Background: The physiological function of cyclin K is poorly defined.
Results: Cyclin K interacts with CDK12 and CDK13, and knockdown of cyclin K, CDK12, or CDK13 causes embryonic stem cell differentiation.
Conclusion: Cyclin K, CDK12, and CDK13 are required for embryonic stem cell self-renewal.
Significance: Novel kinase complexes are identified to maintain embryonic stem cell pluripotency.
Keywords: CDK (Cyclin-dependent Kinase), Cell Differentiation, Cyclins, Embryonic Stem Cell, Transcription/Developmental Factors, CDK12, CDK13, P-TEFb, Cyclin K, Embryonic Stem Cell
Abstract
Protein phosphorylation plays an important role in the regulation of self-renewal and differentiation of embryonic stem cells. However, the responsible intracellular kinases are not well characterized. Here, we discovered that cyclin K protein was highly expressed in pluripotent embryonic stem cells but low in their differentiated derivatives or tissue-specific stem cells. Upon cell differentiation, the level of cyclin K protein was decreased. Furthermore, knockdown of cyclin K led to cell differentiation, which could be rescued by an expression construct resistant to RNA interference. Surprisingly, cyclin K did not interact with CDK9 protein in cells as thought previously. Instead, it associated with CrkRS (also known as CDK12) and CDC2L5 (also known as CDK13). Similar to cyclin K, both CDK12 and CDK13 proteins were highly expressed in murine embryonic stem cells and were decreased upon cell differentiation. Importantly, knockdown of either kinase resulted in differentiation. Thus, our studies have uncovered two novel protein kinase complexes that maintain self-renewal in embryonic stem cells.
Introduction
Pluripotent ES cells are capable of differentiating to essentially all cell lineages, whereas under appropriate tissue culture conditions, they can self-renew indefinitely in vitro (1, 2). Much progress has been made to elucidate the molecular control at the level of transcription (3). The core transcription factors Oct4, Sox2, and Nanog collaborate to activate the expression of genes that promote self-renewal and repress that of lineage-specific genes (4). Yet, our current understanding of other regulatory mechanisms remains incomplete. Protein phosphorylation has emerged recently as an important control of ES cell self-renewal and differentiation. The activity of core transcription factors Oct4 (5), Sox2 (6), and Nanog (7) are controlled tightly by phosphorylation. In addition, dynamic changes in global protein phosphorylation occur during early differentiation of ES cells (8, 9). However, key intracellular kinases that regulate self-renewal as well as differentiation are not well characterized.
The physiological function of cyclin K protein (encoded by CCNK) is defined poorly. This is reflected by the fact that the physiological form of cyclin K protein (CycK) has not been demonstrated convincingly so far. Human CCNK was cloned initially to encode a putative protein of 357 amino acid residues (calculated molecular mass, ∼41 kDa) (10). However, Expressed Sequence Tag profiling studies in genome databases favor a putative alternatively spliced transcript encoding a protein of 580 amino acid residues to be the predominant form (calculated molecular mass, ∼65 kDa). In addition, murine CCNK is predicted to encode only one putative transcript homologous to the longer transcript in humans. Therefore, the physiological form of CycK remains to be determined. Perhaps the most accepted function of CycK is to participate in RNA polymerase II transcription. This is because CycK has long been thought to interact with CDK9 protein, a well established elongation factor in RNA polymerase II transcription (11, 12). This interaction was initially identified in a yeast two-hybrid screen (13) but has never been demonstrated in mammalian cells. In addition, unlike cyclin T1 and T2 (11, 12), two well characterized regulatory subunits of CDK9, CycK does not stimulate transcription when artificially tethered to promoters (14). Nevertheless, CycK-containing protein complex immunoprecipitated from human cells does contain kinase activities in vitro (10, 15).
In this study, we sought to determine the physiological function of CycK protein. Our data are the first to show that the predominant form of CycK protein consists of 554 and 580 amino acid residues in murine and human cells, respectively. We further discovered that cyclin K protein is highly expressed in murine ES cells, and its knockdown results in cell differentiation. Surprisingly, cyclin K does not interact with CDK9 in mammalian cells. Instead, it associates with CDK12 and CDK13 proteins. Similar to cyclin K, both CDK12 and CDK13 are highly expressed in murine ES cells, and their knockdown leads to differentiation. Thus, our studies have uncovered two novel protein kinase complexes that maintain self-renewal in embryonic stem cells.
EXPERIMENTAL PROCEDURES
Cell Culture
Feeder-free R1 murine ES cells were cultured in DMEM containing 15% ES cell-grade fetal bovine serum (Gemini Bio-Products), supplemented with 103 units/ml LIF (Millipore), 2 mm l-glutamine, 0.1 mm 2-mercaptoethanol, and 0.1 mm non-essential amino acids. The pluripotency of R1 ES cell culture was monitored routinely by teratoma formation assay. Briefly, ∼106 ES cells in PBS were injected subcutaneously into the dorsal flank of nude mice. After six to eight weeks, tumors were surgically dissected from the mice, fixed in PBS containing 4% formaldehyde, and embedded in paraffin. Sections were stained with hematoxylin and eosin (HE) and characterized by trained medical pathologists (supplemental Fig. 3). Alkaline phosphatase (AP)3 staining of cells was performed following manufacturer's instructions (Sigma). Derivation and differentiation of dermal stem cells were carried out as described previously (16). Other cell lines were cultured according to ATCC's guidelines. All cell lines were cultured at 37 °C in a 5% CO2 incubator.
Antibodies
Anti-cyclin K and anti-FLAG M2 antibodies were purchased from Sigma; anti-CDK12, CDK9, CycT1, Oct4, Sox2, and HA antibodies were from Santa Cruz; and anti-actin antibody was from Millipore. Anti-CDK13 antibody was a gift from Dr. Geneviere (Universite Pierre et Marie Curie).
Generation of Anti-CycK Antibody
Synthetic peptide GLDPATEARYRREGARF was used to immunize rabbits (Zen Bioscience, Chengdu, China). Crude antiserum was incubated at 54 °C for 20 min and passed over recombinant CycK(1–150) covalently attached to Actigel ADL resin (Sterogene) following the manufacturer's instructions. After washing with PBS, affinity-purified anti-CycK antibody was eluted with 100 mm glycine (pH 2.5), and neutralized with 1 m Tris base. The antibody was aliquoted and stored at −80 °C.
Plasmid Construction
The coding region of murine CycK was cloned from ES cells and verified by sequencing. For rescue experiment in ES cells, three silent mutations (G651A, C654T, and A657G) were introduced by site-directed mutagenesis (Strategene). The coding region of eGFP was fused to the C terminus of CycK after a linker (Gly)5Ala.
Purification of CycK and CDK12-containing Protein Complexes
NIH 3T3-based cell lines stably expressing FLAG-His6-tagged CycK and HEK 293-based cell lines stably expressing CDK12-FLAG were established. Cell extracts were generated as described previously (17). Briefly, cells were collected and washed once in ice-cold PBS supplemented with 0.1% PMSF. After resuspension, cells were lysed by homogenization in ice-cold buffer A (10 mm HEPES, 10 mm KCl, 1.5 mm MgCl2, 1 mm DTT, 0.1% PMSF). Cell lysates were then spun at 4000 rpm/4 °C for 15 min to separate cytoplasm from nuclei. Nuclei were resuspended with ice-cold buffer B (20 mm HEPES, 1.5 mm MgCl2, 0.2 mm EDTA, 25% glycerol, 1 mm DTT, 0.1% PMSF), and supplemented with 5 m NaCl drop-wise to a final concentration of 420 mm. Resuspended nuclei were rotated gently for 30 min at 4 °C, followed by centrifugation at 48,400 × g/4 °C for 60 min. Supernatant was collected and supplemented with an equal volume of buffer A, followed by centrifugation at 48,400 × g/4 °C for 15 min. Cleared lysate was incubated with M2 anti-FLAG-agarose beads (Sigma) for 90 min at 4 °C with rotation. After being washed by 10 volumes of buffer B supplemented with 500 mm KCl, CDK12-containing protein complex was eluted with 2× protein loading buffer. For CycK-containing protein complex, it was eluted with FLAG peptide (Sigma). Eluate was then incubated with nickel-nitrilotriacetic acid-agarose beads (Qiagen) for 60 min at 4 °C with rotation. After being washed by 10 volumes of buffer B supplemented with 500 mm KCl and 25 mm imidazole, CycK-containing protein complex was eluted with buffer B supplemented with 150 mm KCl and 250 mm imidazole. Purified protein complexes were resolved by SDS-PAGE and stained by silver or colloidal blue. Liquid chromatography and tandem MS/MS analyses were carried out by the core facility at Sichuan University.
shRNA Knockdown
Synthetic oligonucleotides were annealed to form doubled strand DNA and cloned into pLKO.1 vector (Open Biosystems), which contains a puromycin-resistant gene for stable selection. The target sequences of each shRNA were listed in supplemental Table 1. shRNA constructs were transfected using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. Stable selections were carried out by supplementing growth medium with 2.5 μg/ml puromycin (Sigma).
RNA Extraction and qPCR Analysis
Total RNA was extracted by TRIzol (Invitrogen) following the manufacturer's instructions, treated with DNase I (Ambion), and reverse-transcribed by random hexamers using SuperScript II (Invitrogen). Quantitative PCR (qPCR) was performed by using EvaGreen Supermix (Bio-Rad) on an iCycler Thermal Cycler (Bio-Rad). Primer sequences are listed in supplemental Table 2. Expression levels of each gene were normalized to actin mRNA and quantified as described previously (18).
Immunoprecipitation
Cells were lysed in ice-cold buffer C (150 mm NaCl, 1.5 mm MgCl2, 10 mm KCl, 20 mm Tris-HCl (pH 7.9), 0.5 mm EDTA, 10% glycerol, 1 mm DTT, 0.1% PMSF, EDTA-free complete protease inhibitor mixture (Roche) and 0.5% Nonidet P-40). Total cell lysates were cleared by centrifugation for 10 min at 17,000 × g/4 °C. Supernatant was then mixed with indicated antibodies immobilized on protein A/G beads (Santa Cruz) and rotated for at least 90 min at 4 °C. Protein A/G beads were then washed by buffer C, supplemented with 5 m NaCl to final concentration of 500 mm. Protein complexes were eluted by 2× protein loading buffer for subsequent Western blot analyses.
Differentiation of ES Cells
ES cells grown on six-well tissue-culture plates were washed in PBS, trypsinized, and resuspended in ES cell growth medium without LIF (ES/−LIF). Cells were then seeded onto a 6-cm bacterial Petri dish and grew in suspension to form embryoid bodies. ES/−LIF medium was refreshed every other day. After 7 days, embryoid bodies were transferred onto gelatin-coated tissue-culture plates and cultured for another seven to 14 days in ES/−LIF medium.
Immunofluorescence
R1 mouse ES cells were grown on six-well tissue-culture plates, fixed for 20 min in 4% paraformaldehyde/PBS, and washed in PBS twice. Cells were permeabilized with 0.3% Triton-X 100 in PBS for 10 min, and then washed in PBS. After being blocked in 3% BSA in PBS for 1 h, cells were incubated with primary antibodies (1:200 dilution) overnight at 4 °C. After washing in PBS for 20 min, cells were incubated in 1:400 dilution of Alexa Fluor 488 goat anti-rabbit IgG or Alexa Fluor 568 goat anti-mouse IgG secondary antibodies (Molecular Probes) for 1 h, followed by washing in PBS for 20 min. To visualize DNA, cells were stained with 0.5 mg/ml of DAPI after secondary antibody incubation.
Tissue Extracts
Tissues (∼0.3 cm3) were surgically dissected from male BALB/c mice and homogenized in radioimmune precipitation assay buffer (50 mm Tris (pH 8.0), 150 mm NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 0.1% PMSF, 1 mm Na3VO4, EDTA-free complete protease inhibitor mixture (Roche), and 50 mm NaF). Lyastes were cleared by centrifugation for 5 min at 17,000 × g/4 °C. Supernatant was then collected for subsequent Western blot analyses.
RESULTS
Determine Physiological Form of CycK Protein
To determine the physiological form of CycK protein, Western blot analysis was carried out using a commercial antibody against the N-terminal region identical in two putative human isoforms. Only one band with a molecular mass of ∼70 kDa was detected in various human and murine cell lines (Fig. 1A), indicating that predominant form of CycK protein contains 580 and 554 amino acid residues in human and murine cells, respectively. To verify this result, an anti-CycK antibody was generated against an epitope identical in putative murine and human isoforms (Fig. 1B). Although the antiserum detected three bands above 50 kDa, the affinity-purified antibody only recognized one band with molecular mass ∼70 kDa in HeLa cell lysate (Fig. 1C). In addition, preincubation of affinity-purified antibody with the epitope peptide abolished this signal (Fig. 1D), confirming specificity of the antibody. Importantly, two short hairpin RNA (shRNA) constructs (hK-1 and hK-2), targeting different regions identical in putative human CCNK isoforms, but not scramble shRNA efficiently reduced the signal detected (Fig. 1E). The specificity of shRNA was further verified by reduction of CCNK mRNA, revealed by a qPCR experiment (Fig. 1F). The corresponding murine cDNA, encoding a protein of 554 amino acid residues, was subsequently cloned and utilized in the rest of experiments. Consistently, transient expression of this cDNA generated one band that overlapped with endogenous CycK in F9 cells (data not shown). Taken together, we concluded that the predominant form of CycK protein contains 580 and 554 amino acid residues in human and murine cells, respectively. In the following experiments, our customer-made, affinity-purified antibody was used to detect the expression of CycK unless otherwise specified.
FIGURE 1.
Identification of the physiological form of CycK protein. A, expression of CycK protein in different human (293 and HeLa) and murine (mES and F9) cell extracts was analyzed by Western blotting using a commercial anti-CycK antibody (Sigma). 293, HEK 293; mES, mouse embryonic stem cell. B, diagram of two putative human CycK isoforms. The two isoforms are identical in the N-terminal 307 amino acid residues. Epitope peptide (40–56 amino acids) was chemically synthesized and used to generate anti-CycK antibody. C, equal amount of HeLa cell lysate was separated in parallel on the same SDS-PAGE gel and probed separately by crude antiserum (Before) or affinity-purified antibody (After). D, detection of CycK protein in HeLa cell extract by affinity-purified antibody without or with prior neutralization by epitope peptide. E, CycK was probed by Western blotting in extracts from untransfected HeLa cells (−), cells stably transfected with scramble (scra), or two shRNA constructs specific for human CycK (hK-1 and hK-2). Actin was used as a loading control. F, quantification of CycK mRNA by qPCR in cells as indicated in E. The expression of CycK was normalized to actin level and relative to the expression level in cells transfected with scramble shRNA. Expression ratios are average measurements from three independent analyses; S.D. are shown.
CycK Is Highly Expressed in ES Cells
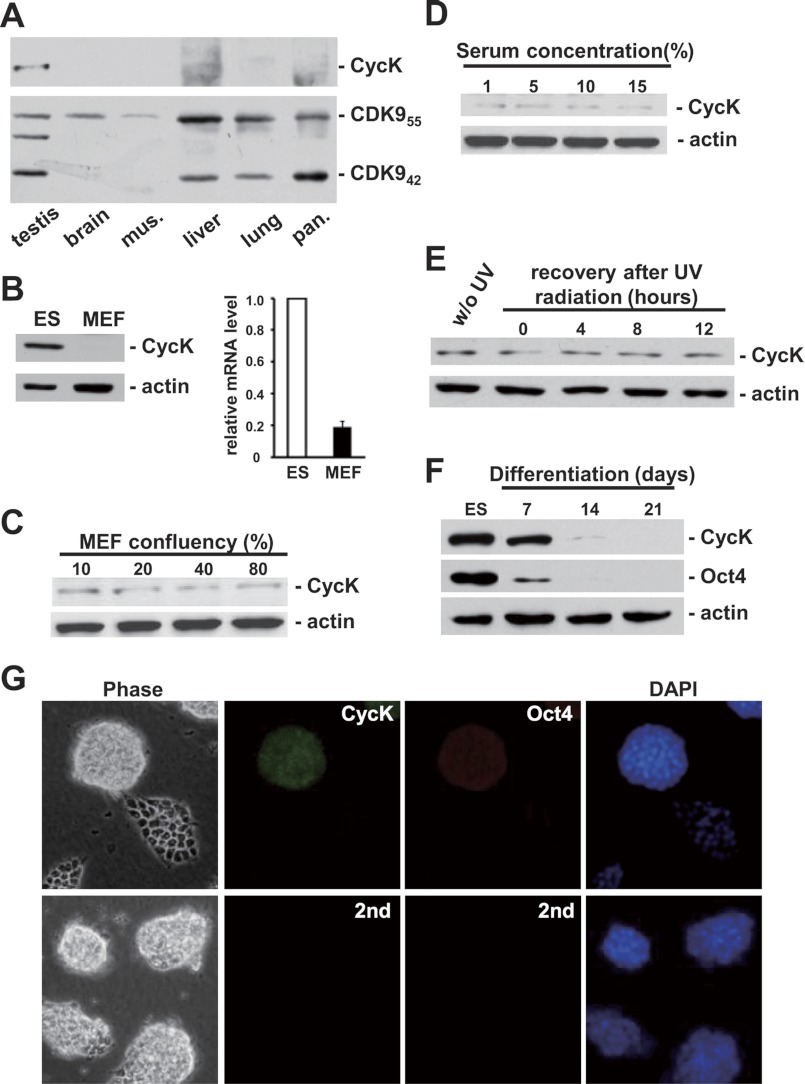
To further characterize the physiological function of CycK, its expression profile was investigated in various murine tissue and cell types. Consistent with the previous report (19), CDK9 was expressed ubiquitously in tissues with a higher ratio of CDK942 isoform in testis and CDK955 in brain and liver (Fig. 2A). Surprisingly, the expression of CycK was only detectable in testis (Fig. 2A). Because tissues contain mostly fully differentiated cells, we reasoned that CycK might be expressed in less differentiated cell types. Indeed, the protein level of CycK was high in murine ES cells but hardly detectable in highly differentiated murine embryonic fibroblasts (MEFs) under the same condition (Fig. 2B). A weak signal could be detected in MEFs only after a much longer exposure time. In addition, various signals including proliferation (Fig. 2C), serum stimulation (Fig. 2D), and UV irradiation (Fig. 2E) failed to increase the level of CycK in MEFs. Furthermore, when ES cells were induced to differentiate in vitro using the standard embryoid body formation procedure, the protein level of CycK was gradually decreased, concomitant with the reduction of Oct4, a master regulator of self-renewal (Fig. 2F). qPCR analyses further revealed a reduction in CycK mRNA level (data not shown), indicative of transcription regulation. The expression of CycK in pluripotent ES cells was also confirmed by immunofluorescence staining, which occurred only in Oct4-positive cells (Fig. 2G).
FIGURE 2.
CycK protein is highly expressed in mouse ES cells. A, The expression profiles of CycK and CDK9 were analyzed in various murine tissues. Equal amount of protein extract from each tissue, determined by BCA protein assay, was probed by Western blotting. B, CycK expression was analyzed by Western blotting (left) and qPCR (right) in ES and MEF cells. The expression of CycK was normalized to actin level, and expression ratios are average measurements from three independent analyses; S.D. are shown. C, MEF cells growing at indicated confluency were collected and analyzed for CycK by Western blotting. D, MEF cells at ∼30% confluency were deprived of serum for 12 h and then stimulated by indicated concentration of serum for another 12 h before harvest. CycK was analyzed by Western blotting. E, MEF cells were treated with Ultraviolet C (40 J/m2) and recovered in growth medium for indicated time before harvest. F, ES cells were induced to differentiate in vitro for 21 days. Cells were collected at the indicated time and analyzed for CycK expression. G, detection of CycK and Oct4 by indirect immunofluorescence. Background fluorescence was shown on the lower panel when only secondary antibodies (2nd) were used. DNA was revealed by DAPI staining. w/o, without. mus., muscle; pan., pancreas.
CycK Is Not Expressed in Dermal Stem Cells
The expression of CycK was further explored in other stem cell types. Dermal stem cells (also known as Skin-derived precursors) (20) were chosen because of its availability in the lab (Fig. 3A). Consistent with literature (20, 21), our dermal stem cells were multipotent in that they could be induced to generate osteoblasts as well as adipocytes (Fig. 3, B and C). However, unlike in ES cells, CycK was hardly detectable in dermal stem cells (Fig. 3D).
FIGURE 3.
CycK protein is not expressed in dermal stem cells. A, the morphology of dermal stem cells observed under microscope. B, dermal stem cells were induced in vitro to generate osteoblasts (indicated by arrows), revealed by Alizarin Red S staining for calcium. C, dermal stem cells were induced in vitro to generate adipocytes (indicated by arrows), revealed by Oil Red O staining for lipid deposits. D, expression of CycK was analyzed in ES and dermal stem cell extracts by Western blotting.
Knockdown of CycK Leads to Differentiation of ES Cells
Based on the above observations, we hypothesized that CycK is required for ES cell self-renewal. To test this, we first asked whether knockdown of CycK by shRNA induces differentiation. The level of CycK in murine ES cells was efficiently reduced by transient transfection with shRNA specific for murine CycK (mK-1), but not with scramble or two different shRNA constructs specific for human CycK (hK-1 and hK-2) (Fig. 4A). Notably, mK-1 and hK-1 differs in only one nucleotide in the seed region, which is important for shRNA to recognize its cognate mRNA, demonstrating that knockdown by mK-1 was highly specific. Undifferentiated ES cells grow in compact colonies and show strong staining for AP. Stable knockdown by scramble shRNA did not change the compact morphology or positive AP staining of ES cells. In sharp contrast, stable knockdown of CycK by mK-1 induced flat cell morphology and negative AP staining, indicating differentiation of ES cells (Fig. 4B). Differentiation was further demonstrated by a great reduction in Sox2, a master regulator of self-renewal (Fig. 4C, lane 3). We noticed that after stable knockdown, a few ES cell-like colonies survived and kept growing. This could be because CycK was not knocked down efficiently in those cells. Alternatively, this might indicate that a subpopulation of ES cells does not require CycK for self-renewal. To distinguish these two possibilities, cells stably transfected with mK-1 were trypsinized and reseeded. Most cells did not attach, consistent with differentiation. The remaining cells grew in an ES cell-like morphology and were stained positive for AP. In addition, they had a similar level of Sox2 as well as CycK to ES cells (Fig. 4C, lane 4). Thus, the reason that these cells did not differentiate was that CycK was not knocked down. Taken together, our results suggest that expression of CycK is strongly correlated with self-renewal of ES cells.
FIGURE 4.
CycK protein maintains self-renewal in mouse ES cells. A, transient knockdown by scramble (scra), human-specific (hK-1 and hK-2), or murine-specific (mK-1) shRNA constructs in murine ES cells. Knockdown efficiency was analyzed by Western blotting. B, stable knockdown by scramble or mK-1 shRNA. Cell differentiation was indicated by negative AP staining as well as flat cell morphology. C, protein expression was analyzed by Western blotting in extracts from untransfected ES cells (−), cells stably transfected with scramble, or mK-1 shRNA. Cells stably transfected with mK-1 were also trypsinized, reseeded, and grown for 7 days before harvest (mK-1-split). Most cells did not attach upon passage. The remaining cells grew in an ES cell-like morphology. D, rescue of CycK expression by an RNAi-resistant CycK expression vector (CycK-R). Cell extracts from untransfected cells and cells transiently transfected with mK-1, CycK-R, or both were analyzed by Western blotting. E, rescue of self-renewal by CycK-R at the presence of mK-1 shRNA. Cells were stably transfected with indicated constructs and stained by AP. F, stable transfection with another CycK-specific shRNA (mK-2) led to differentiation, revealed by loss of Oct4 immunofluorescence staining. Background fluorescence was shown on the lower panel when only secondary antibody (2nd) was used. G, protein expression was examined by Western blotting in extracts from cells stably transfected with scramble or mK-2 shRNA. DNA was revealed by DAPI staining.
To verify the specificity of knockdown, a rescue experiment was carried out. GFP was fused to the C terminus of CycK cDNA (CycK-R) containing silent mutations that would disrupt the recognition by mK-1 shRNA. This fusion protein had a slower mobility than endogenous CycK on SDS-PAGE. When co-transfected with mK-1 shRNA, the expression of CycK-R but not endogenous CycK remained (Fig. 4D, lane 4). As expected, only a few AP-positive colonies remained after stable knockdown by mK-1 shRNA. In contrast, when mK-1 shRNA and CycK-R cDNA in a ratio of 3:1 were co-transfected into ES cells and stably selected, a reproducible, severalfold increase in AP-positive colonies was observed repeatedly (Fig. 4E), demonstrating that self-renewal was rescued by expression of CycK-R.
Knockdown of CycK by a different shRNA, mK-2, targeting the 3′-UTR of CycK mRNA was also carried out. Similarly to mK-1, stable transfection of mK-2 efficiently induced flat cell morphology and loss of Oct4 staining (Fig. 4F). In addition, both Oct4 and Sox2 proteins were reduced greatly (Fig. 4G). Taken together, we concluded that CycK is required to maintain self-renewal in ES cells.
CycK Does Not Interact with CDK9 in Mammalian Cells
Although CycK has long been thought to interact with CDK9, this interaction has never been demonstrated in mammalian cells. To verify this interaction, cDNA encoding FLAG-tagged CycK (the originally cloned form containing 357 amino acid residues) or FLAG-tagged cyclin T1 (CycT1), a well established regulatory subunit of CDK9, was transfected into HEK 293 cells. Endogenous CDK9 efficiently pulled down FLAG-tagged CycT1 but surprisingly not CycK (supplemental Fig. 1).
As we have shown that the physiological form of murine CycK consists of 554 amino acid residues (Fig. 1), we examined the interaction between endogenous CDK9 and this newly identified form of CycK in ES cells. Endogenous CDK9 was immunoprecipitated from ES cell extract and probed for CycT1 or CycK. Again, CycK was not detectable in anti-CDK9 immunoprecipate, whereas CycT1 was pulled down efficiently (Fig. 5A).
FIGURE 5.
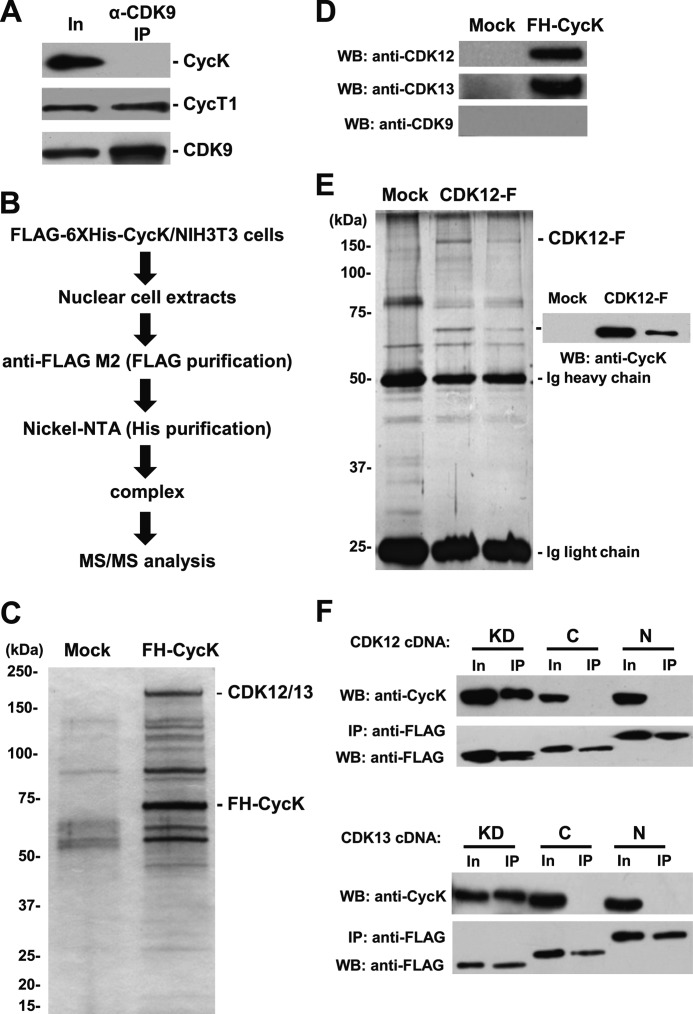
Cyclin K interacts with CDK12 and CDK13 but not CDK9. A, endogenous CDK9 was immunoprecipitated from ES cell extract, followed by Western blotting (WB) with indicated antibodies. B, the experimental flowchart to identify CycK-associated kinases by an unbiased proteomic approach. C, identical purification procedure was carried out using extracts from naïve NIH 3T3 cells (Mock) or cells stably expressing tagged CycK (FH-CycK). Purified materials were visualized by silver. Specific polypeptides in FH-CycK were analyzed by LC-MS/MS. D, purified materials in C were analyzed by Western blotting with indicated antibodies. E, identical purification procedure was carried out using extracts from naïve HEK293 cells (Mock) or two cell lines stably expressing FLAG-tagged CDK12 (CDK12-F). Purified materials were visualized by silver. Specific polypeptides in CDK12-F were analyzed by LC-MS/MS. Identity of CycK was revealed by Western blotting. F, domain mapping of CDK12 and CDK13. cDNAs encoding individual domains were transfected into HEK293 cells, followed by anti-FLAG immunoprecipitation, and a sequential Western blotting with anti-CycK as well as FLAG antibodies. In, Input.
Identify CycK-interacting Kinases by Mass Spectrometry
An unbiased proteomic approach was utilized to identify CycK-associated kinases. For the cost reason, NIH 3T3 instead of ES cells were used. NIH 3T3 cells had a much lower but nevertheless detectable level of endogenous CycK than ES cells. A cell line stably expressing CycK cDNA fused with a FLAG-His6 tag at the N terminus (FH-CycK) was established. The nuclear extract was generated and utilized for a sequential affinity purification procedure using anti-FLAG antibody followed by nickel-nitrilotriacetic acid (Fig. 5B). The purified complex was separated by SDS-PAGE and visualized by silver staining. Compared with mock purification, several specific polypeptides were detected in CycK-containing protein complex (Fig. 5C).
Mass spectrometric analyses of these polypeptides identified CDC2L5 (also known as CDK13) protein. To verify this result, purification was repeated, and an independent mass spectrometric analysis was carried out. Surprisingly, CrkRS (also known as CDK12) but not CDK13 was identified. A close examination of two mass spectrometric data sets revealed that nine of ten unique peptides assigned to either CDK12 or CDK13 each time were actually shared by both kinases. Indeed, both CDK12 and CDK13 but not CDK9 protein were specifically present in purified CycK-containing protein complex, revealed by corresponding antibodies (Fig. 5D).
The identified interactions were further verified by reciprocal purifications. Two independent HEK 293-based cell lines stably expressing FLAG-tagged CDK12 (CDK12-F) were established and utilized for the purification of CDK12-containing protein complex using anti-FLAG antibodies. The purified complex was eluted by protein loading buffer, separated by SDS-PAGE, and visualized by silver (Fig. 5E). Mass spectrometric analyses revealed the presence of CycK in both purifications. The identity of CycK was further verified by Western blot analysis (Fig. 5E). In addition, the originally cloned CycK (357 amino acids) interacted with CDK12 and CDK13 (supplemental Fig. 2). Thus, we concluded that CycK interact with CDK12 and CDK13 but not CDK9.
Kinase Domain of CDK12 and CDK13 Interacts with CycK
CDK12 and CDK13 share >90% sequence identity in their putative kinase domains, whereas they have no obvious homology outside these domains. Therefore, we reasoned that the kinase domain would be the region that interacts with CycK. To test this, FLAG-tagged cDNA expressing the kinase domains (620–1048 amino acids for CDK12, and 604–1030 amino acids for CDK13), C-terminal domains (1049–1490 amino acids for CDK12, and 1031–1452 amino acids for CDK13) and N-terminal domains (1–619 amino acids for CDK12, and 1–603 amino acids for CDK13) were individually transfected into HEK 293 cells. Immunoprecipitation with anti-FLAG antibody was carried out, followed by Western blot analyses with anti-FLAG and CycK antibodies. Indeed, kinase domains of both CDK12 and CDK13 could efficiently pull down endogenous CycK but not the N- or C-terminal domains (Fig. 5F).
Knockdown of CDK12 or CDK13 Leads to ES Cell Differentiation
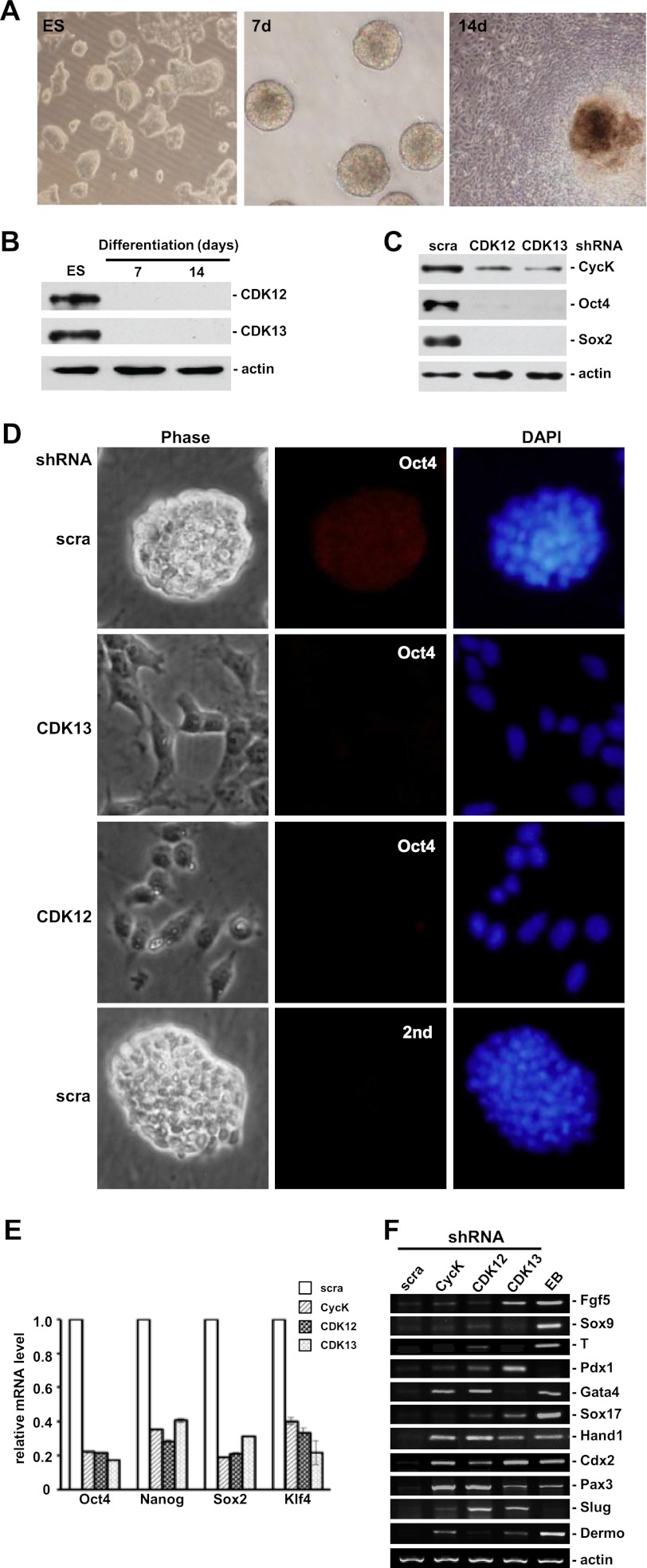
The expression profile of CDK12 and CDK13 was examined in ES cells. ES cells were induced to differentiate in vitro using the standard embryoid body formation procedure for 2 weeks (Fig. 6A). Cells were collected every week and analyzed for the expression of CDK12 and CDK13. Similar to CycK, both CDK12 and CDK13 were highly expressed in ES cells, and this expression was reduced rapidly during differentiation (Fig. 6B).
FIGURE 6.
Knockdown of CDK12 or CDK13 leads to ES cell differentiation. A, differentiation of ES cells in vitro. Cells were induced to differentiate by withdrawing LIF and growing in suspension for 7 days (7d), followed by adherent growth for another 7 days (14d). B, protein expression was analyzed by Western blotting in ES cells and differentiated cells as described in A. C, protein expression was analyzed in cells stably transfected with scramble, CDK12-, or CDK13-specific shRNA. D, Oct4 staining was lost in cells stably transfected with CDK12- or CDK13-specific shRNA but not scramble shRNA. Background fluorescence was shown on the lower panel when only secondary antibody (2nd) was used. DNA was revealed by DAPI staining. E, expression levels of pluripotency genes were analyzed by qPCR in cells stably transfected with scramble (scra), CycK, CDK12, or CDK13-specific shRNA. Expression levels were normalized to actin level and relative to the expression levels of the same transcripts in cells transfected with scramble shRNA. Expression ratios are average measurements from three independent analyses; S.D. are shown. F, expression levels of marker genes representative of differentiation were analyzed by RT-PCR in cells stably transfected with scramble, CycK-, CDK12-, or CDK13-specific shRNA. Fgf5 and Sox9 (ectoderm), T (mesoderm), Pdx1, Gata4 and Sox17 (endoderm), Hand1 and Cdx2 (trophectoderm), Pax3 (myogenesis), Slug (EMT), and Dermo (dermal differentiation).
The role of CDK12 and CDK13 in self-renewal was further investigated. Similar to CycK, stable knockdown of either CDK12 or CDK13 by shRNA efficiently reduced the level of Oct4 and Sox2, master regulators of self-renewal (Fig. 6C). In addition, flattened cell morphology was observed, concomitant with loss of Oct4 staining (Fig. 6D), demonstrating that both CDK12 and CDK13 are required to maintain self-renewal. Expression profiles of a panel of self-renewal as well as differentiation marker genes were analyzed by qPCR and RT-PCR, respectively. Consistent with their roles in maintaining ES cells, knockdown of CDK12, CDK13, or CycK led to reduced expression of self-renewal markers (Fig. 6E) and increased expression of differentiation markers (Fig. 6F). Importantly, the expression profile of differentiation marker genes was different when CDK12 or CDK13 was knocked down, indicating that the functions of these two kinase are not redundant. Taken together, we concluded that CDK12 and CDK13, together with CycK, play an important role in maintaining self-renewal in ES cells.
DISCUSSION
ES cells are self-renewing, pluripotent cells, capable of differentiating into essentially all cell types. These properties make ES cells an ideal model system to study mammalian development and promise future medical applications in regenerative medicine (1, 2). Much of the transcriptional network that controls self-renewal has been elucidated. The core transcription factors Oct4, Sox2, and Nanog collaborate to activate the expression of genes that promote self-renewal and repress that of lineage-specific genes (3, 4). However, our understanding of molecular controls at other levels is behind. Protein phosphorylation is a major transducer of developmental as well as environmental stimuli. For this reason, perhaps it is surprising that intracellular protein kinases important for self-renewal are not well characterized in ES cells.
In this study, we identified two novel protein complexes, CycK-CDK12 and CycK-CDK13. We showed that they are highly expressed in murine ES cells. We further demonstrated that CycK, CDK12, and CDK13 are required to maintain self-renewal in ES cells. The functions of both CDK12 (also known as CrkRS) (22) and CDK13 (also known as CDC2L5) (23) are not well understood. Several lines of evidence indicate that they may regulate certain aspect of transcription. Both proteins contain serine/arginine-rich regions at their N termini, a motif frequently present in splicing factors (22, 24). Indeed, both human CDK12 and CDK13 proteins are colocalized with spliceosome components in nuclear speckles (22, 24). CDK13 can phosphorylate splicing factor ASF/SF2 in vitro (25), although it is not clear whether this happens in vivo or its functional outcomes. Drosophila CDK12 protein has been shown recently to be a transcription elongation-associated kinase (26). As the transcription of many developmental genes is controlled at the step of elongation in ES cells (27), it is tempting to speculate that CDK12 may regulate the expression of these genes. Interestingly, Oct4, Sox2, and Nanog, master regulators of self-renewal, recently have been shown to be regulated by phosphorylation in ES cells, although the responsible kinases are not known (5–7). As there are several consensus CDK phosphorylation sites in these factors, it will be of interest to determine whether CycK-CDK12 and CycK-CDK13 are directly responsible for these phosphorylations.
During the preparation of our manuscript, Blazek et al. (28) showed that CycK interacts with CDK12 and CDK13 but not CDK9 protein, consistent with our results. In addition, in an attempt to generate CycK knock-out mice, they failed to generate any homozygous CycK−/− offspring and did not detect any CycK−/− embryos (28). In light of our findings in this manuscript, we suspect that this early embryonic lethal phenotype might be caused by failure to maintain inner cell mass, from which ES cells are derived (1). Interestingly, they also showed that CDK12 may regulate expression of a subset of genes involved in DNA damage response (28). It will be of interest to determine whether these are downstream effectors of CDK12 in ES cells as genomic stability has previously been linked to ES cell differentiation (29).
In summary, we identified two cyclin K-dependent kinase complexes that are required to maintain self-renewal in ES cells. Identification of their substrates in ES cells in future will be the key to elucidate the underlying molecular mechanisms.
Supplementary Material
Acknowledgments
We thank Jonathon Pines (University of Cambridge) for CDK12 cDNA, Anne-Marie Genevière (Universite Pierre et Marie Curie) for anti-CDK13 antibody and CDK13 cDNA.
This work was supported by grants from National Basic Research Program of China/973 Program (2011CB504200 and 2012CB910700), National Natural Science Foundation of China (30970625 and 31171260), and Program for New Century Excellent Talents in University of Ministry of Education of China (NCET-10-0565 to Q. L.).

This article contains supplemental Tables 1 and 2 and Figs. S1–S3.
- AP
- alkaline phosphatase
- qPCR
- quantitative PCR
- MEF
- murine embryonic fibroblast
- LIF
- leukemia inhibitory factor.
REFERENCES
- 1. Evans M. (2011) Discovering pluripotency: 30 years of mouse embryonic stem cells. Nat. Rev. 12, 680–686 [DOI] [PubMed] [Google Scholar]
- 2. Yu J., Thomson J. A. (2008) Pluripotent stem cell lines. Genes Dev. 22, 1987–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Young R. A. (2011) Control of the embryonic stem cell state. Cell 144, 940–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jaenisch R., Young R. (2008) Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132, 567–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saxe J. P., Tomilin A., Schöler H. R., Plath K., Huang J. (2009) Post-translational regulation of Oct4 transcriptional activity. PloS One 4, e4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jeong C. H., Cho Y. Y., Kim M. O., Kim S. H., Cho E. J., Lee S. Y., Jeon Y. J., Lee K. Y., Yao K., Keum Y. S., Bode A. M., Dong Z. (2010) Phosphorylation of Sox2 cooperates in reprogramming to pluripotent stem cells. Stem Cells 28, 2141–2150 [DOI] [PubMed] [Google Scholar]
- 7. Moretto-Zita M., Jin H., Shen Z., Zhao T., Briggs S. P., Xu Y. (2010) Phosphorylation stabilizes Nanog by promoting its interaction with Pin1. Proc. Natl. Acad. Sci. U.S.A. 107, 13312–13317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Hoof D., Muñoz J., Braam S. R., Pinkse M. W., Linding R., Heck A. J., Mummery C. L., Krijgsveld J. (2009) Phosphorylation dynamics during early differentiation of human embryonic stem cells. Cell Stem Cell 5, 214–226 [DOI] [PubMed] [Google Scholar]
- 9. Brill L. M., Xiong W., Lee K. B., Ficarro S. B., Crain A., Xu Y., Terskikh A., Snyder E. Y., Ding S. (2009) Phosphoproteomic analysis of human embryonic stem cells. Cell Stem Cell 5, 204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Edwards M. C., Wong C., Elledge S. J. (1998) Human cyclin K, a novel RNA polymerase II-associated cyclin possessing both carboxyl-terminal domain kinase and Cdk-activating kinase activity. Mol. Cell. Biol. 18, 4291–4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou Q., Yik J. H. (2006) The Yin and Yang of P-TEFb regulation: Implications for human immunodeficiency virus gene expression and global control of cell growth and differentiation. Microbiol. Mol. Biol. Rev. 70, 646–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peterlin B. M., Price D. H. (2006) Controlling the elongation phase of transcription with P-TEFb. Molecular cell 23, 297–305 [DOI] [PubMed] [Google Scholar]
- 13. Fu T. J., Peng J., Lee G., Price D. H., Flores O. (1999) Cyclin K functions as a CDK9 regulatory subunit and participates in RNA polymerase II transcription. J. Biol. Chem. 274, 34527–34530 [DOI] [PubMed] [Google Scholar]
- 14. Napolitano G., Majello B., Licciardo P., Giordano A., Lania L. (2000) Transcriptional activity of positive transcription elongation factor b kinase in vivo requires the C-terminal domain of RNA polymerase II. Gene 254, 139–145 [DOI] [PubMed] [Google Scholar]
- 15. Lin X., Taube R., Fujinaga K., Peterlin B. M. (2002) P-TEFb containing cyclin K and Cdk9 can activate transcription via RNA. J. Biol. Chem. 277, 16873–16878 [DOI] [PubMed] [Google Scholar]
- 16. Biernaskie J. A., McKenzie I. A., Toma J. G., Miller F. D. (2006) Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat. Protoc. 1, 2803–2812 [DOI] [PubMed] [Google Scholar]
- 17. Abmayr S. M., Yao T., Parmely T., Workman J. L. (2006) Current protocols in molecular biology / edited by Frederick M. Ausubel. [et alChapter 12, Unit 12 11 [DOI] [PubMed] [Google Scholar]
- 18. Peirson S. N., Butler J. N., Foster R. G. (2003) Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 31, e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shore S. M., Byers S. A., Dent P., Price D. H. (2005) Characterization of Cdk9(55) and differential regulation of two Cdk9 isoforms. Gene 350, 51–58 [DOI] [PubMed] [Google Scholar]
- 20. Biernaskie J., Paris M., Morozova O., Fagan B. M., Marra M., Pevny L., Miller F. D. (2009) SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell 5, 610–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toma J. G., Akhavan M., Fernandes K. J., Barnabé-Heider F., Sadikot A., Kaplan D. R., Miller F. D. (2001) Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat. Cell Biol. 3, 778–784 [DOI] [PubMed] [Google Scholar]
- 22. Ko T. K., Kelly E., Pines J. (2001) CrkRS: A novel conserved Cdc2-related protein kinase that colocalizes with SC35 speckles. J. Cell Sci. 114, 2591–2603 [DOI] [PubMed] [Google Scholar]
- 23. Marqués F., Moreau J. L., Peaucellier G., Lozano J. C., Schatt P., Picard A., Callebaut I., Perret E., Genevière A. M. (2000) A new subfamily of high molecular mass CDC2-related kinases with PITAI/VRE motifs. Biochem. Biophys. Res. Commun. 279, 832–837 [DOI] [PubMed] [Google Scholar]
- 24. Even Y., Durieux S., Escande M. L., Lozano J. C., Peaucellier G., Weil D., Genevière A. M. (2006) CDC2L5, a Cdk-like kinase with RS domain, interacts with the ASF/SF2-associated protein p32 and affects splicing in vivo. J. Cell. Biochem. 99, 890–904 [DOI] [PubMed] [Google Scholar]
- 25. Berro R., Pedati C., Kehn-Hall K., Wu W., Klase Z., Even Y., Genevière A. M., Ammosova T., Nekhai S., Kashanchi F. (2008) CDK13, a new potential human immunodeficiency virus type 1 inhibitory factor regulating viral mRNA splicing. J. Virol. 82, 7155–7166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bartkowiak B., Liu P., Phatnani H. P., Fuda N. J., Cooper J. J., Price D. H., Adelman K., Lis J. T., Greenleaf A. L. (2010) CDK12 is a transcription elongation-associated CTD kinase, the metazoan ortholog of yeast Ctk1. Genes Dev. 24, 2303–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bernstein B. E., Mikkelsen T. S., Xie X., Kamal M., Huebert D. J., Cuff J., Fry B., Meissner A., Wernig M., Plath K., Jaenisch R., Wagschal A., Feil R., Schreiber S. L., Lander E. S. (2006) A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 [DOI] [PubMed] [Google Scholar]
- 28. Blazek D., Kohoutek J., Bartholomeeusen K., Johansen E., Hulinkova P., Luo Z., Cimermancic P., Ule J., Peterlin B. M. (2011) The cyclin K/Cdk12 complex maintains genomic stability via regulation of expression of DNA damage response genes. Genes Dev. 25, 2158-2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin T., Chao C., Saito S., Mazur S. J., Murphy M. E., Appella E., Xu Y. (2005) p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat. Cell Biol. 7, 165–171 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.