Background: His-Asp phosphorelay proteins can be components of complex signaling systems.
Results: Two hybrid histidine kinases, EspA and EspC, form a signaling complex; EspA phosphorylates both receiver modules to regulate proteolytic turnover of a regulatory protein.
Conclusion: Two hybrid histidine kinases are integrated by inter- and intra-histidine aspartate phosphotransfer.
Significance: A novel His-Asp phosphorelay signal transduction mechanism was identified.
Keywords: Bacterial Signal Transduction, Development, Histidine Kinases, Protein Turnover, Signaling, Myxococcus xanthus
Abstract
Histidine-aspartate phosphorelay signaling systems are used to couple stimuli to cellular responses. A hallmark feature is the highly modular signal transmission modules that can form both simple “two-component” systems and sophisticated multicomponent systems that integrate stimuli over time and space to generate coordinated and fine-tuned responses. The deltaproteobacterium Myxococcus xanthus contains a large repertoire of signaling proteins, many of which regulate its multicellular developmental program. Here, we assign an orphan hybrid histidine protein kinase, EspC, to the Esp signaling system that negatively regulates progression through the M. xanthus developmental program. The Esp signal system consists of the hybrid histidine protein kinase, EspA, two serine/threonine protein kinases, and a putative transport protein. We demonstrate that EspC is an essential component of this system because ΔespA, ΔespC, and ΔespA ΔespC double mutants share an identical developmental phenotype. Neither substitution of the phosphoaccepting histidine residue nor deletion of the entire catalytic ATPase domain in EspC produces an in vivo mutant developmental phenotype. In contrast, substitution of the receiver phosphoaccepting residue yields the null phenotype. Although the EspC histidine kinase can efficiently autophosphorylate in vitro, it does not act as a phosphodonor to its own receiver domain. Our in vitro and in vivo analyses suggest the phosphodonor is instead the EspA histidine kinase. We propose EspA and EspC participate in a novel hybrid histidine protein kinase signaling mechanism involving both inter- and intraprotein phosphotransfer. The output of this signaling system appears to be the combined phosphorylated state of the EspA and EspC receiver modules. This system regulates the proteolytic turnover of MrpC, an important regulator of the developmental program.
Introduction
Signal transmission via reversible histidine-aspartate (His-Asp) phosphorelay (often termed two-component signal transduction) is a widely used mechanism for coupling specific stimuli to appropriate cellular responses (1). Genes encoding these proteins can be identified in nearly all bacteria, certain eukaryotic microorganisms, plants, and archaea (2). Members of this signal transduction family can be identified by the presence of highly conserved histidine kinase (HK)4 or receiver (REC) signal transmission modules. The HK module consists of a dimerization/histidine phosphotransfer (DHp, also known as HisKA) domain and a catalytic ATP hydrolysis (CA, also known as HATPase_c) domain (3). HK modules are dimeric and interact through the DHp domain; autophosphorylation (HK∼P) occurs by CA-dependent transfer of the γ-phosphoryl group of ATP to an invariant histidine residue in the DHp domain (4). The single “switch” domain REC module catalyzes transfer of a phosphoryl group from an HK∼P, or sometimes from a small molecule phosphodonor, onto an invariant aspartate residue within itself (5, 6). REC phosphorylation is thought to shift the equilibrium between inactive and active conformations. Most HK modules also display phosphatase activity toward the cognate REC∼P, and a phosphatase motif (E/D)XX(N/T) has been identified adjacent to the phosphoaccepting His residue in the DHp domain (7). The CA domain also modulates phosphatase activity (8, 9).
In the paradigm two-component system, the HK and REC modules are organized into two separate proteins as follows: a sensor histidine protein kinase (HPK) containing an N-terminal stimulus-sensing domain that modulates activity of the associated HK module, and a response regulator protein (RR) in which the N-terminal REC domain modulates the activity of an associated effector domain. HPK stimulus-sensing domains are highly variable (10), whereas RR effector domains are most often promoter-binding elements but also include enzymatic or protein-interaction output domains (11). Finally, some RRs, termed “stand alone” receivers lack an associated output domain and mediate signal output by directly interacting with target proteins.
A variation of the simple two-component signal pathway involves hybrid histidine protein kinases (HyHPK) in which one (or sometimes more) REC domain is fused to the HK at the C terminus. Typically, HyHPKs participate in a four-step phosphorelay in which the phosphoryl group is passed from the HyHPK to a histidine phosphotransferase protein and then to an output-mediating RR (12). Multistep phosphorelay systems are proposed to provide additional regulatory sites for fine-tuning signal output. HyHPKs can also function in the absence of histidine phosphotransferase proteins. In these cases, the fused REC domain(s) have been shown to modulate signal transmission to a cognate RR by functioning to inactivate the associated kinase (13) and/or to provide sites for alternative kinase phosphorylation presumably leading to signal integration (14). Thus, HyHPKs provide an excellent example of how the highly modular nature of His-Asp phosphorelays can be exploited to generate complex signaling systems.
HyHPKs represent only 20% of the HKs in bacteria but 90% of the HKs in eukaryotes (2, 15). In general, these more complex signaling systems are favored in organisms that regulate complex behaviors. Perhaps the best example can be observed among the Myxobacteria, a group within the Deltaproteobacteria, which favor a “social” (multicellular) life cycle. Several of the developing myxobacterial species (16–19) encode a large repertoire of signal transduction genes (20–24). Myxococcus xanthus, the best studied of the Myxobacteria, contains 272 His-Asp phosphorelay family homologs (24). Only 29% of the 251 (nonchemosensory related) His-Asp phosphorelay genes are encoded together as HPK-RR pairs (i.e. two-component systems); instead, 58% are genetically orphan, and the remaining 16% encoded in complex gene clusters (24) are indicative of nonlinear complex signaling pathways (9). One of the challenges in analysis of M. xanthus signaling pathways is to assign orphan His-Asp phosphorelay proteins to signaling pathways and to define the wiring networks.
That M. xanthus requires sophisticated signaling systems is perhaps no surprise given its complex life cycle. Under nutrient-limited conditions, the cells enter a complex developmental pathway involving at least three distinct cell fates. Some cells are induced to aggregate into mounds containing ∼105 cells and, exclusively inside these mounds, are triggered to differentiate into quiescent, environmentally resistant spores (25). Other cells instead lyse during development apparently via programmed cell death (26–28). Finally, a minor proportion of the developing population differentiate into a persister-like state termed peripheral rods (29, 30). Formation of fruiting bodies (31–34), sporulation (34, 35), and possibly programmed cell death (28) are regulated by MrpC, a transcriptional regulator that is up-regulated during development and is subject to both negative (33, 36) and positive (31, 34) regulation.
Progression through the developmental program is negatively regulated by several atypical His-Asp phosphorelay proteins (9, 37–39). The Esp (early sporulation) signaling system is an unusually complex multicomponent system consisting of two serine/threonine kinases (PktA5 and PktB8), a putative oligopeptide transport protein (EspB), and a HyHPK (EspA). Based on genetic and biochemical analyses, EspA autophosphorylates and donates a phosphoryl group to its associated receiver to delay developmental progression by preventing accumulation of MrpC (36). It is thought that the delay of development is released by modulation of EspA activity via the upstream signaling module consisting of EspB, PktA5, and PktB8 (40, 41). However, both the exact output and the signals modulating this system are unknown.
In this study, we demonstrate that EspC, a genetic orphan HyHPK, is an essential component of the Esp signaling system. Using a combination of genetic and in vitro biochemical approaches, we demonstrate that EspA and EspC function in a novel HyHPK mechanism involving inter- and intra-HyHPK phosphotransfer. The target of this sophisticated signaling system is the regulated proteolysis of a crucial developmental regulatory protein, MrpC. This signaling system provides an important example of the inherent adaptability and plasticity of the histidine-aspartic acid phosphorelay proteins.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
Bacterial strains and plasmids used in this study are listed in Table 1. M. xanthus strains were grown under vegetative conditions on casitone yeast extract medium broth or agar plates as described previously (36, 42). Plates were supplemented with 100 μg ml−1 kanamycin, where necessary. Escherichia coli cells were grown under standard laboratory conditions in lysogeny broth media (43) supplemented with 50 μg ml−1 kanamycin where necessary. M. xanthus development was induced under submerged culture conditions in either a 16- or 0.5-ml format, as described previously (36, 42). Briefly, exponentially growing cells were diluted to an optical density of 0.035 A550 in casitone yeast extract medium. 16 or 0.5 ml of the diluted culture was seeded in 85-mm Petri dishes or 24-well plates (Sarstedt), respectively. The plates were incubated at 32 °C (without shaking) for 24 h, and the rich media were replaced by MMC starvation buffer (10 mm MOPS, pH 7.6, 4 mm MgSO4, 2 mm CaCl2) to induce development. To enumerate developmental spores, cells were harvested from 1 well of 24-well plates in triplicate, heated to 50 °C for 60 min, sonicated (output 3, 30% duty 2× 20 pulses, Branson Sonifier 250), and then enumerated using a Helber bacterial counting chamber (Hawksley, UK).
TABLE 1.
Strains and plasmids used in this study
| Strain/plasmid | Genotype | Source |
|---|---|---|
| M. xanthus strains | ||
| DZ2 | Wild type | 67 |
| DZ4227 | DZ2 ΔespA | 37 |
| PH1008 | DZ2 espAH407A | 36 |
| PH1009 | DZ2 espAD696A | 36 |
| PH1029 | PH1009 espAH407A | This study |
| PH1044 | DZ2 ΔespC | This study |
| PH1026 | DZ2 espCH461A | This study |
| PH1027 | DZ2 espCD749A | This study |
| PH1028 | PH1026 espCD749A | This study |
| PH1047 | DZ4227 ΔespC | This study |
| PH1032 | DZ2 espCΔCA | This study |
| PH1033 | PH1027 espCΔCA | This study |
| PH1034 | DZ2 espCN465Y | This study |
| PH1035 | PH1027 espCN465Y | This study |
| PH1036 | DZ2 espCN465E | This study |
| PH1037 | PH1027 espCN465E | This study |
| PH1038 | DZ2 espCN465R | This study |
| PH1039 | PH1027 espCN465R | This study |
| PH1040 | DZ2 espCN465C | This study |
| PH1041 | PH1027 espCN465C | This study |
| PH1042 | DZ2 espCE462A | This study |
| PH1043 | PH1027 espCE462A | This study |
| PH1045 | DZ2 espAD411N | This study |
| E. coli strains | ||
| Top10 | F− endA1 recA1 galE15 galK16 nupG rpsL ΔlacX74 Φ80lacZΔM15 araD139 Δ(ara, leu)7697 mcrA Δ(mrr-hsdRMS-mcrBC) λ− | Invitrogen |
| BL21λDE3 | F− ompT gal dcm lon hsdSB (rB− mB−)λ (DE3 [lacI lacUV5-T7 gene 1 ind1 sam7 nin5]) | Novagen |
| Mutagenesis plasmids | ||
| pBJ114 | Suicide plasmid with KmR and galK | 68 |
| pET24a+ | T7-promotor, His6 tag (C-terminal), KmR | Novagen |
| pBS131 | pBJ114 espC (Δcodons 44–797) | This study |
| pPH150 | pBJ114 espAH407A | 36 |
| pAS001 | pBJ114 espCH461A | This study |
| pAS004 | pBJ114 espCD749A | This study |
| pAS029 | pBJ114 espCΔCA (Δcodons 529–674) | This study |
| pAS031 | pBJ114 espCN465Y | This study |
| pAS030 | pBJ114 espCN465E | This study |
| pAS032 | pBJ114 espCN465R | This study |
| pAS033 | pBJ114 espCN465C | This study |
| pAS034 | pBJ114 espCE462A | This study |
| pAS036 | pBJ114 espAD411N | This study |
| Overexpression plasmids | ||
| pAS002 | pET24a+ espCΔMASE1 (aa 311–833) | This study |
| pBS122 | pET24a+ espCREC (aa 690–819) | This study |
| pAS019 | pET24a+ espCREC D749A (aa 690–819) | This study |
| pBS121 | pET24a+ espCHK (aa 451–679) | This study |
| pAS021 | pET24a+ espCHK H461A (aa 451–679) | This study |
| pAS022 | pET24a+ espAHK (aa 390–646) | This study |
| pAS023 | pET24a+ espAHK H407A (aa 390–646) | This study |
Construction of M. xanthus Mutant Strains
In-frame deletions and site-specific point mutations were generated by homologous recombination of the relevant suicide plasmids followed by galK-mediated counter-selection on galactose (44), as described previously in detail (42). All strains were confirmed by sequence analyses of the relevant region (∼1000 bp surrounding the desired substitution/deletion). Specific plasmids used to generate the respective strains are listed in supplemental Table S1. With the exception of pAS036 (see below), all inserts for the mutagenesis plasmids were generated according to the detailed protocol in Ref. 42 using DZ2 genomic DNA as template and the respective primers listed in supplemental Table S2. For pBS131 (ΔespC), the insert was cloned into the XhoI/BamHI sites of pBJ114. For all other mutagenesis plasmids, the insert was cloned into the EcoRI/BamHI sites of pBJ114. Plasmid pAS036 (espAN411D) was generated using a “one-step directed mutagenesis” PCR protocol (45) with outward-facing mutagenesis primers (supplemental Table S2) and pAS035 as a template. pAS035 contains codons 240–571 of espA cloned into pBJ114. All plasmids were sequenced to confirm the absence of PCR-generated errors.
Bioinformatic Analyses
Orthologs for EspA and EspC in the publicly available Myxococcales genomic sequences were identified using reciprocal BlastP analysis as described by Huntley et al. (18). Briefly, the protein sequence of either EspA or EspC was used as query in BlastP analysis against the protein database from the following: Stigmatella aurantiaca DW4/3-1 (accession number CP002271) (18); Myxococcus fulvus HW-1 (accession CP002830) (46); Corallococcus coralloides DSM2259 (accession CP003389) (19); Anaeromyxobacter dehalogenans 2CP-C (accession CP000251.1) (47); Haliangium ochraceum SMP-2 (accession CP001804.1) (48); Sorangium cellulosum So ce 56 (accession AM746676) (17), and Plesiocystis pacifica SIR-1 (accession ABCS00000000). The protein with the best hit (highest bit score) was then used as a query in a second BlastP analysis against the protein database from M. xanthus DK1622 (accession CP000113) (16). If the highest scoring match was identical to the protein sequence originally used for the first BlastP analysis, the two proteins were considered orthologs.
Construction of Protein Overproduction Plasmids
Protein overproduction plasmids and the primer sequences used to generate them are listed in Table 1 and supplemental Table S2, respectively. Overproduction plasmids pAS022 (EspAHK-His6) and pAS023 (EspAHK H407A-His6) encode the DHp and CA domains (collectively referred to as the kinase region (HK)) of espA with a C-terminal hexahistidine (His6) affinity tag. pAS022 and pAS023 were constructed by PCR amplifying the espA (MXAN_0931) codons 390–646 from DZ2 or PH1008 genomic DNA, respectively, and cloned into the EcoRI site of pET24a+. The resulting plasmids were restricted with SacI; overhangs were blunted with T4 DNA polymerase (Fermentas) and religated to bring the His6 affinity tag in-frame with the espAHK fragment. Overproduction plasmids pBS121 (EspCHK-His6) and pAS021 (EspCHK H461A-His6) encode the HK of EspC and kinase-inactive point mutant with C-terminal His6 affinity tags and were constructed by PCR-amplifying espC (MXAN_6855) codons 451–679 from DZ2 or PH1026 genomic DNA, respectively. The resulting fragments were cloned into the EcoRI and XhoI sites of pET24a+. Overproduction plasmids pBS122 (EspCREC-His6) and pAS019 (EspCREC D749A-His6) encode the REC of espC with a C-terminal His6 affinity tag and were constructed by PCR-amplifying espC codons 690–819 from DZ2 or PH1027 genomic DNA, respectively. The resulting fragments were cloned into the EcoRI and XhoI sites of pET24a+. All constructs were sequenced to confirm absence of PCR-generated errors.
Overproduction and Purification of Recombinant Proteins
EspAHK-His6 or EspCHK-His6 (and the respective point mutants) were each produced as inclusion bodies (IBs) from 1 liter of E. coli BL21(λDE3) cultures by induction with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside for 3 h at 37 °C. To isolate IBs, the respective cell cultures were resuspended in 25 ml of TND buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1 mm DTT) and lysed by passage through a French press (SLM-AMINCO/Spectronic) three times at ∼18,000 p.s.i. IBs were collected by centrifugation at 4600 × g for 30 min at 4 °C, resuspended in 25 ml of TND buffer, and treated by French press as above. To remove contaminating proteins, IBs were pelleted as described above and resuspended in 20 ml of TND buffer. Guanidine HCl was added to a final concentration of 1 m, and the solution was incubated for 2 h at RT with rotation. Purified IBs were pelleted as above, washed in TND buffer, and then resuspended in 15 ml of TND buffer. Guanidine HCl was added to a final concentration of 6 m, and the IB protein was solubilized by vortexing and then clarified by centrifugation at 100,000 × g for 30 min at RT. The solution was diluted with TND buffer to achieve a guanidine HCl final concentration of 2 m and a protein concentration of ∼0.1 mm as determined by the absorbance at 280 nm (A280) (ϵ280(EspAHK-His6) = 1490; ϵ280(EspCHK-His6) = 2980). The solution was then dialyzed in TGND buffer (50 mm Tris-HCl, pH 8.0, 10% (v/v) glycerol, 150 mm NaCl, 1 mm DTT) for 2 h at 4 °C, two times, followed by dialysis in fresh buffer overnight. The resulting protein preparation was clarified by centrifugation at 17,000 × g for 5 min at 4 °C and concentrated using a 10 molecular weight cut-off Amicon Ultra column (Millipore). The refolded proteins were stored at −20 °C for further assays.
EspCREC-His6 (and its respective point mutant) were overproduced as soluble proteins in E. coli strain BL21(λDE3) by induction with 1 mm isopropyl 1-thio-β-d-galactopyranoside for 2 h at 37 °C. For purification of the recombinant proteins, the cell cultures were resuspended in lysis buffer (50 mm HEPES, 0.5 m NaCl, 20 mm imidazole, pH 7.4) and lysed by French press as described above. The lysate was clarified by centrifugation at 100,000 × g for 1 h at 4 °C, and the supernatant was subjected to purification by nickel affinity chromatography at 4 °C (ÄKTA purifier, GE Healthcare) using a 1-ml His trap FF1 nickel affinity column (Amersham Biosciences). The proteins were eluted using a 30-ml linear gradient of 20–500 mm imidazole in lysis buffer. A portion of each eluted fraction was analyzed by SDS-PAGE. Elution fractions containing the peak levels of purified protein were pooled, dialyzed, and stored as described above.
Radiolabeled in Vitro Autophosphorylation and Phosphotransfer Assays
In vitro autophosphorylation of 10 μm kinase was carried out in phosphorylation buffer (TGND buffer, 5 mm MgCl2, 50 mm KCl) supplemented with 0.5 mm ATP and 1.7 μm [γ-32P]ATP (222 TBq mmol−1; Hartmann Analytic, Braunschweig) for intervals between 0 and 60 min. At each time point, 10 μl samples were withdrawn, quenched with 2× LSB (125 mm Tris-HCl, 20% (v/v) glycerol, 4% (w/v) SDS, 10% β-mercaptoethanol, 0.02% (w/v) bromphenol blue), and analyzed as described previously (36), except 15% polyacrylamide gels were used to resolve radiolabeled proteins. The gels were exposed to a Storage Phosphor Screen (GE Healthcare) overnight and analyzed using a StormTM 800 imaging system (Amersham Biosciences). 32P-Labeled HK signal intensities from three independent experiments were quantified by ImageQuant (GE Healthcare) with local average background correction and manual background subtraction. The average intensity and associated standard deviation were calculated using Microsoft Excel.
Phosphotransfer reactions were performed by first autophosphorylating 15 μm of either EspAHK-His6 or EspCHK-His6 (or respective point mutants) for 30 min. 6.5 μl of the HK∼P were then added to 3.5 μl of either phosphorylation buffer, 28 μm EspCREC-His6 or EspCREC D749A-His6, leading to an equivalent 10 μm final concentration for each reaction partner. The reactions were quenched after 2 min and analyzed as above, except 20% polyacrylamide gels were used.
Immunoblot Analysis
Protein lysates for immunoblot analyses were prepared from developmental cells grown in 16 ml of submerged culture format. At the indicated time points, the MMC buffer was removed, and the cells were resuspended in 1 ml of ice-cold MMC buffer incubated with an equivalent volume of 26% ice-cold trichloroacetic acid for 15 min on ice, pelleted at 17,000 × g for 5 min at 4 °C, and washed twice with acetone. The pellet was resuspended in 1× clear LSB (62.5 mm Tris-HCl, 10% (v/v) glycerol, 2% (w/v) SDS) and heated for 1 min at 94 °C. The protein concentration of each sample was determined using a BCA protein assay kit (Thermo Scientific) according to manufacturer's instructions. Samples were diluted in 2× LSB to 2 μg μl−1 and stored at −20 °C.
For immunoblot analysis, samples containing 20 μg of protein were resolved by denaturing SDS-PAGE in 8% (anti-EspA and -EspC immunoblots) or in 12% (anti-MrpC immunoblots) polyacrylamide gels. Proteins were transferred to a PVDF membrane using a semi-dry transfer apparatus. Western blot analyses were performed using anti-EspC (supplemental Methods), anti-EspA (41), or anti-MrpC (26) primary antibodies at dilutions of 1:200, 1:1000, or 1:2000, respectively. Goat α-rabbit IgG conjugated to horseradish peroxidase (HRP) secondary antibody (Pierce) was used at a dilution of 1:20,000, and signals were detected with enhanced chemiluminescence substrate (Pierce) followed by exposure to autoradiography film (Thermo Scientific) or detected by LAS 4000 (Fujifilm Life Science). Representative immunoblot patterns are shown, but similar patterns were obtained from at least two biological replicates.
In Vivo MrpC Turnover Assays
Wild type (DZ2) or ΔespA ΔespC mutant (PH1047) strains were developed in 16 ml of submerged culture format for 9 h and treated with 34 μg ml−1 chloramphenicol to block de novo protein synthesis (50). At 0, 10, 20, or 30 min after addition of chloramphenicol, the cells were harvested, pelleted at 4,600 × g for 2 min at 4 °C, resuspended in 400 μl of hot (70 °C) LSB, heated for 5 min at 99 °C, and stored at −20 °C for further analyses. 15 μl of protein samples were analyzed by immunoblot as described above. Band intensities from triplicate experiments were quantified with ImageJ 1.43U (49). The MrpC half-life in wild type cells was calculated assuming a first order kinetic degradation reaction (51–53). Briefly, each background-subtracted band intensity value was first normalized to the intensity at t = 0, and the natural log of the resulting values were plotted versus time in Microsoft Excel. The slope (k) of a linear fit of each graph was used to calculate the t½ using the equation t½ = ln(2)/−k (53).
RESULTS
EspA and EspC Function in Same Signaling Pathway
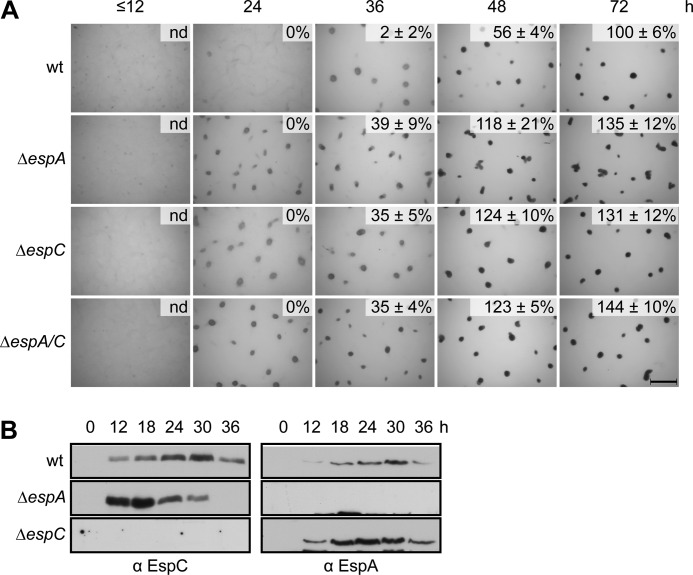
espA and espC each encode orphan HyHPKs and both have a similar mutant phenotype in which progression through the developmental program is accelerated relative to wild type (37, 39). To analyze whether these proteins participated in a linear signaling pathway, we took a genetic epistasis approach. We first generated isogenic ΔespC and ΔespAΔespC strains and compared their developmental phenotypes to the wild type and ΔespA strains. The ΔespA, ΔespC, and ΔespAΔespC strains each yielded an identical developmental phenotype; aggregation centers were visible between 12 and 24 h after induction of starvation, which was ∼12 h earlier than wild type (Fig. 1A). Likewise, all three mutants produce heat- and sonication-resistant spores ∼12 h earlier than the wild type (Fig. 1A). These genetic data suggest that EspA and EspC function in the same signaling pathway to regulate developmental progression.
FIGURE 1.
EspA and EspC are components of a single signaling pathway. A, developmental phenotypes of esp null mutants. Wild type (wt; DZ2), ΔespA (DZ4227), ΔespC (PH1044), and ΔespA ΔespC (PH1047) strains were developed under submerged culture in 24-well culture dishes, and pictures were recorded at the indicated hours of development. Heat- and sonication-resistant spores were isolated at the indicated time points and displayed as the percent of wild type spores at 72 h. Spore numbers are the average and associated standard deviation of three biological replicates. Scale bar, 0.5 mm; nd, not determined. B, EspA and EspC developmental protein accumulation patterns. Anti-EspC (left) and anti-EspA (right) immunoblot analysis of cell lysates prepared from the indicated strains developed for the indicated hours under 16 ml of submerged culture format.
The observation that ΔespAΔespC double mutant phenocopied the single mutants could arise from one of two possibilities. 1) EspA regulates developmental progression, but EspC is necessary for EspA production (or vice versa). 2) EspA and EspC act together to regulate developmental progression. To distinguish between these two possibilities, we examined EspA and EspC production patterns by immunoblot analysis of cell lysates prepared from wild type, ΔespA, or ΔespC stains. The anti-EspC immunosera generated in this study could specifically detect an ∼89-kDa band (similar to the predicted EspC molecular mass of ∼88 kDa) in the wild type, but not ΔespC, lysates (Fig. 1B). EspC was not detected at t = 0, but began to accumulate at ∼12 h, peaked at ∼30 h, and then began to decrease, similar to the EspA accumulation pattern (Fig. 1B). EspC and EspA were present in the ΔespA and ΔespC strains, respectively, and accumulated earlier in the respective backgrounds than in the wild type (Fig. 1B). Together, these data suggest that EspA and EspC act together to regulate developmental progression. Additionally, this dual activity might, directly or indirectly, repress the accumulation of both proteins.
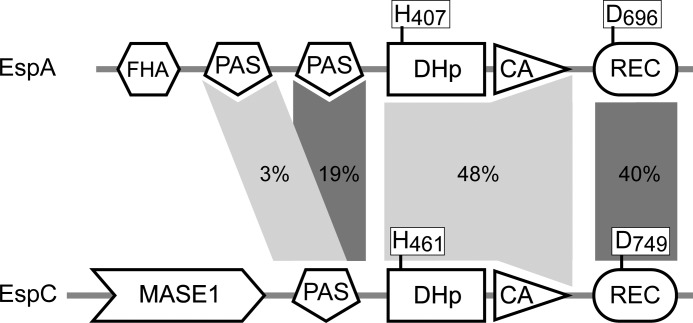
EspA and EspC share 48 and 40% identity in their HK and REC regions, respectively (Fig. 2). These two proteins are not paralogs, because at least three proteins (encoded by Mxan_0195, Mxan_2386, and Mxan_0095) can be identified that display a higher degree of conservation to EspA than does EspC. Orthologs to EspA and EspC can be identified in S. aurantiaca (STAUR_7060 and STAUR_0999, respectively), C. coralloides (COCOR_00882 and COCOR_07432, respectively), and M. fulvus (LILAB_04045 and LILAB_12195, respectively), but orthologs for neither protein could be identified in A. dehalogenans, H. ochraceum, P. pacifica, or S. cellulosum. Thus, if EspA was identified in a genome, then EspC was always additionally present, consistent with a single Esp signaling module in which both proteins act together.
FIGURE 2.
Domain organization of the EspA and EspC hybrid histidine kinases. Percent amino acid identity for the indicated domains is represented by shaded areas. The invariant histidine (H) residues in the dimerization and histidine phosphotransfer domains (DHp) and invariant aspartate (D) residues in the receiver (REC) domains are indicated. FHA, forkhead-associated domain; MASE1, predicted integral membrane sensory domain.
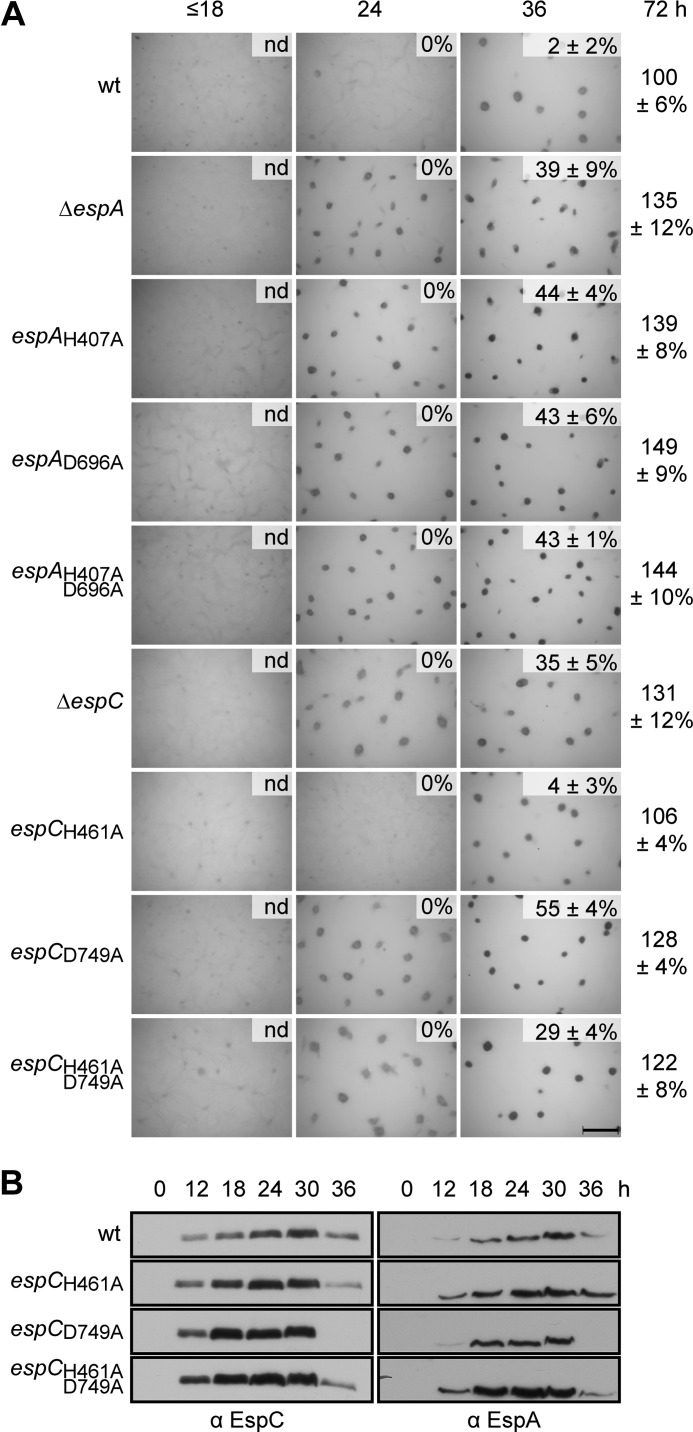
espCD749A but Not espCH461A Mutants Effect Developmental Progression
We have previously demonstrated that both EspA autophosphorylation and phosphotransfer to its associated receiver domain are necessary for appropriate developmental progression; alanine substitution of the kinase autophosphorylation site (H407A) or receiver phosphoaccepting site (D696A) leads to the null mutant phenotype (36) (Fig. 3A). Consistently, we observed an identical phenotype if both residues in espA were mutated (espAH407A,D696A) (Fig. 3A). To determine whether EspC kinase activity is similarly necessary for regulation of developmental progression, we likewise generated an alanine substitution of the predicted autophosphorylation site (His-461) in EspC (espCH461A). Surprisingly, this mutant exhibited the wild type phenotype with respect to timing of aggregation and production of heat- and sonication-resistant spores (Fig. 3A). In contrast, alanine substitution of the conserved phosphoaccepting residue (Asp-749) in the receiver domain of EspC (espCD749A) resulted in the ΔespC phenotype (Fig. 3A). An identical mutant phenotype was also observed in a strain in which both EspC His-461 and Asp-749 were substituted with alanine (espCH461A,D749A) (Fig. 3A).
FIGURE 3.
Phosphorylation of EspC receiver domain, but not EspC autophosphorylation, is required for regulation of development. A, developmental phenotypes of espA and espC signal transmission point mutants. Wild type (wt; DZ2), ΔespA (DZ4227), espAH407A (PH1008), espAD696A (PH1009), espAH407A,D696A (PH1029), ΔespC (PH1044), espCH461A (PH1026), espCD749A (PH1027),and espCH461A,D749A (PH1028) strains were developed under submerged culture in 24-well culture dishes, and pictures were recorded at the indicated hours of development. Heat- and sonication-resistant spores were isolated at the indicated time points and displayed as the percent of wild type spores at 72 h. Spore numbers are the average and associated standard deviation of three biological replicates. Scale bar, 0.5 mm; nd, not determined. B, EspC and EspA developmental protein accumulation patterns. Anti-EspC (left panel) and anti-EspA (right panel) immunoblot analyses of cell lysates were prepared from the indicated strains developed for the indicated hours under 16 ml of submerged culture format.
To determine whether the generated point mutations adversely affect the protein accumulation patterns of either EspA or EspC, we analyzed developmental cell lysates generated from the respective strains by anti-EspC and anti-EspA immunoblot analysis. Neither EspC nor EspA protein accumulation was reduced in the espCH461A,espCD749A or espCH461A,D749A strains (Fig. 3B). Thus, the phenotypes observed are due to disruption of the activity of the mutated proteins rather than to reduction of protein stability for EspA or EspC. The espC mutants that displayed the early developmental phenotype (espCD749A and espCH461A,D749A) also displayed earlier and more rapid accumulation of both EspA and EspC; peak protein levels were detected ∼12 h earlier than in the wild type (Fig. 3B). The espCH461A strain, which does not display a mutant phenotype, may cause slightly earlier production of both EspCH461A and EspA. However, both the rate of accumulation and time point of peak production matched that of the wild type (Fig. 3B). Together, these results suggest that although phosphorylation of the EspC receiver is necessary for appropriate developmental regulation, the phosphoryl group is not donated from EspC kinase.
EspC Autophosphorylates on His-461 in Vitro
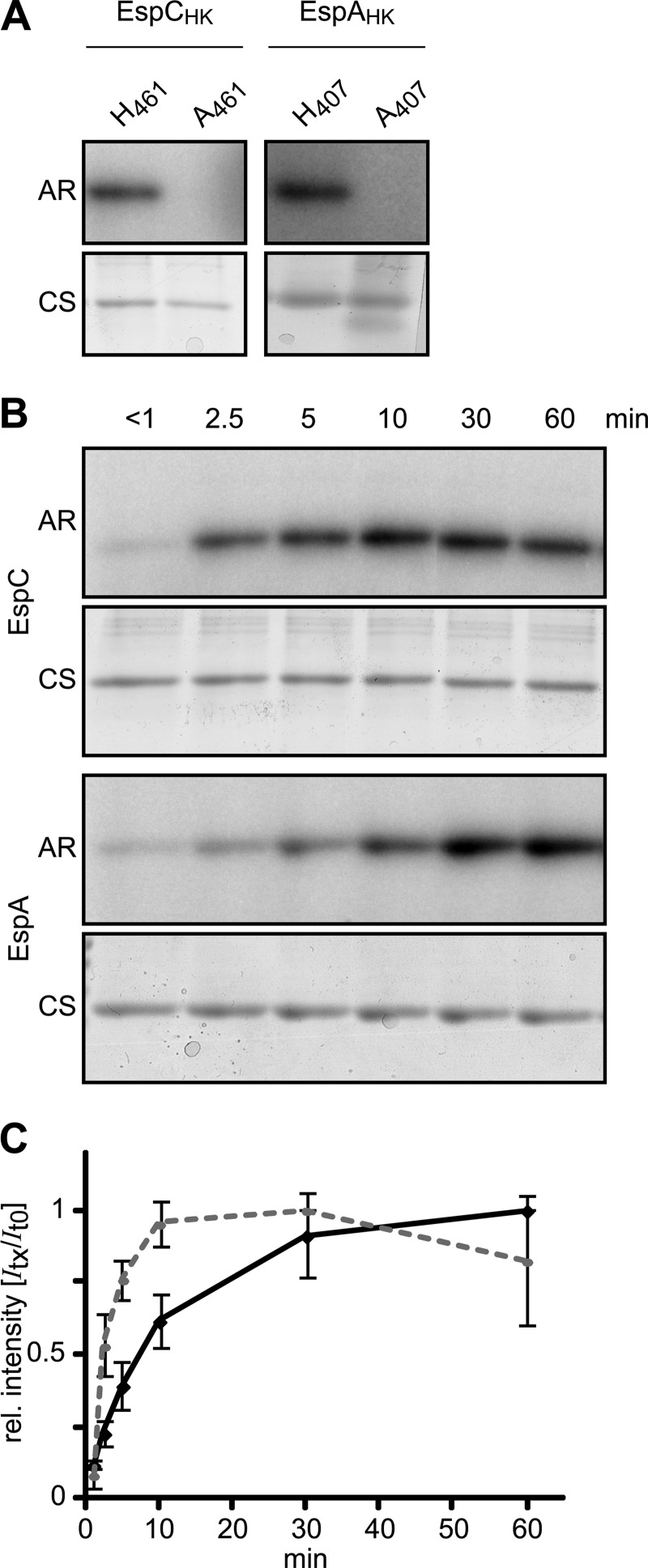
To ascertain whether EspC is capable of autophosphorylation in vitro, we next cloned, overproduced, and purified a recombinant version of the EspC-predicted histidine kinase region (aa 451–679) fused at the C terminus with a hexahistidine affinity tag (EspCHK-His6). We also generated a phosphorylation-deficient H461A version of this protein (EspCHK H461A-His6). As we were unable to find conditions for soluble overproduction, both proteins were refolded from inclusion bodies. To examine whether these two proteins could autophosphorylate, 10 μm of either EspCHK-His6 or EspCHK H461A-His6 was incubated in the presence of [γ-32P]ATP for 60 min and resolved by electrophoresis, and radioactive incorporation onto either protein was analyzed by exposure to a phosphor-imager screen. A radiolabeled band corresponding to EspCHK-His6, but not EspCHK H461A-His6, could be detected (Fig. 4A), suggesting that the EspC kinase region is capable of in vitro autophosphorylation on His-461.
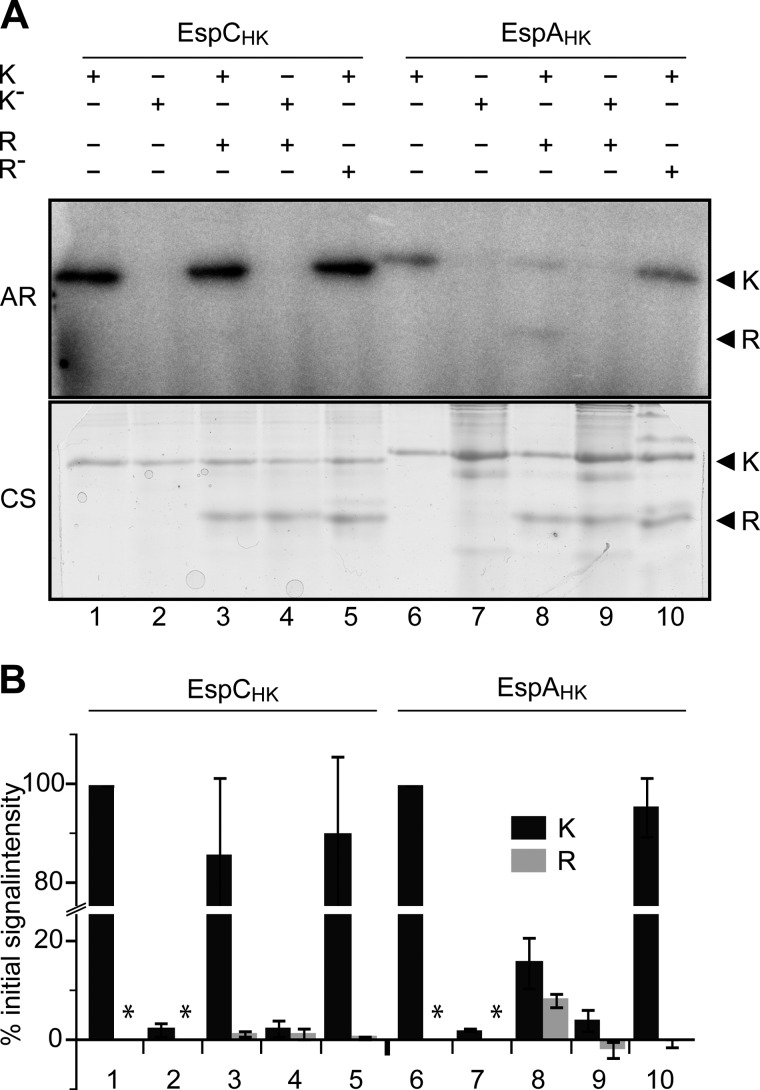
FIGURE 4.
EspC kinase region autophosphorylates in vitro. A, in vitro autophosphorylation of EspAHK-His6, EspCHK-His6, EspAHK H407A-His6, and EspCHK H461A-His6. 10 μm of each recombinant protein was incubated in the presence of [γ-32P]ATP for 60 min, quenched, and resolved by SDS-PAGE, and the radiolabel was detected by exposure to a Storage PhosphoScreen (AR). Total protein was subsequently detected by Coomassie stain (CS). B, relative in vitro autophosphorylation rates of EspC and EspA kinase regions. 10 μm EspAHK-His6 or EspCHK-His6 was incubated in the presence of [γ-32P]ATP; aliquots were removed at the indicated time points and analyzed as above. C, quantification of EspC (dashed line) and EspA (solid line) HK autophosphorylation rates in B. Relative (rel.) signal intensities of the bands from three independent time courses were quantified and normalized to the maximal signal intensity of each protein. The average relative signal intensity and associated standard deviation from each time point was plotted.
To compare the relative activities of the EspC and EspA kinase domains, we generated comparable constructs to produce EspAHK-His6 (aa 390–646) or the kinase-deficient EspAHK H407A-His6. Although these two recombinant proteins could be overproduced as soluble proteins, they were denatured and refolded as for the EspC kinase constructs. As observed previously for a GST-EspAHK construct (36), EspAHK-His6, but not EspAHK H407A-His6, could be labeled by 32P (Fig. 4A). To compare EspAHK-His6 and EspCHK-His6 activity in vitro, 10 μm of the respective proteins was incubated in the presence of [γ-32P]ATP for intervals between ∼0 and 60 min (Fig. 4, B and C). In the case of EspCHK-His6, 32P incorporation could be detected after less than 1 min, and the signal continued to accumulate for 30 min, after which it began to decrease. EspAHK-His6 exhibited slower accumulation of the 32P label that began to saturate between 30 and 60 min of incubation (Fig. 4, B and C). Together, these results indicate that the EspC kinase region is capable of efficient autophosphorylation in vitro with greater activity than that of EspA, despite the observation that the kinase activity is apparently not required during development in vivo.
EspAHK but Not EspCHK Can Transfer a Phosphoryl Group to EspCREC in Vitro
To test our hypothesis that EspA, and not EspC, acts as the phosphodonor to EspCREC, we examined phosphotransfer between EspAHK or EspCHK to the EspC receiver domain in vitro. For this purpose, the EspC receiver domain (aa 690–819), or the putative phosphoaccepting deficient version was each overproduced with C-terminal hexahistidine affinity tags (EspCREC-His6 or EspCREC D749A-His6, respectively). To first examine whether EspCHK could phosphorylate its own receiver in vitro, we allowed for kinase autophosphorylation by incubating EspCHK-His6 (or the EspCHK H461A-His6 control) with [γ-32P]ATP for 30 min. We next added the kinase to either buffer alone or to an equimolar (10 μm) concentration of either EspCREC-His6 or EspCREC D749A-His6 and allowed phosphotransfer reactions to proceed for 2 min. As expected, if either EspCHK-His6 or EspCHK H461A-His6 was first autophosphorylated and subsequently incubated only with buffer, the 32P signal was readily detected on the wild type, but not on the mutated, kinase (Fig. 5, A and B, lanes 1 and 2). If the kinase was instead added to either EspCREC-His6 or EspCREC D749A-His6, no significant change in signal incorporation on EspCHK-His6 could be observed, and no significant 32P label could be detected on either receiver construct (Fig. 5, A and B, lanes 3–5). These results suggest EspCHK-His6 does not act as an efficient phosphodonor for its own receiver in vitro.
FIGURE 5.
EspAHK, but not EspCHK, efficiently phosphorylates EspCREC. A, in vitro phosphotransfer from autophosphorylated EspC or EspA kinase regions to EspC receiver domain (EspCREC). Lanes 1–5, EspCHK-His6 (K) or EspCHK H461A-His6 (K−) was first incubated in the presence of [γ-32P]ATP for 30 min and then incubated with either buffer or equimolar (10 μm) EspCREC-His6 (R) or EspCREC D749A-His6 (R−) for 2 min as indicated. +, indicated component present; −, indicated component absent. Lanes 6–10, EspAHK-His6 (K) or EspAHK H407A-His6 (K−) analyzed as per lanes 1–5. All samples were quenched and resolved by SDS-PAGE, and radiolabel was detected by exposure to a Storage PhosphoScreen (AR). Total protein was subsequently detected by Coomassie stain (CS). B, quantification of the relative signal intensities of radiolabeled EspC or EspA HK (K, black bars), and EspCREC (R, gray bars). Signal intensities are the average and associated standard deviation of three biological replicates of the reactions represented in A. HK and REC intensity values are reported as a percent of the respective Esp HK autophosphorylation controls (represented by lanes 1 and 6, respectively). *, not determined.
We next performed this experiment substituting EspAHK for that of EspCHK. As expected, if either EspAHK-His6 or EspAHK H407A-His6 was first autophosphorylated and subsequently incubated only with buffer, the 32P signal was readily detected on the wild type but not on the mutated kinase (Fig. 5, A and B, lanes 6 and 7). In contrast, if EspCREC-His6 was substituted for the buffer, the signal intensity on EspAHK-His6 was reduced to 16 ± 5, and 8 ± 1% of the original kinase signal could be clearly detected on EspCREC-His6 (Fig. 5, A and B, lane 8). These effects were not observed if the kinase was instead added to EspCREC D749A-His6 (Fig. 5, A and B, lane 10) or if EspAHK H407A-His6 was added instead of the functional HK (Fig. 5, A and B, lane 9). These results indicate that the EspA kinase region can efficiently phosphorylate the receiver of EspC on its predicted phosphoaccepting residue, Asp-749, although under equivalent conditions EspCHK cannot.
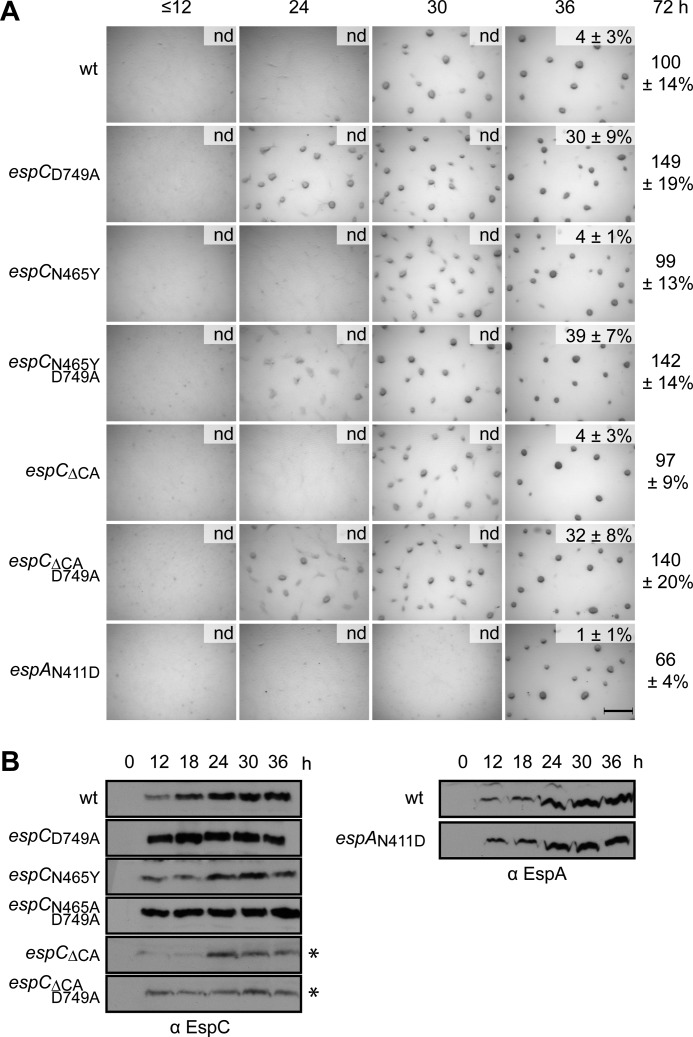
Disruption of a Phosphatase Motif in EspA but Not EspC Delays Development
Our results so far suggested that EspC kinase activity is not necessary for regulation of developmental progression. However, many kinases also display phosphatase activity that is not necessarily disrupted by substitution of the phosphoaccepting histidine residue (9, 54, 55). We next set out to investigate whether EspC may function exclusively as a phosphatase in this system, by generating phosphatase motif (462EXXN465) substitutions that correspond to those producing reduced phosphatase activity in other bifunctional kinases (7, 56). Strains producing EspCN465Y displayed a phenotype indistinguishable from wild type with respect to fruiting body formation and sporulation (Fig. 6A), although the levels of EspCN465Y were slightly reduced relative to that of EspC (Fig. 6B). As expected, if the D749A mutation was combined with the N465Y mutation in EspC (espCN465Y,D749A), the early developmental phenotype was observed, and the double mutant protein levels increased to a similar intensity of EspCD749A (Fig. 6B). Several additional substitutions in the putative phosphatase motif corresponding to substitutions producing reduced phosphatase activity in other bifunctional kinases (espCE462A, espCN465E, espCN465R, or espCN456C) displayed a developmental phenotype indistinguishable from wild type (supplemental Fig. S1A). If these mutations were combined with D749A, all double mutants displayed a phenotype indistinguishable from the espCD749A early developmental phenotype (supplemental Fig. S1A). The single mutants exhibited reduced protein levels relative to the wild type, which were increased in the double mutants (supplemental Fig. S1B).
FIGURE 6.
Disruption of a phosphatase motif in EspA but not EspC effects developmental progression. A, developmental phenotypes of espC and espA phosphatase motif mutants. Wild type (wt; DZ2), espCD749A (PH1027), espCN465Y (PH1034), espCN465Y,D749A (PH1035), espCΔCA (PH1032), espCΔCA,D749A (PH1033), and espAN411D (PH1045) strains were developed under submerged culture in 24-well culture dishes, and pictures were recorded at the indicated hours of development. Heat- and sonication-resistant spores were isolated at the indicated time points and displayed as the percent of wild type spores at 72 h. Spore numbers are the average and associated standard deviation of three biological replicates. Scale bar, 0.5 mm; nd, not determined. B, EspC and EspA developmental protein accumulation patterns. Anti-EspC (left panel) and anti-EspA (right panel) immunoblot analyses of cell lysates were prepared from the indicated strains developed for the indicated hours under 16 ml of submerged culture format. *, 5 times longer exposure is required.
Residues necessary for phosphatase activity have also previously been identified in the CA domain of some kinases, and CA activity has been shown to stimulate phosphatase activity (8, 9, 57). Therefore, we additionally deleted the entire EspC CA domain (aa 529–674) alone or in combination with the D749A mutation. The espCΔCA mutant displayed a phenotype indistinguishable from wild type with respect to fruiting body formation and sporulation, although the espCΔCA,D749A displayed the early developmental phenotype (Fig. 6A). EspCΔCA and EspCΔCA,D749A could be detected by anti-EspC immunoblot at a molecular mass of ∼65 kDa (predicted molecular mass, 73 kDa) (Fig. 6B) and at reduced levels relative to wild type. The reduced intensity of these two mutants relative to EspC in the respective background strains is likely due to loss of some of the antibody epitopes in this protein; anti-EspC antisera were generated against EspC aa 311–833 such that the EspCΔCA mutants lack 28% of the antigen.
To determine whether disruption of the putative phosphatase motif in EspA (408EXXN411) would produce the delayed developmental phenotype consistent with hyper-phosphorylation of EspA and/or EspC receivers preventing developmental progression, we next analyzed the phenotype of an espAN411D mutant. This mutant began to aggregate between 30 and 36 h of development (∼6 h later than the wild type) and produced 66% of wild type spores at 72 h of development.
Together, these results suggest that the putative phosphatase motif in EspA, but not EspC, is important for appropriate regulation of developmental progression. Finally, the observation that the EspCΔCA mutants display a wild type phenotype further confirms that EspC kinase activity is not necessary for in vivo control of the developmental progression.
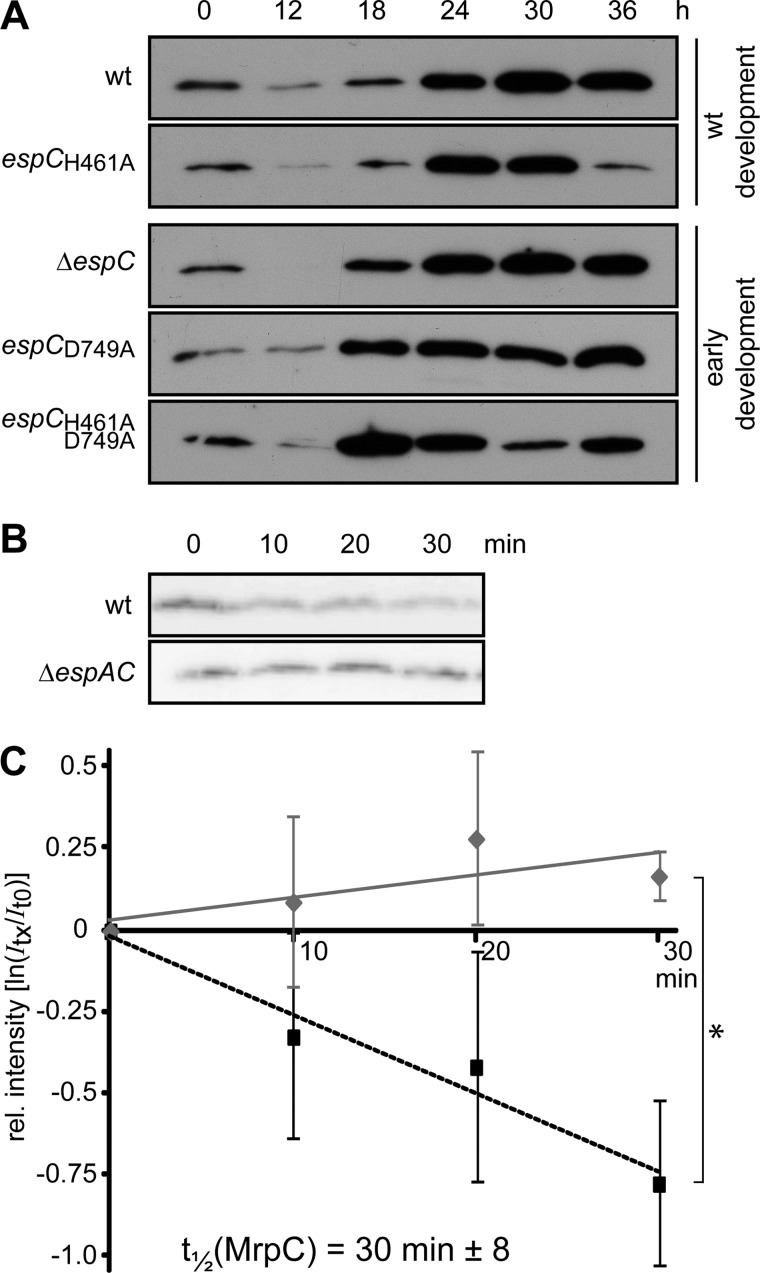
EspA/C System Regulates the Protein Stability of Transcription Factor MrpC
We have previously demonstrated that EspA regulates the accumulation of the key developmental regulator MrpC, because in a ΔespA mutant MrpC protein, but not mrpC transcript, is produced earlier than in the wild type (36). Because our results suggested that EspA and EspC function together, we next examined whether EspC is also involved in regulating MrpC accumulation during development. We used anti-MrpC immunoblot analysis to compare the production of MrpC in the various espC mutants to the wild type. In wild type cells, MrpC could be detected immediately after induction of development (t = 0), decreased between 0 and 12 h, and thereafter gradually increased over at least 36 h of development (Fig. 7A). Consistent with what was observed previously in the ΔespA mutant (36), all early developing EspC mutants (ΔespC, espCD749A, and espCH461A,D749A) produced MrpC more rapidly than the wild type, with MrpC levels at 18 and 24 h higher than the wild type levels (Fig. 7A). In contrast, the espCH461A mutant displayed an accumulation pattern more similar to wild type, although the MrpC level is further reduced at 36 h (Fig. 7A). Taken together, these results suggest that phosphorylation of both EspA and EspC is necessary for appropriate MrpC accumulation during development.
FIGURE 7.
Esp system negatively regulates MrpC protein stability. A, MrpC developmental protein accumulation patterns in the wild type (DZ2, wt), ΔespC (PH1044), ΔespA ΔespC (PH1047), espCH461A (PH1026), espCD749A (PH1027), and espCH461A,D749A (PH1028) strains. 10 μg of total protein lysates developed for the indicated hours in the 16-ml submerged culture format were subject to anti-MrpC immunoblot. B, chloramphenicol chase of MrpC. Wild type (wt) or ΔespA ΔespC cells were developed for 9 h as above and treated with 34 μg ml−1 chloramphenicol for the indicated minutes. Equal proportions of the samples were subject to anti-MrpC immunoblot. C, calculation of the half-life (t½) of MrpC. Triplicate biological replicates of the wild type (black line) and ΔespA ΔespC (gray line) chloramphenicol chase experiments represented in B were performed, and the MrpC band intensity for each time point was normalized to t = 0 of the respective strain and averaged. The natural log of the average intensities was plotted versus min of chloramphenicol treatment and the slope a linear fit of the data were used to calculate the MrpC t½ in wild type cells as described under “Experimental Procedures.” No significant decrease in MrpC signal intensity could be detected in the ΔespA ΔespC strain. Vertical bars, standard deviation of the average from triplicate biological replicates; *, average intensity values are significantly different (p = 0.003).
Given that the mrpC transcriptional pattern in espA mutants matched that of wild type (36), we postulated that the Esp system either effected translation or proteolytic turnover of MrpC. If MrpC is subject to proteolytic turnover, we would predict that MrpC levels would decrease in cells in which de novo protein synthesis is blocked. To examine whether this is the case, we developed wild type cells for 9 h in submerged culture, treated the cells with 34 μg ml−1 chloramphenicol, harvested cells after 0, 10, 20, or 30 min, and examined the MrpC levels by immunoblot (Fig. 7B). In the wild type strain, MrpC levels decreased steadily during the 30 min of treatment with a calculated half-life of 30 ± 8 min (Fig. 7C). To determine whether MrpC turnover was altered in the absence of EspA and EspC, we performed the same experiment with the ΔespA ΔespC strain. Interestingly, in this mutant, MrpC levels did not appreciably decrease (Fig. 7B), and quantification of the immunoblot revealed that no loss in MrpC signal was detected over 30 min of chloramphenicol treatment. Together, these results demonstrate that the MrpC protein is subject to proteolytic turnover, which is not observed in the absence of the Esp system.
DISCUSSION
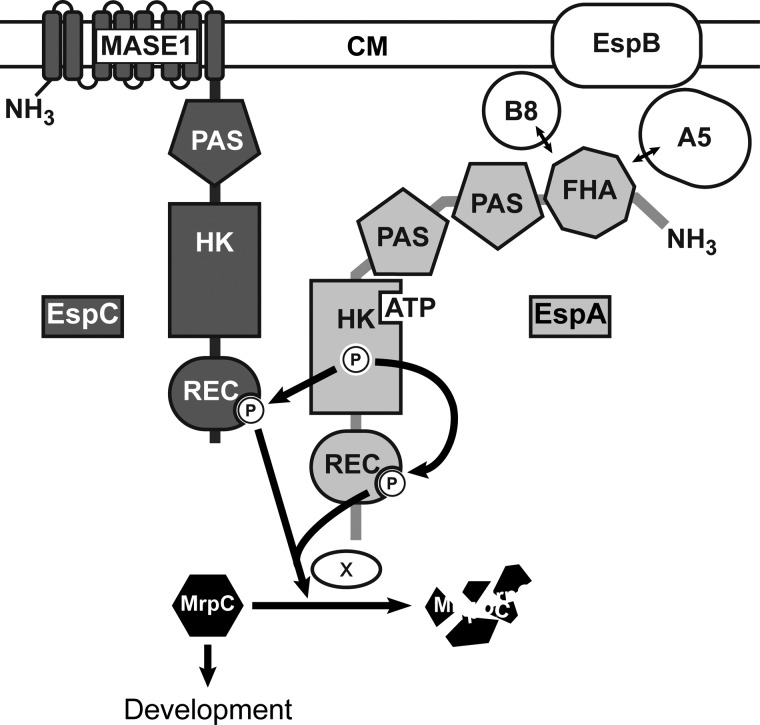
EspA, EspB, PktA5, and PktB8 form an unusually complex bacterial signaling system that negatively regulates progression through the elaborate M. xanthus developmental program (Fig. 8) (36, 37, 41). In this study, we reveal an additional level of complexity in this system by demonstrating that a second HyHPK, EspC, is an obligate component of this system. Together, our data suggest a model in which EspA and EspC function together via an intimate hybrid histidine kinase signaling mechanism involving both an inter-HyHPK phosphorylation (from EspA kinase region to EspC receiver domain) and intra-HyHPK phosphorylation (from EspA kinase to its own receiver) (Fig. 8). The combined phosphorylation of EspA and EspC receiver domains (EspA∼P/EspC∼P) is necessary to stimulate turnover of an important developmental regulator, MrpC.
FIGURE 8.
Model of the Esp signaling system. Two-hybrid histidine kinases EspC (dark gray) and EspA (light gray) regulate the accumulation rate of an important developmental regulator, MrpC, to ensure appropriate and coordinated progression through the M. xanthus developmental program. The combined phosphorylation of EspA and EspC receiver domains (REC) activates an unknown protease or protease targeting factor (×) to stimulate MrpC turnover. EspA histidine kinase region (HK) autophosphorylates and donates a phosphoryl group to both its own and EspC receiver domains. EspA may also act as a phosphatase on EspA and or EspC REC. Autophosphorylation of EspC histidine kinase domain is not required for stimulating MrpC turnover under laboratory developmental conditions. EspA activity is controlled by a signaling module consisting of two serine/threonine kinases, PktA5 (A5) and PktB8 (B8), and a putative transport protein (EspB) predicted to reside in the cytoplasmic membrane (CM) (37, 41). PktA5 and PktB8 are thought to interact with the Forkhead associated (FHA) domain in EspA (41). Two PAS domains in EspA and one in EspC may be involved in sensing internal or membrane-associated redox stimuli (65). EspC is predicted to be anchored in the CM by putative MASE1 sensing domain of unknown function (64). The array of signaling domains may allow cell fate-specific accumulation of MrpC within the developmental program.
The obligatory role of EspC in this signaling system is strongly supported by the observation that the ΔespA, ΔespC, and ΔespA ΔespC mutants display identical developmental phenotypes (Fig. 1). Consistently, we observed that if an espA ortholog could be identified in a sequenced myxobacterial genome, we could also identify an espC ortholog in the same genome. Our data indicate that EspA and EspC function together via an intimate hybrid histidine kinase signaling mechanism involving both an inter- and intra-HyHPK phosphorylation. First, we determined that the EspC receiver must be phosphorylated to negatively regulate development, but that EspC kinase is not the phosphoryl donor, because inactivation of kinase autophosphorylation either by substitution of the phosphoaccepting histidine residues or deletion of the entire CA domain yields the wild type phenotype (Figs. 3 and 6). The EspCHK is functional because it efficiently autophosphorylates in vitro (Fig. 4). EspCHK does not, however, efficiently donate a phosphoryl group to the EspC receiver (Fig. 5). Therefore, the phosphoryl donor must either be a small molecule donor (e.g. the intracellular acetyl-phosphate pools (5, 58) or an alternate kinase. The former is unlikely because the EspC receiver domain does not autophosphorylate in the presence of acetyl-[32P]phosphate in vitro (data not shown). Instead, our data suggest the phosphoryl donor is EspA kinase because of the following: 1) inactivation (EspAH407A) produces a phenotype identical to the EspCD749A mutant (Fig. 3); 2) our deletion mutant analysis indicated EspA and EspC must function together (Fig. 1); and 3) EspAHK∼P efficiently donates a phosphoryl group to the EspC REC in vitro (Fig. 5).
We suggest that the simplest interpretation of our observation of identical mutant phenotypes of the ΔespA, ΔespC, ΔespA ΔespC, espAD696A, and espCD749A strains is that the output from this system is the combined phosphorylation of both EspA and EspC receiver domains (Fig. 8). Given that it has been previously observed that receiver domains can both inhibit (13) or stimulate (59) cognate kinase activities, we also considered alternate scenarios in which one of the receivers plays a phosphorylation-dependent regulatory role to modulate the kinase activity of the other. We immediately discarded scenarios in which either of the receivers inhibit EspA kinase activity, because these models predict a delayed developmental phenotype in the ΔespC mutant or in a mutant where the EspA receiver domain was deleted (espAΔREC), and both of these mutants exhibit early development (this study and Ref. 36). We also discarded a model in which an EspA-phosphorylated receiver is the sole output, but EspA kinase-dependent phosphorylation of the EspC receiver stimulates the EspA kinase because EspA efficiently phosphorylates its own receiver in vitro (36). Finally, we disfavor a model in which the EspC receiver is the sole output, and phosphorylation of the EspA receiver is necessary to stimulate phosphorylation of the EspC receiver. In this last scenario, we would predict that in espAD696A and espAΔREC strains a low level of EspA-kinase phosphotransfer to EspC receiver would produce an intermediate phenotype rather than the null phenotypes observed in the respective mutants (Figs. 1 and 3) (36). It has also been previously proposed that kinases can form heterodimers (60). However, we do not favor a model in which EspA and EspC form obligate heterodimers, because both EspA and EspC kinase domains are functional as individually purified proteins (Fig. 4), and we could detect EspAHK-EspAHK and EspCHK-EspCHK, but not EspAHK-EspCHK, interactions by yeast two-hybrid analysis.5 Thus, we favor the model in which EspA dimers interact with EspC dimers at minimum through the EspAHK and EspCREC domains.
Our data suggest no significant role for the EspCHK domain in control of developmental progression because neither substitution of the phosphoaccepting residue, deletion of the CA domain, nor mutation of several residues predicted to disrupt putative phosphatase activity produce an obvious mutant phenotype. Because we were unable to develop a system to test in vitro phosphatase activity of EspCHK, we cannot test whether EspCHK displays phosphatase activity and whether these mutants indeed disrupt phosphatase activity. However, a disruption of this motive in espA (espAN411D) produced a delayed developmental phenotype (Fig. 6) that is consistent with hyperphosphorylation of EspA and/or EspC receivers. Because the EspCHK module is capable of efficient autophosphorylation in vitro, we hypothesize that EspC kinase activity is retained because it is necessary for modulating the Esp system under conditions not triggered in the laboratory setting.
Our data suggest that the target of the Esp signaling system is the proteolytic turnover of MrpC, a key developmental transcriptional regulator that is necessary to promote aggregation of cells into fruiting bodies (31, 61), and it may play a role in programmed cell death (26, 28). MrpC transcriptional activity is thought to be negatively regulated by a serine/threonine kinase cascade and stimulated in an MrpC isoform thought to lack the 25 N-terminal amino acids (33). We and others have previously demonstrated that premature activation (34) or accumulation (36) of MrpC leads to early accumulation of the MrpC targets and produces an early developmental phenotype. The observation that MrpC accumulates inappropriately rapidly in the ΔespA (36) and ΔespC mutants (Fig. 7) is explained by the demonstration that during early development MrpC is rapidly turned over in wild type (t½ ∼30 min) but not in ΔespA ΔespC mutant cells. Given that the espAD696A and espCD749A point mutants exhibit the same phenotype as the deletion mutants, we propose that the EspA∼P/EspC∼P signaling state stimulates proteolytic turnover of MrpC.
We do not yet understand how EspA∼P/EspC∼P stimulates proteolytic turnover of MrpC, but there are several examples of regulated proteolysis controlled by RRs. For example, E. coli RpoS, the master regulator of the general stress response, is targeted to the ClpXP proteosome by interaction with the phosphorylated RR, RssB (62), and the C. crescentus single domain RR, CpdR, promotes degradation of the master regulator, CtrA, by interacting with ClpXP and promoting its localization to its target (63). We favor the hypothesis that EspA/EspC receiver domains regulate MrpC proteolysis by directly binding target proteins (e.g. sequestering a targeting protein in the unphosphorylated state or by recruiting MrpC together with a protease/protease targeting factor in the phosphorylated state). Alternatively, it may be that the Esp system feeds into a multistep phosphorelay involving an unidentified histidine phosphotransferase protein and soluble RR output protein.
One additional output of EspA∼P/EspC∼P is the repression of EspA and EspC protein accumulation, because early developing espA or espC point mutant strains also accumulate both EspA and EspC earlier than in the wild type (Figs. 1B and 3B) (36). At least in the case of the espAH407A mutant, early EspAH407A production correlates with increased espA transcription (36), suggesting that the system somehow additionally modulates a transcriptional regulator of espAB and likely espC. An intriguing possibility is that this transcriptional regulator is MrpC itself, leading to a negative feedback such that as long as EspA/EspC are phosphorylated their levels and MrpC levels remain low.
What is the advantage of this intricate signaling system? An obvious answer is that regulation of MrpC accumulation can be coupled to stimuli sensing domains of both EspA and EspC. This complex signal sensing capacity may be justified because different patterns of MrpC accumulation may mediate different developmental cell fate decisions in M. xanthus (31, 32). Perhaps different combinations of extra-cytoplasmic and cytoplasmic signals could lead the Esp system to regulate MrpC accumulation differently in cells destined for different developmental cell fates. Both Esp proteins contain multiple sensing domains; EspC contains an integral membrane MAES1 domain of unknown function (64) and a PAS domain; EspA contains a fork-head associated (FHA) domain and two PAS domains (Fig. 2). PAS domains have been implicated in binding of various diverse ligands, mediating protein interactions, protein localization, and signal transmission (65). FHA domains are protein interaction modules that recognize phosphothreonine-containing proteins (66), and the EspA FHA domain is very likely involved in binding to the kinases PktA5 and PktB8 that modulate EspA activity (41). We propose that each of these sensing domains could contribute to modulation of the level of EspA∼P/EspC∼P-generating cell-specific regulation of MrpC accumulation in response to temporal, spatial, and condition-specific signals. In the case of EspC, the sensing domains may regulate EspC kinase activity in response to signals not present under laboratory conditions and/or by simply regulating accessibility of EspC receiver to EspA kinase.
In summary, this study reveals a novel signaling mechanism involving two orphan HyHPKs that participate in an obligate inter- and intraphosphotransfer such that the signaling output is a combined phosphorylation of the two receiver domains. This system represents an excellent example of the inherent plasticity of the His-Asp signaling family, and in particular, it highlights HyHPKs as important players in signal integration.
Supplementary Material
Acknowledgments
We gratefully acknowledge past and present members of the Higgs group for helpful discussions. We also gratefully acknowledge Stuart Huntley and Katharina Schlereth for critical reading of the manuscript.
This work was supported in part by the Max Planck Society.

This article contains supplemental Fig. S1, Tables S1 and S2, and Methods.
T. Jeganathan, A. Schramm, and P. Higgs, unpublished data.
- HK
- histidine kinase region
- HPK
- histidine protein kinase
- HyHPK
- hybrid histidine protein kinase
- REC
- receiver
- RR
- response regulator
- DHp
- dimerization and histidine phosphotransfer domain
- CA
- catalytic ATPase
- FHA
- forkhead associated domain
- PAS
- Per Arnt Sim sensing domain
- MASE1
- membrane-associated sensing domain 1
- IB
- inclusion body
- aa
- amino acid
- LSB
- Laemmli sample buffer.
REFERENCES
- 1. Stock A. M., Robinson V. L., Goudreau P. N. (2000) Two-component signal transduction. Annu. Rev. Biochem. 69, 183–215 [DOI] [PubMed] [Google Scholar]
- 2. Wuichet K., Cantwell B. J., Zhulin I. B. (2010) Evolution and phyletic distribution of two-component signal transduction systems. Curr. Opin. Microbiol. 13, 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dutta R., Qin L., Inouye M. (1999) Histidine kinases. Diversity of domain organization. Mol. Microbiol. 34, 633–640 [DOI] [PubMed] [Google Scholar]
- 4. Casino P., Rubio V., Marina A. (2009) Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell 139, 325–336 [DOI] [PubMed] [Google Scholar]
- 5. Lukat G. S., McCleary W. R., Stock A. M., Stock J. B. (1992) Phosphorylation of bacterial response regulator proteins by low molecular weight phosphodonors. Proc. Natl. Acad. Sci. U.S.A. 89, 718–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bourret R. B. (2010) Receiver domain structure and function in response regulator proteins. Curr. Opin. Microbiol. 13, 142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huynh T. N., Noriega C. E., Stewart V. (2010) Conserved mechanism for sensor phosphatase control of two-component signaling revealed in the nitrate sensor NarX. Proc. Natl. Acad. Sci. U.S.A. 107, 21140–21145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu Y., Qin L., Yoshida T., Inouye M. (2000) Phosphatase activity of histidine kinase EnvZ without kinase catalytic domain. Proc. Natl. Acad. Sci. U.S.A. 97, 7808–7813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jagadeesan S., Mann P., Schink C. W., Higgs P. I. (2009) A novel “four-component” two-component signal transduction mechanism regulates developmental progression in Myxococcus xanthus. J. Biol. Chem. 284, 21435–21445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mascher T., Helmann J. D., Unden G. (2006) Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 70, 910–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galperin M. Y. (2006) Structural classification of bacterial response regulators. Diversity of output domains and domain combinations. J. Bacteriol. 188, 4169–4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Posas F., Wurgler-Murphy S. M., Maeda T., Witten E. A., Thai T. C., Saito H. (1996) Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86, 865–875 [DOI] [PubMed] [Google Scholar]
- 13. Inclán Y. F., Laurent S., Zusman D. R. (2008) The receiver domain of FrzE, a CheA-CheY fusion protein, regulates the CheA histidine kinase activity and downstream signalling to the A- and S-motility systems of Myxococcus xanthus. Mol. Microbiol. 68, 1328–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wegener-Feldbrügge S., Søgaard-Andersen L. (2009) The atypical hybrid histidine protein kinase RodK in Myxococcus xanthus. Spatial proximity supersedes kinetic preference in phosphotransfer reactions. J. Bacteriol. 191, 1765–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wuichet K., Zhulin I. B. (2010) Origins and diversification of a complex signal transduction system in prokaryotes. Sci. Signal. 3, ra50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldman B. S., Nierman W. C., Kaiser D., Slater S. C., Durkin A. S., Eisen J. A., Eisen J., Ronning C. M., Barbazuk W. B., Blanchard M., Field C., Halling C., Hinkle G., Iartchuk O., Kim H. S., Mackenzie C., Madupu R., Miller N., Shvartsbeyn A., Sullivan S. A., Vaudin M., Wiegand R., Kaplan H. B. (2006) Evolution of sensory complexity recorded in a myxobacterial genome. Proc. Natl. Acad. Sci. U.S.A. 103, 15200–15205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schneiker S., Perlova O., Kaiser O., Gerth K., Alici A., Altmeyer M. O., Bartels D., Bekel T., Beyer S., Bode E., Bode H. B., Bolten C. J., Choudhuri J. V., Doss S., Elnakady Y. A., Frank B., Gaigalat L., Goesmann A., Groeger C., Gross F., Jelsbak L., Kalinowski J., Kegler C., Knauber T., Konietzny S., Kopp M., Krause L., Krug D., Linke B., Mahmud T., Martinez-Arias R., McHardy A. C., Merai M., Meyer F., Mormann S., Muñoz-Dorado J., Perez J., Pradella S., Rachid S., Raddatz G., Rosenau F., Rückert C., Sasse F., Scharfe M., Schuster S. C., Suen G., Treuner-Lange A., Velicer G. J., Vorhölter F. J., Weissman K. J., Welch R. D., Wenzel S. C., Whitworth D. E., Wilhelm S., Wittmann C., Blocker H., Puhler A., Muller R. (2007) Complete genome sequence of the Myxobacterium Sorangium cellulosum. Nat. Biotechnol. 25, 1281–1289 [DOI] [PubMed] [Google Scholar]
- 18. Huntley S., Hamann N., Wegener-Feldbrügge S., Treuner-Lange A., Kube M., Reinhardt R., Klages S., Müller R., Ronning C. M., Nierman W. C., Søgaard-Andersen L. (2011) Comparative genomic analysis of fruiting body formation in Myxococcales. Mol. Biol. Evol. 28, 1083–1097 [DOI] [PubMed] [Google Scholar]
- 19. Huntley S., Zhang Y., Treuner-Lange A., Kneip S., Sensen C. W., Søgaard-Andersen L. (2012) Complete genome sequence of the fruiting Myxobacterium corallococcus coralloides DSM 2259. J. Bacteriol. 194, 3012–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inouye S., Nariya H., Munoz-Dorado J. (2008) in Myxobacteria: Multicellularity and Differentiation (Whitworth D. E., ed) pp. 191–210, American Society for Microbiology, Washington, D. C [Google Scholar]
- 21. Pérez J., Castañeda-García A., Jenke-Kodama H., Müller R., Muñoz-Dorado J. (2008) Eukaryotic-like protein kinases in the prokaryotes and the myxobacterial kinome. Proc. Natl. Acad. Sci. U.S.A. 105, 15950–15955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whitworth D. E., Cock P. J. (2008) Two-component systems of the myxobacteria. Structure, diversity, and evolutionary relationships. Microbiology 154, 360–372 [DOI] [PubMed] [Google Scholar]
- 23. Whitworth D. E., Cock P. J. (2008) in Myxobacteria: Multicellularity and Differentiation (Whitworth D. E., ed) pp. 169–189, American Society for Microbiology, Washington, D. C [Google Scholar]
- 24. Shi X., Wegener-Feldbrügge S., Huntley S., Hamann N., Hedderich R., Søgaard-Andersen L. (2008) Bioinformatics and experimental analysis of proteins of two-component systems in Myxococcus xanthus. J. Bacteriol. 190, 613–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zusman D. R., Scott A. E., Yang Z., Kirby J. R. (2007) Chemosensory pathways, motility, and development in Myxococcus xanthus. Nat. Rev. Microbiol. 5, 862–872 [DOI] [PubMed] [Google Scholar]
- 26. Lee B., Holkenbrink C., Treuner-Lange A., Higgs P. I. (2012) Myxococcus xanthus developmental cell fate production: heterogeneous accumulation of developmental regulatory proteins and reexamination of the role of MazF in developmental lysis. J. Bacteriol. 194, 3058–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Janssen G. R., Dworkin M. (1985) Cell-cell interactions in developmental lysis of Myxococcus xanthus. Dev. Biol. 112, 194–202 [DOI] [PubMed] [Google Scholar]
- 28. Nariya H., Inouye M. (2008) MazF, an mRNA interferase, mediates programmed cell death during multicellular Myxococcus development. Cell 132, 55–66 [DOI] [PubMed] [Google Scholar]
- 29. O'Connor K. A., Zusman D. R. (1991) Behavior of peripheral rods and their role in the life cycle of Myxococcus xanthus. J. Bacteriol. 173, 3342–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Connor K. A., Zusman D. R. (1991) Development in Myxococcus xanthus involves differentiation into two cell types, peripheral rods and spores. J. Bacteriol. 173, 3318–3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun H., Shi W. (2001) Genetic studies of mrp, a locus essential for cellular aggregation and sporulation of Myxococcus xanthus. J. Bacteriol. 183, 4786–4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ueki T., Inouye S. (2003) Identification of an activator protein required for the induction of fruA, a gene essential for fruiting body development in Myxococcus xanthus. Proc. Natl. Acad. Sci. U.S.A. 100, 8782–8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nariya H., Inouye S. (2005) Identification of a protein Ser/Thr kinase cascade that regulates essential transcriptional activators in Myxococcus xanthus development. Mol. Microbiol. 58, 367–379 [DOI] [PubMed] [Google Scholar]
- 34. Nariya H., Inouye S. (2006) A protein Ser/Thr kinase cascade negatively regulates the DNA-binding activity of MrpC, a smaller form of which may be necessary for the Myxococcus xanthus development. Mol. Microbiol. 60, 1205–1217 [DOI] [PubMed] [Google Scholar]
- 35. Müller F. D., Treuner-Lange A., Heider J., Huntley S. M., Higgs P. I. (2010) Global transcriptome analysis of spore formation in Myxococcus xanthus reveals a locus necessary for cell differentiation. BMC Genomics 11, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Higgs P. I., Jagadeesan S., Mann P., Zusman D. R. (2008) EspA, an orphan hybrid histidine protein kinase, regulates the timing of expression of key developmental proteins of Myxococcus xanthus. J. Bacteriol. 190, 4416–4426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cho K., Zusman D. R. (1999) Sporulation timing in Myxococcus xanthus is controlled by the espAB locus. Mol. Microbiol. 34, 714–725 [DOI] [PubMed] [Google Scholar]
- 38. Rasmussen A. A., Søgaard-Andersen L. (2003) TodK, a putative histidine protein kinase, regulates timing of fruiting body morphogenesis in Myxococcus xanthus. J. Bacteriol. 185, 5452–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee B., Higgs P. I., Zusman D. R., Cho K. (2005) EspC is involved in controlling the timing of development in Myxococcus xanthus. J. Bacteriol. 187, 5029–5031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cho K., Treuner-Lange A., O'Connor K. A., Zusman D. R. (2000) Developmental aggregation of Myxococcus xanthus requires frgA, an frz-related gene. J. Bacteriol. 182, 6614–6621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stein E. A., Cho K., Higgs P. I., Zusman D. R. (2006) Two Ser/Thr protein kinases essential for efficient aggregation and spore morphogenesis in Myxococcus xanthus. Mol. Microbiol. 60, 1414–1431 [DOI] [PubMed] [Google Scholar]
- 42. Lee B., Schramm A., Jagadeesan S., Higgs P. I. (2010) Two-component systems and regulation of developmental progression in Myxococcus xanthus. Methods Enzymol. 471, 253–278 [DOI] [PubMed] [Google Scholar]
- 43. Maniatis T., Fritsch E. F., Sambrook J. (1982) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 44. Ueki T., Inouye S., Inouye M. (1996) Positive-negative KG cassettes for construction of multigene deletions using a single drug marker. Gene 183, 153–157 [DOI] [PubMed] [Google Scholar]
- 45. Zheng L., Baumann U., Reymond J. L. (2004) An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res. 32, e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li Z. F., Li X., Liu H., Liu X., Han K., Wu Z. H., Hu W., Li F. F., Li Y. Z. (2011) Genome sequence of the halotolerant marine bacterium Myxococcus fulvus HW-1. J. Bacteriol. 193, 5015–5016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thomas S. H., Wagner R. D., Arakaki A. K., Skolnick J., Kirby J. R., Shimkets L. J., Sanford R. A., Löffler F. E. (2008) The mosaic genome of Anaeromyxobacter dehalogenans strain 2CP-C suggests an aerobic common ancestor to the δ-proteobacteria. PLoS ONE 3, e2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ivanova N., Daum C., Lang E., Abt B., Kopitz M., Saunders E., Lapidus A., Lucas S., Glavina Del Rio T., Nolan M., Tice H., Copeland A., Cheng J. F., Chen F., Bruce D., Goodwin L., Pitluck S., Mavromatis K., Pati A., Mikhailova N., Chen A., Palaniappan K., Land M., Hauser L., Chang Y. J., Jeffries C. D., Detter J. C., Brettin T., Rohde M., Göker M., Bristow J., Markowitz V., Eisen J. A., Hugenholtz P., Kyrpides N. C., Klenk H. P. (2010) Complete genome sequence of Haliangium ochraceum type strain (SMP-2). Stand. Genomic Sci. 2, 96–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Abramoff M. D., Magalhaes P. J., Ram S. J. (2004) Image processing wit ImageJ. Biophotonics Int. 11, 36–42 [Google Scholar]
- 50. Nath K., Koch A. L. (1970) Protein degradation in Escherichia coli. I. Measurement of rapidly and slowly decaying components. J. Biol. Chem. 245, 2889–2900 [PubMed] [Google Scholar]
- 51. Segal H. L., Kim Y. S. (1963) Glucocorticoid stimulation of the biosynthesis of glutamic-alanine transaminase. Proc. Natl. Acad. Sci. U.S.A. 50, 912–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arias I. M., Doyle D., Schimke R. T. (1969) Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J. Biol. Chem. 244, 3303–3315 [PubMed] [Google Scholar]
- 53. Belle A., Tanay A., Bitincka L., Shamir R., O'Shea E. K. (2006) Quantification of protein half-lives in the budding yeast proteome. Proc. Natl. Acad. Sci. U.S.A. 103, 13004–13009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hsing W., Silhavy T. J. (1997) Function of conserved histidine-243 in phosphatase activity of EnvZ, the sensor for porin osmoregulation in Escherichia coli. J. Bacteriol. 179, 3729–3735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen Y. E., Tsokos C. G., Biondi E. G., Perchuk B. S., Laub M. T. (2009) Dynamics of two phosphorelays controlling cell cycle progression in Caulobacter crescentus. J. Bacteriol. 191, 7417–7429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dutta R., Yoshida T., Inouye M. (2000) The critical role of the conserved Thr-247 residue in the functioning of the osmosensor EnvZ, a histidine kinase/phosphatase, in Escherichia coli. J. Biol. Chem. 275, 38645–38653 [DOI] [PubMed] [Google Scholar]
- 57. Jiang P., Atkinson M. R., Srisawat C., Sun Q., Ninfa A. J. (2000) Functional dissection of the dimerization and enzymatic activities of Escherichia coli nitrogen regulator II and their regulation by the PII protein. Biochemistry 39, 13433–13449 [DOI] [PubMed] [Google Scholar]
- 58. Wolfe A. J. (2010) Physiologically relevant small phosphodonors link metabolism to signal transduction. Curr. Opin. Microbiol. 13, 204–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Paul R., Jaeger T., Abel S., Wiederkehr I., Folcher M., Biondi E. G., Laub M. T., Jenal U. (2008) Allosteric regulation of histidine kinases by their cognate response regulator determines cell fate. Cell 133, 452–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Goodman A. L., Merighi M., Hyodo M., Ventre I., Filloux A., Lory S. (2009) Direct interaction between sensor kinase proteins mediates acute and chronic disease phenotypes in a bacterial pathogen. Genes Dev. 23, 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sun H., Shi W. (2001) Analyses of mrp genes during Myxococcus xanthus development. J. Bacteriol. 183, 6733–6739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hengge R. (2009) Proteolysis of σS (RpoS) and the general stress response in Escherichia coli. Res. Microbiol. 160, 667–676 [DOI] [PubMed] [Google Scholar]
- 63. Iniesta A. A., McGrath P. T., Reisenauer A., McAdams H. H., Shapiro L. (2006) A phospho-signaling pathway controls the localization and activity of a protease complex critical for bacterial cell cycle progression. Proc. Natl. Acad. Sci. U.S.A. 103, 10935–10940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nikolskaya A. N., Mulkidjanian A. Y., Beech I. B., Galperin M. Y. (2003) MASE1 and MASE2. Two novel integral membrane sensory domains. J. Mol. Microbiol. Biotechnol. 5, 11–16 [DOI] [PubMed] [Google Scholar]
- 65. Henry J. T., Crosson S. (2011) Ligand-binding PAS domains in a genomic, cellular, and structural context. Annu. Rev. Microbiol. 65, 261–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pallen M., Chaudhuri R., Khan A. (2002) Bacterial FHA domains. Neglected players in the phosphothreonine signaling game? Trends Microbiol. 10, 556–563 [DOI] [PubMed] [Google Scholar]
- 67. Campos J. M., Zusman D. R. (1975) Regulation of development in Myxococcus xanthus. Effect of 3′,5′-cyclic AMP, ADP, and nutrition. Proc. Natl. Acad. Sci. U.S.A. 72, 518–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Julien B., Kaiser A. D., Garza A. (2000) Spatial control of cell differentiation in Myxococcus xanthus. Proc. Natl. Acad. Sci. U.S.A. 97, 9098–9103 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.