Background: Macrophages and neutrophils are key phagocytes in regeneration.
Results: Neutrophils are the primary phagocytes in the inflammatory stage and are dispensable for zebrafish fin regeneration, whereas macrophages mainly function in the resolution stage and are required for fin regeneration.
Conclusion: Macrophages and neutrophils behave differently during zebrafish fin injury and regeneration.
Significance: Our study documents that macrophages and neutrophils play distinct functions in tissue regeneration.
Keywords: In Vivo Imaging, Macrophages, Neutrophil, Fin Injury, Fin Regeneration, Zebrafish
Abstract
Macrophages and neutrophils are the pivotal immune phagocytes that enter the wound after tissue injury to remove the cell debris and invaded microorganisms, which presumably facilitate the regrowth of injured tissues. Taking advantage of the regeneration abilities of zebrafish and the newly generated leukocyte-specific zebrafish lines with labeling of both leukocyte lineages, we assessed the behaviors and functions of neutrophils and macrophages during tail fin regeneration. Live imaging showed that within 6 hours post amputation, the inflammatory stage, neutrophils were the primary cells scavenging apoptotic bodies and small cell debris, although they had limited phagocytic capacity and quickly underwent apoptosis. From 6 hours post amputation on, the resolution and regeneration stage, macrophages became the dominant scavengers, efficiently resolving inflammation and facilitating tissue remodeling and regrowth. Ablation of macrophages but not neutrophils severely impaired the inflammatory resolution and tissue regeneration, resulting in the formation of large vacuoles in the regenerated fins. In contrast, removal of neutrophils slightly accelerates the regrowth of injured fin. Our study documents the differing behaviors and functions of macrophages and neutrophils during tissue regeneration.
Introduction
Successful epimorphic regeneration is essential to restore the integrity and function of injured organs (1). During this process, the immune cells play indispensable protective roles for removing dead cells, preventing invasion of microorganisms, and supporting regrowth of damaged tissues (2). Neutrophils and macrophages are two major immune system cells that participate in this process (3). Neutrophils are the first immune cells to arrive at the sites of injury, where they mainly fight against foreign invaders, such as harmful microbes, whereas macrophages emerge later and are thought to participate not only in the removal of dead cells and microbes in the wound but also the remodeling and regeneration of injured tissue (3–9). Nevertheless, despite extensive studies in the past decades (3), the in vivo behavior and function of each type of phagocyte and the communication between them during tissue injury and tissue regeneration are not fully characterized.
Zebrafish have recently emerged as a prominent model organism for the study of tissue regeneration due to their genetic manipulability and potent regeneration ability (10–13). The epimorphic regeneration of tail fin in larva fish is one of the well established tissue regeneration assays (10, 13). Upon tail fin amputation, the wound region undergoes a lesion-induced contraction caused by actin-purse string formation. Shortly after, the epithelium cells cover the lesion to seal the stump surface. From 12 hours post amputation (hpa)3 on, the wound begins to regrow, and the blastema-like cells emerge under the well packed epithelium layer, signifying the regeneration of the injured tail fin. The full recovery of the wound normally requires only 4–5 days and recapitulates the process of tail fin regeneration in adult fish (10). Thus, the larva tail fin regeneration is an ideal system for studying the roles of immune cells in injury-induced tissue regeneration. Moreover, the creations of myeloid lineage-marking transgenic lines (14–16) and myeloid-defective fish (17) have provided unique opportunities to explore the actions of myeloid leukocytes during epimorphic regeneration in a living organism. However, zebrafish lines in which both macrophage and neutrophil lineages are labeled by fluorescent proteins are still limited.
CORONIN 1a, also known as p57 or TACO, is an actin-binding protein that belongs to the CORONIN family (18, 19). Unlike other family members, Coronin 1a RNA is specifically synthesized by leukocytes (20). It has been suggested that Coronin 1a plays an important role in the migration, phagosome formation, and vesicle trafficking of macrophages and neutrophils (21–23). During macrophage phagocytosis, CORONIN 1a rapidly expresses on the leading edge of the lamellipodia and transiently concentrates on the early phagosome, and it quickly disappears when the phagosome is matured or fused with the lysosome (23), suggesting that CORONIN 1a is involved in the formation of phagosome. Intriguingly, continual expression of CORONIN 1a in the phagosome prevents the delivery of mycobacteria from the phagosome to the lysosomes, resulting in the enhancement of mycobacteria survival in macrophages (21). On the other hand, CORONIN 1a in neutrophils is mainly functioned in the chemotaxis (22). Similar to that in mammals, coronin1a (coro1a) in zebrafish is highly enriched in leukocytes, especially in myeloid cells and T lymphocytes, from the early development stage (24). These studies suggest that coro1a may serve as a marker for the full repertoire of myeloid cells in zebrafish.
In this study, the promoter region of coro1a was isolated and utilized to create a stable transgenic line, Tg(coro1a:eGFP), in which both macrophages and neutrophils were marked by fluorescent protein in developing zebrafish embryos. Using a double transgenic line, Tg(coro1a:eGFP;lyz:Dsred), in which macrophages and neutrophils could be distinguished by green and red fluorescence, respectively, combined with morpholino-induced ablation of macrophages and neutrophil-defective mutant runx1w84x, the in vivo functions of macrophages and neutrophils during the epimorphic regeneration of tail fin were analyzed. Live recordings revealed that neutrophils were the major players during the inflammatory stage immediately after tail fin amputation, when they actively engulfed small dead cell debris, but appeared to have an inhibitory role in subsequent regeneration of the fin, whereas macrophages were critical for resolving inflammation and supporting normal regrowth of the regenerating tail fin. This report systematically describes the different functions of macrophages and neutrophils during the process of epimorphic regeneration of the zebrafish tail fin after amputation, and our findings provide clues for further study of the molecules involved.
EXPERIMENTAL PROCEDURES
Fish Lines
Wild-type AB, Tg(lyz:Dsred) (16), Tg(coro1a:eGFP)hkz04t, Tg(coro1a:eGFP)hkz05t, and runx1w84x fish strains were maintained as described (25).
In Vitro Synthesis of Antisense RNA Probe and WISH
The coro1a antisense RNA probe was prepared by in vitro transcription according to the standard protocol. The WISH was performed as described previously (26).
Double Fluorescence Immunohistochemistry Staining
Immunohistochemistry was performed as described previously (27). To examine the co-staining of GFP and DsRed, embryos were first stained with goat anti-GFP and rabbit anti-DsRed antibody (1:250, 4 °C, overnight) and subsequently visualized by Alexa Fluor 488 donkey anti-goat (1:400, 4 °C, overnight) and Alexa Fluor 555 donkey anti-rabbit (1:400, 4 °C, overnight). Similar methods were used for co-staining of GFP and Lcp protein and of GFP and phosphohistone 3 (pH3) (28). Anti-Lcp and anti-pH3 (Upstate) antibody was visualized by Alexa Fluor donkey anti-rabbit 555 (1:400, 4 °C, overnight) (Molecular Probes).
Video-enhanced DIC Imaging
Video-enhanced DIC microscopy was performed as described (28).
Generation of Tg(coro1a:eGFP) and Tg(coro1a:eGFP; lyz:Dsred) Transgenic Lines
7.03-kb DNA sequence upstream of the coro1a translation start site amplified with the primers 5′-AGCAACAACGTTCTAAGAGA-3′/5′-GATGACCTGAGGAAAGACA-3′ was used as a promoter to drive eGFP expression in the pTol2 vector. The pTol2-coro1a-eGFP construct was injected into the wild-type (WT) fish embryos at one-cell stage. The embryos with an appropriate GFP expression were selected and raised to adults. Two founder lines Tg(coro1a:eGFP)hkz04t and Tg(coro1a:eGFP)hkz05t were identified based on their eGFP expression pattern. Tg(coro1a:eGFP;lyz:Dsred) double transgenic line was generated by mating F1 Tg(coro1a:eGFP) with Tg(lyz:Dsred) fish.
Fluorescence-activated Cell Sorting (FACS) and Giemsa Staining
The FACS analysis to isolate GFP-positive cells and Giemsa staining were performed as described previously (29, 30). About 300 Tg(coro1a:eGFP) embryos were used for FACS for each developmental stage and were repeated twice.
Live Image Analysis
The injured fish embryos were anesthetized and mounted in 1% agarose and subsequently imaged under an Olympus confocal microscope (×20 objective) for around 48 h. Images were taken every 3 min, extracted, and converted to the movie using FV10-ASW 1.7 software. Photoshop 6.0 was employed to analyze the moving trace of macrophages and neutrophils.
Acridine Orange (AO) Staining
AO staining was performed as described (31). Embryos of different stages were incubated with 5 μg/ml AO in egg water for 30 min. After washing for 1 h, the embryos were observed under the confocal microscope.
Irf8 (Interferon Regulatory Factor 8) Morpholino-oligonucleotide (MO) Injection
0.6 mm irf8 MOsp (5′-AATGTTTCGCTTACTTTGAAAATGG-3′) or 0.3 mm irf8 MOatg (5′-TCAGTCTGCGACCGCCCGAGTTCAT-3′) and control morpholino-oligonucleotide were injected into the one-cell stage embryos (28).
Statistical Methods
The calculated data were recorded and analyzed by Excel software. Student's t test was mainly used as the statistical method.
RESULTS
Tg(coro1a:eGFP) Fish Mark both Macrophages and Neutrophils
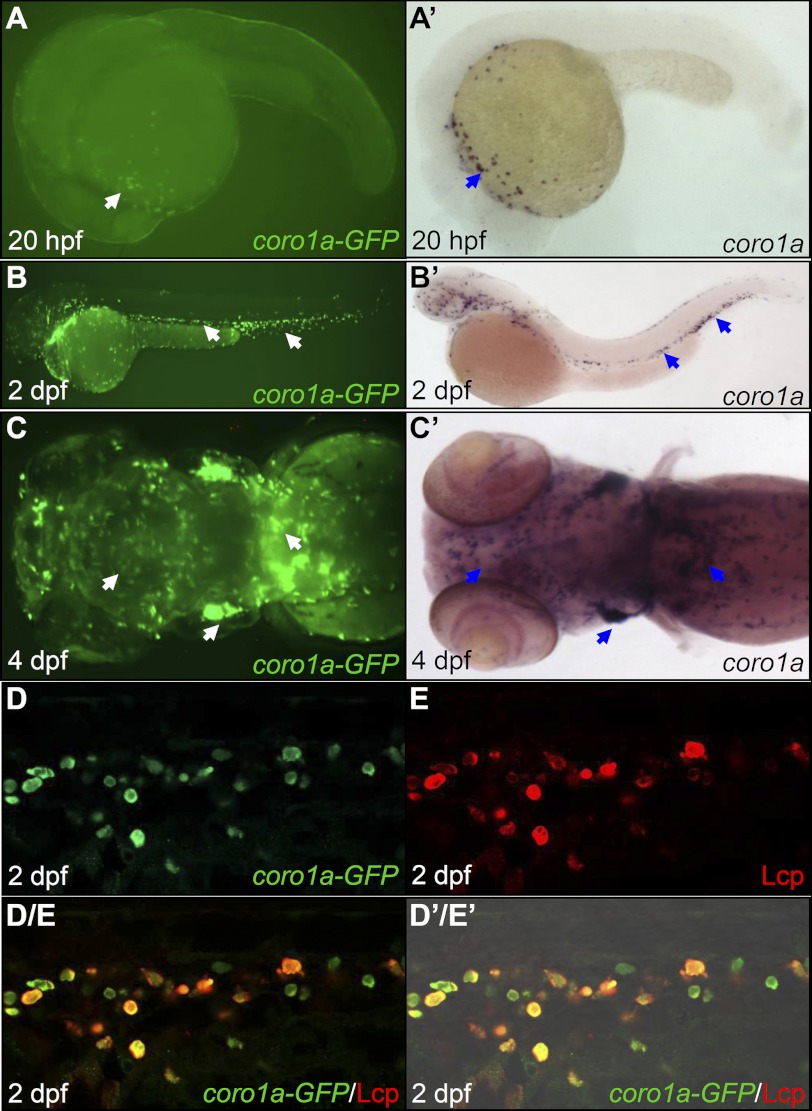
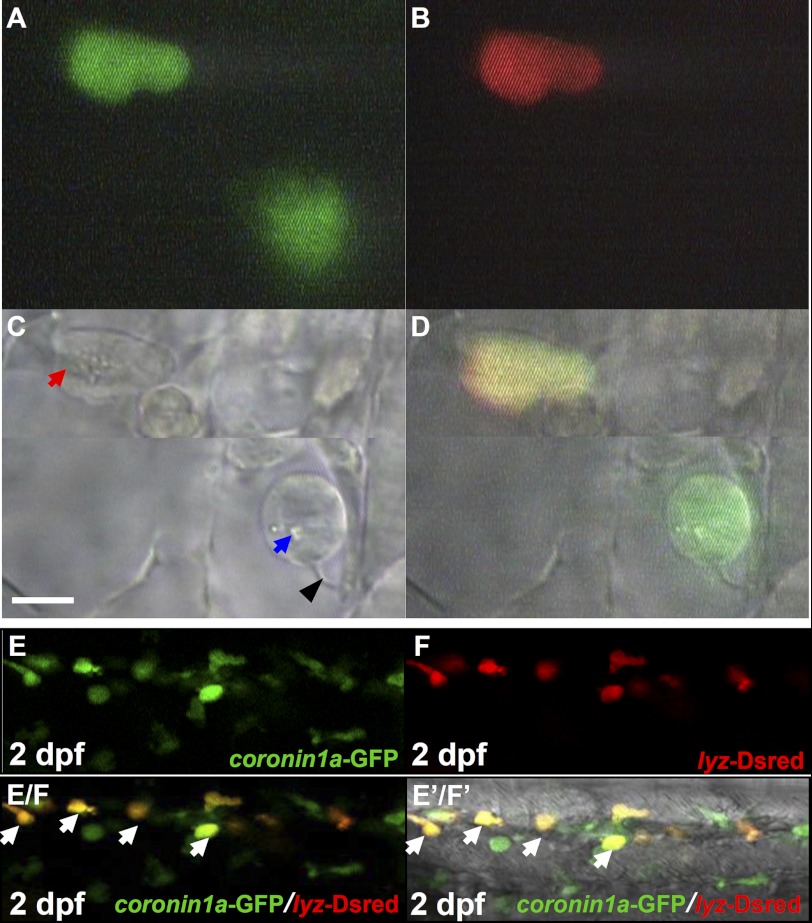
To generate zebrafish in which both macrophages and neutrophils can be marked by fluorescent protein, we isolated a 7.03-kb DNA fragment upstream of the coro1a translation start site and fused it with the enhanced green fluorescent protein (eGFP) gene. When this reporter construct pTol2-coro1a-eGFP was transiently injected into one-cell stage wild-type embryos, it displayed a typical myeloid lineage expression pattern (data not shown), suggesting that this 7.03-kb upstream sequence is sufficient to direct GFP expression in myeloid cells. Two independent stable transgenic lines, Tg(coro1a:eGFP)hkz04t and Tg(coro1a:eGFP)hkz05t, were recovered. Detailed characterization of both transgenic lines revealed that coro1a-GFP expression recapitulated the pattern of endogenous coro1a, which predominantly expresses in myeloid cells and T lymphocytes (24). As shown in Fig. 1, in Tg(coro1a:eGFP) fish (Tg(coro1a:eGFP)hkz04t will be used hereafter), GFP was first detected as early as 20 hours post fertilization (hpf) in myeloid cells dispersed in the yolk sac (Fig. 1A, white arrow, n = 40/40). As the embryos developed, the GFP-positive (GFP+) cells were evident in the trunk, the tail region (Fig. 1B, white arrows, n = 40/40), the central nervous system (presumably representing microglia-specialized macrophages (31), the thymus, and the kidneys (Fig. 1C, white arrows, n = 40/40). The co-localization of GFP with Lcp, a pan-myeloid marker (32), in 2 day post fertilization (dpf) Tg(coro1a:eGFP) embryos (Fig. 1, D–D′/E′, n = 31/31) suggests that GFP marks both macrophages and neutrophils. To confirm that this is indeed the case, we performed video-enhanced DIC imaging analysis, which distinguishes macrophages from neutrophils based on their morphological appearances (33, 34). Examination of 2 dpf live Tg(coro1a:eGFP) embryos revealed that neutrophils defined as cells with mobile cytoplasmic granules (Fig. 2, A, C, and D, red arrow, n = 12/12) and macrophages defined as cells with lysosomes/phagosomes and long filopodia but a lack of cytoplasmic granules (Fig. 2, A, C, and D, blue arrow and black arrowhead, n = 12/12) were positive for GFP. From these observations, we conclude that GFP marks both macrophages and neutrophils in Tg(coro1a:eGFP) embryos.
FIGURE 1.
Tg(coro1a:eGFP) marks both macrophages and neutrophils. A–C, fluorescence imaging shows GFP signals in live Tg(coro1a:eGFP) embryos (n = 40/40) in the yolk sac at 20 hpf (A); in the AGM, the CHT, and the circulation at 2 dpf (B); and in the thymus, the kidney, and the brain at 4 dpf (C). A′–C′, WISH reveals coro1a RNA expression in the yolk sac at 20 hpf (n = 40/40) (A′); in the AGM, the CHT, and the circulation at 2 dpf (n = 40/40) (B′); and in the thymus, the kidney, and the brain at 4 dpf (n = 40/40) (C′). White arrows in A–C indicate the GPF+ cells, whereas blue arrows in A′–C′ represent WISH signals. D and E, GFP and Lcp double antibody staining shows the co-localization of coro1a-GFP and Lcp in the CHT of 2 dpf Tg(coro1a:eGFP) embryos (n = 31/31). D/E, superimposed image of D and E. D′/E′, merged view of D/E with the DIC image, AGM, aorta-gonad-mesonephros; CHT, caudal hematopoietic tissue.
FIGURE 2.
Tg(coro1a:eGFP;lyz:Dsred) distinct macrophages and neutrophils. A–C, live imaging (×60) of two GFP+ cells in 2 dpf Tg(coro1a:eGFP;lyz:Dsred) embryos under a video-enhanced DIC microscope at the GFP channel (A), Dsred channel (B), and bright field (BF) channel (C) (n = 12/12). D, superimposed image of A, B, and C. The blue arrow and black arrowhead in C indicate the lysosome/phagosome inside the macrophage and the long filopodia of macrophage, respectively. The red arrow in C represents granules in neutrophils. Scale bar, 5 μm. E and F, confocal fluorescence imaging (×40) of the CHT region in 2 dpf Tg(coro1a:eGFP;lyz:Dsred) live embryos (n = 40/40) using the GFP (E) and Dsred channel (F), respectively. E/F, superimposed image of E and F. E′/F′, merged view of E/F with the DIC image. White arrows in E/F and E′/F′ represent yellow neutrophils resulting from the merge of GFP and Dsred.
The Cellular Development of Embryonic Macrophages and Neutrophils
The early coro1a-eGFP expression in myeloid cells in Tg(coro1a:eGFP) transgenic lines prompted us to define the development stages of embryonic myeloid cells. The GFP+ cells from Tg(coro1a:eGFP) embryos were isolated at various developmental time points by FACS and subjected to cytological analysis. Giemsa staining revealed that at 20 hpf, all of the GFP+ cells uniformly had a round morphology with large nuclei and scant basophilic cytoplasm (supplemental Fig. 1, A and G, n ≥ 300), a characteristic of myeloid progenitors (35). By 24 hpf, a majority (∼67%) of the GFP+ cells remained as myeloid progenitors. However, about 14% of the GFP+ cells harbored beanlike nuclei and a bubbled contour (supplemental Fig. 1B, n ≥ 300), which is similar to the young macrophage reported previously (33, 36), and around 19% of the GFP+ cells developed into functional macrophages defined as cells with large cytoplasmic vacuoles and some of the vacuoles loaded with dead debris (supplemental Fig. 1C, n ≥ 300). No typical neutrophils were detected at this stage. At 2 dpf, in addition to the myeloid progenitors (∼48%) and monocyte/macrophage lineage (∼34%) (supplemental Fig. 1, E and G, n ≥ 300), neutrophils (∼17%) with the typical segmented nuclear lobes were discernible (supplemental Fig. 1, D and G, n ≥ 300). This result is consistent with the previous studies showing that the functional monocytes/macrophages emerged at around 24 hpf (33, 36) and maturing neutrophils were not detected until 36 hpf (34). Notably, the dendritic-like cells (less than 1%) characterized by the typical dendritic filaments (37) were also detected at 2 dpf (supplemental Fig. 1G, n ≥ 300), and the dendritic-like cells increased to about 1–2% at 3 dpf and displayed a more arborized morphology (supplemental Fig. 1F, n ≥ 300).
Neutrophils Are the Primary Scavengers during Inflammation Stage after Tail Fin Amputation
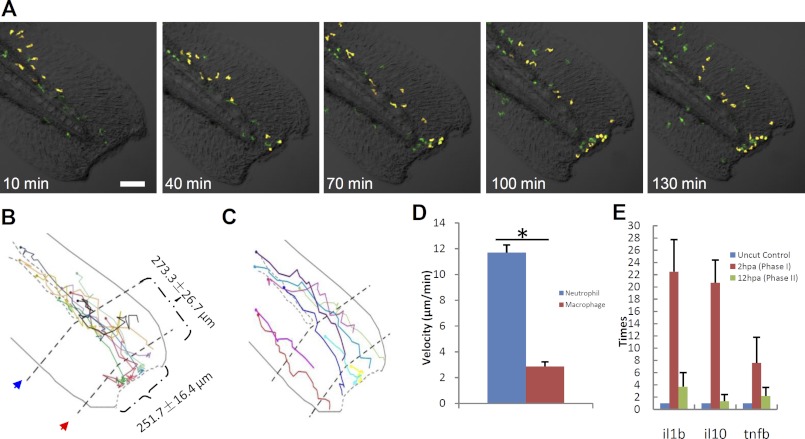
The capability of Tg(coro1a:eGFP) marking both macrophages and neutrophils but Tg(lyz:Dsred) (16) marking only neutrophils at the embryonic stage provides an ideal system to directly assess the behaviors and functions of macrophages and neutrophils simultaneously. To achieve this, we outcrossed Tg(coro1a:eGFP) fish with Tg(lyz:Dsred). In the double transgenic line Tg(coro1a:eGFP;lyz:Dsred), neutrophils co-expressed lyz-Dsred and coro1a-GFP (lyz+/coro1a+) and displayed yellow fluorescence, whereas macrophages expressed coro1a-GFP (coro1a+) only and displayed green fluorescence (Fig. 2, E–E′/F′, n = 40/40). To study the real-time actions of macrophages and neutrophils in tissue regeneration, the well established tail fin regeneration assay (14, 15, 38, 39) was employed in 72 hpf Tg(coro1a:eGFP;lyz:Dsred) embryos, making a lesion 251.7 ± 16.4 μm in length and 273.3 ± 26.7 μm from the end of the circulation (Fig. 3B, n = 13). The injured embryos were immediately viewed under a confocal microscope at 3-min intervals for 48 h. Results showed that, similar what was noted in the previous reports (14, 15), both types of myeloid cells responded immediately, but they migrated into the wound at different speeds (Fig. 3, A–D, n ≥ 9; supplemental Movie 1, n = 3). Although a few localized resident macrophages were activated and migrated toward the lesion (Fig. 3A), neutrophils, with a higher mobility (Fig. 3D, n ≥ 9; supplemental Movie 1, n = 3), were the first phagocytes to arrive at the wound, and they functioned as the major scavengers within 6 hpa (Fig. 5L, n ≥ 13), during which time the inflammatory cytokines were increased to their maximum levels (Fig. 3E, n = 42). This period was thus referred to as the inflammatory stage (3, 9).
FIGURE 3.
Neutrophil and macrophage behavior upon fin transaction. A, frames of different time points following tail transaction in a 72 hpf Tg(coro1a:eGFP;lyz:Dsred) embryo (green, macrophages; yellow, neutrophils). Scale bar, 100 μm. B and C, schematic diagrams show the wound size and cell migration trajectory of the first 10 neutrophils (B) and nine macrophages (C) to arrive at the wound, followed for 5 h after tail transaction. The circle in the end of each trajectory line represents the initial site of each myeloid phagocyte. The red arrow indicates the first line near the cut edge, which is the end of the notochord, and the blue arrow represents the second line, which is the end of circulation. The dotted curved lines in B and C represent vessels. D, histogram reveals that the velocity of neutrophils is much faster than macrophages during the process toward the wound edge (n ≥ 9, mean ± S.E. (error bars)). *, statistical differences with corresponding control (t test, p < 0.05). E, histogram showing that the RNA level of cytokines increased dramatically in inflammatory stage but decreased thereafter until the regeneration stage (n = 42).
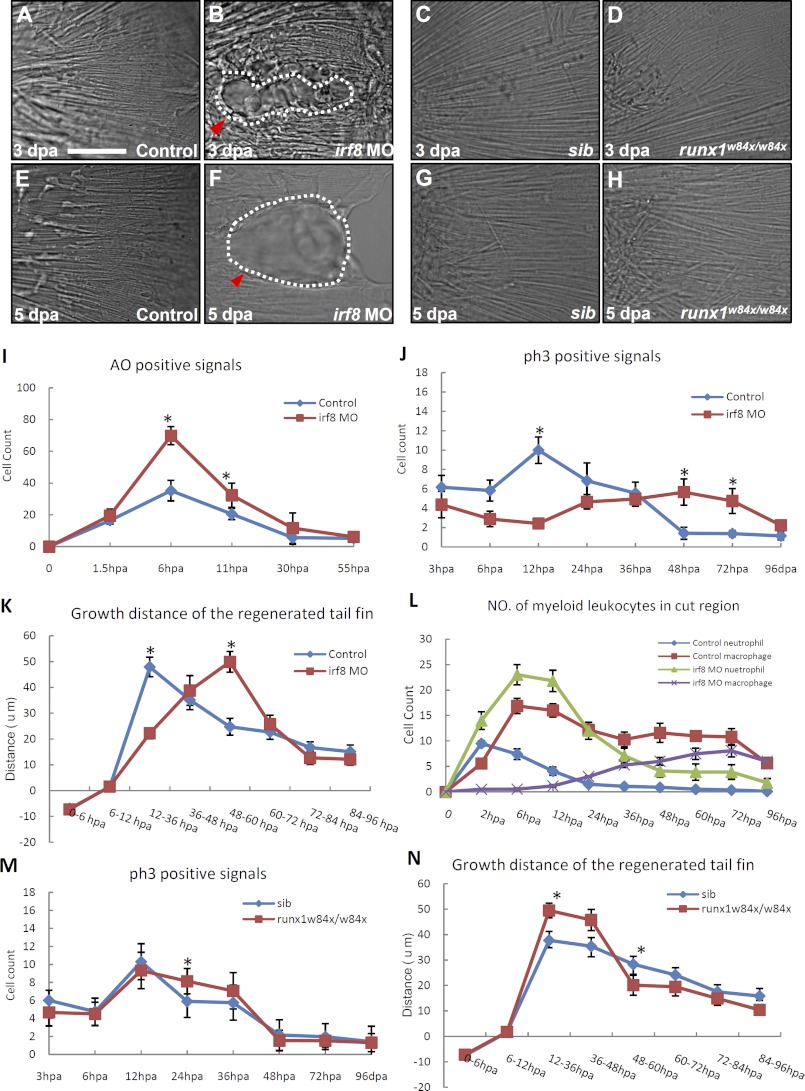
FIGURE 5.
Macrophages and neutrophils play different roles in the regeneration of tail fin. A–H, DIC images show the morphology of newly regenerated fin in 3 dpa control (n = 20/20) (A), 3 dpa irf8 morphants (n = 6/11) (B), 3 dpa siblings (sib) (n = 12/12) (C), 3 dpa runx1w84x mutant (n = 7/7) (D), 5 dpa control (n = 20/20) (E), 5 dpa irf8 morphants (n = 4/11) (F), 5 dpa siblings (n = 11/11) (G), and 5 dpa runx1w84x mutants (n = 6/6) (H). Scale bar, 50 μm. The white dashed lines in B and F indicate the outline of the vacuoles (red arrowheads). I, histogram reveals the number of pieces of AO+ cell debris at different time points after the transaction of tail fin in 72 hpf control (n ≥ 15, mean ± S.E. (error bars)) and irf8 morphants (n ≥ 16, mean ± S.E.) Tg(coro1a:eGFP;lyz:Dsred) embryos. J, histogram reveals the number of pH3+ cells at different time points after the transaction of tail fin in 72 hpf control (n ≥ 10, mean ± S.E.) and irf8 morphants (n ≥ 10, mean ± S.E.) Tg(coro1a:eGFP;lyz:Dsred) embryos. K, histogram quantifies the grow distance (μm) of regenerated fin at different stages after transaction in 72 hpf control (n ≥ 17, mean ± S.E.) and irf8 morphants (n ≥ 15, mean ± S.E.) Tg(coro1a:eGFP;lyz:Dsred) embryos. L, histogram reveals the number of myeloid phagocytes in the wound at different time points after transaction of tail fin in 72 hpf control (n ≥ 13, mean ± S.E.) and irf8 morphants (n ≥ 14, mean ± S.E.) Tg(coro1a:eGFP;lyz:Dsred) embryos. M, histogram reveals the number of pH3+ cells at different time points after the transaction of tail fin in 72 hpf sibling (n ≥ 11, mean ± S.E.) and runx1w84x mutant (n ≥ 6, mean ± S.E.) embryos. N, histogram quantifies the grow distance (μm) of regenerated fin at different stages after transaction in 72 hpf sibling (n ≥ 11, mean ± S.E.) and runx1w84x mutant (n ≥ 6, mean ± S.E.) embryos. The asterisks in I–K, M, and N indicate statistical differences with corresponding control (t test, p < 0.05).
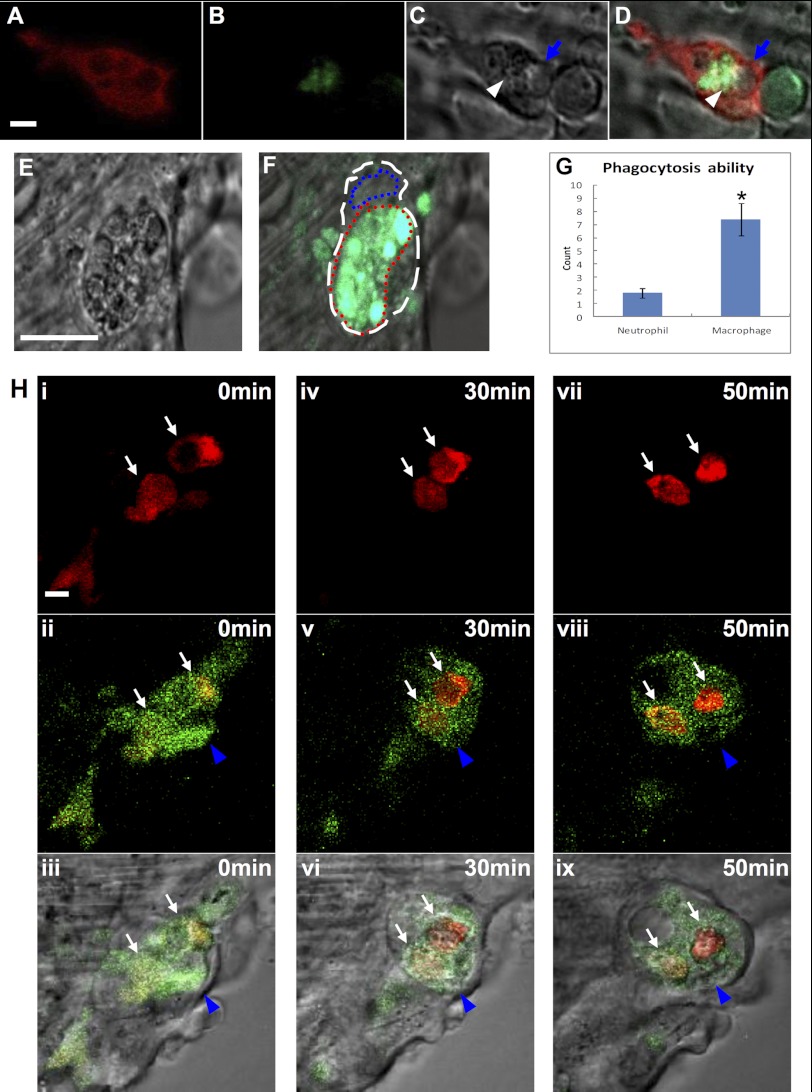
In the initial phase of the inflammatory stage, a substantial number of cells died near the edge of the wound, most of which underwent fragmentation, a characteristic of necrosis (Fig. 4, B–D, white arrowhead and blue arrow, n = 13), leading to retraction of the cut edge of the tail fin (Fig. 5K, n ≥ 15). Incubation of the wounded embryos with AO (31) showed that more than half of the cell debris were vacuolar cell debris that were negative for AO (Fig. 4, B–D, blue arrow, n = 13), whereas others were particulate cell debris positive for AO (Fig. 4, B–D, white arrowhead, n = 13). Shortly after the injury, neutrophils infiltrated the wound and began to engulf both vacuolar and particulate cell debris up to a size of 0.94 ± 0.09 μm (Fig. 4, B–D, n = 13; supplemental Movie 2, n = 5). The phagocytotic ability of neutrophils was limited, and one neutrophil could engulf less than four pieces of cell debris (Fig. 4G, n = 12). About 20 min later, these neutrophils changed from amoeboid appearance to round shape and subsequently underwent a series of morphological alterations that resembled the characteristics of apoptosis, including membrane blebbing, chromatin condensation, and nuclear fragmentation (supplemental Fig. 2A, n = 7; supplemental Movie 2, n = 5) (40–42). The apoptotic neutrophils, in which the engulfed cell debris was usually not fully digested, were subsequently removed by the infiltrated macrophages (Fig. 4H, n = 7).
FIGURE 4.
Macrophages and neutrophils behaviors in the wound after amputation. A–D, live imaging (×40) shows that lyz+ neutrophils engulf both AO+ particle cell debris and AO− vacuolar cell debris at 2 hpa (n = 13). The white arrowhead and blue arrow indicate the AO+ particle and AO− vacuolar cell debris, respectively. Scale bar, 1 μm. E and F, live imaging (×40) reveals a macrophage with a large amount of cell debris (E, n = 12), including AO+ particles (green) (F, n = 12). The white and blue dashed lines indicate the shape and the nucleus of macrophage, respectively, whereas the red dashed line manifests the large vacuole full of ingested debris. Scale bar, 10 μm. G, histogram reveals the phagocytosis ability (represented by the number of dead particles ingested) of macrophages and neutrophils (n = 12, mean ± S.E. (error bars)). The asterisk in G indicates statistical differences with corresponding control (t test, p < 0.05). H, frames (×100) of different time points of two neutrophils (indicated by white arrows) engulfed by a macrophage (represented by blue arrowheads) in a 72 hpf Tg(coro1a:eGFP;lyz:Dsred) embryo (n = 7). The bottom panels are merges of DIC with the middle panels. Scale bar, 1 μm.
Macrophages Emerge Later in the Wound and Are Essential for Regeneration of the Injured Tail Fin
From 6 hpa onward, macrophages became the dominant myeloid cells at the wound site (Fig. 5L, n ≥ 13). Immediately after their arrival, macrophages, usually with a beanlike morphology (supplemental Fig. 2C, n = 13), displayed professional phagocytosis. An activated macrophage could engulf more than six pieces of large cell debris (0.8–3.2 μm) (Fig. 4, E–G, n = 12), including the apoptotic neutrophils (Fig. 4H, n = 7). As a consequence, the number of AO+ apoptotic bodies (Fig. 5I, n ≥ 15) and the number of neutrophils (Fig. 5L, n ≥ 13) in the wound were rapidly reduced, and so were the levels of inflammatory cytokines (Fig. 3E, n = 42). This process, referred to as the resolution stage, lasted for 5–6 h.
To the end of the resolution stage (∼12 hpa), cells in the wound began to proliferate, signifying the initiation of regeneration (Fig. 5J, n ≥ 10). During the regeneration stage, the wound surface became smoother (supplemental Fig. 2, B and D, n = 13), and the cells around the site of amputation proliferated extensively (Fig. 5J, n ≥ 10). The regeneration process continued for 4–5 days (Fig. 5, J, n ≥ 10 and K, n ≥ 15). During this stage, macrophages were the predominant phagocytes in the wound; they were concentrated along the growing edge and displayed a long and thin morphology (supplemental Fig. 2, B–F, n = 13 and Fig. 5L, n ≥ 13).
The continuing invigilation of macrophages in the growing edge during the regeneration stage and the findings that macrophages are essential for muscle regeneration in mice (4) prompted us to speculate that macrophages might play a crucial role in the remodeling and the regrowth of the zebrafish tail fin. To support this hypothesis, we depleted macrophages by knocking down irf8 (interferon regulatory factor 8) in zebrafish by specific MOs (28). In the irf8 MO-injected embryos (morphants), neutrophils responded normally to tail amputation (supplemental Fig. 3, A and B and supplemental Movie 3, n = 7) and effectively phagocytized the small dead cell debris (supplemental Fig. 3, D–F, n = 11). However, macrophages were hardly seen in the irf8 morphants (supplemental Fig. 3C, n = 15). As a result, the removal of cell debris was delayed in the irf8 morphants (Fig. 5I, n ≥ 15). Likewise, the cell proliferation and growth rate of the regenerating tail fin in the irf8 morphants were significantly reduced compared with that in controls (Fig. 5, J, n ≥ 10 and K, n ≥ 15). Although the injured tail was eventually regrown in the irf8 morphants, the regenerated fin formed large vacuoles (Fig. 5, A, B, E, and F, n ≥ 11). These vacuoles emerged at 3 dpa with 82 ± 23 μm in length, and by 5 dpa, some of the vacuoles enlarged to 103 ± 48 μm in length (Fig. 5F, n = 4/11). In contrast, the tail fin regeneration in the homozygous runx1w84x mutant embryos (25), in which neutrophils but not macrophages are significantly reduced (43), was normal (Fig. 5, D and H, n ≥ 6). Unexpectedly, the regeneration speed in the runx1w84x mutants was slightly accelerated (Fig. 5N, n ≥ 6), reflected by the increased cell proliferation and faster growth of the injured fin in mutant embryos compared with that in siblings (Fig. 5, M and N, n ≥ 6), suggesting that neutrophils have a negative effect on fin regeneration. From these observations, we conclude that macrophages are essential for the regeneration of zebrafish tail fin, whereas neutrophils appear to have an inhibitory role.
DISCUSSION
In the present study, we created a Tg(coro1a:eGFP) transgenic line in which the GFP reporter is driven by a 7.03-kb coro1a regulatory sequence. We demonstrated that coro1a-GFP expressed in myeloid cells (macrophages, neutrophils, and dendritic-like cells) and lymphocytes in early development (Fig. 1) as well as in adulthood (data not shown). To our knowledge, this is the first zebrafish reporter line that marks all of the leukocytes. The early expression of GFP in Tg(coro1a:eGFP) transgenic fish allows us to isolate embryonic myeloid cells for cytological analysis. Giemsa staining of coro1a+ cells shows that functional monocytes/macrophages emerge early at 24 hpf, whereas mature neutrophils are evident until 2 dpf (supplemental Fig. 1). Notably, a small number of dendritic-like cells, which have an arborized morphology and dendritic filaments, are also detected from 2 dpf onward (supplemental Fig. 1). This result is consistent with previous studies using molecular markers and functional analysis (33, 36).
By utilizing the Tg(coro1a:eGFP;lyz:Dsred) double transgenic lines, the in vivo behaviors and functions of macrophages and neutrophils in the larva tail fin regeneration (14, 15, 38, 39) were directly assayed in a living embryo. We showed that upon injury, the local resident macrophages and neutrophils (Fig. 3A and supplemental Movie 1) were the first phagocytes to be activated. Shortly after, distant/circulation-derived neutrophils were recruited to the wound, and finally the macrophages distant from the lesion or monocytes in the circulation arrived. The consequential colonization of the wound by the local resident macrophages/neutrophils, distant/circulation-recruited neutrophils, and distant/circulation-derived macrophages is partially in agreement with the previous study in mice, which suggested that the local resident macrophages may facilitate the activation and recruitment of the distant/circulation-derived neutrophils and macrophages (44). However, contrary to this notion, our study showed that the activation and recruitment of both local resident and distant/circulation neutrophils are not affected in the irf8 morphants, in which resident macrophages and circulating monocytes are absent (28), indicating that the local resident macrophages are not essential for the activation and recruitment of distant/circulation-derived neutrophils. Further study is needed to clarify the role of the local resident macrophages.
In the inflammation stage during the first several hours after fin amputation, neutrophils are the first group of cells that reach the wound, and they are the predominant functional phagocytes during this stage, suggesting the conservational response process of myeloid cells after injury (9). In addition to removing micro-organisms (45), we show that neutrophils can engulf cell debris during tissue damage, suggesting that neutrophils are also involved in the resolution of inflammation and perhaps subsequent tissue regeneration as suggested previously (9, 45, 46). Unexpectedly, we found that the regeneration speed of the fin is slightly faster in the neutrophil-deficient runx1w84x (44) (Fig. 5), indicating that neutrophils may play an inhibitory role in the regeneration stage. This result is consistent with the previous findings in mice, in which the depletion of neutrophils leads to faster epidermal healing when measured by wound closure (3, 46). It will be of great interest to investigate how neutrophils inhibit the tissue regeneration in general.
In the resolution and regeneration stages, macrophages appear to be dominant effect cells to remove large cell debris as well as apoptotic neutrophils, demonstrating that macrophages but not neutrophils are the key scavengers for resolving inflammation and facilitating tissue regrowth. Consistent with previous studies in mice (4, 7), we further document that the depletion of macrophages in zebrafish leads to the delay of the clearance of cell debris, decrease of regeneration speed, and formation of vacuoles in the regenerating fin. Further investigation is required for elucidating the underlying mechanisms.
Supplementary Material
Acknowledgment
We thank Dr. Phil Crosier for providing the Tg(lyz:Dsred) transgenic line.
This work was supported by Research Grants Council of the Hong Kong Special Administrative Region General Research Fund Grants 663109 and HKUST6/CRF/09, National Basic Research Program of China Grant 2012CB945102, and National Natural Science Foundation of China Grants 31171403 and 30828020.

This article contains supplemental Figs. 1–3 and Movies 1–3.
- hpa
- hours post amputation
- dpa
- day(s) postamputation
- eGFP
- enhanced GFP
- hpf
- hour(s) postfertilization
- dpf
- day(s) postfertilization
- AO
- acrine orange
- MO
- morpholino-oligonucleotides
- morphant
- MO-injected embryo
- pH3
- phosphohistone 3
- DIC
- differential interference contrast
- WISH
- whole mount in situ hybridization.
REFERENCES
- 1. Poss K. D., Keating M. T., Nechiporuk A. (2003) Tales of regeneration in zebrafish. Dev. Dyn. 226, 202–210 [DOI] [PubMed] [Google Scholar]
- 2. Medzhitov R. (2007) Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 [DOI] [PubMed] [Google Scholar]
- 3. Martin P., Leibovich S. J. (2005) Inflammatory cells during wound repair. The good, the bad, and the ugly. Trends Cell Biol. 15, 599–607 [DOI] [PubMed] [Google Scholar]
- 4. Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R. K., Chazaud B. (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leibovich S. J., Ross R. (1975) The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am. J. Pathol. 78, 71–100 [PMC free article] [PubMed] [Google Scholar]
- 6. Leibovich S. J., Ross R. (1976) A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am. J. Pathol. 84, 501–514 [PMC free article] [PubMed] [Google Scholar]
- 7. London A., Itskovich E., Benhar I., Kalchenko V., Mack M., Jung S., Schwartz M. (2011) Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J. Exp. Med. 208, 23–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rappolee D. A., Mark D., Banda M. J., Werb Z. (1988) Wound macrophages express TGF-α and other growth factors in vivo. Analysis by mRNA phenotyping. Science 241, 708–712 [DOI] [PubMed] [Google Scholar]
- 9. Serhan C. N., Brain S. D., Buckley C. D., Gilroy D. W., Haslett C., O'Neill L. A., Perretti M., Rossi A. G., Wallace J. L. (2007) Resolution of inflammation. State of the art, definitions, and terms. FASEB J. 21, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawakami A., Fukazawa T., Takeda H. (2004) Early fin primordia of zebrafish larvae regenerate by a similar growth control mechanism with adult regeneration. Dev. Dyn. 231, 693–699 [DOI] [PubMed] [Google Scholar]
- 11. Poss K. D., Shen J., Nechiporuk A., McMahon G., Thisse B., Thisse C., Keating M. T. (2000) Roles for Fgf signaling during zebrafish fin regeneration. Dev. Biol. 222, 347–358 [DOI] [PubMed] [Google Scholar]
- 12. Poss K. D., Wilson L. G., Keating M. T. (2002) Heart regeneration in zebrafish. Science 298, 2188–2190 [DOI] [PubMed] [Google Scholar]
- 13. Yoshinari N., Ishida T., Kudo A., Kawakami A. (2009) Gene expression and functional analysis of zebrafish larval fin fold regeneration. Dev. Biol. 325, 71–81 [DOI] [PubMed] [Google Scholar]
- 14. Ellett F., Pase L., Hayman J. W., Andrianopoulos A., Lieschke G. J. (2011) mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 117, e49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gray C., Loynes C. A., Whyte M. K., Crossman D. C., Renshaw S. A., Chico T. J. (2011) Simultaneous intravital imaging of macrophage and neutrophil behavior during inflammation using a novel transgenic zebrafish. Thromb. Haemost. 105, 811–819 [DOI] [PubMed] [Google Scholar]
- 16. Hall C., Flores M. V., Storm T., Crosier K., Crosier P. (2007) The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev. Biol. 7, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hogan B. M., Layton J. E., Pyati U. J., Nutt S. L., Hayman J. W., Varma S., Heath J. K., Kimelman D., Lieschke G. J. (2006) Specification of the primitive myeloid precursor pool requires signaling through Alk8 in zebrafish. Curr. Biol. 16, 506–511 [DOI] [PubMed] [Google Scholar]
- 18. de Hostos E. L. (1999) The coronin family of actin-associated proteins. Trends Cell Biol. 9, 345–350 [DOI] [PubMed] [Google Scholar]
- 19. Uetrecht A. C., Bear J. E. (2006) Coronins. The return of the crown. Trends Cell Biol. 16, 421–426 [DOI] [PubMed] [Google Scholar]
- 20. Gatfield J., Albrecht I., Zanolari B., Steinmetz M. O., Pieters J. (2005) Association of the leukocyte plasma membrane with the actin cytoskeleton through coiled coil-mediated trimeric coronin 1 molecules. Mol. Biol. Cell 16, 2786–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferrari G., Langen H., Naito M., Pieters J. (1999) A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 97, 435–447 [DOI] [PubMed] [Google Scholar]
- 22. Yan M., Di Ciano-Oliveira C., Grinstein S., Trimble W. S. (2007) Coronin function is required for chemotaxis and phagocytosis in human neutrophils. J. Immunol. 178, 5769–5778 [DOI] [PubMed] [Google Scholar]
- 23. Yan M., Collins R. F., Grinstein S., Trimble W. S. (2005) Coronin-1 function is required for phagosome formation. Mol. Biol. Cell 16, 3077–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song H. D., Sun X. J., Deng M., Zhang G. W., Zhou Y., Wu X. Y., Sheng Y., Chen Y., Ruan Z., Jiang C. L., Fan H. Y., Zon L. I., Kanki J. P., Liu T. X., Look A. T., Chen Z. (2004) Hematopoietic gene expression profile in zebrafish kidney marrow. Proc. Natl. Acad. Sci. U.S.A. 101, 16240–16245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jin H., Sood R., Xu J., Zhen F., English M. A., Liu P. P., Wen Z. (2009) Definitive hematopoietic stem/progenitor cells manifest distinct differentiation output in the zebrafish VDA and PBI. Development 136, 647–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Westerfield M., Doerry E., Kirkpatrick A. E., Douglas S. A. (1999) Zebrafish informatics and the ZFIN database. Methods Cell Biol. 60, 339–355 [DOI] [PubMed] [Google Scholar]
- 27. Jin H., Xu J., Qian F., Du L., Tan C. Y., Lin Z., Peng J., Wen Z. (2006) The 5′ zebrafish scl promoter targets transcription to the brain, spinal cord, and hematopoietic and endothelial progenitors. Dev. Dyn. 235, 60–67 [DOI] [PubMed] [Google Scholar]
- 28. Li L., Jin H., Xu J., Shi Y., Wen Z. (2011) Irf8 regulates macrophage versus neutrophil fate during zebrafish primitive myelopoiesis. Blood 117, 1359–1369 [DOI] [PubMed] [Google Scholar]
- 29. Dobson J. T., Da'as S., McBride E. R., Berman J. N. (2009) Fluorescence-activated cell sorting (FACS) of whole mount in situ hybridization (WISH) labeled hematopoietic cell populations in the zebrafish. Br. J. Haematol. 144, 732–735 [DOI] [PubMed] [Google Scholar]
- 30. Qian F., Zhen F., Xu J., Huang M., Li W., Wen Z. (2007) Distinct functions for different scl isoforms in zebrafish primitive and definitive hematopoiesis. PLoS. Biol. 5, e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peri F., Nüsslein-Volhard C. (2008) Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 133, 916–927 [DOI] [PubMed] [Google Scholar]
- 32. Meijer A. H., van der Sar A. M., Cunha C., Lamers G. E., Laplante M. A., Kikuta H., Bitter W., Becker T. S., Spaink H. P. (2008) Identification and real-time imaging of a myc-expressing neutrophil population involved in inflammation and mycobacterial granuloma formation in zebrafish. Dev. Comp Immunol. 32, 36–49 [DOI] [PubMed] [Google Scholar]
- 33. Herbomel P., Thisse B., Thisse C. (1999) Ontogeny and behavior of early macrophages in the zebrafish embryo. Development 126, 3735–3745 [DOI] [PubMed] [Google Scholar]
- 34. Le Guyader D., Redd M. J., Colucci-Guyon E., Murayama E., Kissa K., Briolat V., Mordelet E., Zapata A., Shinomiya H., Herbomel P. (2008) Origins and unconventional behavior of neutrophils in developing zebrafish. Blood 111, 132–141 [DOI] [PubMed] [Google Scholar]
- 35. Traver D., Paw B. H., Poss K. D., Penberthy W. T., Lin S., Zon L. I. (2003) Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat. Immunol. 4, 1238–1246 [DOI] [PubMed] [Google Scholar]
- 36. Lieschke G. J., Oates A. C., Paw B. H., Thompson M. A., Hall N. E., Ward A. C., Ho R. K., Zon L. I., Layton J. E. (2002) Zebrafish SPI-1 (PU.1) marks a site of myeloid development independent of primitive erythropoiesis. Implications for axial patterning. Dev. Biol. 246, 274–295 [DOI] [PubMed] [Google Scholar]
- 37. Lugo-Villarino G., Balla K. M., Stachura D. L., Bañuelos K., Werneck M. B., Traver D. (2010) Identification of dendritic antigen-presenting cells in the zebrafish. Proc. Natl. Acad. Sci. U.S.A. 107, 15850–15855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Niethammer P., Grabher C., Look A. T., Mitchison T. J. (2009) A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459, 996–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Y., Bai X. T., Zhu K. Y., Jin Y., Deng M., Le H. Y., Fu Y. F., Chen Y., Zhu J., Look A. T., Kanki J., Chen Z., Chen S. J., Liu T. X. (2008) In vivo interstitial migration of primitive macrophages mediated by JNK-matrix metalloproteinase 13 signaling in response to acute injury. J. Immunol. 181, 2155–2164 [DOI] [PubMed] [Google Scholar]
- 40. Duffin R., Leitch A. E., Fox S., Haslett C., Rossi A. G. (2010) Targeting granulocyte apoptosis. Mechanisms, models, and therapies. Immunol. Rev. 236, 28–40 [DOI] [PubMed] [Google Scholar]
- 41. Hallett J. M., Leitch A. E., Riley N. A., Duffin R., Haslett C., Rossi A. G. (2008) Novel pharmacological strategies for driving inflammatory cell apoptosis and enhancing the resolution of inflammation. Trends Pharmacol. Sci. 29, 250–257 [DOI] [PubMed] [Google Scholar]
- 42. Leitch A. E., Duffin R., Haslett C., Rossi A. G. (2008) Relevance of granulocyte apoptosis to resolution of inflammation at the respiratory mucosa. Mucosal Immunol. 1, 350–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jin H., Li L., Xu J., Zhen F. H., Zhu L., Liu P. P., Zhang M., Zhang W., Wen Z. L. (2012) Runx1 regulates embryonic myeloid fate choice in zebrafish through a negative feedback loop that confines Pu.1 expression. Blood, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Soehnlein O., Lindbom L. (2010) Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 10, 427–439 [DOI] [PubMed] [Google Scholar]
- 45. Nathan C. (2006) Neutrophils and immunity. Challenges and opportunities. Nat. Rev. Immunol. 6, 173–182 [DOI] [PubMed] [Google Scholar]
- 46. Dovi J. V., He L. K., DiPietro L. A. (2003) Accelerated wound closure in neutrophil-depleted mice. J. Leukoc. Biol. 73, 448–455 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.