Background: The naturally occurring α5(D398N) variant alters smoking behavior, but functional differences have not been detected between α3β4α5 nAChR harboring these variants.
Results: ACh-induced α3β4α5 nAChR function is lower when α5(Asn-398) substitutes for α5(Asp-398).
Conclusion: The α5 variant-induced change in α3β4α5 nAChR function may underlie some of the phenotypic changes associated with this polymorphism.
Significance: α3β4α5 nAChR function may be a useful target for smoking cessation pharmacotherapies.
Keywords: Genetic Polymorphism, Ion Channels, Molecular Pharmacology, Nicotinic Acetylcholine Receptors, Pharmacology, α5(D398N), Receptor Function
Abstract
Genome-wide studies have strongly associated a non-synonymous polymorphism (rs16969968) that changes the 398th amino acid in the nAChR α5 subunit from aspartic acid to asparagine (D398N), with greater risk for increased nicotine consumption. We have used a pentameric concatemer approach to express defined and consistent populations of α3β4α5 nAChR in Xenopus oocytes. α5(Asn-398; risk) variant incorporation reduces ACh-evoked function compared with inclusion of the common α5(Asp-398) variant without altering agonist or antagonist potencies. Unlinked α3, β4, and α5 subunits assemble to form a uniform nAChR population with pharmacological properties matching those of concatemeric α3β4* nAChRs. α5 subunit incorporation reduces α3β4* nAChR function after coinjection with unlinked α3 and β4 subunits but increases that of α3β4α5 versus α3β4-only concatemers. α5 subunit incorporation into α3β4* nAChR also alters the relative efficacies of competitive agonists and changes the potency of the non-competitive antagonist mecamylamine. Additional observations indicated that in the absence of α5 subunits, free α3 and β4 subunits form at least two further subtypes. The pharmacological profiles of these free subunit α3β4-only subtypes are dissimilar both to each other and to those of α3β4α5 nAChR. The α5 variant-induced change in α3β4α5 nAChR function may underlie some of the phenotypic changes associated with this polymorphism.
Introduction
Nicotinic acetylcholine receptors (nAChR)2 are prototypical members of the ligand-gated ion channel superfamily of neurotransmitter receptors. nAChR exist as a diverse family of molecules composed of different pentameric combinations of homologous subunits derived from at least 17 genes (α1-α10, β1-β4, γ, δ, ϵ). The properties of nAChR are determined by their subunit composition, giving rise to multiple subtypes with a range of overlapping pharmacological and biophysical properties (1). It also has become apparent that different stoichiometries of the same subunits can produce subtypes with distinctly different characteristics, a phenomenon observed in both heterologous and natural expression systems (1–5).
Recently, genome-wide association studies have indicated that single-nucleotide polymorphisms (SNPs) within nAChR subunits can substantially affect nAChR-mediated smoking behavior in humans. Most prominent among these single-nucleotide polymorphisms have been those located in the CHRNA5/CHRNA3/CHRNB4 locus, located on chromosome 15q25, which encodes the α5, α3 and β4 subunits of nicotinic receptors. This locus was first associated with nicotine dependence (6). Subsequent studies confirmed associations of single-nucleotide polymorphisms at this locus with heavy smoking (>25 cigarettes smoked daily), Fagerström Test for Nicotine Dependence scores and age dependent severity of nicotine dependence (7–11). One non-synonymous polymorphism (rs16969968), which changes the 398th amino acid from aspartic acid to asparagine (D398N) in the α5 subunit, is particularly strongly associated with greater risk for increased nicotine consumption. Interestingly, variants at this locus also are associated with increased liability for lung cancer (8, 12, 13), and possibly with decreased risk for alcoholism (7, 14) and cocaine dependence (15).
These observations raise the question of what the functional effects of the D398N mutation might be. The α5 subunit can only assemble into functional nAChR when expressed with at least two other subunits (1). In the central nervous system, most α5 subunit expression occurs in combination with α4 and β2 subunits (16, 17). Experiments using heterologous expression systems have demonstrated that α4β2* nAChR containing α5 subunits harboring the risk (Asn-398) variant have lower function than those that incorporate α5 subunits with the common (Asp-398) variant (11, 18). This provides a mechanism through which the α5(D398N) mutation could produce phenotypic effects. Notably, a restricted set of brain regions (most prominently in the habenuolopeduncular pathway) express α5 subunits in combination with α3 and β4 subunits (19, 20), as often occurs in autonomic α3β4* nAChR (21–23). A recent study showed that increased expression of α3β4* nAChR in the habenulopeduncular tract of mice increases nicotine aversion, an effect that can be reduced by the introduction and expression of additional α5(Asn-398) subunits in the same pathway (20). Furthermore, α3, β4, and α5 nAChR subunits are commonly expressed in bronchial, epithelial, and lung cancer cells, where nAChR activation by nicotine has been proposed as a mechanism that may increase tumor initiation and/or growth (24). However, heterologous expression studies done to date have not identified functional differences induced by α5 variant incorporation into α3β4* nAChR (18, 25).
Other observations may help to explain this discrepancy between in vitro observations and in vivo phenotypes. It has been shown that α3β4 nAChR can be expressed in multiple stoichiometries, with different functional properties (26–28). Moreover, α5 subunits can “compete” with β4 subunits for incorporation into assembled nAChR (29), possibly forcing formation of non-functional nAChR subunit assemblies as “dead end intermediates” (30). Thus, the effect(s) of common α5(Asp-398) versus risk α5(Asn-398) variant subunit incorporation into α3β4* nAChR may be obscured by changes, attendant on any α5 subunit incorporation, in the overall level of α3β4 nAChR functional expression and/or the balance of functional stoichiometric isoforms expressed. This complication in experimental interpretation is compounded when various mixtures of nAChR subtypes with specific subunit ratios are expressed from “loose” subunits assembled under host cell, and not investigator, control.
To overcome these difficulties in interpretation, we employed a concatemeric nAChR approach (Fig. 1). Here, nAChR constructs are assembled that encode all five subunits of the desired α3β4* nAChR subtypes joined by short peptide linkers. The advantage of this approach is that complex nAChR subtypes can be expressed with native nAChR-like properties and with completely defined subunit ratios and orders of assembly (5, 31). Using concatemeric α3β4α5 nAChR, we demonstrate that, as is true for α4β2* nAChR, incorporation of the α5(Asn-398) variant reduces maximal acetylcholine-induced function when compared with the α5(Asp-398) variant. The properties of the defined concatemeric nAChR also were compared with those of α3β4* nAChR allowed to assemble freely from loose individual subunits. These comparisons confirmed that concatemeric and freely assembled α3β4α5 nAChR have essentially indistinguishable pharmacological properties. Interestingly, these comparisons also suggested that loose α3 and β4 subunits associate quite differently in the presence or absence of α5 subunits.
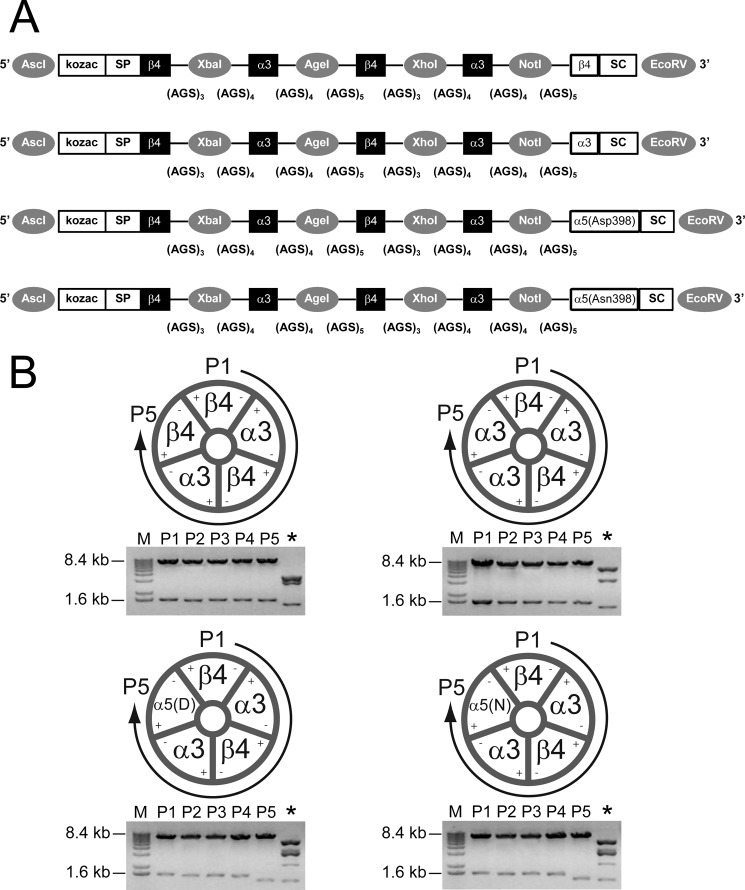
FIGURE 1.
Linked (concatemeric) subunit design of α3β4α5 receptors. A, shown is a schematic illustration of (from top to bottom) β4α3β4α3β4, β4α3β4α3α3, β4α3β4α3α5(Asp-398), and β4α3β4α3α5(Asn-398) constructs. Each construct is flanked with AscI and EcoRV restriction sites (5′ and 3′, respectively; indicated by gray circles) for subcloning into high expression oocyte vectors. Kozac and the β4 signal peptide (SP) were retained only for the 1st position. Flanking each subunit position are unique restriction sites (indicated by gray circles) used in concatemer design (for example, AscI and XbaI used in exchanging nAChR subunits at position 1; XbaI and AgeI sites were used in exchanging nAChR subunits at position 2; etc.). Concatemers varied in composition only at position 5, containing either the β4, α3, or two naturally occurring variants of the α5 nAChR subunit (aspartic acid (Asp-398) or asparagine (Asn-398)). Stop codons (SC) were added at the 3′ end of subunit position 5. The number of AGS repeats flanking each subunit is listed below each linker region. B, shown is stoichiometry of β4α3β4α3β4, β4α3β4α3α3, β4α3β4α3α5(Asp-398), and β4α3β4α3α5(Asn-398) constructs. Concatemers form pentameric receptors by joining the positive interface of the nAChR subunit at position 1 (P1) and the negative interface of position 5 (P5). Restriction digest (below each schematic) using unique restriction sites (as mentioned above) was used to verify each subunit within its respective position (P1-P5). An additional restriction digest (*) using ScaI was performed to diagnose correct subunit composition and order. M, molecular mass markers.
EXPERIMENTAL PROCEDURES
Chemicals
All buffer components and pharmacological reagents (acetylcholine, atropine, cytisine, nicotine, and mecamylamine) were purchased from Sigma. Fresh stock drug solutions were made daily and diluted as required.
Constructs for Individual α3, β4, and α5 nAChR Subunits
Native human subunit protein sequences for α3, β4, and α5 (both Asn-398 and Asp-398 variants) nAChR subtypes were encoded by nucleotide sequences optimized for expression in vertebrate expression systems (synthesized by GeneArt AG; Invitrogen). Optimizations included minimization of high GC content sequence segments, improved codon usage, reduction of predicted RNA secondary structure formation, and removal of sequence repeats and possible alternative start and splice sites. Sequences were subcloned into the pSGEM oocyte high expression vector (a kind gift of Prof. Michael Hollmann; Ruhr-Universitaet, Bochum, Germany).
Concatemeric α3β4 and α3β4α5 Constructs
Fully pentameric nAChR concatemers were constructed from human nAChR subunits. cDNAs encoding concatemers were created using the same subunit layout as successfully used to encode high and low agonist sensitivity α4β2* nAChR isoforms (5). Subunits were arranged in the order β4-α3-β4-α3-X, where X was either β4, α3, α5(Asp-398) or α5(Asn-398); Fig. 1A. Kozac and signal peptide sequences were removed from all subunit sequences with the exception of subunits expressed in the first position of the concatemer. As previously demonstrated, the initial β-α subunit protein pairs of the constructs will assemble to form an orthosteric binding site between the complementary (−) face of the initial β4 subunit and the principal (+) face of the following α3 subunit (4). The assembled α3β4* nAChR concatemers thus contain orthosteric agonist binding pockets at the β4(−)/(+)α3 interfaces between the first and second and between the third and fourth subunits (5). As for individual subunits, native human subunit protein sequences were encoded by nucleotide sequences optimized for expression in vertebrate expression systems (synthesized by GeneArt AG). Optimizations fell in the same categories as those previously described. Subunits were linked by alanine-glycine-serine (AGS) repeats designed to provide a complete linker length (including the C-terminal tail of the preceding subunit) of 40 ± 2 amino acids. At the nucleotide level, linker sequences were designed to contain unique restriction sites that allow easy removal and replacement of individual α3, β4, and α5 subunits (Fig. 1A). Sequences of all subunits together with their associated partial linkers were confirmed by DNA sequencing (Geneart AG). Each concatemer was subcloned into the pSGEM oocyte high expression vector. Correct assembly of the concatemers into the expression vector was verified by restriction digest (Fig. 1, A and B). Additionally, concatemers were digested with ScaI to further diagnose the stoichiometry of each construct (* as indicated; Fig. 1B).
RNA Synthesis
Plasmids containing single α3, β4, or α5 (Asn-398 and Asp-398 variants) nAChR subunits or α3β4 and α3β4α5 concatemeric constructs were linearized with NheI (2 h at 37 °C) and treated with proteinase K (30 min at 50 °C). cRNAs were transcribed using mMessage mMachine T7 kit (Applied Biosystems/Ambion, Austin, TX). Reactions were treated with TURBO DNase (1 unit for 15 min at 37 °C), and cRNAs were purified using Qiagen RNeasy Clean-up kit (Valencia, CA). cRNA purity was confirmed on a 1% agarose gel (Fig. 1B), and preparations were stored at −80 °C.
Oocyte Preparation and RNA Injection
Methods of oocyte isolation and processing for receptor expression have previously been described (32, 33) but were modified as follows. Lobes were digested with 0.75 units/ml LiberaseTM (Roche Applied Science), and oocytes were incubated at 13 °C. The tips of pulled glass micropipettes were broken to achieve an outer diameter of ∼40 μm (resistance of 2–6 milliohms), and pipettes were used to inject 20–60 nl containing 10 ng of cRNA/oocyte.
Expression of α3β4 and α3β4α5 Constructs in Xenopus Oocytes
Seven days after injection, Xenopus oocytes expressing loose α3 and β4 with or without α5 (Asn-398 and Asp-398 variants) subunits from individual cRNAs at the indicated ratios or α3β4 and α3β4α5 concatemers were voltage-clamped at −70 mV with an Axoclamp 900A amplifier (Molecular Devices, Sunnyvale, CA). Recordings were sampled at 10 kHz (low-pass Bessel filter, 40 Hz; high pass filter, DC), and the resulting traces were saved to disk (Molecular Devices Clampex v10.2). Data from oocytes with leak currents (Ileak) >50 nA were excluded from recordings. Known nAChR agonists and antagonists were applied using a 16 channel, gravity-fed, perfusion system with automated valve control (AutoMate Scientific, Inc.; Berkeley, CA). All solutions contained atropine sulfate (1.5 μm) to ensure that muscarinic responses were not recorded. Oocytes-expressing loose subunits and/or concatemeric α3β4α5 nAChR were perfused with receptor agonist (e.g. ACh, cytisine, and nicotine) or antagonist (e.g. mecamylamine) for 5 s with 60 s washout times between each subsequent application of drug.
Data Analysis
EC50 or IC50 values and peak current amplitudes (Imax) were determined from individual oocytes. All stimulation protocols began with stimulation by a maximally efficacious dose of ACh (1 mm). This ensured that oocytes were indeed expressing functional nAChR before we did further recording, and it provided an internal control response for each oocyte. Relative agonist efficacies were calculated by comparison to this internal ACh control response. EC50 and IC50 values were determined through non-linear least squares curve-fitting (GraphPad Prism 4.0, GraphPad Software, Inc., La Jolla, CA) using unconstrained, monophasic logistic equations to fit all parameters, including Hill slopes. Additional normalization was used to compare absolute agonist efficacy between the concatemeric nAChR constructs. As for the nAChR expressed from loose subunits, all peak current response data were collected at 7 days post-injection. Function produced by oocytes expressing (α3β4)2α5(Asp-398) concatemers was chosen as the internal reference point for each batch of injected oocytes, as α5(Asp-398) is the more-common variant. Responses to 1 mm ACh, which is a maximally effective concentration for all of the constructs studied here, were measured. The mean function produced by oocytes injected with (α3β4)2α5(Asp-398) concatemers on each experimental day was used to normalize all of the data collected on that day. All four concatemeric constructs were tested in each experiment. In this way, any residual batch-to-batch oocyte variation could be accounted for.
EC50 and IC50 values are presented as the mean ± 95% confidence interval (CI). Data were analyzed using Student's t test to compare pairs of groups or by one-way or two-way ANOVA and Tukey's multiple comparison test to compare the means of three or more groups (PRISM, GraphPad Software, Inc.).
RESULTS
Introduction of the α5 Subunit Produces α3β4* Receptors with Distinct Pharmacological Responses
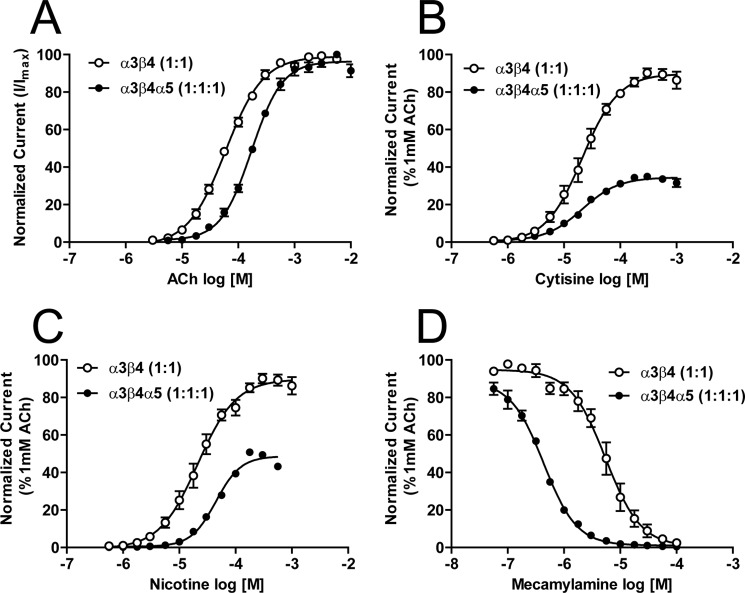
In an initial experiment to assess if co-injection of α5 subunits altered functional responses of α3β4* nAChR, oocytes were injected with equal amounts of α3 and β4 RNA (1:1 injection ratio) or with equal amounts of α3, α4, and α5 RNA (1:1:1 injection ratio). The concentration-response profiles of the resulting nAChR populations are illustrated in Fig. 2. The introduction of α5 significantly decreased ACh potency at the α3β4* nAChR population (Fig. 2A; from 59 μm (CI, 54–65 μm) to 172 μm (CI, 158–187 μm; p < 0.05)) and approximately halved the maximum ACh-induced nAChR function (data not shown due to normalization in Fig. 2). In contrast, the introduction of α5 had no effect on cytisine potency at α3β4* nAChR (Fig. 2B; mean α3β4 and α3β4α5 EC50 values, 21 μm (CI, 18–24.8 μm) and 20.5 μm (CI, 17.7–24 μm), respectively; p > 0.05). However, the addition of α5 subunits significantly reduced relative cytisine efficacy when compared with the internal, maximally effective, 1 mm ACh control (Fig. 2B; α3β4 (1:1) = 90.0 ± 1.9% and α3β4α5 (1:1:1) = 34.1 ± 0.7%, p < 0.05). The α3β4-only nAChR population also differed slightly from the α3β4α5 population in sensitivity to nicotine (21 μm (CI, 18–26 μm) to 45 μm (CI, 40–51 μm) respectively; p < 0.001). Similar to the situation with cytisine, introduction of the α5 subunit also reduced the relative efficacy of nicotine (% of internal ACh control per oocyte = 89.8 ± 2.2% for α3β4-nAChR compared with 48.7 ± 1.2% for α3β4α5-nAChR; p < 0.001). Most strikingly, introduction of the α5 subunit increased the sensitivity of α3β4* nAChR to the non-competitive antagonist mecamylamine by more than an order of magnitude (α3β4-only IC50 = 5.5 μm (CI, 4.4–6.7 μm); α3β4α5 IC50 = 0.40 μm (CI, 0.38–0.49 μm); p < 0.0001).
FIGURE 2.
Concentrations response profiles for α3β4α5 nAChR expressed as loose subunits. Oocytes injected with RNA for α3, β4, and α5 subunits in a 1:1 or 1:1:1 ratio were perfused with nAChR agonists acetylcholine (10−5.5 to 10−2; n = 6) (A), cytisine (10−6.25 to 10−2.5; n = 6) (B), nicotine (10−6.25 to 10−2; n = 6) (C), or the α3β4 nAChR antagonist mecamylamine (10−8 to 10−4; n = 6) (D). Data points represent averages (±S.E.). Differences in drug potency and efficacy between groups were analyzed using one-way ANOVA with Tukey's post hoc comparison (see “Experimental Procedures”).
Effects of Common (Asp-398) and Risk (Asn-398) Variant α5 Subunit Integration into Concatenated α3β4 Receptors
As noted in the introduction, α5 subunits could potentially affect nAChR function in a variety of ways. These could include “competition” with β4 subunits for incorporation into assembled nAChR (29) and/or trapping as dead end intermediates of subunits that might otherwise assemble into functional nAChR (30). Self-assembly of individual nAChR subunits could also result in mixed populations of α3β4 and α3β4α5 nAChR subtypes, possibly in proportions that vary between individual oocytes. To remove these confounds, we designed pentameric concatemers that enforce precise subunit ratios and assembly orders. The resulting constructs encoded functional nAChR as (α3β4)2α5(Asn-398) and (α3β4)2α5(Asp-398) forms. We also produced concatemers as (α3β4)2β4 and (α3β4)2α3 isoforms for comparison to concatemers containing α5 subunits. These constructs are displayed schematically in Fig. 1A.
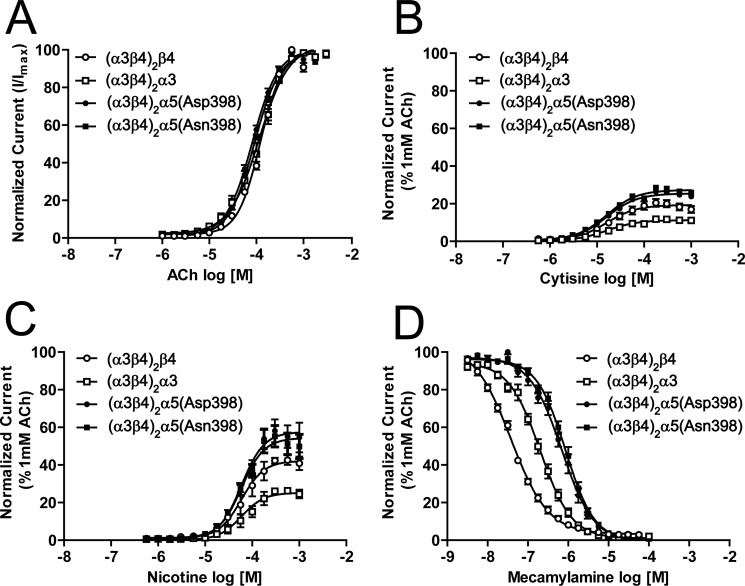
No significant differences in agonist sensitivity (EC50) were observed among concatemers when β4, α3, or α5 nAChR subunits were present in the 5th pentameric position (Fig. 3A; p > 0.05). In fact, EC50 values for multiple agonists were similar across each of the concatemeric constructs and to those measured for α3β4α5 nAChR assembled from individual subunits at 1:1:1 cRNA ratios (Fig. 3A-C, Table 1; ACh, p > 0.05; cytisine, p > 0.05; nicotine, p > 0.05).
FIGURE 3.
Positional effects and functional sensitivity of concatenated α3, β4, α5(Asp-398) and α5(Asn-398) nAChR subunits. Concentration response curves were generated for linked α3β4 nAChR containing β4 at position 5 (open circles), α3 at position 5 (open boxes), α5(Asp-398) variant at position 5 (filled circles), and α5(Asn-398) variant at position 5 (filled boxes). Concentration response curves were generated for known α3β4 nAChR agonists acetylcholine (α3β4)2(β4); n = 16; (α3β4)2(α3); n = 11; (α3β4)2α5(Asp-398); n = 17; (α3β4)2α5(Asn-398); n = 17) (A), cytisine (α3β4)2(β4); n = 6; (α3β4)2(α3); n = 6; (α3β4)2α5(Asp-398); n = 10; (α3β4)2α5(Asn-398); n = 10) (B), and nicotine (α3β4)2(β4); n = 6; (α3β4)2(α3); n = 6; (α3β4)2α5(Asp-398); n = 8; (α3β4)2α5(Asn-398); n = 8) (C). D, concentration response curves were also obtained using the α3β4 antagonist, mecamylamine (α3β4)2(β4); n = 6; (α3β4)2(α3); n = 6; (α3β4)2α5(Asp-398); n = 8; (α3β4)2α5(Asn-398); n = 8). Data points represent averages (±S.E.). For cytisine, nicotine, and mecamylamine, comparisons were made by normalizing current responses as % 1 mm ACh (see “Experimental Procedures”). Differences in drug potency and efficacy between groups were analyzed using one-way ANOVA with Tukey's post hoc comparison (see “Experimental Procedures”).
TABLE 1.
Pharmacological parameters calculated from concatemeric α3β4* nAChR and from α3β4α5 nAChR expressed from loose subunits
Oocytes injected with RNA encoding either concatenated α3β4* nAChR or single α3, β4, and α5 subunits in a 1:1:1 ratio were perfused with the nAChR agonists acetylcholine (10−6 to 10−2 m), cytisine (10−6.25 to 10−3 m), nicotine (10−6.5 to 10−3.5 m), or the nAChR antagonist mecamylamine (10−7.5 to 10−4 m). Data are presented as the mean ± S.E., with numbers of individual oocytes tested (n) as indicated. * denotes concatemeric constructs.
| Expressed nAChR | Acetylcholine |
Cytisine |
Nicotine |
Mecamylamine |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Log EC50 | nH ± S.E. | n | Log EC50 | nH ± S.E. | n | Log EC50 | nH ± S.E. | n | Log IC50 | nH ± S.E. | |
| β4α3β4α3β4* | 16 | −3.9 ± 0.02 | 1.8 ± 0.11 | 6 | −4.8 ± 0.07 | 1.6 ± 0.4 | 6 | −4.2 ± 0.07 | 1.6 ± 0.4 | 6 | −7.4 ± 0.04 | −0.9 ± 0.05 |
| β4α3β4α3α3* | 11 | −4.0 ± 0.03 | 1.4 ± 0.13 | 6 | −4.7 ± 0.04 | 1.2 ± 0.12 | 6 | −4.1 ± 0.07 | 1.8 ± 0.4 | 6 | −6.7 ± 0.03 | −1.1 ± 0.08 |
| β4α3β4α3α5(Asp-398)* | 17 | −4.0 ± 0.01 | 1.6 ± 0.06 | 10 | −4.8 ± 0.04 | 1.3 ± 0.15 | 8 | −4.2 ± 0.05 | 1.8 ± 0.4 | 8 | −6.2 ± 0.03 | −1.1 ± 0.08 |
| β4α3β4α3α5(Asn-398)* | 17 | −4.0 ± 0.02 | 1.6 ± 0.09 | 10 | −4.8 ± 0.03 | 1.3 ± 0.13 | 8 | −4.3 ± 0.06 | 1.9 ± 0.4 | 8 | −6.0 ± 0.03 | −1.2 ± 0.08 |
| α3β4α5 (1:1:1) | 6 | −3.8 ± 0.02 | 1.6 ± 0.10 | 6 | −4.7 ± 0.03 | 1.5 ± 0.2 | 6 | −4.4 ± 0.03 | 2.0 ± 0.2 | 6 | −6.4 ± 0.02 | −1.5 ± 0.09 |
Cytisine consistently demonstrated partial agonism across the concatemeric α3β4* nAChR constructs (Fig. 3B). The efficacy of cytisine (normalized to responses to 1 mm ACh) was indistinguishable between (α3β4)2α5(Asp-398) and (α3β4)2α5(Asn-398) concatemers (25.5 ± 0.6 to 27.3 ± 0.5%; respectively; p > 0.05). However, cytisine efficacy was significantly reduced at (α3β4)2β4 tethered pentamers (Fig. 3B; 19.1 ± 0.8%; p < 0.001) and still further at (α3β4)2α3 constructs (Fig. 3B; 6.0 ± 0.3%; p < 0.001). Similarly, nicotine consistently demonstrated partial agonism across the set of concatemeric α3β4* nAChR constructs but evoked progressively weaker responses (as a % of 1 mm ACh control responses) in oocytes expressing (α3β4)2α5(Asp-398) (57.4 ± 2.7%) or (α3β4)2α5(Asn-398) (53.5 ± 2.5%) assemblies as opposed to (α3β4)2β4 (43.3 ± 2.6%) or (α3β4)2α3 (26.6 ± 1.7%) concatemers (Fig. 3C; latter two reductions p < 0.001). Overall, the presence of an α5 subunit in the concatemers resulted in expression of nAChR with increased partial agonist efficacy by cytisine and nicotine compared with that for actions at non-α5 α3β4* concatemeric nAChR.
In contrast, the potency of the non-competitive antagonist mecamylamine decreased significantly on the order (α3β4)2β4 > (α3β4)2α3 > (α3β4)2α5 (Fig. 3D, Table 1; p < 0.0001). However, no significant differences in IC50 values were observed between (α3β4)2α5(D398) and (α3β4)2α5(N398) concatemers (p > 0.05). Again, the mecamylamine IC50 values recorded from α3β4α5 nAChR were very similar regardless of whether these nAChR were assembled from individual subunits or from concatemeric constructs (see last three lines of Table 1).
Only Intact nAChR Concatemers Contribute to Recorded Function
In some cases, the covalent linkers within concatemeric constructs have been observed to break down. This liberates smaller products that can assemble to form functional byproducts (4, 34, 35). To determine if this potential confound was present in our system, the α5(V9′S) “gain-of-function” mutant was coinjected with either a concatemeric construct (α3β4)2β4 or with individual α3 and β4 nAChR subunits. Assembly of the α5(V9′S) subunit with either single subunits or abridged concatemers would result in a substantial gain of function (34, 36). Co-expression of α5(V9′S) with unlinked α3 and β4 subunits produced a significant increase in function (peak current amplitude elicited by 1 mm ACh; Fig. 4A). This demonstrates that the α5(V9′S) subunit can assemble with non-linked subunits as predicted. As previously noted, co-injection of a non-gain-of-function α5 subunit at a 1:1:1 ratio approximately halves α3β4* function. This suggests that comparing nAChR function between oocytes injected with α3 and β4 subunits at a 1:1 ratio to that after injection with α3, β4, and α5(V9′S) subunits at a 1:1:1 ratio may underestimate the effect of the gain-of-function mutation. In contrast, co-injection of the α5(V9′S) subunit with the concatemeric construct, even at a 3:1 α5(V9′S):concatemer ratio, produced no change in function (Fig. 4B). These data demonstrate that at least the great majority of nAChR function arising from injection of the concatemeric construct mRNAs must be mediated by intact, pentameric nAChR concatemers.
FIGURE 4.
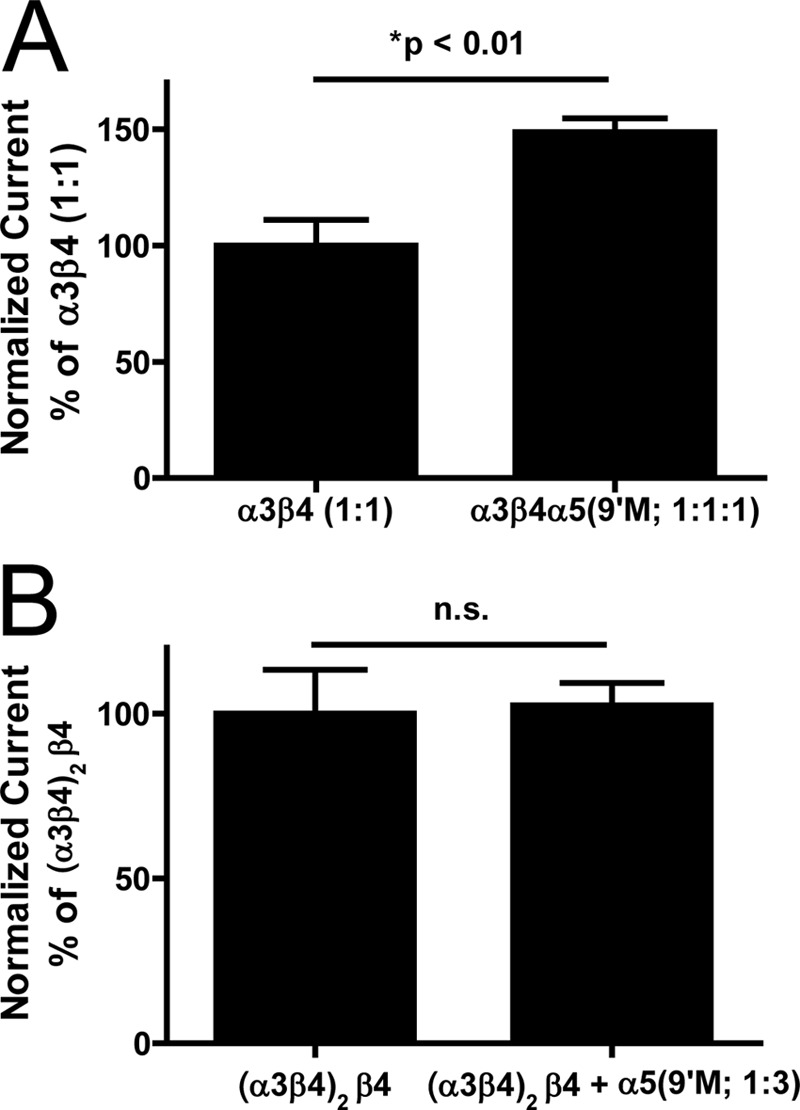
α3β4α5 concatemers are expressed as functional pentamers and are not byproducts of fractional assembly. A, co-injection (1:1:1) of loose α3 and β4 nAChR subunits with the α5 gain of function subtype (V9′S) enhances receptor function (p < 0.01; n = 4 oocytes per group). B, overexpression of the α5(V9′S) subunit has no effect on (α3β4)2(β4) concatemer function (n.s.; n = 4 oocytes per group). Panels A and B represent normalized currents (% of α3β4 (1:1) or β4(p5), respectively. Normalized currents were analyzed using one-way ANOVA (see “Experimental Procedures”).
Absolute Efficacy Comparisons between (α3β4)2α5(Asp-398) and (α3β4)2α5(Asn-398) nAChR Concatemers
The studies above describe partial agonist efficacies normalized to ACh. However, we wanted to compare absolute agonist efficacies between constructs containing either the α5(Asp-398) or α5(Asn-398) variants. The use of concatemeric constructs allows these comparisons to be made without uncertainty related to the subunit makeup of the functional receptors. However, efficiency of functional nAChR expression varies across oocyte preparations and as a function of time post-injection. To compensate for this form of variation, we used a batch-to-batch normalization strategy (described in detail under “Experimental Procedures”).
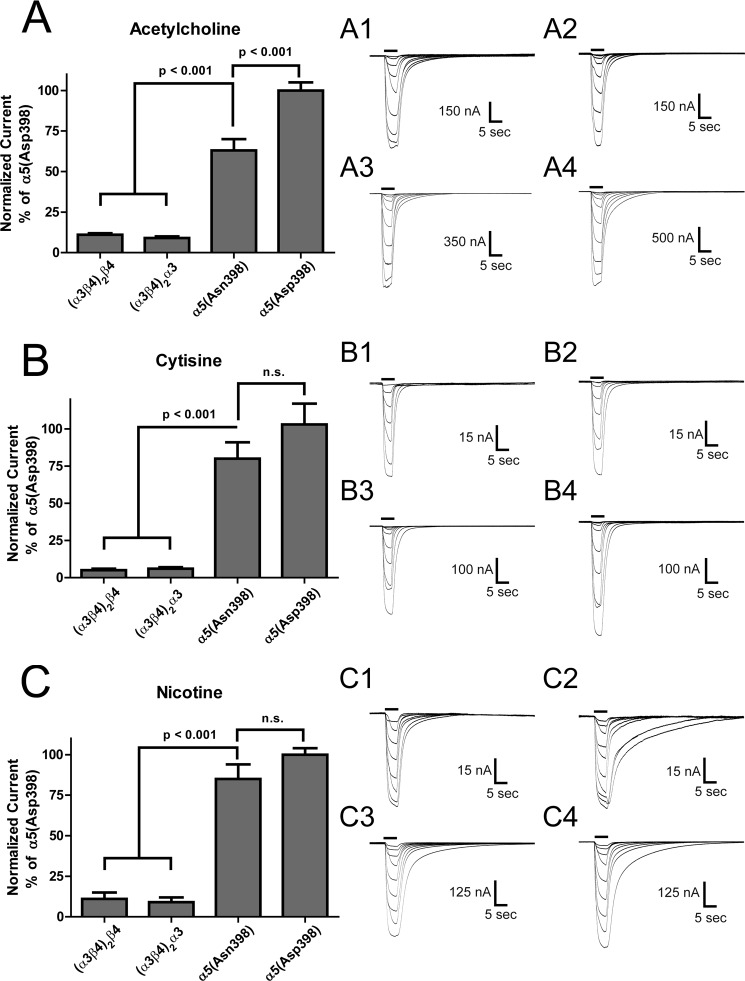
As shown in Fig. 5A, peak ACh responses mediated by (α3β4)2α5(Asn-398) concatemers were significantly lower than those evoked by stimulation of (α3β4)2α5(Asp-398) concatemers (p < 0.001). Thus, although no differences in ACh potency were observed between α3β4* nAChR containing the α5(Asp-398) and α5(Asn-398) variants (as measured by their EC50 values; see Fig. 3A), significant differences were observed in levels of receptor function (as measured by current magnitudes; Fig. 5A). Similar trends were observed when absolute levels of function were measured in response to maximally efficacious concentrations of cytisine (Fig. 5B; 300 μm) or nicotine (Fig. 5C; 300 μm), although in neither case did these apparent differences attain statistical significance (p > 0.05).
FIGURE 5.
Maximum function comparison between (α3β4)2α5(Asp-398) and (α3β4)2α5(Asn-398) nAChR concatemers. Maximal currents (normalized to (α3β4)2α5(Asp-398); see “Experimental Procedures”) were compared between α3β4 and α3β4α5-containing concatemers for acetylcholine (A); panels A1–A4 represent averaged traces for ACh doses (10−6 to 10−2.25) for (α3β4)2(β4) (n = 16), (α3β4)2(α3) (n = 11), (α3β4)2α5(Asn-398) (n = 17), and (α3β4)2α5(Asp-398) (n = 17), respectively. Peak ACh responses mediated by (α3β4)2α5(Asn-398) concatemers were significantly lower than those of evoked by stimulation of (α3β4)2α5(Asp-398) concatemers (p < 0.001). Maximal currents were also compared between α3β4 and α3β4α5 containing concatemers for cytisine (B); panels B1–B4 represent averaged traces for cytisine doses (10−6.25 to 10−3) for (α3β4)2(β4) (n = 6), (α3β4)2(α3) (n = 6), (α3β4)2α5(Asn-398) (n = 10), and (α3β4)2α5(Asp-398), (n = 8), respectively. However, peak cytisine responses mediated by (α3β4)2α5(Asn-398) concatemers were not significantly lower than those of evoked by stimulation of (α3β4)2α5(Asp-398) concatemers (p > 0.05). Additionally, comparisons were made between concatemers for nicotine (C); panels C1–C4 represent averaged traces for nicotine doses (10−6.25 to 10−3) for (α3β4)2(β4) (n = 6), (α3β4)2(α3) (n = 6), (α3β4)2α5(Asn-398) (n = 8), and (α3β4)2α5(Asp-398) (n = 8), respectively. Again, no differences in peak nicotine responses were observed between (α3β4)2α5(Asn-398)-containing concatemers and (α3β4)2α5(Asp-398)-containing concatemers (p > 0.05). Maximum responses recorded from (α3β4)2(β4) and (α3β4)2(α3) nAChR were much smaller than those measured from oocytes expressing either of the α5 variant constructs (p < 0.001). Comparisons between groups were analyzed using one-way ANOVA with Tukey's post hoc comparison (see “Experimental Procedures”).
One of the more striking findings was that for each agonist the maximum responses recorded from (α3β4)2α3 or (α3β4)2β4 nAChR were much smaller than those measured from oocytes expressing either of the (α3β4)2α5 variant constructs (Figs. 5, A–C; see Table 3). Peak currents were indistinguishable between (α3β4)2α3 and (α3α4)2α4 nAChR for each agonist. This increased function for α5-containing concatemers is the opposite of the decreased function observed previously when α5 subunits are co-injected with unlinked α3 and β4 subunits. This observation may indicate that enforcing correct assembly of α5 subunits by use of a concatemeric construct reduces inefficiencies of assembly or the formation “dead-end intermediates” that have previously been observed when attempting to use self-assembly of individual subunits (30).
TABLE 3.
Pharmacological parameters calculated from α3β4-only nAChR expressed from different injected subunit ratios
Oocytes injected with RNA for α3 and β4 subunits in a 1:1, 1:20, or 20:1 molar ratio were perfused with the nAChR agonists acetylcholine (10−6 to 10−2 m), cytisine (10−6.25 to 10−3 m), nicotine (10−6.5 to 10−3.5 m), or the nAChR antagonist mecamylamine (10−7.5 to 10−4 m). Data are presented as the mean ± S.E., with numbers of individual oocytes tested (n) as indicated.
| Expressed nAChR | Acetylcholine |
Cytisine |
Nicotine |
Mecamylamine |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Log EC50 | nH ± S.E. | n | Log EC50 | nH ± S.E. | n | Log EC50 | nH ± S.E. | n | Log IC50 | nH ± S.E. | |
| α3β4 (1:1) | 6 | −4.2 ± 0.02 | 1.3 ± 0.07 | 6 | −4.7 ± 0.03 | 1.3 ± 0.13 | 6 | −4.7 ± 0.04 | 1.3 ± 0.14 | 6 | −5.3 ± 0.04 | −1.4 ± 0.18 |
| α3β4 (1:20) | 4 | −4.3 ± 0.02 | 0.96 ± 0.07 | 4 | −5.1 ± 0.05 | 1.1 ± 0.13 | 4 | −4.6 ± 0.05 | 1.3 ± 0.17 | 4 | −6.0 ± 0.03 | −1.2 ± 0.1 |
| α3β4 (20:1) | 4 | −3.7 ± 0.01 | 1.8 ± 0.06 | 4 | −4.2 ± 0.03 | 1.5 ± 0.14 | 4 | −4.3 ± 0.04 | 2.2 ± 0.3 | 4 | −6.9 ± 0.1 | −1.2 ± 0.14 |
Divergent α3:β4 RNA Injection Ratios Produce nAChR with Different Functional Properties
As previously noted, pharmacological parameters measured for α3β4α5 nAChR were very similar regardless of whether they were expressed from loose subunits or from concatemeric constructs. The same was not true, however, for α3β4-only subtypes expressed from single subunits injected at a 1:1 ratio or as concatemers (Figs. 2 and 3). It has recently been noted that oocytes injected with different α3:β4 mRNA ratios express α3β4 nAChR populations with differing pharmacological properties (27, 28). By analogy to more extensively studied high and low agonist sensitivity α4β2 nAChR isoforms, it has been speculated that these populations may correspond to (α3β4)2β4 and (α3β4)2α3 stoichiometries (27, 28) as expressed by the concatemeric constructs produced in this study.
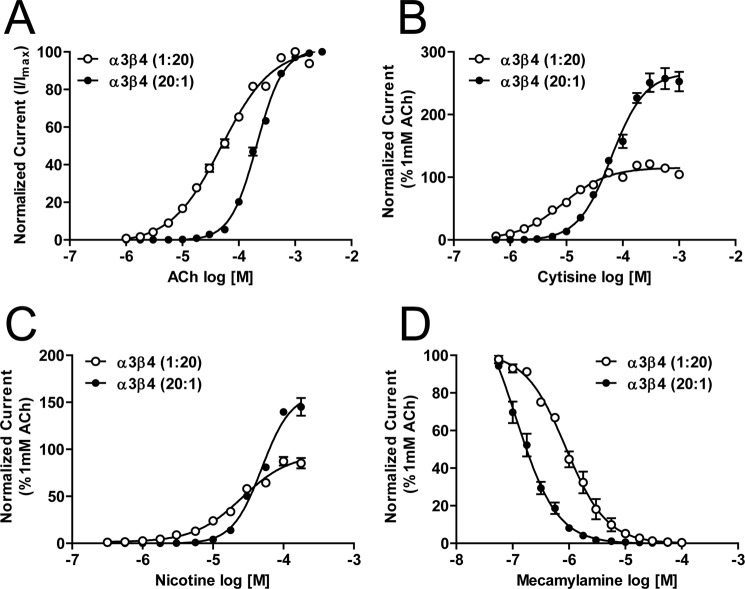
The pharmacological properties of nAChR arising from co-injection of α3 and β4 subunits at 1:20 and 20:1 ratios are very different (Fig. 6). Oocytes injected with α3β4 in a 20:1 cRNA injection ratio expressed receptors that were less sensitive to ACh than those injected with α3β4 in a 1:20 cRNA injection ratio (Fig. 6A; EC50 values of 210 μm (CI, 199–218 μm) versus 50 μm (CI, 43–57 μm), respectively; p < 0.0001). Cytisine concentration response profiles also differed, yielding lower sensitivity responses for nAChR in oocytes injected with 20:1 α3:β4 subunit cRNAs (Fig. 6B, EC50 = 63 μm; CI, 54–74 μm) when compared with those injected with 1:20 subunit ratios (Fig. 6B, EC50 = 8.6 μm; CI, 6.7 to 11 μm; p < 0.01). In addition, cytisine efficacy (normalized to 1 mm ACh) was 2.3× greater for oocytes injected with α3:β4 cRNAs at a 20:1 ratio than for oocytes injected with a 1:20 cRNA ratio (Fig. 6B, 266.5 ± 7.5 to 115.3 ± 2.5%, respectively; p < 0.0001). Moreover, nicotine EC50 values and normalized efficacy values were lower for oocytes injected with α3:β4 subunit cRNAs in a 1:20 ratio than in a 20:1 ratio (Table 2; 25 μm (CI, 20–33 μm) to 46 μm (CI, 39–55 μm) respectively, p < 0.05). Nicotine was more efficacious for oocytes injected with α3:β4 cRNAs at a 20:1 ratio than for oocytes injected with a 1:20 cRNA ratio (Fig. 6C, 151.9 ± 5.4% compared with 95.1 ± 8.2% of 1 mm ACh control, respectively; p > 0.01). Finally, oocytes injected with α3:β4 cRNA at a 20:1 ratio were more sensitive to antagonism by mecamylamine than oocytes injected at a 1:20 ratio (Fig. 6D, 0.12 μm (CI, 0.07–0.18 μm) to 0.89 μm (CI, 0.8–1.0 μm) respectively, p < 0.001).
FIGURE 6.
Concentration response profiles for α3β4-only nAChR expressed as loose subunits. Oocytes injected with RNA for α3 and β4 subunits in a 1:20 or 20:1 ratio were perfused with nAChR agonists acetylcholine (10−6 to 10−2, n = 4) (A), cytisine (10−6.25 to 10−3; n = 4) (B), nicotine (10−6.5 to 10−3.5; n = 4) (C), or the nAChR antagonist mecamylamine (10−7.5 to 10−4; n = 4) (D). Data points represent averages (±S.E.). Differences in drug potency and efficacy between groups were analyzed using one-way ANOVA with Tukey's post hoc comparison (see “Experimental Procedures”).
TABLE 2.
Agonist efficacies compared between concatemeric (α3β4)2X nAChR, where X is either β4, α3, α5(Asn-398) or α5 (Asp-398)
Maximal currents (normalized to (α3β4)2α5(Asp-398); see “Experimental Procedures”) were compared between α3β4* nAChR concatemers for acetylcholine (10−6 to 10−2.25 m), cytisine (10−6.25 to 10−3 m), and nicotine (10−6.25 to 10−3 m). Data are presented as the mean ± S.E., with numbers of individual oocytes tested (n) as indicated.
| Compound | n | (α3β4)2β4(% of Asp-398) | n | (α3β4)2α3(% of Asp-398) | n | (α3β4)2α5(Asn-398)(% of Asp-398) | n | (α3β4)2α5(Asp-398) concatemer |
|---|---|---|---|---|---|---|---|---|
| Acetylcholine | 16 | 11 ± 1.0 | 11 | 9 ± 1.0 | 17 | 63 ± 7.0 | 17 | 100 ± 5.0 |
| Cytisine | 6 | 5 ± 1.0 | 6 | 6 ± 1.0 | 10 | 80 ± 11.0 | 10 | 100 ± 14.0 |
| Nicotine | 6 | 11 ± 4.0 | 6 | 9 ± 3.0 | 8 | 85 ± 9.0 | 8 | 100 ± 4.0 |
Overall, the pharmacological properties measured from oocytes injected with α3:β4 cRNAs in a 1:1 or a 1:20 ratio more closely resembled each other than those recorded from oocytes injected at a 20:1 ratio (Table 3). The very similar properties of nAChR arising from 1:1 or 1:20 α3:β4 mRNA injection ratios indicate that the same subunit assembly pattern predominates in both cases. This confirms previous reports that Xenopus oocytes injected with 1:1 and 1:9 α3:β4 mRNA ratios express similar α3β4 nAChR populations, whereas high α3:β4 mRNA injection ratios result in expression of a distinctly different α3β4 nAChR isoform (27, 28). However, none of the outcomes observed from oocytes injected with any ratio of α3:β4 mRNAs closely resembled the results obtained from the concatemeric constructs or after co-injection of single α3, β4, and α5 subunits (Table 1). We conclude that the presence of an α5 subunit or the use of concatemeric constructs results in the assembly of functional nAChR with similar pharmacological properties. These properties are likely the hallmark of assembly into a format containing two (α3/β4) subunit interfaces, with the addition of a fifth subunit in a non-ligand binding role. This conclusion is supported by a very recent study showing similar pharmacological profiles of (α3β4)2X nAChR assembled from α3-β4 dimeric concatemers with the addition of single α3, β4, or α5 subunits (37). Without the constraints imposed by the concatemeric linkers or by the need to integrate a non-ligand binding α5 subunit, it seems possible that α3 and β4 subunits are free to assemble into at least two other formats. The relative proportions of the two formats expressed in the Xenopus oocyte system can be altered by biasing the α3:β4 nAChR subunit mRNA injection ratio.
DISCUSSION
The pentameric concatemer approach allows accurate and consistent reproduction of complex nAChR subtypes, with complete control over subunit ratios and associations (5, 31, 38, 39). It also allows for mutagenesis of a single subunit within an entire nAChR complex even where multiple copies of the target subunit may be present. These unique advantages were central to the work presented in this study. Using concatemeric α3β4α5 nAChR, we show that α5 subunit risk variant (Asn-398) incorporation reduces ACh-evoked function when compared with inclusion of the α5 common variant (Asp-398). Coexpression of unlinked α3, β4, and α5 subunits enforces assembly of an apparently uniform nAChR population with very similar pharmacological properties to those of concatemeric α3β4* nAChR. In addition, either variant of the α5 subunit is capable of reducing the overall amount of α3β4* nAChR function after coinjection with non-concatenated α3 and β4 subunits. Further observations suggested that removing the constraints imposed by either concatemerization or by co-expression with unlinked α5 subunits allows loose α3 and β4 subunits to assemble into at least two further subtypes. These α3β4-only subtypes have substantially different pharmacological profiles from each other, from unlinked subunit α3β4α5 nAChR, and from any of the concatenated α3β4 or α3β4α5 nAChR.
Critically, the pharmacological properties of α3β4α5 nAChR expressed using pentameric concatemers were similar to those of the same subtype expressed from unlinked subunits. This finding indicates that the addition of the concatemeric linkers did not noticeably alter nAChR function. It also reinforces further that pentameric concatemers faithfully replicate the ligand sensitivity of the equivalent subunit arrangement when formed from loose subunits. It has been suggested that α5 subunits compete with β4 subunits (20, 29), reducing expression of functional α3β4α5 nAChR, possibly by encouraging the formation of dead-end intermediates that become trapped inside the cell (30). Our observations support this concept. Coinjection of non-concatenated α5 subunit mRNA approximately halved α3β4* functional expression in Xenopus oocytes compared with injection of loose α3 and β4 subunits only (1:1:1 or 1:1 ratios were used; see the legend to Fig. 2). In contrast, if the α5 subunit is forced by concatemerization to assemble only as part of a pentameric nAChR complex, its incorporation substantially increases functional expression (Fig. 5). Together, these observations suggest that a reduction in function is not caused by the incorporation of α5 subunits per se. Instead, the presence of loose α5 subunits likely adversely affects the efficiency of unlinked α3 and β4 nAChR subunit assembly into functional nAChR.
In contrast, α3β4-only nAChR expressed from pentameric concatemers had different pharmacological properties from those expressed from loose subunits (Table 1, top two rows, and Table 2). This discrepancy could be explained in several ways. One possibility is that covalent linkers may alter the properties of concatemeric nAChR by constraining structural transitions that are essential for normal function. This concern is mitigated by previous publications (5, 31, 38, 39) indicating that well designed pentameric nAChR concatemers can accurately reproduce the properties of multiple native nAChR subtypes (which assemble from unlinked subunits). In addition, the linkers in each of the pentameric concatemers used in this study are of the same length and composition; it is unlikely that only the non-α5* concatemers used in this study would suffer from linker-induced functional alterations. Furthermore, if the non-α5* concatemers were uniquely affected by the presence of the linkers, it would be expected that this would strongly alter agonist potencies and relative efficacies when compared with those of the α5* concatemers. This is not the case; the pharmacological parameters measured from all four of the concatemers tested here are strikingly similar. A second possibility is that the covalent linkers within the concatemers might break down. This would release sub-pentameric products that could assemble to form unintended, but functional, byproducts (4, 34, 35). The presence of such degradation products was checked for by coinjection with an α5(V9′S) mutant subunit. Assembly of this mutant subunit with either single α3 and β4 subunits or subpentameric concatemers would result in a substantial gain of function (34, 36). No change in function was noted when α5(V9′S) was co-injected with a concatemeric construct. This confirms that all, or nearly all, of the function in oocytes injected with pentameric nAChR mRNA constructs arises from fully-pentameric concatemeric nAChR. Finally, and most likely, the precise subunit associations imposed by concatemeric constructs may, or may not, correspond to those favored during association of loose subunits. Our data suggest that the α3β4α5 concatemers accurately reproduce the conformation adopted when the relevant individual subunits assemble freely. However, the same is not true for the α3β4-only constructs when compared with nAChR assembled from loose α3 and β4 subunits. This would indicate that one role of the α5 subunit is to impose a particular subunit composition on α3β4* nAChR expressed from loose subunits. If α5 is a true “accessory” subunit (i.e. does not interact directly with ligands), this may be unavoidable; a (α3β4)2α5 conformation is the only one in which two pairs of α3+β4 subunits would be available to provide agonist binding pockets and thus to assemble a functional α3β4α5 nAChR.
The preceding observations raise the question of which nAChR subtype(s) is expressed after coinjection of only α3 and β4 subunits. This study confirms prior reports that at least two α3β4 nAChR populations may be formed and that their relative expression levels depend on the molar injection ratio of the subunit mRNAs (1:20 versus 20:1). The pharmacology observed in this study matches that reported in other recent publications (27, 28) that used less-extreme injection ratios (1:9 versus 9:1 or 1:10 versus 10:1). The lack of further changes in observed pharmacology after adoption of more extreme subunit ratios indicates that, as for α4 and β2 subunits (2, 40, 41), relatively pure populations of two different α3β4 subunit assemblies are produced at the injection ratios used in this study. The same studies proposed again by analogy to the well-studied α4β2 nAChR that the different nAChR isoforms might correspond to (α3β4)2β4 and (α3β4)2α3 nAChR (27).
Accordingly we constructed (α3β4)2β4 and (α3β4)2α3 concatemers using the same subunit arrangements as used successfully to encode high and low agonist sensitivity pentameric α4β2 nAChR concatemers (5). We initially anticipated that these concatemers would have similar pharmacological profiles to α3β4-only nAChR formed after injection of loose α3 and β4 subunits at 1:20 and 20:1 ratios, respectively. However, the pharmacology observed after injection of loose α3 and β4 subunits at either 1:20 or 20:1 ratios (Fig. 6, Table 2) was strikingly different from the concatemeric “(α3β4)2X-type” measurements. The precise arrangements adopted by loose α3 and β4 subunits injected at different ratios remain unknown. It certainly seems probable that 1:20 and 20:1 α3:β4 injection ratios may give rise to nAChR with different stoichiometries (27, 28). In addition, as demonstrated for GABAA receptors, the precise order of subunit incorporation (even for identical subunit stoichiometries) can affect receptor function (42). The emerging awareness that agonist binding to non-canonical nAChR interfaces can strongly affect function underlines this point (5, 41, 43). Determining whether different α3:β4 subunit mRNA injection ratios produce nAChR with different stoichiometries, different arrangements of the same subunit stoichiometries, or both will require a great deal more investigation. The concatemeric pentamer approach is uniquely well suited to addressing this question.
Unlike agonist EC50 values, IC50 values for mecamylamine inhibition were greatly affected by the identity of the fifth subunit in each pentameric concatemer. This suggests that mecamylamine (a non-competitive antagonist) interacts with the resulting nAChR in a position where it can be influenced by the presence of alternate subunits in the fifth, non-agonist-binding position. This sensitivity to α3β4* nAChR composition was also evident when comparing mecamylamine IC50 values between α3β4 and α3β4α5 nAChR expressed from loose subunits (Table 1). These observations indicate that non-competitive ligands may provide the best opportunities to pharmacologically distinguish between different subunit arrangements of α3β4* isoforms. Importantly, this category could also include positive allosteric modulators and/or allosteric agonists in addition to non-competitive antagonists. Given the association of α5 subunit variants with a variety of substance abuse behaviors (see introduction), selective manipulation of α3β4α5 nAChR activity could have valuable therapeutic implications.
Functional effects of the α5(D398N) mutation are hard to distinguish without using a fully pentameric concatemer approach. The previously described effects of α5 subunits on the efficiency of α3β4* nAChR expression and possibly also on subunit associations/assembly could outweigh and obscure the effects of the α5(D398N) mutation. This could explain previous studies' conclusions that the effects of α5(Asp-389) incorporation were the same as those of α5(Asn-389) (18, 25, 37). However, using a pentameric concatemer approach, we were able to compare the function of uniform populations of (α3β4)2α5(Asp-389) versus (α3β4)2α5(Asn-389) nAChR. The maximum ACh-induced function produced by (α3β4)2α5(Asp-389) nAChR was significantly greater than that measured for (α3β4)2α5(Asn389) nAChR (Fig. 5). Increased function for α5(Asp-398)* versus α5(Asn-398)* nAChR with little difference in pharmacological profile matches previous observations regarding α5 variant incorporation into α4β2* nAChR (11, 18).
It appears that, as previously proposed (20), α5 subunit expression may act to modulate the amount of α3β4* nAChR function in the habenulopeduncular tract and in other tissues that express α3β4α5 nAChR. This study indicates that the presence of the α5(Asp-398) or α5(Asn-398) variant will impose an additional layer of functional modulation. As noted previously (18), the concentrations of nicotine present in smokers are too low to significantly activate or desensitize α3β4α5 nAChR. However, the activity induced by synaptic or perisynaptic ACh release onto α3β4* nAChR could be strongly affected by the integration of α5(Asp-398) or α5(Asn-398) subunits. This in turn could result in compensatory changes either at the neurotransmitter/receptor level or at the circuit activity level, which may explain some of the phenotypic variations attributed to the α5(D398N) mutation. Given the established role of the habenulopeduncular pathway α3β4α5 nAChR function in nicotine dependence and aversive behavior (20, 44, 45), it seems likely that selective manipulation of α3β4α5 function mediated by this subtype could represent a valuable smoking cessation strategy. Our current findings indicate that non-competitive/allosteric compounds may be the most promising category of potential therapeutic agents for such an approach.
Acknowledgment
We thank Minoti Bhakta for technical assistance with concatemer design and construction.
This work was supported, in whole or in part, by National Institutes of Health Grants R21 DA027070–02S1 (to P. W., X. C., M. I. D., and R. J. L.) and R21 DA026627 (to P. W.). This work was also supported by an endowment and capitalization funds from the Men's and Women's Boards of the Barrow Neurological Foundation (to P. W.). Portions of this work were presented as a Society for Neuroscience abstract (46).
- nAChR
- nicotinic acetylcholine receptor(s)
- ACh
- acetylcholine
- CI
- confidence interval
- ANOVA
- analysis of variance.
REFERENCES
- 1. Gotti C., Clementi F., Fornari A., Gaimarri A., Guiducci S., Manfredi I., Moretti M., Pedrazzi P., Pucci L., Zoli M. (2009) Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem. Pharmacol. 78, 703–711 [DOI] [PubMed] [Google Scholar]
- 2. Zwart R., Vijverberg H. P. (1998) Four pharmacologically distinct subtypes of α4β2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol. Pharmacol. 54, 1124–1131 [PubMed] [Google Scholar]
- 3. Nelson M. E., Kuryatov A., Choi C. H., Zhou Y., Lindstrom J. (2003) Alternate stoichiometries of α4β2 nicotinic acetylcholine receptors. Mol. Pharmacol. 63, 332–341 [DOI] [PubMed] [Google Scholar]
- 4. Zhou Y., Nelson M. E., Kuryatov A., Choi C., Cooper J., Lindstrom J. (2003) Human α4β2 acetylcholine receptors formed from linked subunits. J. Neurosci. 23, 9004–9015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carbone A. L., Moroni M., Groot-Kormelink P. J., Bermudez I. (2009) Pentameric concatenated (α4)(2)(β2)(3) and (α4)(3)β2)(2) nicotinic acetylcholine receptors. Subunit arrangement determines functional expression. Br. J. Pharmacol. 156, 970–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saccone S. F., Hinrichs A. L., Saccone N. L., Chase G. A., Konvicka K., Madden P. A., Breslau N., Johnson E. O., Hatsukami D., Pomerleau O., Swan G. E., Goate A. M., Rutter J., Bertelsen S., Fox L., Fugman D., Martin N. G., Montgomery G. W., Wang J. C., Ballinger D. G., Rice J. P., Bierut L. J. (2007) Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum. Mol. Genet. 16, 36–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schlaepfer I. R., Hoft N. R., Collins A. C., Corley R. P., Hewitt J. K., Hopfer C. J., Lessem J. M., McQueen M. B., Rhee S. H., Ehringer M. A. (2008) The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol. Psychiatry 63, 1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thorgeirsson T. E., Geller F., Sulem P., Rafnar T., Wiste A., Magnusson K. P., Manolescu A., Thorleifsson G., Stefansson H., Ingason A., Stacey S. N., Bergthorsson J. T., Thorlacius S., Gudmundsson J., Jonsson T., Jakobsdottir M., Saemundsdottir J., Olafsdottir O., Gudmundsson L. J., Bjornsdottir G., Kristjansson K., Skuladottir H., Isaksson H. J., Gudbjartsson T., Jones G. T., Mueller T., Gottsäter A., Flex A., Aben K. K., de Vegt F., Mulders P. F., Isla D., Vidal M. J., Asin L., Saez B., Murillo L., Blondal T., Kolbeinsson H., Stefansson J. G., Hansdottir I., Runarsdottir V., Pola R., Lindblad B., van Rij A. M., Dieplinger B., Haltmayer M., Mayordomo J. I., Kiemeney L. A., Matthiasson S. E., Oskarsson H., Tyrfingsson T., Gudbjartsson D. F., Gulcher J. R., Jonsson S., Thorsteinsdottir U., Kong A., Stefansson K. (2008) A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 452, 638–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weiss R. B., Baker T. B., Cannon D. S., von Niederhausern A., Dunn D. M., Matsunami N., Singh N. A., Baird L., Coon H., McMahon W. M., Piper M. E., Fiore M. C., Scholand M. B., Connett J. E., Kanner R. E., Gahring L. C., Rogers S. W., Hoidal J. R., Leppert M. F. (2008) A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet 4, e1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berrettini W., Yuan X., Tozzi F., Song K., Francks C., Chilcoat H., Waterworth D., Muglia P., Mooser V. (2008) α-5/α-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Molecular psychiatry 13, 368–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bierut L. J., Stitzel J. A., Wang J. C., Hinrichs A. L., Grucza R. A., Xuei X., Saccone N. L., Saccone S. F., Bertelsen S., Fox L., Horton W. J., Breslau N., Budde J., Cloninger C. R., Dick D. M., Foroud T., Hatsukami D., Hesselbrock V., Johnson E. O., Kramer J., Kuperman S., Madden P. A., Mayo K., Nurnberger J., Jr., Pomerleau O., Porjesz B., Reyes O., Schuckit M., Swan G., Tischfield J. A., Edenberg H. J., Rice J. P., Goate A. M. (2008) Variants in nicotinic receptors and risk for nicotine dependence. Am. J. Psychiatry 165, 1163–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spitz M. R., Amos C. I., Dong Q., Lin J., Wu X. (2008) The CHRNA5-A3 region on chromosome 15q24–25.1 is a risk factor both for nicotine dependence and for lung cancer. J. Natl. Cancer Inst. 100, 1552–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hung R. J., McKay J. D., Gaborieau V., Boffetta P., Hashibe M., Zaridze D., Mukeria A., Szeszenia-Dabrowska N., Lissowska J., Rudnai P., Fabianova E., Mates D., Bencko V., Foretova L., Janout V., Chen C., Goodman G., Field J. K., Liloglou T., Xinarianos G., Cassidy A., McLaughlin J., Liu G., Narod S., Krokan H. E., Skorpen F., Elvestad M. B., Hveem K., Vatten L., Linseisen J., Clavel-Chapelon F., Vineis P., Bueno-de-Mesquita H. B., Lund E., Martinez C., Bingham S., Rasmuson T., Hainaut P., Riboli E., Ahrens W., Benhamou S., Lagiou P., Trichopoulos D., Holcátová I., Merletti F., Kjaerheim K., Agudo A., Macfarlane G., Talamini R., Simonato L., Lowry R., Conway D. I., Znaor A., Healy C., Zelenika D., Boland A., Delepine M., Foglio M., Lechner D., Matsuda F., Blanche H., Gut I., Heath S., Lathrop M., Brennan P. (2008) A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 452, 633–637 [DOI] [PubMed] [Google Scholar]
- 14. Wang J. C., Grucza R., Cruchaga C., Hinrichs A. L., Bertelsen S., Budde J. P., Fox L., Goldstein E., Reyes O., Saccone N., Saccone S., Xuei X., Bucholz K., Kuperman S., Nurnberger J., Jr., Rice J. P., Schuckit M., Tischfield J., Hesselbrock V., Porjesz B., Edenberg H. J., Bierut L. J., Goate A. M. (2009) Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol. Psychiatry 14, 501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grucza R. A., Wang J. C., Stitzel J. A., Hinrichs A. L., Saccone S. F., Saccone N. L., Bucholz K. K., Cloninger C. R., Neuman R. J., Budde J. P., Fox L., Bertelsen S., Kramer J., Hesselbrock V., Tischfield J., Nurnberger J. I., Jr., Almasy L., Porjesz B., Kuperman S., Schuckit M. A., Edenberg H. J., Rice J. P., Goate A. M., Bierut L. J. (2008) A risk allele for nicotine dependence in CHRNA5 is a protective allele for cocaine dependence. Biol. Psychiatry 64, 922–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown R. W., Collins A. C., Lindstrom J. M., Whiteaker P. (2007) Nicotinic α5 subunit deletion locally reduces high affinity agonist activation without altering nicotinic receptor numbers. J. Neurochem. 103, 204–215 [DOI] [PubMed] [Google Scholar]
- 17. Mao D., Perry D. C., Yasuda R. P., Wolfe B. B., Kellar K. J. (2008) The α4β2α5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J. Neurochem. 104, 446–456 [DOI] [PubMed] [Google Scholar]
- 18. Kuryatov A., Berrettini W., Lindstrom J. (2011) Acetylcholine receptor (AChR) α5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (α4β2)2α5 AChR function. Mol. Pharmacol. 79, 119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zoli M., Le Novère N., Hill J. A., Jr., Changeux J. P. (1995) Developmental regulation of nicotinic ACh receptor subunit mRNAs in the rat central and peripheral nervous systems. J. Neurosci. 15, 1912–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frahm S., Slimak M. A., Ferrarese L., Santos-Torres J., Antolin-Fontes B., Auer S., Filkin S., Pons S., Fontaine J. F., Tsetlin V., Maskos U., Ibañez-Tallon I. (2011) Aversion to nicotine is regulated by the balanced activity of β4 and α5 nicotinic receptor subunits in the medial habenula. Neuron 70, 522–535 [DOI] [PubMed] [Google Scholar]
- 21. David R., Ciuraszkiewicz A., Simeone X., Orr-Urtreger A., Papke R. L., McIntosh J. M., Huck S., Scholze P. (2010) Biochemical and functional properties of distinct nicotinic acetylcholine receptors in the superior cervical ganglion of mice with targeted deletions of nAChR subunit genes. Eur. J. Neurosci. 31, 978–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Conroy W. G., Berg D. K. (1995) Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. J. Biol. Chem. 270, 4424–4431 [DOI] [PubMed] [Google Scholar]
- 23. Vernallis A. B., Conroy W. G., Berg D. K. (1993) Neurons assemble acetylcholine receptors with as many as three kinds of subunits while maintaining subunit segregation among receptor subtypes. Neuron 10, 451–464 [DOI] [PubMed] [Google Scholar]
- 24. Egleton R. D., Brown K. C., Dasgupta P. (2008) Nicotinic acetylcholine receptors in cancer. Multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol. Sci. 29, 151–158 [DOI] [PubMed] [Google Scholar]
- 25. Li P., McCollum M., Bracamontes J., Steinbach J. H., Akk G. (2011) Functional characterization of the α5 (Asn-398) variant associated with risk for nicotine dependence in the α3β4α5 nicotinic receptor. Mol. Pharmacol. 80, 818–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Papke R. L., Wecker L., Stitzel J. A. (2010) Activation and inhibition of mouse muscle and neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 333, 501–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krashia P., Moroni M., Broadbent S., Hofmann G., Kracun S., Beato M., Groot-Kormelink P. J., Sivilotti L. G. (2010) Human α3β4 neuronal nicotinic receptors show different stoichiometry if they are expressed in Xenopus oocytes or mammalian HEK293 cells. PLoS ONE 5, e13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grishin A. A., Wang C. I., Muttenthaler M., Alewood P. F., Lewis R. J., Adams D. J. (2010) α-Conotoxin AuIB isomers exhibit distinct inhibitory mechanisms and differential sensitivity to stoichiometry of α3β4 nicotinic acetylcholine receptors. J. Biol. Chem. 285, 22254–22263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gahring L. C., Rogers S. W. (2010) Nicotinic receptor subunit α5 modifies assembly, up-regulation, and response to pro-inflammatory cytokines. J. Biol. Chem. 285, 26049–26057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuryatov A., Onksen J., Lindstrom J. (2008) Roles of accessory subunits in α4β2(*) nicotinic receptors. Mol. Pharmacol. 74, 132–143 [DOI] [PubMed] [Google Scholar]
- 31. Kuryatov A., Lindstrom J. (2011) Expression of functional human α6β2β3* acetylcholine receptors in Xenopus laevis oocytes achieved through subunit chimeras and concatamers. Mol. Pharmacol. 79, 126–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang Y., Ghansah E., Chen Y., Ye J., Weiss D. S. (2002) Desensitization mechanism of GABA receptors revealed by single oocyte binding and receptor function. J. Neurosci. 22, 7982–7990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dash B., Chang Y., Lukas R. J. (2011) Reporter mutation studies show that nicotinic acetylcholine receptor (nAChR) α5 subunits and/or variants modulate function of α6*-nAChR. J. Biol. Chem. 286, 37905–37918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Groot-Kormelink P. J., Broadbent S. D., Boorman J. P., Sivilotti L. G. (2004) Incomplete incorporation of tandem subunits in recombinant neuronal nicotinic receptors. J. Gen. Physiol. 123, 697–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nicke A., Rettinger J., Schmalzing G. (2003) Monomeric and dimeric byproducts are the principal functional elements of higher order P2X1 concatamers. Mol. Pharmacol. 63, 243–252 [DOI] [PubMed] [Google Scholar]
- 36. Labarca C., Nowak M. W., Zhang H., Tang L., Deshpande P., Lester H. A. (1995) Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature 376, 514–516 [DOI] [PubMed] [Google Scholar]
- 37. Stokes C., Papke R. L. (2012) Neuropharmacology, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mazzaferro S., Benallegue N., Carbone A., Gasparri F., Vijayan R., Biggin P. C., Moroni M., Bermudez I. (2011) Additional acetylcholine (ACh) binding site at α4/α4 interface of (α4β2)2α4 nicotinic receptor influences agonist sensitivity. J. Biol. Chem. 286, 31043–31054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Groot-Kormelink P. J., Broadbent S., Beato M., Sivilotti L. G. (2006) Constraining the expression of nicotinic acetylcholine receptors by using pentameric constructs. Mol. Pharmacol. 69, 558–563 [DOI] [PubMed] [Google Scholar]
- 40. Moroni M., Vijayan R., Carbone A., Zwart R., Biggin P. C., Bermudez I. (2008) Non-agonist binding subunit interfaces confer distinct functional signatures to the alternate stoichiometries of the α4β2 nicotinic receptor. An α4-α4 interface is required for Zn2+ potentiation. J. Neurosci. 28, 6884–6894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harpsøe K., Ahring P. K., Christensen J. K., Jensen M. L., Peters D., Balle T. (2011) Unraveling the high and low sensitivity agonist responses of nicotinic acetylcholine receptors. J. Neurosci. 31, 10759–10766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sigel E., Baur R., Boulineau N., Minier F. (2006) Impact of subunit positioning on GABAA receptor function. Biochem. Soc. Trans. 34, 868–871 [DOI] [PubMed] [Google Scholar]
- 43. Seo S., Henry J. T., Lewis A. H., Wang N., Levandoski M. M. (2009) The positive allosteric modulator morantel binds at noncanonical subunit interfaces of neuronal nicotinic acetylcholine receptors. J. Neurosci. 29, 8734–8742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fowler C. D., Lu Q., Johnson P. M., Marks M. J., Kenny P. J. (2011) Habenular α5 nicotinic receptor subunit signaling controls nicotine intake. Nature 471, 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salas R., Orr-Urtreger A., Broide R. S., Beaudet A., Paylor R., De Biasi M. (2003) The nicotinic acetylcholine receptor subunit α 5 mediates short term effects of nicotine in vivo. Mol. Pharmacol. 63, 1059–1066 [DOI] [PubMed] [Google Scholar]
- 46. George A. A., Bhakta M., Lucero L. M., Lukas R. J., Whiteaker P. (2011) Functional properties of concatenated α3β4 and α3β4α5 nicotinic receptors. Soc. Neurosci. Abst. 34, 864.20 [Google Scholar]