signature were found to increase in a statistically significant manner (p value < 0.001) in response to the mAb1-microspheres.
FIGURE 3.
High particle numbers correlate with and are required for aggregate activation. PBMC from eight donors were challenged with either mAb1-microspheres or mAb1 aggregates and assessed for cytokine secretion by multiplex cytokine analysis at the innate phase (20 h). A, number of particles in the mAb1-microspheres and aggregated samples were determined by light obscuration. Responses were compared between samples that had either an equal concentration of the mAb1 protein or equal particle numbers. B, average dose response of two donors to mAb1-microspheres. The average SI above background and standard deviation representing the variability of the population tested are shown. The number of particles at each concentration of mAb1-microspheres tested is shown, where the black arrow indicates the approximate threshold of particles for activation. C and D, average SI of positive donors (SI ≥2.0, colored bars) and percentage of donors that responded (% donors, gray bars) to mAb1-microspheres and mAb1 aggregates above monomeric mAb1. Black dots depict responding individuals.
The relationship between the number of particles in the 2–150 μm range and the observed innate immune response was assessed by light obscuration. At an equal concentration of mAb1, the level of response varied based on the aggregate type but seemed to correlate with the number of particles detected (Fig. 3A, left graph). For example, the mAb1-microspheres had the highest number of particles and also induced a much higher response than any of the other aggregate types. However, when the mAb1-microspheres and stir-20h samples that had an equal number of particles were compared, the relative response of PBMC appears similar (Fig. 3A, right graph). These results suggest that particle number may be a dominant factor in determining if an immune response occurs. A dose response to mAb1-microspheres revealed that high particle numbers may be required to break the threshold of activation in vitro as a response could not be detected in samples that had ≤3 × 104 particle numbers/ml (Fig. 3B).
Specific Aggregate Characteristics Correlate with Immune Reactivity
Additional biophysical characterization of mAb1, mAb2, and mAb3 aggregates was performed to determine the correlation of aggregate characteristics with the induction of the innate immune response. Table 2 shows a comparison between the relative response of PBMC to different aggregate types and the biophysical characteristics of these aggregates. The response of PBMC to aggregates is depicted as + symbols, where the number of symbols depends on the relative magnitude of the response and the number of donors that responded (see Fig. 2). Particle counting in the nanometer range (20–1000 nm) was determined by nanoparticle tracking analysis, detection of particles in the micron-sized range (≥2 μm) was achieved by light obscuration, and changes in secondary structure were assessed by Fourier transform infrared (FTIR) spectroscopy, as described previously (26). The relative response of PBMC was found to correlate with the number of particles in the 2–10 μm size range and the retention of at least a partially folded mAb secondary structure (Table 2). The high response seen to the mAb1-microspheres, which are 5 μm in diameter, may further indicate that this is close to the optimum size range. As a final indication that there is an optimum size range for enhancing the innate immune response at least in this assay, mAb1-coated nanospheres (50 nm diameter) were generated and were found to stimulate PBMC at a level well below the mAb1-microspheres (5 μm diameter) (data not shown). Aggregates that maintained a higher degree of their folded structure were also associated with a higher immune response. For example, stir-20h aggregates maintained the highest percentage of their folded secondary structure (average across molecules of 42–46%) of all aggregates types and also induced the highest immune response (Table 2). The mAb1-microspheres also maintained the highest percentage of the mAb1 folded structure (70%) of all samples tested (Table 2 and Fig. 4).
TABLE 2.
Particle characteristics (2–10 μm size; partially folded conformation) correlate with the induction of the innate immune response
| Molecule | Stress treatment | Response of PBMCa | Particle concentrationb |
Folded secondary structurec | p valued | ||
|---|---|---|---|---|---|---|---|
| <2 μm | 2–10 μm | >10 μm | |||||
| % | |||||||
| mAb1 | microspheres | +++++e | – | +++++ | NDf | 70g | *** |
| stir-20h | ++++ | ++++ | ++++ | ++ | 42 | *** | |
| stir-3d | +++ | + | +++ | +++ | 20 | * | |
| 65C/pH 8.5 | +++ | ++ | ++++ | +++ | 26 | *** | |
| syringe-so+ | +++ | ++++ | +++ | ++ | 32 | * | |
| monomer | ++ | + | + | ND | 100 | ** | |
| mAb2 | stir-20h | +++++ | – | ++++ | ++ | 46 | *** |
| stir-3d | ++++ | – | ++++ | +++ | 33 | *** | |
| 65C/pH 8.5 | +++ | – | ++++ | ++++ | 12 | *** | |
| syringe-so+ | + | – | +++ | ++ | 49 | / | |
| monomer | + | – | ++ | + | 100 | / | |
| mAb3 | stir-20h | ++++ | ++++ | ++++ | ++ | – | *** |
| stir-3d | +++ | ++ | ++++ | ++ | – | ** | |
| 65C/pH 8.5 | +++ | + | +++ | ++++ | – | / | |
| syringe-so+ | +++ | ++++ | +++ | +++ | – | / | |
| monomer | ND | + | + | + | 100 | / | |
a The relative response of PBMC from 22 donors at the innate phase to mAb1, mAb2, and mAb3 monomer or aggregated samples is shown. The + symbol indicates the relative strength of the aggregate-induced response in PBMC as measured by the magnitude of the response and the number of donors that responded (Fig. 2).
b Particle concentration in discrete size ranges was determined by nanoparticle tracking analysis (<2 μm) or HIAC (2–10 μm; >10 μm). The + symbol indicates the relative fold increase (26).
c Correlation coefficient (%) between the spectra of the monomeric mAb and pellet fraction of the aggregated mAb is shown (26).
d p values generated by a mixed effect statistical model were used to indicate statistically significant differences between 22 donors treated with aggregated mAbs as compared with the background response. MCP-1 was used as a representative cytokine. Eight donors were used for the microsphere calculation. Statistically significant increases are denoted with one asterisk (p value < 0.048), two asterisks (p value < 0.010), or three asterisks (p value < 0.001). / indicates no significant differences (p value >0.048).
e + indicates the relative fold increase or extent of change, and – means not tested.
f ND means not detected.
g Average of several analyses is shown. Data showed variability due to low concentration of protein on the mAb1-microspheres and because the microspheres themselves have a spectra. All of the mAb1 aggregates, but not the mAb1-microspheres, displayed a shift in the minima compared with the mAb1 monomer spectra.
FIGURE 4.
mAb1-microspheres retain a high percentage of their mAb1 folded structure. A, overlaid second derivative FTIR spectra of the monomeric mAb1 (black line), aggregated mAb1 pellet fractions (red, orange, and green lines), and mAb1-microspheres (purple line) show varying degrees of structural changes. B, % correlation coefficient (CC) was calculated between the spectra of the monomeric protein and the aggregated samples or mAb1-microspheres.
Aggregates Interact with FcγRs, TLRs, and Complement to Induce an Innate Response
To investigate if the mechanism of aggregate activation of PBMC is mediated by specific surface receptors or is nonspecific, we evaluated the role of several cell receptor classes. PBMC were incubated with mAbs against different FcγRs (FcγRI, FcγRII, and FcγRIII) or TLRs (TLR-2 and TLR-4) (47) and then challenged with mAb1, mAb2, and mAb3 monomeric and stir-20h aggregated samples (Fig. 5). Supernatants were assessed at the innate phase (20 h) by multiplex cytokine analysis. We observed partial inhibition in the presence of inhibitors of FcγRs, which seemed to be dependent on the class of receptor and molecule IgG subtype (Fig. 5B). Anti-FcγRIII inhibited more donors (50–75%) than anti-FcγRI or anti-FcγRII (25% for most cases). Anti-FcγRI showed more inhibition of the mAb3 stir-20h sample as compared with the other mAbs, perhaps due to the higher affinity of FcγRI for the IgG1 subtype. It is important to note that anti-FcγRII alone increased the background response of PBMC, so to minimize this effect, lower concentrations of FcγRII were tested, but no inhibition was observed. A larger reduction in cytokine release was detected in response to stir-20h aggregates in the presence of inhibitors of several of the TLRs (TLR-2 and TLR-4) (Fig. 5, A and B). Inhibition was seen for all molecules tested (mAb1, mAb2, and mAb3) and in the majority of donors for both TLR-2 (75–100% donors) and TLR-4 (100% donors) (Fig. 5B). A similar inhibitory effect was observed for cells challenged with the known TLR agonist, LPS (48), in the presence of anti-TLR-2 and anti-TLR-4 (data not shown).
FIGURE 5.
Mechanism of antibody aggregate activation is mediated by cell surface receptors. A, PBMC were challenged with stir-20h aggregates in the absence and presence of inhibitory antibodies of cell surface receptors (FcγRI, FcγRII, FcγRIII, TLR-2, and TLR-4) or stir-20h aggregates that had been opsonized with catalytically active C3b or heat-inactivated C3b (56 °C). Supernatants were assessed by multiplex cytokine analysis at the innate phase (20 h). Cytokine release was calculated as a percentage of the total response to stir-20h aggregates in the absence of inhibitory antibodies or heat inactivation. Values shown represent the average of duplicate tests from one representative donor. B, percentage of donors (four donors tested) that were inhibited from responding to stir-20h aggregates by blocking antibodies or heat inactivation.
The innate immune response could also occur through the complement pathway by opsonization of Fc-containing aggregates by complement proteins. To test the involvement of the complement system, stir-20h aggregates were opsonized with C3b fragments (generated from trypsinized C3) and then added to PBMC. Stir-20h aggregates that had been opsonized with heat-inactivated C3b were also tested to assess the requirement of catalytically active C3b. Although C3b-opsonized stir-20h aggregates that were catalytically active showed maximal cytokine release, heat inactivation of C3b before opsonization of the aggregates resulted in ≥80% reduction in release of the cytokines shown (Fig. 5A). Inhibition was observed for all molecules tested (mAb1, mAb2, and mAb3) and in the majority of donors (75–100%) (Fig. 5B).
Innate Signal to Aggregates Can Progress to the Adaptive Phase
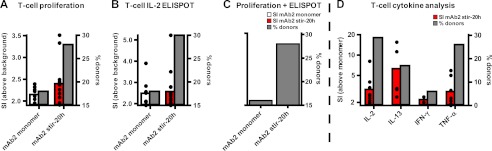
To determine whether the innate signal could advance to an adaptive T-cell response, the mAb2 stir-20h sample was tested in ex vivo T-cell assays (41–43). CD4+ T-cell responses from 50 healthy individuals were monitored over 8 days in the presence of mAb2 monomeric and stir-20h samples, by T-cell proliferation ([3H]thymidine uptake), IL-2 ELISPOT, and multiplex cytokine assessment (Fig. 6). The average CD4+ T-cell response increased in the presence of the mAb2 stir-20h sample compared with the mAb2 monomeric sample in all assays. T-cell proliferative responses were found to increase in donors treated with mAb2 stir-20h (average SI above background = 2.35; % donors = 28%), as compared with monomeric mAb2 (average SI above background = 2.15; % donors = 18%) (Fig. 6A). IL-2 secretion by CD4+ T-cells as detected by ELISPOT was greater in donors treated with mAb2 stir-20h (average SI above background = 2.57; % donors = 30%), as compared with the mAb2 monomeric sample (average SI above background = 2.49; % donors = 18%) (Fig. 6B). Overall, the number of donors that responded in both the T-cell proliferation and IL-2 ELISPOT assays increased for mAb2 stir-20h (28%) compared with the monomeric form (16%) (Fig. 6C). Multiplex cytokine assessment of the supernatants on day 7 showed the increase of several T-cell effector cytokines (IL-2, IL-13, IFN-γ, and TNF-α) in donors treated with mAb2 stir-20h above the monomeric form (Fig. 6D). These results suggest that the innate immune response to aggregates has the potential to develop into an adaptive T-cell mediated response.
FIGURE 6.
Average CD4+ T-cell response from 50 individuals was enhanced when challenged with aggregated biotherapeutic. The CD4+ T-cell response from 50 healthy donors to mAb2 monomeric and stir-20h samples was assessed at the adaptive phase (up to 8 days) using T-cell proliferation assays ([3H]thymidine uptake) (A) and IL-2 cytokine secretion (ELISPOT) (B). C, percentage of donors that responded positively in both the T-cell proliferation and IL-2 ELISPOT assays. The average SI of positive donors (SI ≥1.9) above the background response and the percentage of donors that responded are shown. D, supernatants were assessed for T-cell effector cytokines by multiplex cytokine analysis. The average SI of positive donors (SI ≥1.9) above the response to the monomeric mAb is shown. Black dots depict responding individuals.
DISCUSSION
Aggregates and particles are intrinsic to manufacturing and storage of biotherapeutics. We have studied the impact of different types of aggregated mAb therapeutics on the in vitro induction of potential biomarkers of the innate and adaptive immune responses in human PBMC. Overall, aggregated mAbs enhanced the innate and adaptive immune responses in vitro; however, this response was dependent on the inherent immunogenicity of the monomeric mAb, the specific properties of the aggregate type tested, and the responsiveness of the individual donor, and it required a very high number of particles, well beyond that seen in manufactured products, at least in this in vitro system.
A cytokine signature was identified as a potential biomarker for the immune response to aggregates that could be helpful in predicting clinical immunogenic risk of a protein therapeutic. Cytokine signatures were established based on the following four parameters: breadth of the response (number of cytokines induced), magnitude of the response (amount of cytokine induced), diversity of the response (percentage of responding subjects), and statistical significance of the response. General statistically derived cut-points were previously established for spontaneous and monomeric-biotherapeutic induced cytokine secretion (39). At least one type of aggregate tested for each mAb induced a higher cytokine signature than the respective monomeric form. Three types of cytokine signatures were observed as biomolecular markers for an immune response as follows. The first was a signature specific to the identity of the mAb. For example, mAb1, which has a target-mediated immunomodulatory effect, induced the following cytokines: ICAM-1, IFN-γ, IL-1ra, IL-7, MCP-1, MIP-1α, MIP-1β, MMP-2, TNF-α, and TNF-β; p value <0.048. The second was a signature induced by the majority of aggregates tested, not dependent on IgG subclass. This included the following: MCP-1, MIP-1α, MIP-1β, MMP-2 and TNF-α; p value <0.048. The third was a signature identified in response to the stir-20h aggregates, also not dependent on IgG subclass. This included the following: IL-1β, IL-6, IL-10, MCP-1, MIP-1α, MIP-1β, MMP-2 and TNF-α; p value <0.048. Upon examination of these signatures, it appears that there is a loss of some of the monomer induced cytokines upon aggregation, possibly due to aggregate formation masking the epitopes or due to changes in epitopes following unfolding of the molecule. Furthermore, additional cytokines were identified in the stir-20h aggregate signature, perhaps implicating the formation of neoepitopes resulting from a partial conformational change within a molecule and the involvement of new pathways. The functional consequences induced by these cytokines range from activation of danger signals, generating chemokine gradients, and control of the migration of inflammatory cells (49).
This study does not address whether the proposed cytokine biomarkers are predictive of an adverse response due to aggregates in formulations administered in the clinic. It is worth noting that some of the pro-inflammatory cytokines proposed as part of the cytokine signature to aggregates (IL-1β, TNF-α, IL-6, and IL-8) are also implicated in the underlying mechanism for immunogenicity-related adverse events following clinical administration (50, 51). Hence, the signature observed in the in vitro assay might be predictive of an immune response to protein aggregates if these species were present in sufficient amounts. Our study also indicates that, similar to adverse events in clinical studies, cytokine release is a consequence of direct activation of immunocompetent cells. Monitoring the correlation of adverse events in the clinic with the amount of protein aggregate in different lots of a commercial product will be useful for determining whether the aggregate biomarker proposed here is predictive in humans in vivo. Also worth noting is that the overall cytokine levels induced by the aggregates were much lower than that induced by the positive control, LPS, in all cases.
High particle numbers appear to be a necessary and dominant factor in an aggregate-induced immune response in vitro. It should be noted that a high number of particles might not be representative of therapeutics administered in vivo. In this set of experiments, all of the aggregated solutions tested contained very high amounts of particles, well beyond what would be typically found in marketed products. The highest response was detected to the mAb1-microspheres that also had the highest number of particles in the 2–150 μm range. In addition, the response elicited from each aggregated sample appeared to directly correlate with the total number of particles between 2 and 150 μm (see Fig. 3A). For example, the stir-20h aggregated sample had the greatest number of subvisible particles next to the mAb1-microspheres, and it also induced the highest innate response of all aggregated samples. When an equal number of particles was compared for the mAb1-microspheres and the stir-20h samples, the relative SI appeared similar, further indicating that the number of particles present is an important factor in eliciting an innate immune response. It is important to mention that there are additional differences between these two samples that could affect their ability to induce an immune response, including the following: degree of heterogeneity, size distribution, chemical modifications and composition, shape, and formation of a regular array, to name a few. Furthermore, a response to even the most immune reactive sample, mAb1-microspheres, could only be detected at particle numbers >104/ml, which is much higher than that found in drugs used therapeutically. Thus, the threshold for activation of the immune response to aggregates appears to be quite high. It is worth noting that the threshold of activation by aggregates may differ for intravenous and subcutaneous administration based on the nature of APCs being targeted as well as the “depo effect” associated with subcutaneous delivery.
Diverse kinds of aggregates induce distinct innate responses based on their biophysical attributes. Aggregates induced by stirring for 20 h elicited the maximal cytokine response by both magnitude (breadth of cytokines induced), strength (SI), and diversity (percentage subjects responding). These aggregates had a particle size predominantly in the 2–10 μm range. Previous studies have shown that particles of this size range are efficiently taken up by the phagocytic activity of monocytes (52, 53). These observations were confirmed in our experiments that showed that 100% of donors responded to the mAb1-microspheres, which were 5 μm in diameter. Likewise, aggregates made by syringe stress had an equal number of nanoparticles but 10-fold fewer particles in the 2–10 μm range as compared with aggregates made by stirring for 20 h and induced a lower immune response. Aggregates generated by stirring for 20 h also retained the largest percentage of their folded structure (average across molecules of 42–46%) compared with all other aggregate types tested (26). In contrast, aggregates that were made by prolonged stirring over 3 days, or by heat and pH denaturation, were larger in size, had lost a greater percentage of their folded structure, and were also less effective in inducing an immune response. As further support of the importance of conformation, the protein on the mAb1-microspheres retained most of the mAb1 folded structure (70%). These results indicate that a high number of particles in the 2–10 μm size range combined with the preservation of at least some folded structure are required to induce a robust cytokine response. Furthermore, the classification of these aggregates as described in our previous report (26) suggests that members of class 3 and 5 (defined as small sized, at least partially folded, and partially reversible) would induce the highest response as they possess both of these characteristics.
Our observations suggest that aggregated mAb therapeutics may signal through specific cell surface receptors to contribute to the mechanism of induction of an innate cytokine response. Aggregates could induce an innate cytokine response in PBMC by at least four mechanisms as follows: 1) interaction with specific receptors (e.g. TLRs) on APC and inflammatory cells that recognize repeated motifs, which mimic pathogen-associated molecular patterns; 2) interaction with Fc receptors; 3) interaction with the complement component of the innate immune system, and 4) increased relative dosage of epitopes during the efficient processing and presentation of T-cell epitopes, due to the increased number of mAb molecules present in a single ingested aggregate versus a single monomer. Cytokine secretion induced by any of these mechanisms has the potential to induce clinical responses that range from no clinical effect, induction of danger signals for activation of antigen-specific immune responses (54–57), to tissue inflammation. In our experiments, the largest inhibition of aggregate activation was seen in the presence of inhibitors of the TLRs (TLR-2 and TLR-4). This inhibition occurred across a range of cytokines in the signature, in all three mAbs, and in the majority of donors tested, implicating a role of the TLR family in the mechanism of the response to aggregates. In addition, partial inhibition was observed in the presence of inhibitors of the FcγRs (58, 59), which seemed to be dependent on the class of receptors and IgG subtypes. Anti-FcγRIII showed the largest inhibition of the FcγRs, which is consistent with other reports implicating this receptor class in responding to soluble IgG aggregates (60), IgG1 complexes (61), and immune complexes (62). Inhibition of opsonized aggregates was also seen upon heat inactivation of complement, implicating the contribution of the complement pathway to the mechanism of inflammatory responses.
The innate immune response to aggregates has the potential to progress to the adaptive phase as shown in ex vivo T-cell assays (41). mAb2 stir-20h aggregates were found to induce T-cell proliferation and a T-cell inflammatory cytokine profile above the monomeric form of the protein (see supplemental material). mAb2 aggregates were also very effective inducers of the innate immune response. These observations suggest that an efficient priming of APC during the innate phase by aggregates could propagate to the adaptive phase where the same aggregates can now induce T-cell specific cytokines. The strength and nature of the signal delivered through the aggregated biotherapeutics can drive the adaptive response. If the signal is derived from a molecule known to have significant immunogenicity, the aggregate could potentially present a higher amount of immunogenic epitopes to the APCs as more epitopes would be present in each protein aggregate internalized. Alternatively, the signal from an aggregated biotherapeutic with low numbers of immunogenic epitopes might induce a nonspecific bystander T-cell response. In the absence of co-stimulation and antigen presentation, the signals through the T-cell receptor might not propagate to a fully developed antigen specific T-cell response. The nature of the adaptive T-cell responses (antigen-specific versus bystander) is currently being investigated. Our study indicates that aggregation of a molecule with intrinsic immunogenic epitopes can enhance an innate response that can propagate to a late stage adaptive response mediated by T-cells. However, the ability of this response to mature to a T-cell driven B-cell response is not known. It is also important to note that the nature of the connection between the innate and adaptive immune responses to aggregates has not been established and requires further evaluation.
The type of antigen that an aggregate contains might drive the T-cell response and determine whether the T-cell response is inflammatory or immunosuppressive. For example, aggregated forms of ovalbumin, ProDer P (63), and influenza vaccines (64), can produce an inflammatory T-cell response signified by IFN-γ secretion, whereas aggregated factor VIII has been shown to induce IL-10 by immunosuppressive T-cells instead of IFN-γ. This suggests that aggregate challenge is not always immunogenic and can instead be immunosuppressive, possibly due to the masking of immunogenic epitopes. In our study, IL-10 secretion from PBMC was found to increase in response to aggregated mAb1, mAb2, and mAb3 above the relevant monomeric mAb at the innate phase (Fig. 2 and supplemental Fig. 1). The detection of the immunosuppressive cytokine IL-10 at the innate phase may implicate immunological tolerance, leading to the generation of aggregate-specific tolerance in memory T-cells (23, 65) (see supplemental material).
The experiments presented in this study were used to understand the impact of attributes of aggregates/particulates in monoclonal antibody-based formulations on potential biomarkers of the innate and adaptive immune responses. It is worth noting that these results are based on an in vitro model and may not be representative of what occurs in vivo. Additional assays using a wider array of molecules and in a broader scope of testing systems will be essential for establishing the signature. The use of in vitro cytokine signatures in PBMC, the ability of biotherapeutics to induce in vitro bystander T-cell responses, and the evaluation of in silico MHC-binding epitopes could comprise a series of biomarkers that have the possibility of predicting the potential immunogenicity of aggregates/particles in biotherapeutics.
Supplementary Material
Acknowledgments
We thank Taruna Arora, Vivian Bi, Gloria Juan, Arunan Kaliyaperumal, Dan Mytych, Jilin Sun, Jette Wypych, and Jiansong Xie for useful discussions, Cynthia Li and Danika Wullner for technical assistance, and David Brems, Joseph Phillips, and Steve Swanson for critically reading the manuscript.

This article contains supplemental Experimental Procedures, Fig. 1, and additional references.
- APC
- antigen presenting cell
- PBMC
- peripheral blood mononuclear cell
- ADA
- anti-drug antibody
- TLR
- toll-like receptor
- SI
- stimulation index
- ELISPOT
- enzyme-linked immunosorbent spot
- MCP-1
- monocyte chemotactic protein-1
- MIP-1α
- MIP-1β, macrophage inflammatory protein-1α, -1β
- MMP-2
- matrix metalloproteinase-2.
REFERENCES
- 1. Carpenter J. F., Randolph T. W., Jiskoot W., Crommelin D. J., Middaugh C. R., Winter G., Fan Y. X., Kirshner S., Verthelyi D., Kozlowski S., Clouse K. A., Swann P. G., Rosenberg A., Cherney B. (2009) Overlooking subvisible particles in therapeutic protein products. Gaps that may compromise product quality. J. Pharm. Sci. 98, 1201–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. den Engelsman J., Garidel P., Smulders R., Koll H., Smith B., Bassarab S., Seidl A., Hainzl O., Jiskoot W. (2011) Strategies for the assessment of protein aggregates in pharmaceutical biotech product development. Pharm. Res. 28, 920–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenberg A. S. (2006) Effects of protein aggregates. An immunologic perspective. AAPS J. 8, E501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh S. K. (2011) Impact of product-related factors on immunogenicity of biotherapeutics. J. Pharm. Sci. 100, 354–387 [DOI] [PubMed] [Google Scholar]
- 5. Demeule B., Gurny R., Arvinte T. (2006) Where disease pathogenesis meets protein formulation. Renal deposition of immunoglobulin aggregates. Eur. J. Pharm. Biopharm. 62, 121–130 [DOI] [PubMed] [Google Scholar]
- 6. Reichert J. M. (2010) Antibodies to watch in 2010. MAbs 2, 84–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reichert J. M. (2011) Antibody-based therapeutics to watch in 2011. MAbs 3, 76–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahler H. C., Friess W., Grauschopf U., Kiese S. (2009) Protein aggregation. Pathways, induction factors, and analysis. J. Pharm. Sci. 98, 2909–2934 [DOI] [PubMed] [Google Scholar]
- 9. Singh S. K., Afonina N., Awwad M., Bechtold-Peters K., Blue J. T., Chou D., Cromwell M., Krause H. J., Mahler H. C., Meyer B. K., Narhi L., Nesta D. P., Spitznagel T. (2010) An industry perspective on the monitoring of subvisible particles as a quality attribute for protein therapeutics. J. Pharm. Sci. 99, 3302–3321 [DOI] [PubMed] [Google Scholar]
- 10. Roberts J. P. (2011) Delving deeper into marker development. Genet. Eng. Biotechnol. News 31, 7 [Google Scholar]
- 11. Matzinger P. (2002) The danger model. A renewed sense of self. Science 296, 301–305 [DOI] [PubMed] [Google Scholar]
- 12. Matzinger P. (2007) Friendly and dangerous signals: is the tissue in control? Nat. Immunol. 8, 11–13 [DOI] [PubMed] [Google Scholar]
- 13. Patten P. A., Schellekens H. (2003) The immunogenicity of biopharmaceuticals. Lessons learned and consequences for protein drug development. Dev. Biol. 112, 81–97 [PubMed] [Google Scholar]
- 14. Schellekens H. (2002) Immunogenicity of therapeutic proteins. Clinical implications and future prospects. Clin. Ther. 24, 1720–1740 [DOI] [PubMed] [Google Scholar]
- 15. Wang W., Kelner D. N. (2003) Correlation of rFVIII inactivation with aggregation in solution. Pharm. Res. 20, 693–700 [DOI] [PubMed] [Google Scholar]
- 16. Wills R. J., Ferraiolo B. L. (1992) The role of pharmacokinetics in the development of biotechnologically derived agents. Clin. Pharmacokinet 23, 406–414 [DOI] [PubMed] [Google Scholar]
- 17. Braun A., Kwee L., Labow M. A., Alsenz J. (1997) Protein aggregates seem to play a key role among the parameters influencing the antigenicity of interferon α (IFN-α) in normal and transgenic mice. Pharm. Res. 14, 1472–1478 [DOI] [PubMed] [Google Scholar]
- 18. Hermeling S., Aranha L., Damen J. M., Slijper M., Schellekens H., Crommelin D. J., Jiskoot W. (2005) Structural characterization and immunogenicity in wild-type and immune tolerant mice of degraded recombinant human interferon α2b. Pharm. Res. 22, 1997–2006 [DOI] [PubMed] [Google Scholar]
- 19. Hermeling S., Schellekens H., Maas C., Gebbink M. F., Crommelin D. J., Jiskoot W. (2006) Antibody response to aggregated human interferon α2b in wild-type and transgenic immune tolerant mice depends on type and level of aggregation. J. Pharm. Sci. 95, 1084–1096 [DOI] [PubMed] [Google Scholar]
- 20. Maas C., Hermeling S., Bouma B., Jiskoot W., Gebbink M. F. (2007) A role for protein misfolding in immunogenicity of biopharmaceuticals. J. Biol. Chem. 282, 2229–2236 [DOI] [PubMed] [Google Scholar]
- 21. Fradkin A. H., Carpenter J. F., Randolph T. W. (2011) Glass particles as an adjuvant: a model for adverse immunogenicity of therapeutic proteins. J. Pharm. Sci. 100, 4953–4964 [DOI] [PubMed] [Google Scholar]
- 22. Fradkin A. H., Carpenter J. F., Randolph T. W. (2009) Immunogenicity of aggregates of recombinant human growth hormone in mouse models. J. Pharm. Sci. 98, 3247–3264 [DOI] [PubMed] [Google Scholar]
- 23. Purohit V. S., Middaugh C. R., Balasubramanian S. V. (2006) Influence of aggregation on immunogenicity of recombinant human factor VIII in hemophilia A mice. J. Pharm. Sci. 95, 358–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reipert B. M., van Helden P. M., van den Helden P. M., Schwarz H. P., Hausl C. (2007) Mechanisms of action of immune tolerance induction against factor VIII in patients with congenital hemophilia A and factor VIII inhibitors. Br. J. Haematol. 136, 12–25 [DOI] [PubMed] [Google Scholar]
- 25. Kiese S., Papppenberger A., Friess W., Mahler H. C. (2008) Shaken, not stirred. Mechanical stress testing of an IgG1 antibody. J. Pharm. Sci. 97, 4347–4366 [DOI] [PubMed] [Google Scholar]
- 26. Joubert M. K., Luo Q., Nashed-Samuel Y., Wypych J., Narhi L. O. (2011) Classification and characterization of therapeutic antibody aggregates. J. Biol. Chem. 286, 25118–25133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luo Q., Joubert M. K., Stevenson R., Ketchem R. R., Narhi L. O., Wypych J. (2011) Chemical modifications in therapeutic protein aggregates generated under different stress conditions. J. Biol. Chem. 286, 25134–25144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiang S. D., Scholzen A., Minigo G., David C., Apostolopoulos V., Mottram P. L., Plebanski M. (2006) Pathogen recognition and development of particulate vaccines. Does size matter? Methods 40, 1–9 [DOI] [PubMed] [Google Scholar]
- 29. Morefield G. L., Sokolovska A., Jiang D., HogenEsch H., Robinson J. P., Hem S. L. (2005) Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine 23, 1588–1595 [DOI] [PubMed] [Google Scholar]
- 30. Fifis T., Gamvrellis A., Crimeen-Irwin B., Pietersz G. A., Li J., Mottram P. L., McKenzie I. F., Plebanski M. (2004) Size-dependent immunogenicity. Therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 173, 3148–3154 [DOI] [PubMed] [Google Scholar]
- 31. Ryff J. C., Schellekens H. (2002) Immunogenicity of rDNA-derived pharmaceuticals. Trends Pharmacol. Sci. 23, 254–256 [DOI] [PubMed] [Google Scholar]
- 32. Schellekens H. (2002) Bioequivalence and the immunogenicity of biopharmaceuticals. Nat. Rev. Drug Discov. 1, 457–462 [DOI] [PubMed] [Google Scholar]
- 33. Rosenberg A. (2011) in Immunogenicity 2011: Predictive Science of the Immunogenicity Aspects of Particles in Biopharmaceutical Products, pp. 6–19, CASSS, Gaithersburg, MD [Google Scholar]
- 34. Seaver S. (2008) Immunogenicity of therapeutic proteins. Questions and controversies still abound. BioQuality 13, 3–4 [Google Scholar]
- 35. Bachmann M. F., Zinkernagel R. M. (1997) Neutralizing antiviral B cell responses. Annu. Rev. Immunol. 15, 235–270 [DOI] [PubMed] [Google Scholar]
- 36. Denis J., Majeau N., Acosta-Ramirez E., Savard C., Bedard M. C., Simard S., Lecours K., Bolduc M., Pare C., Willems B., Shoukry N., Tessier P., Lacasse P., Lamarre A., Lapointe R., Lopez Macias C., Leclerc D. (2007) Immunogenicity of papaya mosaic virus-like particles fused to a hepatitis C virus epitope. Evidence for the critical function of multimerization. Virology 363, 59–68 [DOI] [PubMed] [Google Scholar]
- 37. Brinks V., Jiskoot W., Schellekens H. (2011) Immunogenicity of therapeutic proteins. The use of animal models. Pharm. Res. 28, 2379–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bussiere J. L. (2003) Animal models as indicators of immunogenicity of therapeutic proteins in humans. Dev. Biol. 112, 135–139 [PubMed] [Google Scholar]
- 39. Wullner D., Zhou L., Bramhall E., Kuck A., Goletz T. J., Swanson S., Chirmule N., Jawa V. (2010) Considerations for optimization and validation of an in vitro PBMC-derived T-cell assay for immunogenicity prediction of biotherapeutics. Clin. Immunol. 137, 5–14 [DOI] [PubMed] [Google Scholar]
- 40. Laborde E. A., Vanzulli S., Beigier-Bompadre M., Isturiz M. A., Ruggiero R. A., Fourcade M. G., Catalan Pellet A. C., Sozzani S., Vulcano M. (2007) Immune complexes inhibit differentiation, maturation, and function of human monocyte-derived dendritic cells. J. Immunol. 179, 673–681 [DOI] [PubMed] [Google Scholar]
- 41. Jaber A., Baker M. (2007) Assessment of the immunogenicity of different interferon β-1a formulations using ex vivo T-cell assays. J. Pharm. Biomed. Anal. 43, 1256–1261 [DOI] [PubMed] [Google Scholar]
- 42. Jaber A., Driebergen R., Giovannoni G., Schellekens H., Simsarian J., Antonelli M. (2007) The Rebif new formulation story. It's not trials and error. Drugs R D 8, 335–348 [DOI] [PubMed] [Google Scholar]
- 43. Perry L. C., Jones T. D., Baker M. P. (2008) New approaches to prediction of immune responses to therapeutic proteins during preclinical development. Drugs R D 9, 385–396 [DOI] [PubMed] [Google Scholar]
- 44. Tilg H., Dinarello C. A., Mier J. W. (1997) IL-6 and APPs. Anti-inflammatory and immunosuppressive mediators. Immunol. Today 18, 428–432 [DOI] [PubMed] [Google Scholar]
- 45. Higgins S. C., Lavelle E. C., McCann C., Keogh B., McNeela E., Byrne P., O'Gorman B., Jarnicki A., McGuirk P., Mills K. H. (2003) Toll-like receptor 4-mediated innate IL-10 activates antigen-specific regulatory T-cells and confers resistance to Bordetella pertussis by inhibiting inflammatory pathology. J. Immunol. 171, 3119–3127 [DOI] [PubMed] [Google Scholar]
- 46. Popi A. F., Lopes J. D., Mariano M. (2004) Interleukin-10 secreted by B-1 cells modulates the phagocytic activity of murine macrophages in vitro. Immunology 113, 348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ravetch J. V., Bolland S. (2001) IgG Fc receptors. Annu. Rev. Immunol. 19, 275–290 [DOI] [PubMed] [Google Scholar]
- 48. Lin Y., Lee H., Berg A. H., Lisanti M. P., Shapiro L., Scherer P. E. (2000) The lipopolysaccharide-activated toll-like receptor (TLR)-4 induces synthesis of the closely related receptor TLR-2 in adipocytes. J. Biol. Chem. 275, 24255–24263 [DOI] [PubMed] [Google Scholar]
- 49. Corry D. B., Kiss A., Song L. Z., Song L., Xu J., Lee S. H., Werb Z., Kheradmand F. (2004) Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. FASEB J. 18, 995–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vultaggio A., Maggi E., Matucci A. (2011) Immediate adverse reactions to biologicals. From pathogenic mechanisms to prophylactic management. Curr. Opin. Allergy Clin. Immunol. 11, 262–268 [DOI] [PubMed] [Google Scholar]
- 51. Descotes J., Vial T. (2007) in Cytokines in Human Health: Immunotoxicology, Pathology and Therapeutic Applications (House R. V., Descotes J., eds) pp. 193–204, Humana Press Inc., Totowa, NJ [Google Scholar]
- 52. Catelas I., Huk O. L., Petit A., Zukor D. J., Marchand R., Yahia L. (1998) Flow cytometric analysis of macrophage response to ceramic and polyethylene particles. Effects of size, concentration, and composition. J. Biomed. Mater. Res. 41, 600–607 [DOI] [PubMed] [Google Scholar]
- 53. Prior S., Gander B., Blarer N., Merkle H. P., Subirá M. L., Irache J. M., Gamazo C. (2002) In vitro phagocytosis and monocyte-macrophage activation with poly(lactide) and poly(lactide-co-glycolide) microspheres. Eur. J. Pharm. Sci. 15, 197–207 [DOI] [PubMed] [Google Scholar]
- 54. Brightbill H. D., Libraty D. H., Krutzik S. R., Yang R. B., Belisle J. T., Bleharski J. R., Maitland M., Norgard M. V., Plevy S. E., Smale S. T., Brennan P. J., Bloom B. R., Godowski P. J., Modlin R. L. (1999) Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285, 732–736 [DOI] [PubMed] [Google Scholar]
- 55. Hertz C. J., Kiertscher S. M., Godowski P. J., Bouis D. A., Norgard M. V., Roth M. D., Modlin R. L. (2001) Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J. Immunol. 166, 2444–2450 [DOI] [PubMed] [Google Scholar]
- 56. Krutzik S. R., Tan B., Li H., Ochoa M. T., Liu P. T., Sharfstein S. E., Graeber T. G., Sieling P. A., Liu Y. J., Rea T. H., Bloom B. R., Modlin R. L. (2005) TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat. Med. 11, 653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Medzhitov R., Preston-Hurlburt P., Janeway C. A., Jr. (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 [DOI] [PubMed] [Google Scholar]
- 58. Anderson C. L., Abraham G. N. (1980) Characterization of the Fc receptor for IgG on a human macrophage cell line, U937. J. Immunol. 125, 2735–2741 [PubMed] [Google Scholar]
- 59. Canfield S. M., Morrison S. L. (1991) The binding affinity of human IgG for its high affinity Fc receptor is determined by multiple amino acids in the CH2 domain and is modulated by the hinge region. J. Exp. Med. 173, 1483–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Robinson J. J., Watson F., Bucknall R. C., Edwards S. W. (1994) Role of Fcγ receptors in the activation of neutrophils by soluble and insoluble immunoglobulin aggregates isolated from the synovial fluid of patients with rheumatoid arthritis. Ann. Rheum Dis. 53, 515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hazenbos W. L., Heijnen I. A., Meyer D., Hofhuis F. M., Renardel de Lavalette C. R., Schmidt R. E., Capel P. J., van de Winkel J. G., Gessner J. E., van den Berg T. K., Verbeek J. S. (1998) Murine IgG1 complexes trigger immune effector functions predominantly via FcγRIII (CD16). J. Immunol. 161, 3026–3032 [PubMed] [Google Scholar]
- 62. Coxon A., Cullere X., Knight S., Sethi S., Wakelin M. W., Stavrakis G., Luscinskas F. W., Mayadas T. N. (2001) FcγRIII mediates neutrophil recruitment to immune complexes. a mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity 14, 693–704 [DOI] [PubMed] [Google Scholar]
- 63. Magi M., Garcia L., Vandenbranden M., Palmantier R., Jacquet A. (2004) Heat denaturation affects the ProDer p 1 IgE reactivity and down-regulates the development of the specific allergic response. J. Allergy Clin. Immunol. 114, 545–552 [DOI] [PubMed] [Google Scholar]
- 64. Babiuk S., Skowronski D. M., De Serres G., HayGlass K., Brunham R. C., Babiuk L. (2004) Aggregate content influences the Th1/Th2 immune response to influenza vaccine. Evidence from a mouse model. J. Med. Virol. 72, 138–142 [DOI] [PubMed] [Google Scholar]
- 65. Rönnelid J., Tejde A., Mathsson L., Nilsson-Ekdahl K., Nilsson B. (2003) Immune complexes from SLE sera induce IL10 production from normal peripheral blood mononuclear cells by an FcγRII-dependent mechanism. Implications for a possible vicious cycle maintaining B cell hyperactivity in SLE. Ann. Rheum. Dis. 62, 37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.