Background: TpeL is a member of the family of clostridial glucosylating toxins, produced by Clostridium perfringens.
Results: TpeL enters target cells by self-mediated entry and mono-glycosylates Ras proteins at Thr-35.
Conclusion: TpeL inhibits Ras signaling and induces apoptosis in target cells.
Significance: TpeL is a new glucosylating toxin produced by C. perfringens.
Keywords: Apoptosis, Bacterial Pathogenesis, Bacterial Toxins, Glycosyltransferases, Ras, Clostridium perfringens, Ras Signaling, Bacterial Glucosyltransferases, Clostridial Glucosylating Toxins
Abstract
TpeL is a member of the family of clostridial glucosylating toxins produced by Clostridium perfringens type A, B, and C strains. In contrast to other members of this toxin family, it lacks a C-terminal polypeptide repeat domain, which is suggested to be involved in target cell binding. It was shown that the glucosyltransferase domain of TpeL modifies Ras in vitro by mono-O-glucosylation or mono-O-GlcNAcylation (Nagahama, M., Ohkubo, A., Oda, M., Kobayashi, K., Amimoto, K., Miyamoto, K., and Sakurai, J. (2011) Infect. Immun. 79, 905–910). Here we show that TpeL preferably utilizes UDP-N-acetylglucosamine (UDP-GlcNAc) as a sugar donor. Change of alanine 383 of TpeL to isoleucine turns the sugar donor preference from UDP-GlcNAc to UDP-glucose. In contrast to previous studies, we show that Rac is a poor substrate in vitro and in vivo and requires 1–2 magnitudes higher toxin concentrations for modification by TpeL. The toxin is autoproteolytically processed in the presence of inositol hexakisphosphate (InsP6) by an intrinsic cysteine protease domain, located next to the glucosyltransferase domain. A C-terminally extended TpeL full-length variant (TpeL1–1779) induces apoptosis in HeLa cells (most likely by mono-O-GlcNAcylation of Ras), and inhibits Ras signaling including Ras-Raf interaction and ERK activation. In addition, TpeL blocks Ras signaling in rat pheochromocytoma PC12 cells. TpeL is a glucosylating toxin, which modifies Ras and induces apoptosis in target cells without having a typical C-terminal polypeptide repeat domain.
Introduction
Clostridium perfringens is a widespread pathogen, which is responsible for a number of severe diseases in humans and animals (1, 2). In 70 to 80% of the cases it is the causative agent of the gas gangrene syndrome. The bacteria are equipped with a broad arsenal of toxins (3–6). C. perfringens is further classified into five serotypes A to E. C. perfringens type C produces the two major toxins α- and β-toxin, but not ϵ- and ι-toxin. Strains of this serotype cause a severe life-threatening necrotic enteritis of the jejunum and ileum mainly due to the production of β-toxin (7).
Recently TpeL was identified and isolated from the supernatant of C. perfringens type C strain MC18 (8). TpeL is a C-terminally truncated homologue of clostridial glucosylating toxins (CGTs),2 encompassing toxin A and B from C. difficile, lethal and hemorrhagic toxin from C. sordellii and α-toxin from C. novyi (8–11). CGTs share a similar structure-function relationship. The toxins consist of at least 4 major domains (ABCD model) (12). The N terminus harbors the biological active glucosyltransferase activity (domain A) (13). This region is followed by a cysteine protease domain (C domain) (14). The receptor binding domain (B domain), which consists of polypeptide repeats (15, 16), is located C-terminally and was shown to bind certain carbohydrates. Between the C domain and the putative receptor binding domain, a toxin part is localized, which is probably responsible for the delivery (D domain) of the glucosyltransferase into the cytosol of target cells (17). Toxin up-take is suggested to start with receptor binding and clathrin-mediated endocytosis of the toxin-receptor complex into endosomal compartments (18). At low pH of endosomes, insertion into membranes is accomplished and toxin translocation initiated. After autoproteolytical processing by the intrinsic cysteine protease domain only the glucosyltransferase domain is released into the cytosol. Once in the cytosol, the toxins catalyze the mono-O-glucosylation of a threonine residue of Rho/Ras proteins, which is crucial for function of the small GTPases (19, 20). Notably, while C. difficile toxins A, B, and C. sordellii lethal toxin utilize UDP-glucose as a sugar donor (19, 21–23), C. novyi toxin transfers N-acetylglucosamine from UDP-N-acetylglucosamine onto the target protein (20). The molecular basis for these differences in the donor substrate specificity were recognized to be due to changes of 2 amino acid residues in the glucosyltransferase domain of the toxins (25, 26).
Recently, Nagahama et al. proposed that in contrast to other members of the CGTs, TpeL could utilize both UDP-Glc and UDP-GlcNAc as a donor substrate to modify certain Rho- and Ras-GTPases (27). Here we characterized the enzymatic activities of TpeL in detail and show that Ras is the preferred acceptor and N-acetylglucosamine the preferred donor substrate. The donor substrate specificity of TpeL is defined by amino acid residue alanine-383. A cysteine protease domain, located next to the glucosyltransferase domain, mediates InsP6-dependent autocatalytic cleavage of the TpeL toxin. Furthermore, we report that a TpeL full-length variant, which is ∼15 kDa larger than previously described, causes cytotoxic effects in HeLa cells, induces apoptosis and inhibits Ras signaling pathways in rat pheochromocytoma PC12 cells. Intoxication of HeLa cells with TpeL followed by mass spectrometric analysis of immunoprecipitated Ras indicates that N-acetylglucosamine is the preferred modification of Ras in living cells.
EXPERIMENTAL PROCEDURES
Cultivation of Mammalian and Bacterial Cells
C. perfringens type C strain CN3685 was cultivated in BHI medium at 37 °C. Escherichia coli (strains BL21 (DE3), XL1-Blue, Rosetta) and Bacillus megaterium WH320 (MoBiTec, Göttingen, Germany) were cultivated in LB medium at 37 °C. HeLa cells were grown at 37 °C with 5% CO2 in DMEM (Biochrom, Berlin, Germany) supplemented with 10% FCS (Biochrom), 1% nonessential amino acids (PAA Laboratories, Pasching, Austria), and 4 mm penicillin/streptomycin (PAN Biotech, Aidenbach, Germany). PC12 cells were grown at 37 °C with 5% CO2 in RPMI 1640 (Biochrom) supplemented with 10% horse serum (PAA Laboratories), 5% FCS (Biochrom), 1% sodium pyruvate (PAN Biotech), and 4 mm penicillin/streptomycin (PAN Biotech).
Chemicals and Reagents
UDP-Glc, UDP-GlcNAc, and staurosporine were ordered from Sigma-Aldrich (Taufkirchen, Germany). EGF was obtained from Bachem (Bubendorf, Switzerland). NGF was ordered from Biomol (Hamburg, Germany).
Constructs, Cloning, and Mutagenesis
For cloning of TpeL543–805, TpeL1–805, TpeL1–1651, and TpeL1–1779 in the B. megaterium expression vector pHIS1522 (MoBiTec), the respective DNA sequences were amplified by PCR with oligonucleotides generating a 5′-BsrGI and a 3′-KpnI restriction site, and by using genomic DNA from C. perfringens type C strain CN3685 as template. TpeL1–542 was cloned into the E. coli expression vector pET28a (Merck, Darmstadt, Germany) using the restriction sites BamHI and XhoI. Eventually, mutations were generated by site-directed mutagenesis (QuikChange, Stratagene, La Jolla, CA).
Expression and Purification of Recombinant Proteins
TpeL543–805, TpeL1–805, TpeL1–805(C696A), TpeL1-1651, TpeL1–1779, lethal toxin, and toxin A were expressed in B. megaterium as C-terminally His6-tagged proteins and purified by nickel affinity chromatography as described previously (28). The proteins were finally stored in 20 mm Tris/HCl (pH 7.5), 150 mm NaCl and 10% glycerol. TpeL1–542, TpeL1–542 (A383I), and TpeL1–542 (DXD mut.) were expressed as C-terminally His6-tagged proteins in E. coli BL21 (DE3), purified by nickel affinity chromatography and finally stored in 50 mm Hepes (pH 7.5), 150 mm KCl, and 10% glycerol. Small GTPases used in this study and the glucosyltransferase domain of lethal toxin (lethal toxin1–546) were expressed as GST-tagged fusion proteins (pGEX expression system) in E. coli BL21 (DE3) and purified with Glutathione-Sepharose™ 4B (GE Healthcare, Munich, Germany) as described previously (13, 21). The proteins were eluted as GST fusion proteins with buffer containing 50 mm Tris/HCl (pH 8) and 10 mm glutathione, or, eventually, without the GST moiety, by thrombin cleavage.
In Vitro Glucosylation Assay
At indicated concentrations, recombinant toxins or toxin fragments were incubated for 10–45 min at 37 °C in glucosylation buffer (50 mm Hepes (pH 7.5), 2 mm MgCl2, 1 mm MnCl2, 100 mm KCl, and 0.1 mg/ml BSA) in the presence of 10 μm UDP-[14C]glucose (Biotrend) or UDP-[14C]-N-acetylglucosamine (Biotrend) and together with the indicated amounts of the respective small GTPase. The reaction was stopped by addition of Laemmli buffer and by heating for 5 min at 95 °C. Radiolabeled proteins were separated by SDS-PAGE and visualized by autoradiography.
UDP-sugar Hydrolase Assay
Toxin fragments or mutants were incubated at 37 °C with 20 μm UDP-[14C]glucose or UDP-[14C]-N-acetylglucosamine and 100 μm of the respective unlabeled UDP-sugar in reaction buffer (50 mm Hepes (pH 7.5), 2 mm MgCl2, 100 μm MnCl2, 100 mm KCl, and 0.1 mg/ml BSA). Samples were subjected to thin layer chromatography with silica gel 60 F254 -plates (Merck) and n-butanol/acetic acid/water (volume ratio 5:2:3) as mobile phase. The dried plates were analyzed by autoradiography.
Tryptophan Fluorescence Quenching
Tryptophan fluorescence of 1 μm toxin fragments or mutants was measured at room temperature in the presence of indicated concentrations of UDP-Glc or UDP-GlcNAc in buffer containing 50 mm Hepes (pH 7.5), 150 mm KCl, and 5 mm CaCl2, by using the Infinite M200 (Tecan, Männedorf, Switzerland) microplate reader (excitation at 295 nm, emission at 340 nm).
Mass Spectrometry (LC-MS/MS)
For in-gel digestion the excised gel bands were destained with 30% acetonitrile, shrunk with 100% acetonitrile, and dried in a Vacuum Concentrator 5301 (Eppendorf). Digests with protease were performed overnight at 37 °C (trypsin) or at 60 °C for 2.5 h (thermolysin), respectively, in 0.1 m NH4HCO3 (pH 8). For each gel band, ∼0.1 μg of protease was used. Peptides were extracted from gel slices with 5% formic acid. All LC-MS/MS analyses were performed on the Q-TOF mass spectrometer Agilent 6520 (Agilent Technologies, Böblingen, Germany) coupled to an 1200 Agilent nanoflow system via an HPLC-Chip cube ESI interface. Peptides were separated on a HPLC-Chip with an analytical column of 75 μm internal diameter and 150 mm length and a 40 nl trap column (both packed with Zorbax 300SB C-18 (5 μm particle size)). Peptides were eluted with a linear acetonitrile gradient with 1%/min at a flow rate of 300 nl/min (starting with 3% acetonitrile). The Q-TOF was operated in the 2 GHz extended dynamic range mode. MS/MS analyses were performed using the data-dependent acquisition mode. After an MS scan (2 spectra/s), a maximum of three peptides was selected for MS/MS (2 spectra/s). Singly charged precursor ions were excluded from selection. Internal calibration was applied. Mascot Distiller 2.3 was used for raw data processing and for generating peak lists, essentially with standard settings for the Agilent Q-TOF. Mascot Server 2.3 was used for database searching with the following parameters: peptide mass tolerance: 20 ppm, MS/MS mass tolerance: 0.05 Da, enzyme: trypsin/thermolysin with 2 uncleaved sites allowed. For protein identification, the SwissProt protein database was used.
Intoxication of Cultured Cells
HeLa and PC12 cells were cultivated in their respective media to form subconfluent monolayers, prior to washing with PBS and intoxication by the direct addition of toxins into the medium at indicated concentrations. Eventually, upon onset of intoxication characteristics, images were taken using an Axiovert 25 inverted microscope (Carl Zeiss, Jena, Germany).
Actin Staining and Live Cell Imaging
Cells were washed with warmed PBS, fixed with 4% formaldehyde in PBS, washed again and permeabilized with 0.15% Triton X-100 in PBS. Permeabilized cells were then incubated with 1% BSA and 0.05% Tween-20 in PBS for 30 min. Actin was stained for 1 h at room temperature with TRITC-conjugated phalloidin (Invitrogen, diluted 1:200 from a 50 μg/ml stock solution). Subsequently, cells were washed again with PBS and finally embedded in Mowiol supplemented with DABCO (Sigma). For life cell imaging, cells were seeded on glass bottom dishes (Mattek, Ashland, MA). Cells were kept at 37 °C in a stage incubator that was mounted on an Axiovert 200 m inverted microscope (Carl Zeiss). Differential interference contrast (DIC) images were acquired with a digital camera (Coolsnap HQ, Roper Scientific, Tucson) driven by MetaMorph® imaging software (Universal Imaging, Downingtown).
Preparation of Whole Cell Lysates and Immunoblotting
Whole-cell lysates were prepared by washing cells twice in PBS and lysis on ice with buffer 50 mm Tris/HCl (pH 7.5), 150 mm NaCl, 10% glycerol, 2 mm EGTA, 2 mm MgCl2, 1% Nonidet P-40, 0.5% Na-desoxycholate, Complete protease inhibitor mixture (Roche, Basel, Switzerland), and, eventually, Na3VO4 (ERK-phosphorylation), by using a Potter Homogenizer. The cell debris was removed by centrifugation and the protein concentration was measured with BCA assay kit (Uptima, Montluçon, France). Lysate proteins were separated by SDS-PAGE and transferred onto a PVDF membrane for antigen detection using specific antibodies. Rac1 and Ras signals were either detected with the glucosylation-sensitive antibodies Mab 102 (BD Biosciences, Heidelberg, Germany) and Mab 27H5 (Cell Signaling, Danvers), respectively, or with the glucosylation-insensitive antibodies Mab 23A8 (Millipore, Eschborn, Germany) and Mab Y13–259 (Calbiochem, Darmstadt, Germany), respectively. Phospho-ERK1/2 (Thr202/Tyr204) was obtained from Cell Signaling Technology (Danvers). GAPDH and PARP1 were detected with the antibodies Mab 374 (Millipore) and #9542 (Cell Signaling Technology), respectively. The secondary, HRP-linked antibodies used in this study were anti-mouse IgG (Vector Laboratories, Burlingame), anti-rabbit IgG (Rockland, Gilbertsville), and anti-rat IgG (Santa Cruz Biotechnology, Santa Cruz, CA).
Ras Pull-down Assay
Glutathione-Sepharose™ 4B beads coated with a GST-Raf-1-Ras-binding domain-fusion protein (expressed in E. coli Rosetta with the pGEX expression system) were incubated with HeLa whole-cell lysate (1 mg total protein) for 30 min at 4 °C. The beads were then washed four times with buffer containing 50 mm Tris/HCl (pH 7.4), 100 mm NaCl, 2 mm MgCl2, 10% glycerol, and 1% Nonidet P-40, and then boiled in 10 μl of Laemmli buffer for 5 min at 95 °C. Bound proteins were separated by SDS-PAGE and Ras was detected by immunoblotting.
In Vitro Cleavage Assay
In vitro cleavage of TpeL1–1779, TpeL1–805, or TpeL1–805(C696A) was performed with 2 μg of protein that was incubated in buffer containing 100 mm Tris-HCl (pH 7.4) for 1 h at 37 °C. Prior to incubation, cleavage was eventually initiated by the addition of the indicated concentrations of InsP6. The reaction was stopped by the addition of Laemmli buffer to the samples and boiling for 5 min at 95 °C. Cleavage products were visualized as Coomassie-stained protein bands after SDS-PAGE.
Isothermal Titration Calorimetry
The KD value of InsP6 binding to the CPD of TpeL (TpeL543–805) was determined by isothermal titration calorimetry (ITC 200, GE Healthcare). The titration was carried out twice at 25 °C and at neutral pH conditions. The protein was diluted to a final concentration of 70 μm in a buffer containing 20 mm Tris-HCl (pH 7.4), 150 mm NaCl, and 10% glycerol. During the measurement InsP6 was injected at 10-fold higher concentrations. As a control, InsP6 was injected alone into the cell filled with buffer only. Data were evaluated with the manufacturer's software.
Immunoprecipation of Ras
20 mg of total protein of HeLa whole-cell lysates was incubated with with 20 μg of Ras-specific antibody (anti-v-H-Ras Mab Y13–259) and 80 μl Protein G-Sepharose® (Sigma-Aldrich) at 4 °C overnight. Beads were then washed four times with buffer containing 50 mm Tris/HCl (pH 7.5), 150 mm NaCl, 10% glycerol, 2 mm EGTA, 2 mm MgCl2, 1% Nonidet P-40, 0.5% Na-desoxycholate, and Complete protease inhibitor mixture (Roche) and then boiled in Laemmli buffer for 5 min at 95 °C. Bound proteins were separated by SDS-PAGE and immunoprecipitated Ras was detected by immunoblotting.
RESULTS
Donor and Acceptor Substrate Specificity of TpeL Toxin in Vitro
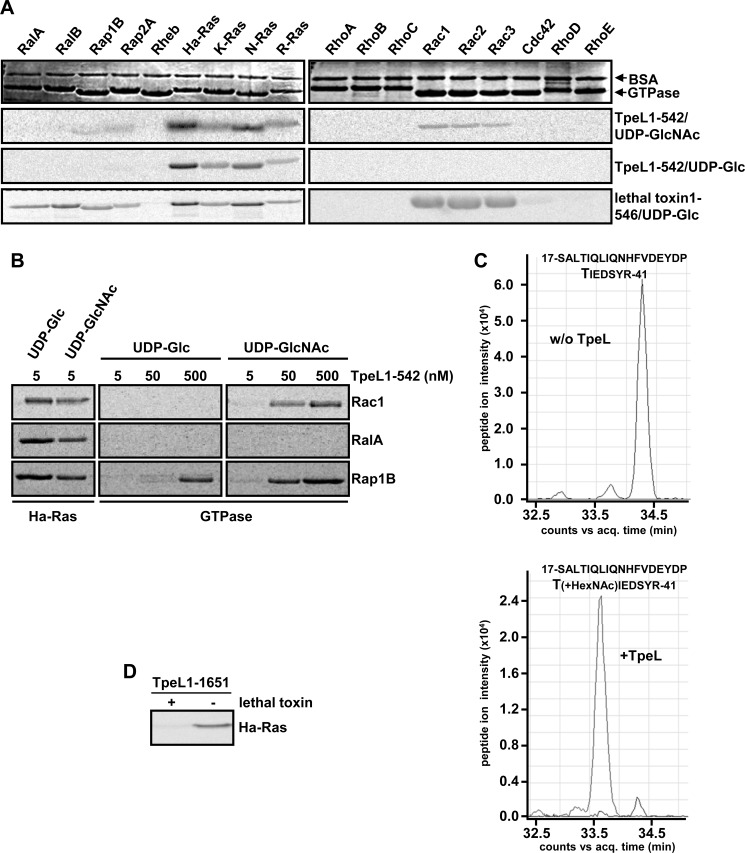
To study the donor and acceptor substrate specificity of TpeL toxin, we used a toxin fragment, comprising only the glucosyltransferase domain of TpeL (TpeL1–542). In a series of glucosylation reactions, including either the sugar donor UDP-Glc or UDP-GlcNAc, several purified small GTPases were tested for modification by the glucosyltransferase domain of TpeL. As a result, TpeL1–542 was capable of incorporating UDP-GlcNAc into all tested Ras (Ha-, K-, R-Ras) and Rac (Rac1, Rac2, Rac3) isoforms, and, to a very low extent, into Rap1B and Rap2A (Fig. 1A). In the case where UDP-Glc was employed as a sugar donor, we observed only modification of the Ras isoforms by TpeL1–542 (Fig. 1A). In vitro Ha-Ras modification by TpeL1–542 was strictly dependent on the presence of a DXD motif, a short conserved motif found in many glucosyltransferases and among clostridial glucosylating toxins (supplemental Fig. S1). Therefore, in line with the observation of Nagahama and colleagues (27), TpeL is a unique member of the family of clostridial glucosylating toxins, in respect of utilizing two different UDP-sugars for modification of small GTPases. However, concerning the substrate specificity, TpeL shares an obvious similarity with Clostridium sordellii lethal toxin that also modifies members of the Ras and Rac family (Fig. 1A).
FIGURE 1.
Ras-GTPases are the preferred substrates of TpeL in vitro. A, protein substrate specificity of TpeL. Glucosylation of the indicated GST-GTPases (3 μg) with recombinant TpeL1–542 or lethal toxin1–546 (each 5 nm) in the presence of radiolabeled UDP-GlcNAc or UDP-Glc (each 10 μm) for 10 min at 37 °C. The samples were subjected to SDS-PAGE and modified GTPases were visualized by autoradiography. B, comparative analysis of the in vitro glucosylation of Ha-Ras with Rac1, RalA and Rap1B by TpeL1–542. GST-GTPases (3 μg) were incubated with indicated concentrations of TpeL1–542 in the presence of radiolabeled UDP-GlcNAc or UDP-Glc (each 10 μm) for 10 min at 37 °C. C. Acceptor amino acid in Ha-Ras. Ha-Ras (5 μg) was incubated with or without TpeL1–1651 (250 nm) in the presence of UDP-GlcNAc (100 μm) for 1 h at 37 °C. Following SDS-PAGE, the respective protein bands were excised and analyzed by tandem mass spectrometry. The extracted ion chromatograms of the unmodified peptide (acq. time: 34.3 min, m/z = 989.8172 (3+)) and the GlcNAc-modified peptide (acq. time: 33.7 min, m/z = 1057.5103 (3+)) are shown. D, lethal toxin and TpeL modify threonine 35 in Ha-Ras. Ha-Ras was eventually preincubated with lethal toxin (100 nm) (as indicated) and UDP-Glc (10 μm) for 30 min at 37 °C. Subsequently, in vitro glucosylation with TpeL1–1651 (100 nm) and radiolabeled UDP-GlcNAc (10 μm) was performed for 45 min at 37 °C. The samples were subjected to SDS-PAGE and modified Ha-Ras was visualized by autoradiography.
Ras Family Proteins Are the Preferred Substrate of TpeL
To determine the preferred substrate of TpeL, we incubated Ha-Ras with 5 nm TpeL1–542 and Rac1, RalA, and Rap1B with 5, 50, and 500 nm TpeL1–542, respectively, and compared the incorporation of radiolabeled UDP-sugars (UDP-Glc and UDP-GlcNAc) in the respective GTPases by autoradiography after SDS-PAGE (Fig. 1B). TpeL1–542 modified Ha-Ras in the presence of UDP-Glc or UDP-GlcNAc already at a concentration of 5 nm. At least a 10-fold higher concentration of TpeL1–542 (50 nm) was required for modification of Rac1 and Rap1B with UDP-GlcNAc. A 100-fold concentration of TpeL1–542 (500 nm) was required for the modification of Rap1B with UDP-Glc. RalA that was previously described by Nagahama and co-workers as a substrate of the TpeL toxin (27), was not modified by TpeL1–542 neither with UDP-Glc nor with UDP-GlcNAc, even at high concentrations of the glucosyltransferase domain (500 nm).
TpeL Glucosylates Ha-Ras at Threonine 35
We next aimed to validate glucosylation of Ha-Ras with a full-length variant of TpeL and to determine the acceptor amino acid of Ha-Ras that is modified by TpeL. To this end, we produced recombinant TpeL1–1651 (a full-length variant from C. perfringens type C strain MC18) described by Amimoto et al. (8) and performed an in vitro glucosylation reaction with Ha-Ras and UDP-GlcNAc as sugar donor. Following tryptic digestion of the Ha-Ras protein for mass spectrometric analysis, a mass shift equivalent to an N-acetyl hexose (HexNAc) moiety (203 Da) was found in a single peptide that includes threonine-35 of Ha-Ras (Fig. 1C), which is also the site of glucosylation by the C. sordellii lethal toxin. This finding could be confirmed by successively incubating Ha-Ras with lethal toxin and TpeL1–1651 in glucosylation reactions. In vitro glucosylation of Ha-Ras with lethal toxin in the presence of non-labeled UDP-Glc impaired the incorporation of radiolabeled N-acetyl glucosamine into the Ha-Ras protein, when TpeL1–1651 was added in a second reaction (Fig. 1D).
UDP-GlcNAc Is the Preferred Donor Substrate of the TpeL
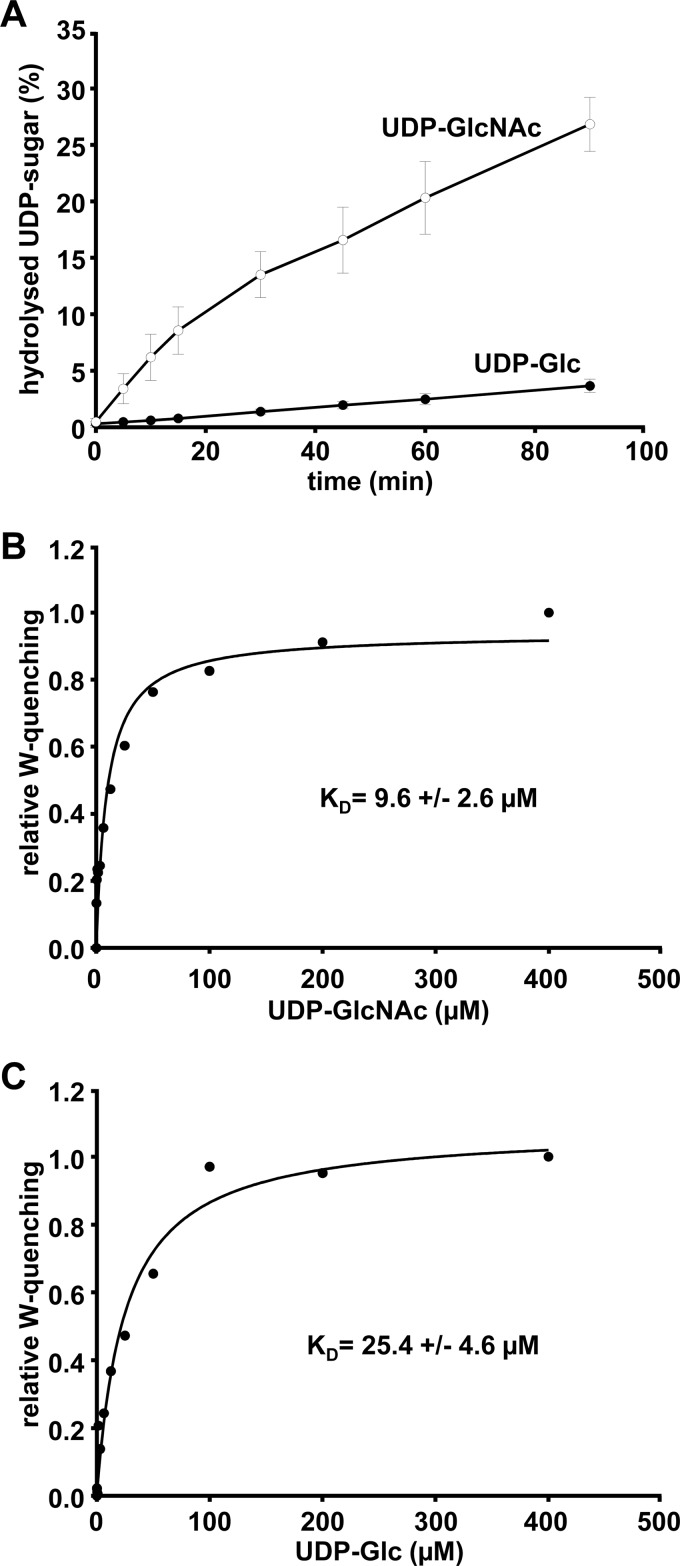
We next aimed to determine which activated sugar, UDP-Glc or UDP-GlcNAc, is preferentially used by the glucosyltransferase domain of TpeL. In the absence of a protein substrate, glucosyltransferases bind and hydrolyze their donor substrate into UDP and the sugar moiety. The rate of UDP-Glc and UDP-GlcNAc hydrolysis was determined after addition of TpeL1–542 and incubation for increasing time intervals (Fig. 2A), resulting in the kcat values 17.4 ± 1.4 h−1 (S.E., n = 3) and 397.3 ± 13.9 h−1 (S.E., n = 3) for UDP-Glc and UDP-GlcNAc, respectively. Accordingly, TpeL1–542 hydrolyzes UDP-GlcNAc ∼20 times more efficient than UDP-Glc. To compare the binding affinity of UDP-Glc and UDP-GlcNAc to TpeL1–542, we incubated TpeL1–542 with increasing concentrations of the respective UDP-sugar and measured the tryptophan fluorescence quenching efficiency (Fig. 2, B and C). The calculated KD values, 25.4 ± 4.6 μm (S.E., n = 3) and 9.6 ± 2.6 μm (S.E., n = 3) for UDP-Glc and UDP-GlcNAc, respectively, indicate that TpeL1–542 exhibits a higher binding affinity for UDP-GlcNAc. From this data, we argue that both UDP-sugars, UDP-Glc and UDP-GlcNAc, are donor substrates for TpeL, with a preference of the toxin for utilizing UDP-GlcNAc.
FIGURE 2.
UDP-GlcNAc is the preferred donor substrate of TpeL in vitro. A, donor substrate specificity of TpeL. UDP-sugar hydrolase assay of TpeL1–542 with radiolabeled UDP-Glc and UDP-GlcNAc, respectively. At indicated time points samples were subjected to thin layer chromatography. The hydrolyzed sugars were visualized by autoradiography and the signal intensities quantified with ImageQuant. Calculation of kcat values was done by using time points 5, 10, and 15 min. B and C, determination of the binding affinity of UDP-GlcNAc (B) and UDP-Glc (C) to the glucosyltransferase of TpeL (TpeL1–542) by ligand-induced quenching of the intrinsic tryptophan fluorescence. The maximal quenching efficiency of the respective UDP-sugars was set to 1.0 and the increase of the relative tryptophan quenching (W-quenching) is represented as a function of the UDP-sugar concentration.
Amino Acids of TpeL Involved in UDP-sugar Binding
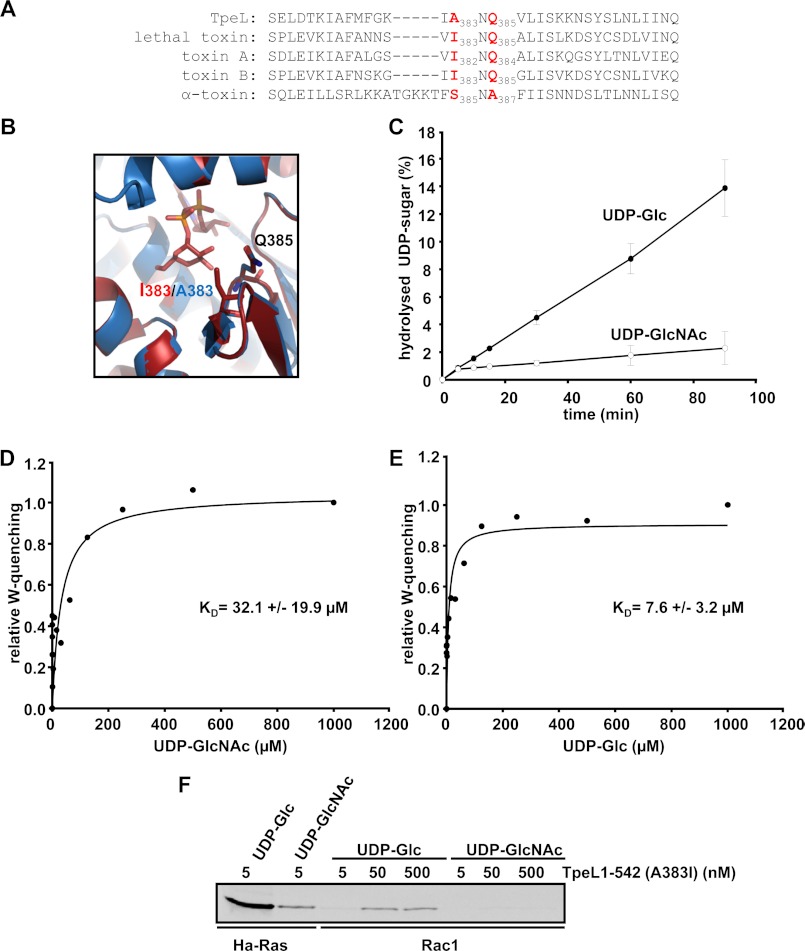
TpeL is a unique member of the family of clostridial glucosylating toxins, since it utilizes both activated sugars, UDP-Glc or UDP-GlcNAc, for glucosylation of target GTPases. We therefore aimed to clarify the structural prerequisites for the dual-specific donor sugar binding and aligned the region that determines donor substrate specificity in several clostridial glucosylating toxins (25). For instance, toxins that utilize only UDP-Glc for modification of their substrates (e.g. C. difficile toxin A and B and C. sordellii lethal toxin) harbor an INQ-motif (isoleucine-asparagine-glutamine), whereas C. novyi α-toxin that uses only UDP-GlcNAc possesses the SNA-motif (serine-asparagine-alanine). TpeL differs in respect of its donor substrate binding motif as it possesses a non-uniform ANQ-motif (alanine-asparagine-glutamate) (Fig. 3A). We performed a protein structure homology modeling of the glucosyltransferase domain of TpeL by using the respective domain of lethal toxin as basis. Fig. 3B represents a magnified view into the donor substrate binding pocket of the glucosyltransferase domain of TpeL (in blue) and lethal toxin (in red), respectively, with bound UDP-Glc. Since UDP-Glc is the only sugar donor of lethal toxin, one can assume that the isoleucine-383 residue of lethal toxin disturbs binding of UDP-GlcNAc due to its additional acetyl amino group. The presence of an alanine residue at this position in the donor substrate pocket of TpeL may permit binding of both UDP-sugars, UDP-Glc and UDP-GlcNAc. To test this hypothesis we generated a TpeL glucosyltransferase mutant that possesses the donor substrate binding motif of lethal toxin (TpeL1–542 (A383I)). Interestingly, the TpeL mutant A383I exhibited increased hydrolysis of UDP-Glc as compared with UDP-GlcNAc (kcat for UDP-Glc: 114.3 ± 22.2 h−1 versus kcat for UDP-GlcNAc: 20.0 ± 4.0 h−1) (Fig. 3C). Moreover, tryptophan fluorescence quenching measurements uncovered preferred binding of UDP-Glc rather than UDP-GlcNAc to the TpeL mutant A383I (KD for UDP-Glc: 7.6 ± 3.2 μm (S.E., n = 3) versus KD for UDP-GlcNAc: 32.1 ± 19.9 μm (S.E., n = 3)) (Fig. 3, D and E). Finally, we investigated the glucosylation of Ha-Ras and Rac1 with wild type TpeL1–542 in comparison to the A383I mutant by using either UDP-Glc or UDP-GlcNAc as donor substrate. Importantly, Ha-Ras was modified by the TpeL1–542 (A383I) mutant to a higher extent when UDP-Glc was used as donor substrate (Fig. 3F). Accordingly, in an in vitro glucosylation reaction, Rac1 was modified by mutant TpeL1–542 only when UDP-Glc was used as a sugar donor, in contrast to wild type TpeL1–542 that modifies Rac1 only in the presence of UDP-GlcNAc (Figs. 3F and 1A). We, therefore, conclude that the dual-specific sugar donor binding property of TpeL originates from the presence of a different sugar binding motif.
FIGURE 3.
Amino acids involved in UDP-sugar binding of TpeL. A, alignment of primary sequences of TpeL and other CGTs representing the putative amino acid sequence segment responsible for the donor substrate specificity. The involved amino acids were numbered and highlighted in red. B, depiction of the amino acid motif responsible for donor substrate specificity of TpeL and lethal toxin. Structural alignment of the glucosyltransferase domain of lethal toxin (in red) and the glucosyltransferase domain of TpeL (in blue). The UDP-sugar binding site of lethal toxin and TpeL is shown. The structure model of the glucosyltransferase domain of TpeL was created by SWISS-MODEL (alignment mode) using as matrix the crystal structure of the glucosyltransferase domain of lethal toxin in complex with Mn2+-ion and UDP-Glc. The glucosyltransferase domains are shown with ribbons. Mn2+-ion, UDP-Glc, and isoleucine 383, alanine 383, and glutamine 385 are shown with sticks. C, donor substrate specificity of TpeL1–542 (A383I). UDP-sugar hydrolase assay of TpeL1–542 (A383I) with radiolabeled UDP-Glc and UDP-GlcNAc, respectively. At indicated time points samples were subjected to thin layer chromatography. The hydrolyzed sugars were visualized by autoradiography and the signal intensities quantified with ImageQuant. Calculation of kcat values was done by using time points 5, 10, and 15 min. D and E, determination of the binding affinity of UDP-GlcNAc (B) and UDP-Glc (C) to TpeL1–542 (A383I) by ligand-induced quenching of the intrinsic tryptophan fluorescence. The maximal quenching efficiency of the respective UDP-sugars was set to 1.0 and the increase of the relative tryptophan quenching (W-quenching) is represented as a function of the UDP-sugar concentration. F, substrate specificity of TpeL1–542 (A383I). Glucosylation of the indicated GST-GTPases (3 μg) with TpeL1–542 (A383I) (for Ha-Ras 5 nm, for Rac1 5, 50, 500 nm, respectively) in the presence of radiolabeled UDP-GlcNAc or UDP-Glc (each 10 μm) for 10 min at 37 °C. The samples were subjected to SDS-PAGE and modified GTPases were visualized by autoradiography.
Autocatalytic Processing of TpeL
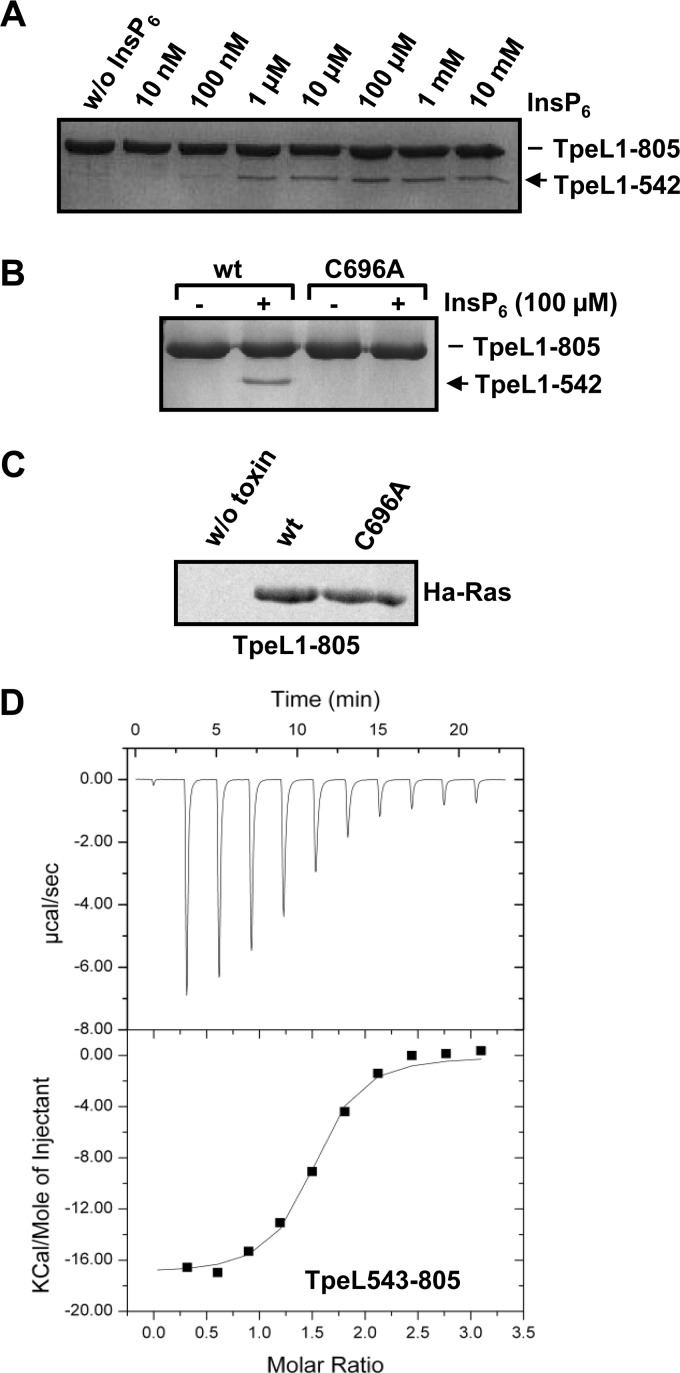
The autocatalytic processing of clostridial glucosylating toxins depends on an intrinsic cysteine protease domain (CPD) that directly follows the N-terminal glucosyltransferase domain. Binding of the small cytosolic compound inositol hexakisphosphate (InsP6) to the CPD, activates the protease and triggers autocatalytic cleavage (29). A CPD-like domain can be found also next to the glucosyltransferase domain of TpeL. The respective amino acid sequence of this domain exhibits 51% identity and 70% similarity to the cysteine protease domains of C. difficile toxin A and B. We, therefore, aimed to study, whether this domain is responsible for autocatalytic processing of the TpeL toxin. For this purpose, we synthesized a TpeL variant comprising only the glucosyltransferase domain and the CPD-like domain (TpeL1–805) and performed an in vitro cleavage assay with increasing concentrations of InsP6 at 37 °C for 1 h. Interestingly, significant processing of TpeL1–805 was observed with physiological concentrations of InsP6 (≤ 100 μm) (Fig. 4A). Moreover, InsP6-induced processing of TpeL1–805 was impaired in a TpeL1–805 (C696A) mutant, where the conserved catalytic cysteine residue that is crucial for the function of the cysteine protease domains of all clostridial glucosylating toxins was exchanged by alanine (Fig. 4B). The C696A mutation does not alter the general folding of the TpeL fragment, because glucosyltransferase activity is not changed, when compared with the wild type TpeL fragment (Fig. 4C). These combined data argue that TpeL shares a functional, InsP6-activatable cysteine protease domain with other members of the clostridial glucosylating toxin family. Finally, we attempted to determine the binding affinity of InsP6 to a TpeL fragment comprising only the cysteine protease domain (TpeL543–805) by isothermal titration calorimetry (Fig. 4D). Based on the obtained KD values (2.1 and 2.7 μm), InsP6 bound with a similar affinity to the CDP of TpeL when compared with the CPDs of other clostridial glucosylating toxins (30).
FIGURE 4.
Autoprocessing of TpeL by the intrinsic cysteine protease domain. A, InsP6-induced autoprocessing of TpeL1–805. TpeL1–805 (2 μg) was incubated with increasing concentrations of InsP6 (as indicated) for 1 h at 37 °C. Cleavage products were then separated by SDS-PAGE and stained with Coomassie. Arrow indicates the cleavage product TpeL1–542 (glucosyltransferase domain). B, identification of the catalytic cysteine residue of the CPD of TpeL. TpeL1–805 and TpeL1–805 (C696A) (each 2 μg), were incubated in the presence of InsP6 (100 μm) for 1 h at 37 °C. Cleavage products were then separated by SDS-PAGE and stained with Coomassie. Arrow indicates the cleavage product TpeL1–542 (glucosyltransferase domain). C, in vitro glucosylation of Ha-Ras with TpeL1–805 and TpeL1–805 (C696A) (each 3 μg) in the presence of radiolabeled UDP-GlcNAc for 30 min at 37 °C. The samples were subjected to SDS-PAGE and modified GTPases were visualized by autoradiography. D, binding affinity of InsP6 to the CPD of TpeL measured by isothermal calorimetry. Isothermal calorimetry was carried out twice with TpeL543–805 (70 μm) at pH 7.4 in the presence InsP6 (700 μm).
Intoxication of Cells by TpeL
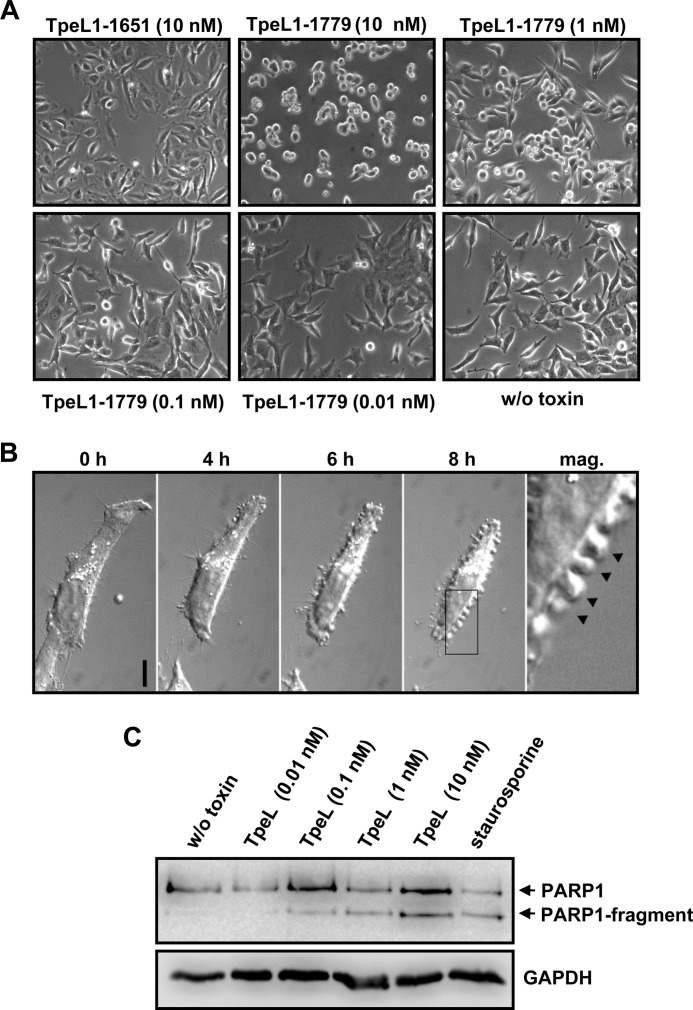
To analyze the cytotoxic effects of the TpeL toxin in more detail, we aimed to recombinantly produce a full-length version of the toxin in the bacterial expression host B. megaterium. Initially, by using genomic DNA from the TpeL-producing C. perfringens type C strain CN3685 as template, we cloned a TpeL full-length version into the B. megaterium expression vector pHIS1522 that was based on the DNA sequence of TpeL from C. perfringens type C strain MC18, reported by Amimoto et al. (8). According to this report, TpeL represents a protein of 1651 amino acids in total. However, overnight incubation of the purified TpeL1–1651 protein (MC18 version) with cultured HeLa cells did not induce any cytotoxic effects in these cells (Fig. 5A), although the toxin was functional in terms of glucosylating Ha-Ras in vitro (Fig. 1D). By analyzing the publicly available genome sequence of C. perfringens type C strain JGS1495, we noticed a TpeL gene that is 384 base pairs longer, when compared with the TpeL gene that was reported for the MC18 strain. The extended sequence encodes additional 128 amino acids at the C terminus of the TpeL JGS1495 version, resulting in a protein with 1779 amino acids in total. Consequently, again by using genomic DNA from C. perfringens type C strain CN3685 as template, we were able to clone and to express the TpeL JGS1495 version (TpeL1–1779) in B. megaterium and observed morphological changes of HeLa cells after overnight incubation, when the toxin was added at nanomolar concentrations (Fig. 5A). Investigation of the sensitivity of several other cell lines (e.g. Swiss-3T3, H1-HeLa, KBM7-derived Hap1 cells, Platinum-E, NG cells (mouse neuroblastoma X rat glioma hybrid cells), and MCF 10A) toward the two TpeL versions confirmed cytotoxic potential only for the TpeL JGS1495 variant (supplemental Fig. S2). We argue from this result that the TpeL full-length variant from strain MC18 may lack C-terminal elements that participate in receptor-binding (supplemental Fig. S3). Therefore, we consider that TpeL from strain JGS1495 represents the complete ORF for this toxin. The following experiments in the present study were thus done with the recombinant JGS1495 version of full-length TpeL.
FIGURE 5.
Intoxication characteristics of full-length TpeL in living cells. A, HeLa cells were intoxicated with or without TpeL1–1651 (10 nm) or with indicated concentrations of TpeL1–1779 overnight at 37 °C, prior to microscopy to analyze the cell morphology. B, TpeL-induced apoptotic blebbing in HeLa cells. HeLa cells were intoxicated with TpeL (10 nm) at 37 °C and observed over time by live cell imaging using an inverted microscope. Representative images were taken at indicated time points. The black box (image after 8 h) represents a magnified image area where apoptotic blebbs were observed (marked with arrows). Scale bar represents 10 μm. C, PARP1 cleavage upon treatment with TpeL. HeLa cells were treated with staurosporine (1 μm) or were left untreated, or were intoxicated with increasing concentrations of TpeL (as indicated) overnight at 37 °C. The next day, cells were lysed, followed by SDS-PAGE of whole cell lysate proteins and PARP1 immunoblotting for the detection of PARP1 cleavage products. Equal loading of samples was monitored by the detection of GAPDH.
To test whether TpeL is a short-trip toxin that enters the cytosol from early endosomes, we preincubated HeLa cells with bafilomycin A1 to prevent endosomal acidification. TpeL-induced morphological changes in HeLa cells were strongly reduced in bafilomycin A1-treated cells (supplemental Fig. S4). Therefore, TpeL shares the same entry route as the other members of the CGT family that involves a low pH-dependent membrane insertion and translocation step for delivery of the enzyme portion into the host cell cytosol.
To analyze the cytotoxic effects of TpeL in more detail, we intoxicated HeLa cells with 10 nm of the toxin and performed live cell imaging. Membrane blebbing, suggesting the execution phase of apoptosis, was evident after 8 h of incubation for cells treated with toxin (Fig. 5B). TpeL-induced apoptosis in HeLa cells could be confirmed by analyzing the cleavage of the caspase-substrate PARP1 in whole cell lysates. The exposure of HeLa cells to increasing concentrations of TpeL correlated with an increase in PARP1 cleavage (Fig. 5C). Moreover, TpeL-induced apoptosis in HeLa cells was inhibited by the pan-caspase inhibitor Z-VAD-fmk (supplemental Fig. S5).
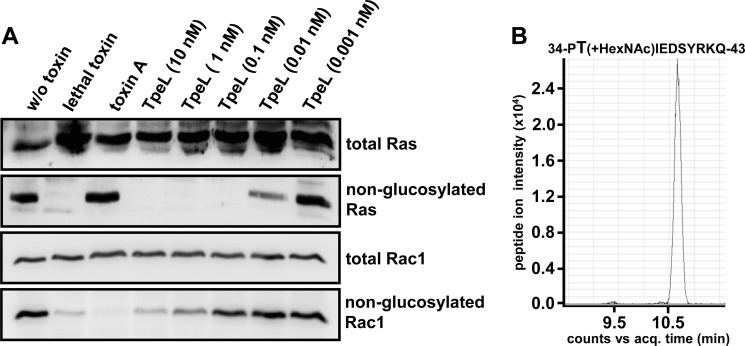
In Vivo Substrate Specificity of TpeL
Our next aim was to validate that TpeL modifies Rac and Ras proteins also in vivo. For this purpose, we incubated HeLa cells with increasing concentrations of TpeL for 24 h at 37 °C. Subsequently, we analyzed the glucosylation status of Rac1 and Ras in cytosolic extracts with antibodies recognizing either total Rac1 and Ras levels or only the non-modified versions of the GTPases. C. sordellii lethal toxin (glucosylates Rac1 and Ras) and C. difficile toxin A (modifies Rac1 but not Ras) were used as controls in this experiment. Importantly, the entire amount of Ras protein was already modified with 0.1 nm TpeL, whereas almost complete modification of Rac1 required incubation with a hundred times higher concentration of TpeL (10 nm) (Fig. 6A). Therefore, at low concentrations, TpeL selectively glucosylates Ras rather than Rac proteins.
FIGURE 6.
Substrate specificity of TpeL in living cells. A, HeLa cells were intoxicated with lethal toxin, toxin A (each 1 nm) or with increasing concentrations of TpeL (as indicated) overnight at 37 °C. The next day, cells were lysed, followed by SDS-PAGE of whole cell lysate proteins and immunoblotting for the detection of Ras and Rac with glucosylation-sensitive antibodies (input control; total Ras or Rac, respectively) or with glucosylation-insensitive antibodies (non-glucosylated Ras or Rac, respectively). B, TpeL modifies Ras in living cells by GlcNAcylation. HeLa cells were intoxicated with TpeL (10 nm) overnight at 37 °C, followed by lysis of cells and immunoprecipitation of Ras with a Ras-specific antibody. Following SDS-PAGE, the protein band corresponding to Ras was excised and analyzed by tandem mass spectrometry. The extracted ion chromatogram of the GlcNAc-modified peptide (acq. time: 10.6 min, m/z = 480.5719 (3+)) is shown.
In addition, we employed mass spectrometry to study the sugar donor specificity of TpeL in vivo. HeLa cells were treated with TpeL (10 nm) overnight at 37° C, then cells were lysed and Ras was immunoprecipitated with an anti-Ras-antibody. Precipitated proteins were analyzed by SDS-PAGE. Protein bands migrating with the apparent mass of Ras were cut out, digested with thermolysin, and the resulting peptides subsequently supplied to LC-MS/MS. MS data revealed attachment of an N-acetyl hexose (HexNAc) residue (203 Da) to threonine-35 of Ras (Fig. 6B). No attachment of a hexose was detected, supporting the view that TpeL modifies Ras in living cells by mono-O-GlcNAcylation.
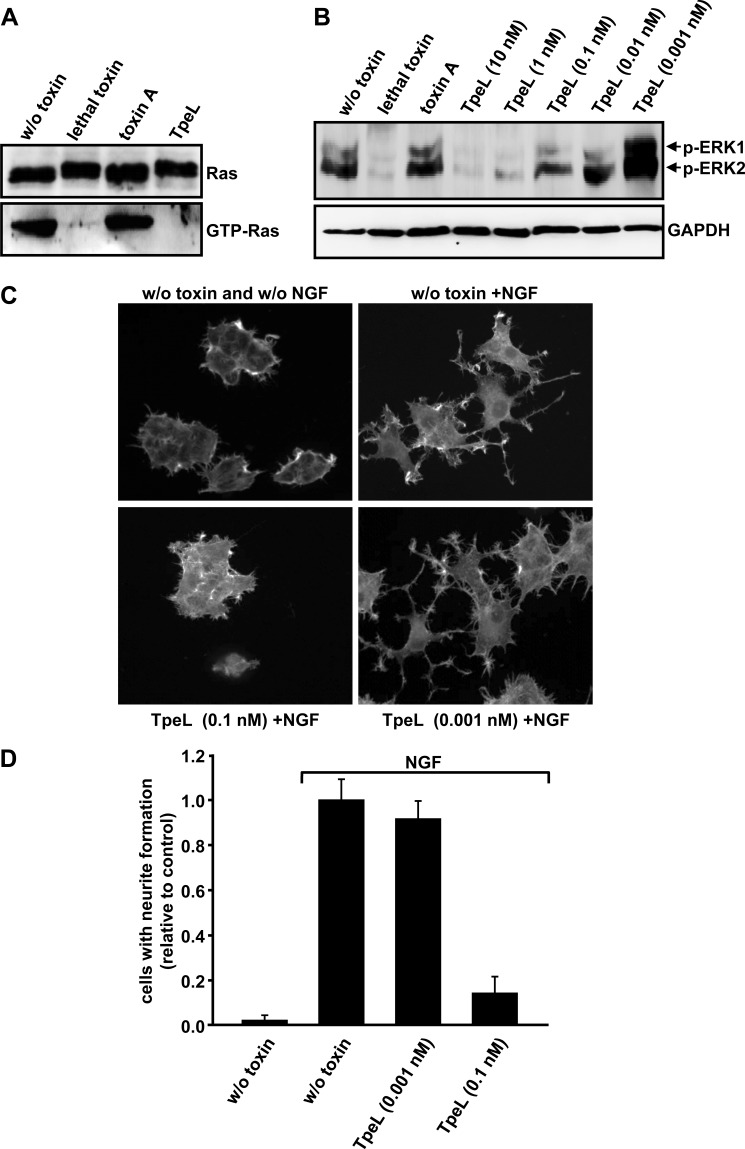
Effects of TpeL on Ras Signaling
So far, our data suggested that TpeL specifically modifies Ras by GlcNAcylation. We next analyzed whether TpeL-dependent glucosylation of Ras affects the interaction with downstream effectors of the Raf/MEK/ERK pathway. To this end, a GST-tagged Ras-binding domain (RBD) of Raf-1 that binds only to activated, GTP-loaded Ras protein, was immobilized on glutathione-Sepharose™ beads and cytosolic extracts of TpeL-intoxicated or non-intoxicated HeLa cells were applied. Whereas Ras protein from non-intoxicated cells co-precipitated with the RBD of Raf-1, this was not the case for the TpeL-treated cells (Fig. 7A). The missing interaction of Ras to its downstream effector Raf would inhibit the Raf/MEK/ERK signaling pathway after stimulation of cells with the growth factor EGF (31). In this context, we intoxicated HeLa cells with increasing concentrations of TpeL or left the cells untreated and analyzed the phosphorylation state of ERK1/2 in cellular extracts with specific antibodies after activation of the Raf/MEK/ERK signaling pathway with EGF. As expected, the decrease of phosphorylated ERK1/2 correlated with increasing concentrations of TpeL that were used to intoxicate HeLa cells (Fig. 7B).
FIGURE 7.
Effects of TpeL intoxication on Ras signaling. A, functional consequence of the glucosylation of Ras by TpeL. HeLa cells were intoxicated with lethal toxin, toxin A (each 1 nm) or TpeL (10 nm) overnight at 37 °C. Following cell lysis, equal amounts of whole cell lysate proteins were separated by SDS-PAGE and Ras was detected by immunoblotting (input control; upper panel). Glutathione-Sepharose beads coated with GST-Raf-1-RBD were used for pull down of endogenous Ras from whole-cell lysate proteins of intoxicated cells. Precipitated Ras-GTP was then detected by immunoblotting (lower panel). B, influence of TpeL on ERK1/2 phosphorylation. Starved HeLa cells were intoxicated with lethal toxin, toxin A (each 1 nm) or with increasing concentrations of TpeL (as indicated) overnight at 37 °C. The next day, cells were stimulated by the addition of EGF (10 ng/ml) for 3 min at 37 °C. Finally, cells were lysed, followed by SDS-PAGE of whole-cell lysate proteins and immunoblotting for the detection of phosphorylated ERK1/2. Equal loading of samples was monitored by the detection of GAPDH. C, neurite formation in PC-12 cells was stimulated by adding 100 ng/ml NGF, prior to intoxication of cells with the indicated concentrations of TpeL for 2 days at 37 °C. In parallel samples, PC12 cells were either not treated with toxin and NGF (w/o toxin and w/o NGF) or were treated only with NGF (w/o toxin +NGF). Then, actin cytoskeleton of PC12 cells was stained with TRITC-phalloidin to visualize cell morphology and neurite formation of PC12 cells by fluorescence microscopy. D, quantification of the neurite formation from PC12 cells shown in C. For each represented bar (S.E., n = 3), a minimum of 100 cells was analyzed. Cells with neurites with at least the length of the cell diameter were regarded as positive. The number of non-intoxicated, NGF-stimulated cells producing neurites was set to 1.0 (control). Shown is the relative number of cells with neurite formation in comparison to the control.
Ras-dependent signaling pathways are involved in the differentiation of rat pheochromocytoma cells (PC12 cells) (32). Accordingly, addition of NGF that binds to Ras signaling-linked receptor tyrosine kinase TrkA, induces neurite formation in PC12 cells (Fig. 7C). As expected, NGF-induced stimulation of neurite formation was prevented when PC12 cells were cotreated with 0.1 nm TpeL. This effect of TpeL was concentration-dependent, since a 100-fold lower concentration (0.001 nm) was not capable of disturbing the Ras-related signaling pathway that controls neurite formation (Fig. 7, C and D). Taken together, the results obtained with HeLa and PC12 cells confirmed the finding that TpeL acts predominantly as a Ras-specific glucosyltransferase that renders this GTPase into an inactive state.
DISCUSSION
TpeL is the most recently discovered member of the family of CGTs (8, 27). Accordingly, important amino acids in the glucosyltransferase domain (e.g. DXD motif, donor substrate binding motif) and the cysteine protease domain (catalytic DHC triad) are conserved among the different TpeL variants described in this study and C. difficile toxin B, a typical member of CGTs (supplemental Fig. S6). Here we characterized the toxin in respect to its substrate specificity, autocatalytic activation and cytotoxic effects in intact cells. Depending on the toxin type, the various CGTs specifically modify Rho/Ras proteins by glucosylation or GlcNAcylation. Mainly based on studies with toxin fragments, a recent report suggests that TpeL possesses both glucosylation and GlcNAcylation activity to modify Rac and Ras (27). Using the recombinant glucosyltransferase domain of TpeL (TpeL1–542) we observed the modification of Ha-, K-, N-, and R-Ras in the presence of UDP-Glc and UDP-GlcNAc with a slight preference for Ha-Ras and N-Ras. By using the indirect method of differential glucosylation, Nagahama et al. suggested that Ras is modified in threonine 35 (27). We confirmed this suggestion by direct mass spectrometry analysis. Moreover, we observed that Rap1B and Rap2A as well as the Rho family GTPases Rac1, Rac2, and Rac3 were modified in the presence of UDP-GlcNAc but not with UDP-Glc. However, RalA that was described as a substrate of TpeL by the recent study of Nagahama et al. (27), was not modified by the TpeL glucosyltransferase domain in our study.
The activity of TpeL1–542 to hydrolyze UDP-GlcNAc was almost 20-fold higher than the hydrolysis of UDP-Glc and the KD value of the toxin fragment for binding of UDP-GlcNAc was ∼3-fold lower than for UDP-Glc. Therefore, TpeL is an enzyme which prefers UDP-GlcNAc. The structural determinants for donor sugar specificity of CGTs have been identified as a short motif in the catalytic site, covering residues 383INQ385 of toxin A or lethal toxin (utilizing UDP-Glc) and 385SNA387 of α-toxin (utilizing UDP-GlcNAc) (25). This motif is 383ANQ385 in TpeL. In line with an essential role of this sequence, the mutation of this motif to 383INQ385 in TpeL resulted in the change of the UDP-sugar preference. TpeL1–542 (A383I) exhibited higher glucohydrolase and glucotransferase activities as well as higher binding affinity with UDP-Glc than with UDP-GlcNAc. This mutant modified Rac1 in vitro only in the presence of UDP-Glc but not with UDP-GlcNAc. The data indicate that the 383ANQ385 motif of TpeL is a major determinant of its donor substrate specificity.
Downstream of the glucosyltransferase domain of all CGTs is a cysteine protease domain located, which is involved in the autoproteolytical activation of the toxins (14). This domain is activated by InsP6 (33) and causes the release of the glucosyltransferase domain into the cytosol (34). Our study highlights that TpeL also undergoes InsP6-dependent autocatalytic processing and that a cysteine protease domain-like region (TpeL543–805), located next to the glucosyltransferase domain, is sufficient for cleavage. Accordingly, the KD value for the interaction of InsP6 with TpeL543–805, determined by isothermal titration calorimetry, was ∼2.5 μm. This sensitivity characteristics are very similar to those of C. difficile toxin B, C. sordellii lethal toxin, and C. novyi α-toxin (30).
Finally, we studied the biological activity of TpeL in intact cells. Amimoto et al. described a full-length variant of TpeL that is produced by C. perfringens strain MC18 (8). The same group reported on the poor expression of this TpeL variant in E. coli (27). We were able to express reasonable amounts of this TpeL full-length variant in B. megaterium. However, we noticed that although the recombinant protein was active in vitro, no signs of intoxication where observed when added to a variety of cultured cells. Genomic analysis of C. perfringens type C strain JGS1495 uncovered a TpeL full-length variant, which is C-terminally extended and ∼15 kDa larger than the TpeL from the MC18 strain. This recombinant toxin was much more stable, was well expressed in B. megaterium and caused cytotoxic effects in different cell lines and apoptosis in HeLa cells. We conclude that the TpeL full-length variant from strain MC18 may lack C-terminal elements that participate in receptor-binding. Therefore, we believe that TpeL from strain JGS1495 represents the complete ORF for this toxin.
Treatment of HeLa cells with the toxin caused cytotoxic effects and induced apoptosis of target cells. Toxin-induced apoptosis was characterized by blebbing and cleavage of PARP1. What is the reason for induction of apoptosis? By using glucosylation specific antibodies, we observed that Ras is the preferred substrate of TpeL in intact cells. Moreover, spectrometric analysis definitely show that Ras is modified by attachment of GlcNAc in intact cells. Interestingly, also C. sordellii lethal toxin, which modifies Ras and Rac by glucosylation, prefers Ras in vitro (35, 36) and in intact cells (36, 37). Importantly, our studies on the apoptosis of HeLa cells were performed at rather high concentrations of TpeL (10 nm), where Ras as well as Rac1 is probably modified by the toxin. However, PARP1 cleavage could also be observed with concentrations of TpeL that were not sufficient for Rac1 modification. We propose that the modification of Ras is most important for induction of apoptosis. This notion is supported by recent findings with C. sordellii lethal toxin and the related toxin B variant TcdBF, which shares all protein targets with lethal toxin with the exception of Ras and is not able to induce apoptosis (24). Therefore, inhibition of the Ras signaling pathway is most likely essential for induction of apoptosis. Our present studies show that modification of Ras by TpeL blocks interaction with the Ras effector Raf and prevents activation of ERK. Another well-studied Ras-dependent signaling pathway is the formation of neurite-like processes in PC12 cells stimulated by nerve growth factor NGF (32). In line with the inhibition of the Ras pathway, TpeL blocked formation of neurites in PC12 cells.
Taken together, our data indicate that TpeL modifies Ras preferentially by GlcNAcylation at threonine 35. Rac is a poor substrate of TpeL and is modified in vitro and in vivo at 1–2 magnitudes higher toxin concentrations. An intrinsic cysteine protease domain is responsible for the autocatalytic cleavage of TpeL in the presence of InsP6. TpeL induces apoptosis in target cells most likely by inhibition of Ras signaling. Effects of the toxin on intact cells depend on TpeL1–1779, a toxin variant, which is extended at the C terminus but lacks the typical C-terminal polypeptide repeat domain that is present in all members of the CGT family.
Supplementary Material
Acknowledgments
We thank Otilia Wunderlich for excellent technical assistance, Andreas Schlosser and Ulrike Lanner (Core Facility Proteomics, ZBSA, Freiburg, Germany) for mass spectrometric analysis, Alfred Wittinghofer (Max-Planck-Institut für molekulare Physiologie, Dortmund, Germany) and Tilman Brummer (Institut für Biologie III und Zentrum für Biosystemanalyse (ZBSA), Freiburg, Germany) for sharing plasmids, Julian Rood (Monash University, Victoria, Australia) for providing C. perfringens strains, Thijn Brummelkamp (Netherlands Cancer Institute) for providing Hap1 cells, and Thomas Wilmes and Björn Schorch for valuable advices.
This work was supported by the Deutsche Forschungsgemeinschaft (AK6/16-3) and the Center for Biological Signaling Studies (BIOSS) in Freiburg (Germany).

This article contains supplemental Figs. S1–S6.
- CGTs
- clostridial glucosylating toxins
- UDP-Glc
- UDP-glucose
- CPD
- cysteine protease domain
- InsP6
- inositol hexakisphosphate
- UDP-GlcNAc
- UDP-N-acetylglucosamine
- DABCO
- 1,4-diazabicyclo[2.2.2]octane.
REFERENCES
- 1. Borriello S. P., Aktories K. (2005) in Clostridium perfringens, Clostridium difficile, and other Clostridium species, Topley Wilson's Microbiology & Microbial Infections (Borriello S. P., Murray P. R., Funke G., eds) Hodder Arnold, London [Google Scholar]
- 2. Hatheway C. L. (1990) Toxigenic clostridia. Clin. Microbiol. Rev. 3, 66–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smedley J. G., 3rd, Fisher D. J., Sayeed S., Chakrabarti G., McClane B. A. (2004) The enteric toxins of Clostridium perfringens. Rev. Physiol Biochem. Pharmacol. 152, 183–204 [DOI] [PubMed] [Google Scholar]
- 4. Rood J. I., Cole S. T. (1991) Molecular genetics and pathogenesis of Clostridium perfringens. Microbiol. Rev. 55, 621–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rood J. I. (1998) Virulence genes of Clostridium perfringens. Annu. Rev. Microbiol. 52, 333–360 [DOI] [PubMed] [Google Scholar]
- 6. Petit L., Gibert M., Popoff M. R. (1999) Clostridium perfringens: toxinotype and genotype. Trends Microbiol. 7, 104–110 [DOI] [PubMed] [Google Scholar]
- 7. Stevens D. L. (1997) in Necrotizing Clostridial Soft Tissue Infections. The Clostridia, Molecular Biology and Pathogenesis (Rood J., McClane B. A., Songer J. G., Titball R. W., eds) Academic Press, San Diego [Google Scholar]
- 8. Amimoto K., Noro T., Oishi E., Shimizu M. (2007) A novel toxin homologous to large clostridial cytotoxins found in culture supernatant of Clostridium perfringens type C. Microbiology 153, 1198–1206 [DOI] [PubMed] [Google Scholar]
- 9. Jank T., Giesemann T., Aktories K. (2007) Rho-glucosylating Clostridium difficile toxins A and B: new insights into structure and function. Glycobiology 17, 15R-22R [DOI] [PubMed] [Google Scholar]
- 10. Just I., Gerhard R. (2004) Large clostridial cytotoxins. Rev. Physiol Biochem. Pharmacol. 152, 23–47 [DOI] [PubMed] [Google Scholar]
- 11. Voth D. E., Ballard J. D. (2005) Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18, 247–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jank T., Aktories K. (2008) Structure and mode of action of clostridial glucosylating toxins: the ABCD model. Trends Microbiol. 16, 222–229 [DOI] [PubMed] [Google Scholar]
- 13. Hofmann F., Busch C., Prepens U., Just I., Aktories K. (1997) Localization of the glucosyltransferase activity of Clostridium difficile toxin B to the N-terminal part of the holotoxin. J. Biol. Chem. 272, 11074–11078 [DOI] [PubMed] [Google Scholar]
- 14. Egerer M., Giesemann T., Jank T., Satchell K. J., Aktories K. (2007) Auto-catalytic cleavage of Clostridium difficile toxins A and B depends on cysteine protease activity. J. Biol. Chem. 282, 25314–25321 [DOI] [PubMed] [Google Scholar]
- 15. von Eichel-Streiber C., Sauerborn M. (1990) Clostridium difficile toxin A carries a C-terminal repetitive structure homologous to the carbohydrate binding region of streptococcal glycosyltransferases. Gene 96, 107–113 [DOI] [PubMed] [Google Scholar]
- 16. Greco A., Ho J. G., Lin S. J., Palcic M. M., Rupnik M., Ng K. K. (2006) Carbohydrate recognition by Clostridium difficile toxin A. Nat. Struct. Mol. Biol. 13, 460–461 [DOI] [PubMed] [Google Scholar]
- 17. Genisyuerek S., Papatheodorou P., Guttenberg G., Schubert R., Benz R., Aktories K. (2011) Structural determinants for membrane insertion, pore formation and translocation of Clostridium difficile toxin B. Mol. Microbiol. 79, 1643–1654 [DOI] [PubMed] [Google Scholar]
- 18. Papatheodorou P., Zamboglou C., Genisyuerek S., Guttenberg G., Aktories K. (2010) Clostridial glucosylating toxins enter cells via clathrin-mediated endocytosis. PLoS. One. 5, e10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Just I., Selzer J., Wilm M., von Eichel-Streiber C., Mann M., Aktories K. (1995) Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375, 500–503 [DOI] [PubMed] [Google Scholar]
- 20. Selzer J., Hofmann F., Rex G., Wilm M., Mann M., Just I., Aktories K. (1996) Clostridium novyi α-toxin-catalyzed incorporation of GlcNAc into Rho subfamily proteins. J. Biol. Chem. 271, 25173–25177 [DOI] [PubMed] [Google Scholar]
- 21. Just I., Wilm M., Selzer J., Rex G., von Eichel-Streiber C., Mann M., Aktories K. (1995) The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J. Biol. Chem. 270, 13932–13936 [DOI] [PubMed] [Google Scholar]
- 22. Just I., Selzer J., Hofmann F., Green G. A., Aktories K. (1996) Inactivation of Ras by Clostridium sordellii lethal toxin-catalyzed glucosylation. J. Biol. Chem. 271, 10149–10153 [DOI] [PubMed] [Google Scholar]
- 23. Popoff M. R., Chaves-Olarte E., Lemichez E., von Eichel-Streiber C., Thelestam M., Chardin P., Cussac D., Antonny B., Chavrier P., Flatau G., Giry M., de Gunzburg J., Boquet P. (1996) Ras, Rap, and Rac small GTP-binding proteins are targets for Clostridium sordellii lethal toxin glucosylation. J. Biol. Chem. 271, 10217–10224 [DOI] [PubMed] [Google Scholar]
- 24. Dreger S. C., Schulz F., Huelsenbeck J., Gerhard R., Hofmann F., Just I., Genth H. (2009) Killing of rat basophilic leukemia cells by lethal toxin from Clostridium sordellii: critical role of phosphatidylinositide 3′-OH kinase/Akt signaling. Biochemistry 48, 1785–1792 [DOI] [PubMed] [Google Scholar]
- 25. Jank T., Reinert D. J., Giesemann T., Schulz G. E., Aktories K. (2005) Change of the donor substrate specificity of Clostridium difficile toxin B by site-directed mutagenesis. J. Biol. Chem. 280, 37833–37838 [DOI] [PubMed] [Google Scholar]
- 26. Reinert D. J., Jank T., Aktories K., Schulz G. E. (2005) Structural basis for the function of Clostridium difficile toxin B. J. Mol. Biol. 351, 973–981 [DOI] [PubMed] [Google Scholar]
- 27. Nagahama M., Ohkubo A., Oda M., Kobayashi K., Amimoto K., Miyamoto K., Sakurai J. (2011) Clostridium perfringens TpeL glycosylates the Rac and Ras subfamily proteins. Infect. Immun. 79, 905–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang G., Zhou B., Wang J., He X., Sun X., Nie W., Tzipori S., Feng H. (2008) Expression of recombinant Clostridium difficile toxin A and B in Bacillus megaterium. BMC. Microbiol. 8, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Egerer M., Satchell K. J. (2010) Inositol hexakisphosphate-induced autoprocessing of large bacterial protein toxins. PLoS. Pathog. 6, e1000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guttenberg G., Papatheodorou P., Genisyuerek S., Lü W., Jank T., Einsle O., Aktories K. (2011) Inositol hexakisphosphate-dependent processing of Clostridium sordellii lethal toxin and Clostridium novyi α-toxin. J. Biol. Chem. 286, 14779–14786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karnoub A. E., Weinberg R. A. (2008) Ras oncogenes: split personalities. Nat. Rev. Mol. Cell Biol. 9, 517–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vaudry D., Stork P. J., Lazarovici P., Eiden L. E. (2002) Signaling pathways for PC12 cell differentiation: making the right connections. Science 296, 1648–1649 [DOI] [PubMed] [Google Scholar]
- 33. Reineke J., Tenzer S., Rupnik M., Koschinski A., Hasselmayer O., Schrattenholz A., Schild H., von Eichel-Streiber C. (2007) Autocatalytic cleavage of Clostridium difficile toxin B. Nature 446, 415–419 [DOI] [PubMed] [Google Scholar]
- 34. Pfeifer G., Schirmer J., Leemhuis J., Busch C., Meyer D. K., Aktories K., Barth H. (2003) Cellular uptake of Clostridium difficile toxin B. Translocation of the N-terminal catalytic domain into the cytosol of eukaryotic cells. J. Biol. Chem. 278, 44535–44541 [DOI] [PubMed] [Google Scholar]
- 35. Busch C., Hofmann F., Selzer J., Munro S., Jeckel D., Aktories K. (1998) A common motif of eukaryotic glycosyltransferases is essential for the enzyme activity of large clostridial cytotoxins. J. Biol. Chem. 273, 19566–19572 [DOI] [PubMed] [Google Scholar]
- 36. Huelsenbeck S. C., Klose I., Reichenbach M., Huelsenbeck J., Genth H. (2009) Distinct kinetics of (H/K/N)Ras glucosylation and Rac1 glucosylation catalyzed by Clostridium sordellii lethal toxin. FEBS Lett. 583, 3133–3139 [DOI] [PubMed] [Google Scholar]
- 37. Genth H., Just I. (2011) Functional implications of lethal toxin-catalysed glucosylation of (H/K/N)Ras and Rac1 in Clostridium sordellii-associated disease. Eur. J. Cell Biol. 90, 959–965 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.