Background: p115-RhoGEF can be regulated by activated Gα13.
Results: Both RGS and DH domains of p115-RhoGEF interact with Gα13.
Conclusion: The binding of RGS to Gα13 facilitates direct association of Gα13 to DH to regulate its exchange activity.
Significance: RGS domains can act cooperatively with other domains to mediate effector regulation by G proteins.
Keywords: G Proteins, Guanine Nucleotide Exchange Factor (GEF), RGS Proteins, Rho GTPases, Signal Transduction
Abstract
RGS-containing RhoGEFs (RGS-RhoGEFs) represent a direct link between the G12 class of heterotrimeric G proteins and the monomeric GTPases. In addition to the canonical Dbl homology (DH) and pleckstrin homology domains that carry out the guanine nucleotide exchange factor (GEF) activity toward RhoA, these RhoGEFs also possess RGS homology (RH) domains that interact with activated α subunits of G12 and G13. Although the GEF activity of p115-RhoGEF (p115), an RGS-RhoGEF, can be stimulated by Gα13, the exact mechanism of the stimulation has remained unclear. Using combined studies with small angle x-ray scattering, biochemistry, and mutagenesis, we identify an additional binding site for activated Gα13 in the DH domain of p115. Small angle x-ray scattering reveals that the helical domain of Gα13 docks onto the DH domain, opposite to the surface of DH that binds RhoA. Mutation of a single tryptophan residue in the α3b helix of DH reduces binding to activated Gα13 and ablates the stimulation of p115 by Gα13. Complementary mutations at the predicted DH-binding site in the αB-αC loop of the helical domain of Gα13 also affect stimulation of p115 by Gα13. Although the GAP activity of p115 is not required for stimulation by Gα13, two hydrophobic motifs in RH outside of the consensus RGS box are critical for this process. Therefore, the binding of Gα13 to the RH domain facilitates direct association of Gα13 to the DH domain to regulate its exchange activity. This study provides new insight into the mechanism of regulation of the RGS-RhoGEF and broadens our understanding of G protein signaling.
Introduction
RGS-RhoGEFs3 are a homologous subfamily of RhoGEFs (guanine nucleotide exchange factors for Rho proteins) that contain regulator of G protein signaling (RGS) domains. There are three members of this subfamily: p115-RhoGEF (p115), PDZ-RhoGEF (PRG), and leukemia-associated RhoGEF (LARG). They represent potential regulatory links between G protein-coupled receptors that activate the G12 class of heterotrimeric G proteins and RhoA-mediated pathways that lead to cytokinesis and transformation (1, 2). RGS-RhoGEFs catalyze the exchange of GDP for GTP on RhoA, a small GTPase of the Ras superfamily (3). Activated RhoA bound to GTP can then engage downstream effectors and influence cellular functions. Like all members of the large family of RhoGEFs (about 70 in the human genome), the GEF activity of RGS-RhoGEFs resides in their tandemly linked Dbl homology (DH) and pleckstrin homology (PH) domains (4, 5). The RGS homology (RH) domains are situated N-terminal to the DH/PH domains. The RH domains of p115 and LARG function as GTPase-activating proteins (GAPs) for Gα13 and Gα12 subunits, and binding of Gα subunits to their respective RhoGEFs stimulates their guanine nucleotide exchange activity toward RhoA (6–9). In addition to the RH domain, PRG and LARG also contain an N-terminal PDZ domain that has been shown to mediate interaction of the RGS-RhoGEFs with regulatory proteins (10–12).
The exact mechanism by which Gα13 stimulates the exchange activity of p115 remains elusive (7–9). Studies on interactions between RGS-RhoGEFs and activated Gα13 have focused primarily on the RH domain, which led to elucidation of crystal structures of the RH domain alone and complexes between RH and Gα13 (13–16). The RH domains in RGS-RhoGEFs share low sequence similarity to the canonical RGS domain and require elements outside of the consensus RGS box for proper folding and binding to Gα13. In p115, these include the 23IIG and 27EDEDF motifs located N-terminal to the consensus RGS box and the 163MGM motif C-terminal to the RGS box (see Fig. 1A). When bound to Gα13, RH occupies both the regulator binding and effector binding sites on the GTPase, mainly through direct interaction with its Ras-like domain. The presence of RH is crucial for stimulation of the GEF activity by Gα13, as the activity of a p115 fragment missing the RH domain could not be regulated by Gα13 (17). As expected, mutations within the 27EDEDF motif resulted in the loss of GAP activity and a reduction in binding affinity of p115 toward Gα13. The same mutations, however, had little impact on the stimulation of GEF activity by Gα13 (18). This raises the question of whether direct association between RH and Gα13 is actually required for the stimulation of GEF activity; it also suggests that regions outside of RH in p115 might interact with Gα13 during the activation process. There is evidence that activated Gα13 binds weakly to regions outside of RH; however, it has not been shown that the GEF activity of p115 is regulated by such interactions (9, 17).
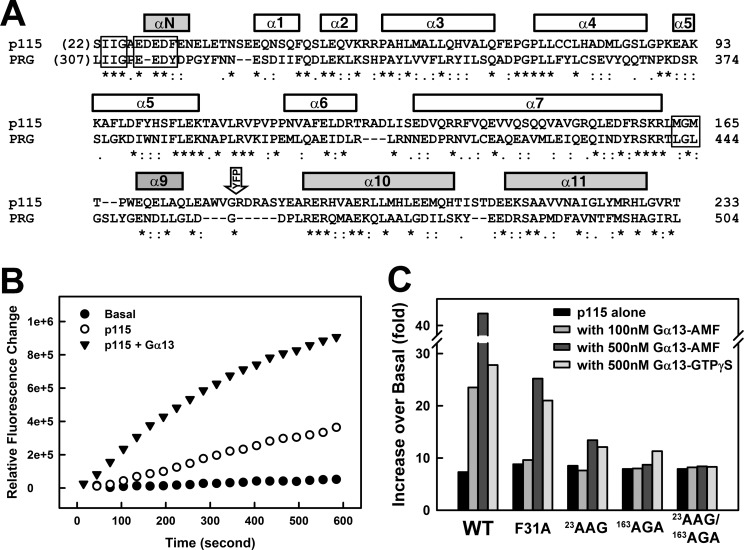
FIGURE 1.
Two hydrophobic anchors in the RH domain of p115 are critical for stimulation of GEF activity by Gα13. A, structural based sequence alignment of RH domains from p115 and PRG. The helices are represented with bars on top of the amino acid sequences. The sequence alignment was carried out using the program Clustal W (35). The shaded bars represent additional elements N- and C-terminal to the consensus RGS box (white bars). The 23IIG motif and the 163MGM motif, as well as the 27EDEDF motif critical for the GAP activity of p115, are boxed. The open arrow labeled YFP indicates the position of the insertion of YFP between helices α9 and α10. B, nucleotide exchange assays with p115RH-L-DH/PH and RhoA. For each time course, 2 μm RhoA was mixed with 5 μm mant-GTP, and the exchange reaction was started at room temperature by the addition of buffer (basal, solid circles), 100 nm p115 (open circles), or 100 nm p115 with 100 nm Gα13 bound to GDP·AlF4−·Mg2+ (GDP·AMF, closed triangles). The subsequent increase in fluorescence (λex = 356 nm, λem = 445 nm) was measured for 10 min. C, stimulation of the GEF activity of RH-L-DH/PH by activated Gα13 bound to GDP·AMF or GTPγS. Binding of mant-GTP to RhoA was measured as shown in B. The initial rates were approximated by linear regression and plotted as the fold increase over basal exchange on RhoA for the indicated conditions: WT, wild-type p115; F31A, Phe-31 in 27EDEDF mutated to alanine; 23AAG, 23IIG mutated to AAG; 163AGA, 163MGM mutated to AGA; and 23AAG/163AGA, combination of both mutations.
Here, a combination of molecular cloning, biochemical assays and small angle x-ray scattering (SAXS) was used to examine the interaction of activated Gα13 with a C-terminally truncated p115 molecule that includes the tandemly linked DH and PH domains in addition to RH. We show that the 23IIG and the 163MGM hydrophobic motifs, respectively, located N- and C-terminal to the consensus RGS box rather than the 27EDEDF motif are crucial for the stimulation of the GEF activity of p115 by activated Gα13. Furthermore, activated Gα13 interacts with the DH domain via a novel effector binding site located within its helical domain. Mutations of residues in the αB-αC loop of the helical domain negatively affect stimulation of p115 by Gα13. The additional binding site in p115 for Gα13 is located on the side of DH opposite to the binding site for the substrate RhoA. Mutations of residues in the predicted binding site for Gα13 on DH abolished stimulation of GEF activity by Gα13. These observations provide a comprehensive model for the molecular mechanism by which the intrinsic exchange activity of RGS-RhoGEFs is regulated by activated G proteins.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
Coding regions of human p115-RhoGEF were subcloned into a pGEX-KG vector containing the protease recognition site for the tobacco etch virus (pGEX-KG-TEV) for proteolytic cleavage of the expressed domains from glutathione S-transferase as described previously (19, 20). His6 tags were also inserted at the C termini of the p115 coding sequences. The proteins were expressed and purified from Escherichia coli strain BL21(DE3) as described (20). The expression and purification of a C-terminally truncated human RhoA (residues 1–181) was carried out as described previously (19). The N-terminally truncated Gα13 (41–377) was expressed and purified from insect cells as described (13).
Nucleotide Exchange Assay
Fluorescence assays measuring the binding of N-methylanthraniloyl-GTP (mant-GTP; Invitrogen) were performed on a Fluorolog-3 spectrofluorometer at room temperature (λex = 356 nm, λem = 445 nm, slits = 1/1 nm), as described previously (20). In each assay, 1–2 μm RhoA was incubated with 5 μm mant-GTP in reaction buffer (25 mm NaHEPES, pH 8.0, 50 mm NaCl, 1 mm DTT, and 5 mm MgCl2) in a 200 μl cuvette. The exchange reaction was started by the addition of 100 nm p115, in the presence or absence of activated Gα13.
Binding of GTPγS to Gα13
Purified Gα13 was exchanged into binding buffer (20 mm NaHEPES, pH 8, 1 mm EDTA, 1 mm DTT, 50 mm NaCl, and 10 μm GDP) and concentrated to 100–250 μm. The concentrate was adjusted to 0.5 mm MgSO4 and 1 mm GTPγS and incubated at 25 °C for 48–72 h.
Size Exclusion Chromatography with p115 and Gα13-GTPγS
Fragments of p115 were mixed with activated Gα13 bound to GTPγS, in a buffer containing 25 mm NaHEPES, pH 8.0, 100 mm NaCl, 1 mm DTT, 1 mm EDTA, and 5 mm MgCl2. The mixture was concentrated by Amicon-Ultra 4 (10 kDa) concentrators (Millipore) to a final volume of less than 1 ml and then loaded onto Superdex 200/75 columns (Amersham Biosciences) that had been pre-equilibrated with the same buffer.
Collection of SAXS Data
The purified RH-L-DH/PH fragment of p115 was dialyzed overnight in 25 mm NaHEPES, pH 8.0, 200 mm NaCl, 5 mm β-mercaptoethanol, 1 mm EDTA, 10 mm DTT, and 5% glycerol to ensure a precise buffer match for background subtraction of the scattering arising with the buffer alone from that of the protein sample. The complex of p115 RH-L-DH/PH and activated Gα13 bound to GDP·AlF4−·Mg2+ was dialyzed overnight in 25 mm NaHEPES, pH 8.0, 200 mm NaCl, 5 mm β-mercaptoethanol, 1 mm EDTA, 10 mm DTT, 0.05 mm AlCl3, 5 mm MgCl2, 5 mm NaF, and 5% glycerol. Triton X-100 was added to the sample to a final concentration of 0.5% before the measurement to prevent aggregation. The addition of Triton X-100 had no effect on the stimulation of GEF activity by activated Gα13 (supplemental Fig. S1). The samples for SAXS were at a concentration between 1 and 4 mg/ml. Measurements were taken at 10 °C using the SAXS instrument at the BioCAT beamline of the Advanced Photon Source at the Argonne National Laboratory as described previously (20). The samples were analyzed by size exclusion chromatography before and after the SAXS measurements to determine concentration and oligomerization state. Scattering profiles (intensity I versus scattering vector Q) were reduced, and the SAXS data were merged using IGOR Pro software (WaveMetrics, Inc.) with macros written by the BioCAT staff. Structural parameters and the distance distribution functions, P(r), were calculated with GNOM (21) using data up to a Q of 0.30 and 0.31 Å−1, for p115 alone and the p115-Gα13 complex, respectively.
Ab Initio Modeling
Low resolution molecular shape reconstructions from the experimental scattering data were performed with GASBOR (22). GASBOR searches a chain-compatible spatial distribution of an exact number of dummy residues, corresponding to the Cα atoms of protein amino acids. The Cα chain is folded to minimize the discrepancy between the scattering curve calculated from the folded model and the experimental scattering curve. More than 80 GASBOR calculations were performed, and 20 calculations with the smallest standard deviation relative to the experimental data were selected for averaging by DAMAVER (23) to generate the final model; this represents the most probable conformation reconstruction for the protein. The molecular envelopes of RH-L-DH/PH, alone or in a complex with activated Gα13, were calculated based on SAXS data using the program Situs (24), and the crystal structures of DH/PH (20) and the Gα13-RH complex (14) were then fit into the envelope using the program Chimera (25).
RESULTS
Two Conserved Hydrophobic Motifs outside of the Canonical RGS Box in p115-RhoGEF Are Critical for Stimulation of GEF Activity by Gα13
The GEF activity of RGS-RhoGEFs can be stimulated by activated G12 class Gα subunits in vivo, and some of the RGS-RhoGEFs can be stimulated in vitro by activated Gα13 (1). In the case of p115, Gα13 bound to GDP·AlF4−·Mg2+ (GDP·AMF), which mimics the transition state of GTP hydrolysis, is more efficient at stimulating the GEF activity of p115 than Gα13 bound to GTPγS, a nonhydrolyzable analog of GTP (Fig. 1C). This is anticipated because p115 is a GAP for Gα13, and it has a much higher affinity toward Gα13 bound to GDP·AMF than toward Gα13 bound to GTP. The GAP activity of RGS-RhoGEFs is conveyed by the RH domain (14), particularly by a series of acidic residues followed by a phenylalanine located N-terminal to the RGS box (the 27EDEDF motif; Fig. 1A). A recent study of the interfaces observed in various complexes of RH-Gα13 revealed two conserved hydrophobic anchors in RH (13): the 23IIG motif at the very N terminus of RH and the 163MGM motif just C-terminal to the RGS box (Fig. 1A). Mutation of either one of the two hydrophobic anchors greatly diminished binding between RH and activated Gα13 (data not shown) and greatly reduced stimulation of p115 by Gα13 (Fig. 1C). However, the reduction can be at least partially recovered by using higher concentrations of activated Gα13 (Fig. 1C). Gα13 could not activate p115 containing mutations of both isoleucines of 23IIG and the two methionines of 163MGM, even in the presence of higher concentrations of activated Gα13 (Fig. 1C). In contrast, the F31A mutation had much less impact on Gα13-stimulated GEF activity of p115 (Fig. 1C). This is consistent with an earlier study demonstrating that mutations within the 27EDEDF motif led to a loss of GAP activity and a reduction in binding affinity between RH and Gα13, but the same mutations did not diminish Gα13-stimulated GEF activity in the background of full-length p115 (18). In fact, the GAP activity is not required at all for the stimulation of GEF activity by Gα13. The replacement of RH in p115 with that from PRG, which is not a GAP, had little effect on Gα13-stimulated GEF activity (supplemental Fig. S2) (17). Taken together, these data show that the physical association of RH and Gα13, mediated by the two conserved hydrophobic anchors outside of the RGS box, is critical for stimulation of GEF activity by activated Gα13.
A Binding Site for Activated Gα13 outside of the RH Domain
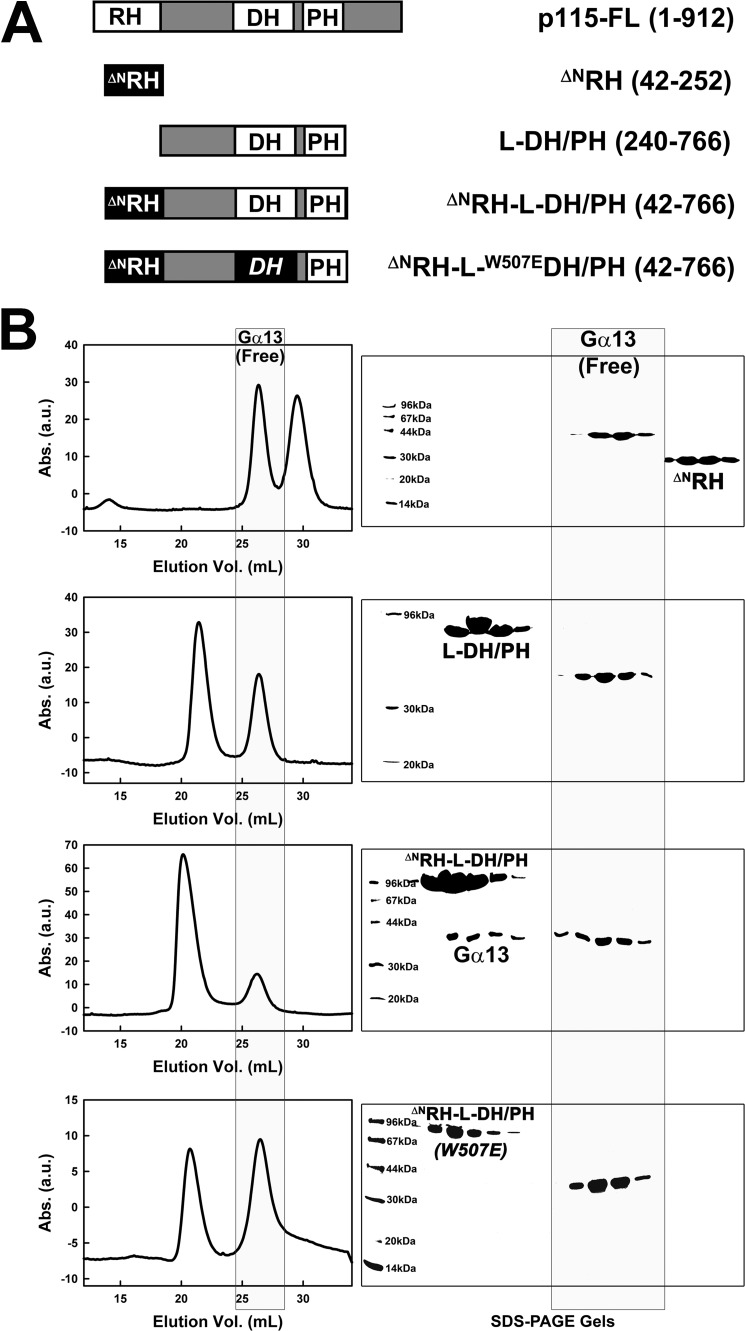
The interaction between RH domains and activated Gα13 has been well characterized (13, 14). The binding affinities between RH and Gα13 range between 10 nm to 1 μm, depending on the specific RH domain and the state of the GTPase.4 Binding of Gα13 to regions outside of the RH domain has been suggested (9, 17), but the evidence is limited. The affinity between truncated p115 missing RH and activated Gα13 is low, such that no physical association between the two could be observed by size exclusion chromatography (Fig. 2). In contrast, activated Gα13 and RH form a stable complex that is dependent on two interfaces (13, 14). The N-terminal 41 amino acids in RH include one of these interfaces, the 23IIG motif and the 27EDEDF motif, which are located N-terminal to the consensus RGS box. Truncation of this N-terminal segment (ΔNRH; Fig. 2A) results in a sharp decrease in the affinity of the RH domain itself toward activated Gα13. Although the intact RH domain readily forms a stable complex with activated Gα13, ΔNRH failed to form a complex with Gα13-GTPγS that can be detected by size exclusion chromatography (Fig. 2B). A fragment of p115 consisting of L-DH/PH also failed to bind Gα13-GTPγS (Fig. 2B). However, the fragment consisting of both ΔNRH and L-DH/PH can form a weak but readily observable complex with the activated α subunit (Fig. 2B). This strongly suggests the existence of a weak binding site for activated Gα13 within the L-DH/PH region in addition to the known site in ΔNRH.
FIGURE 2.
An additional binding site for activated Gα13 outside of RH. A, schematic representation of truncated forms of p115 used; the included amino acids are listed in parentheses. B, left panels, chromatograms from size exclusion chromatography with p115 and Gα13-GTPγS are aligned based on elution volumes; peaks corresponding to free (unbound) Gα13 with elution volumes around 26 ml are outlined in a gray box. Right panels, SDS-PAGE gels showing components of eluted peaks of protein. Lanes on gels are aligned based on volumes of elution. Molecular masses of protein standard makers (first lanes on the left) are labeled. Fractions corresponding to free (unbound) Gα13 are outlined in a gray box. The same amount of Gα13, which was premixed with equal molar of the p115 fragment, was used in all four experiments. The scale of absorption (Abs.) in the last panel (at bottom) was changed because of variations in detector efficiency.
Solution Structure of the C-terminally Truncated p115
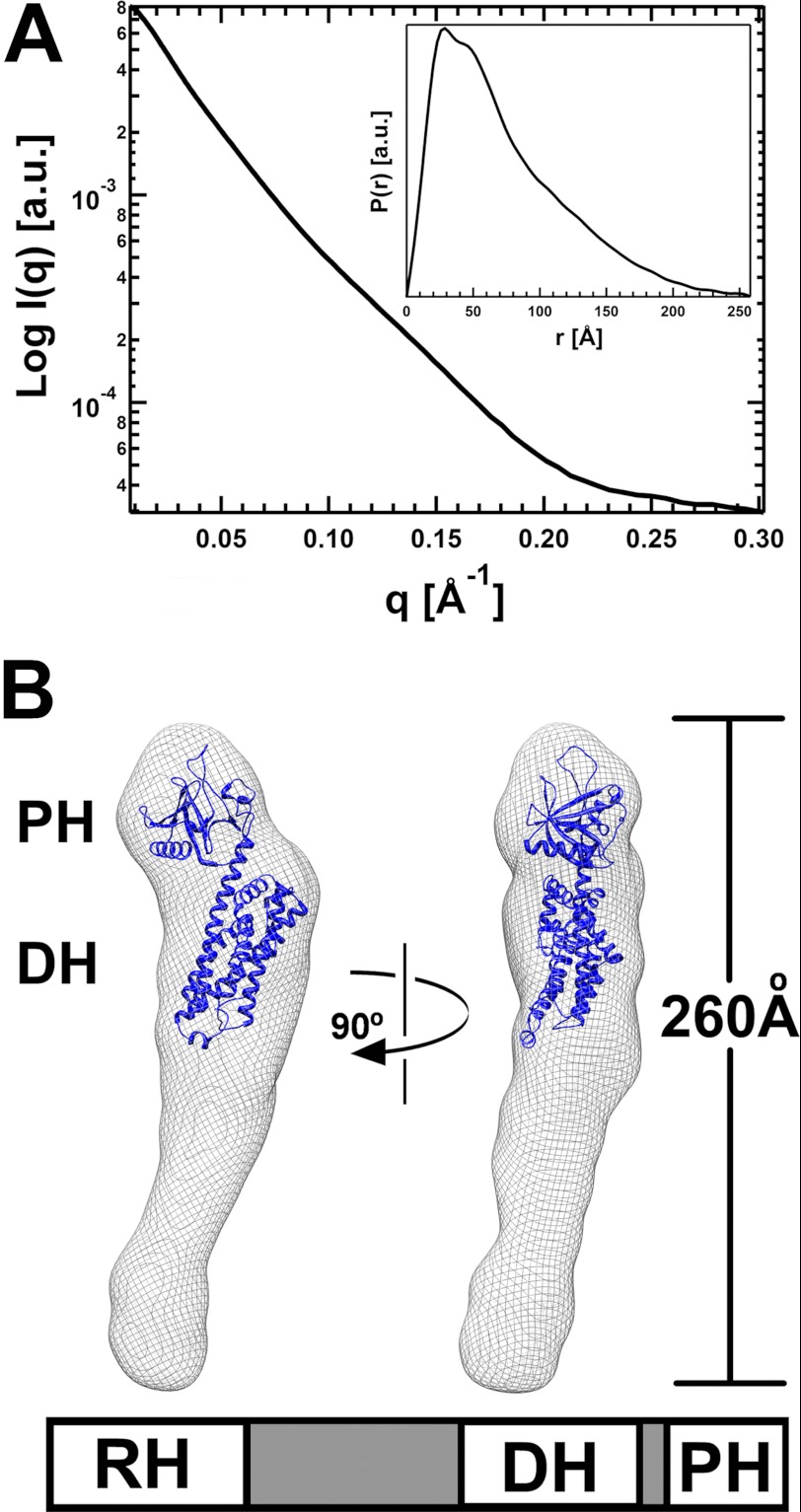
Dimerization of p115 in solution is dependent on the domain C-terminal to PH (1). Thus, the C-terminally truncated proteins used in Figs. 1 and 2 are monomeric. We determined the solution structure of the C-terminally truncated p115 (RH-L-DH/PH) at 20 Å resolution using SAXS. The molecular envelope of RH-L-DH/PH was obtained by ab initio shape reconstruction from experimental SAXS data (22). The experimental scattering profile is shown in Fig. 3A. Low angle scattering intensity calibration with cytochrome c indicated that RH-L-DH/PH exists as a monomer in solution, with a distance distribution function, P(r), that is characteristic of an elongated molecule (Fig. 3A, inset). The calculated molecular envelope of RH-L-DH/PH confirms this, with the longest dimension of the molecule at ∼260 Å. The crystal structure of p115 DH/PH (20) fits well into the calculated molecular envelope (Fig. 3B), occupying half of the envelope. The shape and volume of this region closely resembles those calculated for solution envelopes of the DH/PH domains in the absence of RH (20) or without the linker (data not shown). The linker region and RH putatively occupy the volume of the molecular envelope below the DH domain.
FIGURE 3.
Solution structures of p115 RH-L-DH/PH. A, solution x-ray scattering profile for p115 RH-L-DH/PH. The distance distribution function (inset), P(r), of RH-L-DH/PH was computed from the x-ray scattering using the program GNOM (21). B, solution structure of RH-L-DH/PH of p115. The solution structure (molecular envelope) is depicted as a mesh and superimposed onto the crystal structure of the p115 DH/PH domains (blue ribbon). The large unoccupied region in the molecular envelope beneath the DH domain presumably contains the linker region and RH. The schematic representation of RH-L-DH/PH of p115 is depicted underneath the molecular envelope.
The spatial relationship between DH/PH and RH is not evident. The linker region is disordered in the crystal structure of L-DH/PH and partially disordered in solution (20). Crystal structures of RH domains are available; however, the exact location of RH within the volume below DH (Fig. 3B) could not be unambiguously determined. To identify the location of RH, we inserted YFP between helices α9 and α10 of RH (Fig. 1A) and determined the molecular envelope of the YFPRH-L-DH/PH fusion protein by SAXS (supplemental Fig. S3). The YFP fusion protein is elongated, with the longest dimension similar to that of the native p115. The portion of the YFPRH-L-DH/PH envelope containing the DH/PH domains can be overlapped relatively well with that of the native protein. There is almost no change in shape or volume at the distal end (supplemental Fig. S3) of the envelope, suggesting that YFP, and hence the RH domain, is not located in this area. The major difference between the two envelopes is evident in the center region underneath the DH domain. Insertion of YFP in RH caused a 20° bend of the molecule in the middle, a strong indication that RH (and YFP) is located in this region, near the bottom of the DH domain. The close proximity of RH to DH is also supported by the calculated molecular envelope of RH-L-DH/PH bound to activated Gα13.
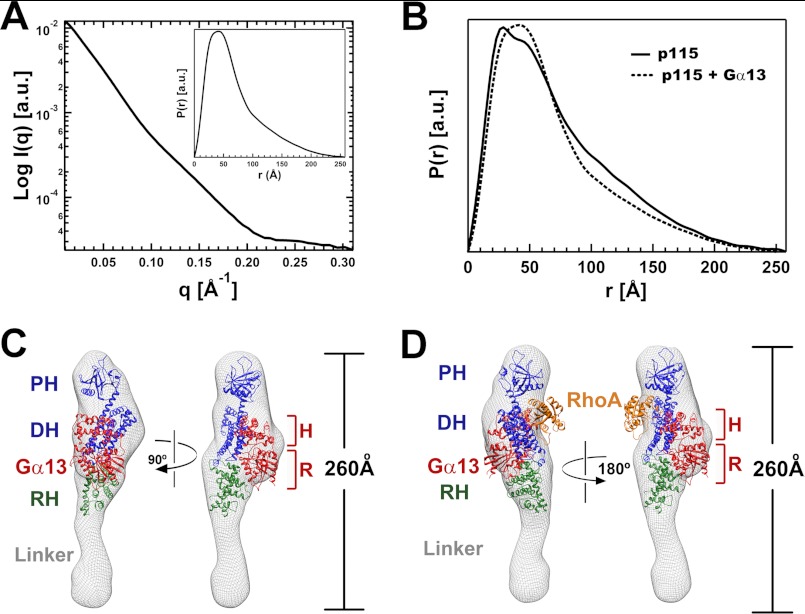
Solution Structure of a p115-Gα13 Complex
The solution structure of RH-L-DH/PH from p115 (Fig. 3B) in a complex with activated Gα13 bound to GDP·AMF was determined by SAXS at a resolution of 20 Å. The experimental scattering profile is shown in Fig. 4A. Low angle scattering intensity calibration with cytochrome c indicated that the complex is monomeric in solution. Comparison of the P(r) function of p115 alone and that of the complex indicates that the overall shapes of the two are similar, with the longest dimension being 260 Å in both cases (Fig. 4B). The molecular envelope of the RH-L-DH/PH-Gα13 complex was obtained by ab initio shape reconstruction from experimental SAXS data. Although the overall shape of the complex resembles that of the RH-L-DH/PH alone, a large increase in volume (∼65,000 Å3) was observed in the midsection of the molecular envelope, near the bottom of the modeled DH domain. This additional volume can accommodate a Gα13 molecule (calculated at ∼50,000 Å3). As discussed above, binding of the RH domain to activated Gα13 is critical for both the activation of p115 and the formation of a stable complex between p115 and Gα13 in solution (Fig. 2B). It is therefore reasonable to assume that the RH domain remains bound to Gα13 in this complex. Hence, the RH domain is located near the DH domain where the increase in mass is observed, as concluded from inspection of the molecular envelope of the YFP fusion protein (supplemental Fig. S3). The crystal structure of the p115-RH-Gα13 complex (14) can be fit into the calculated solution envelope together with the DH/PH domains (Fig. 4C). In this model, activated Gα13 contacts the DH domain, opposite to the site at which RhoA binds (Fig. 4D). Importantly, the binding site for Gα13 on the DH domain does not overlap with the binding site for RhoA. The DH domain appears to form an interface with the helical domain of Gα13 rather than the Ras-like domain, which is engaged with the RH domain as observed in the structures of RH-Gα13 complexes (13, 14). Thus, Gα13 utilizes both the helical and the Ras-like domains for interaction with p115. We do note that the positioning of RhoA in this model (Fig. 4D) is based exclusively on crystal structures of DH/PH-RhoA complexes (26, 27). A solution structure of the ternary complex may provide further insight into the orientation of RhoA in the presence of Gα13.
FIGURE 4.
Solution structures of the complex of p115 RH-L-DH/PH bound to Gα13-GDP·AMF. A, solution x-ray scattering profile for the complex, with the distance distribution function (inset), P(r). B, comparison of the distance distribution functions of p115 alone and its complex with Gα13. The overall shapes of the two are similar, with the longest dimensions being identical at ∼260 Å. C, solution structure of RH-L-DH/PH of p115 bound to Gα13-GDP·AMF. The solution structure (molecular envelope) is depicted as a mesh and superimposed onto the crystal structures of the p115 DH/PH domains (blue ribbon) and the complex of RH (green ribbon) bound to Gα13-GDP·AMF (red ribbon). The helical domain and the Ras-like domain of Gα13 are outlined with brackets and labeled with H and R, respectively. The unoccupied region in the molecular envelope beneath the RH domain is likely to be the location for most of the linker region. D, a model for the ternary complex of RH-L-DH/PH bound to activated Gα13 and nucleotide-free RhoA. RhoA (orange ribbon) was modeled into the binding site on DH/PH of p115 based on crystal structures of PRG-DH/PH or LARG-DH/PH bound to nucleotide-free RhoA (26, 27). The location of RhoA was not experimentally determined by SAXS in this study.
Mutations in a Predicted Binding Site for Gα13 on DH Abolish Gα13-stimulated GEF Activity
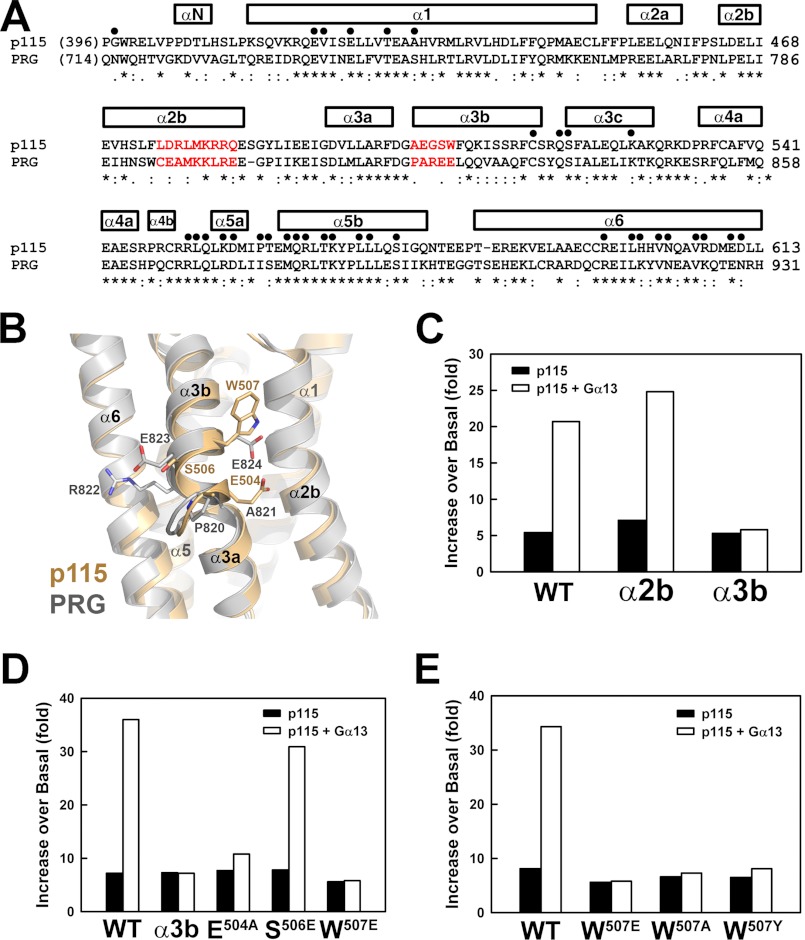
The model of the RH-L-DH/PH-Gα13 complex derived from interpretation of SAXS data features an interface between the DH domain of p115 and the helical domain of Gα13 (Fig. 4C). Structures of DH domains from RGS-RhoGEFs (20, 26, 27) consist of six major α-helices (Fig. 5A). Unlike p115, the GEF activity of PRG, a member of the RGS-RhoGEF family, cannot be stimulated by activated Gα13 in vitro, even though its RH domain binds tightly to the α subunit (13). Thus, differences in the amino acid sequences and tertiary structures of the DH domains of p115 and PRG provide clues to the location of the binding site at which Gα13 exerts GEF stimulatory activity toward p115. Two DH segments that are poorly conserved between p115 and PRG were identified at the putative interface between DH and Gα13 in the SAXS model of the RH-L-DH/PH-Gα13 complex (Fig. 5, A and B). The first segment is located in the α2b helix, consisting of amino acids 475–483 (α2b). The second segment is located within the α3b helix, including residues 503–507 (α3b). None of these residues in α2b or α3b are involved in binding to RhoA (Fig. 5A). Replacement of residues in α2b or α3b in p115 with those from PRG had little effect on the basal exchange activity of p115 toward RhoA, indicating that these substitutions do not affect the structure of the DH recognition site for RhoA (Fig. 5C and supplemental Fig. S4). Although changes in α2b had no effect on GEF activity stimulated by Gα13, swapping of residues from PRG into α3b in p115 abolished stimulation by Gα13 in vitro (Fig. 5C).
FIGURE 5.
Mutations at the predicted Gα13-binding site on DH abolish the Gα13-stimulated GEF activity of p115. A, sequence alignment of DH domains from p115 and PRG. Residues involved in contacts with nucleotide-free RhoA are marked with dots over the alignment. Helices are represented with bars on top of the amino acid sequences. The sequence alignment was carried out using the program Clustal W (35). The two potential binding sites for Gα13 targeted for mutagenesis are colored red. B, ribbon diagrams with superimposed structures of DH domains from p115 (wheat) and PRG (gray). Side chains in α3b are depicted as sticks. Oxygen and nitrogen atoms are colored red and blue, respectively. C, stimulation of the GEF activity of RH-L-DH/PH by activated Gα13. Binding of mant-GTP to RhoA was measured as shown in Fig. 1B. Initial rates were approximated by linear regression, and the increases of exchange rate over basal RhoA were plotted for the following proteins: WT, wild-type p115; α2b, mutant p115 where residues 475–483 were substituted with corresponding residues from PRG; and α3b, mutant p115 where residues 503–507 were substituted with corresponding residues from PRG. D, effects of single point mutations in the α3b region on stimulation by Gα13. E, effects of single point mutations of Trp507 on stimulation by Gα13.
Three of the five amino acids in α3b are exposed to solvent in the crystal structures of p115 and PRG DH domains and are not conserved in the amino acid sequences of the domains (Fig. 5B). These include Glu504 (Ala821 in PRG), Ser506 (Glu823 in PRG), and Trp507 (Glu824 in PRG). Single point mutations at any of these three positions in p115 had varied effects on stimulation of GEF activity by Gα13 (Fig. 5D). Mutation of Ser506 to glutamate in p115 had no effect on this regulation. The change of glutamate 504 to alanine decreased the response of p115 to activated Gα13. Mutation of the exposed tryptophan 507 to glutamate, the corresponding residue in PRG, completely abolished Gα13-stimlated GEF activity of p115. None of these point mutations affected the basal exchange activity of p115 in the absence of Gα13. The p115 fragment, ΔNRH-L-DH/PH, bearing the W507E mutation also failed to bind activated Gα13 as assayed by size exclusion chromatography (Fig. 2). Mutations of Trp507 to either a tyrosine or an alanine also greatly reduced Gα13-stimulated GEF activity of p115 (Fig. 5E).
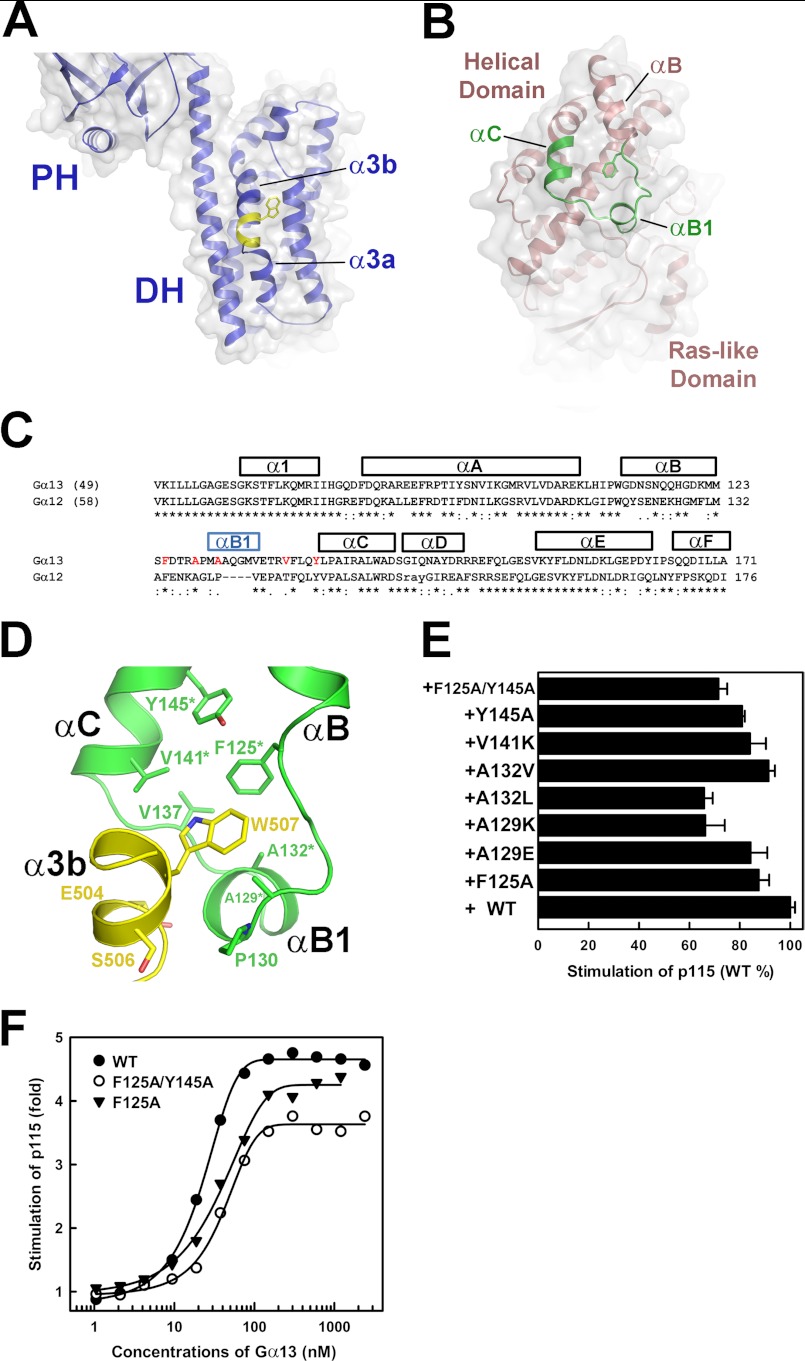
The αB-αC Loop in the Helical Domain of Gα13 Is a Novel Effector Binding Motif
The α3b helix in the DH domain of p115, which includes Trp507, forms a protruding bulge on the surface of DH that is predicted to interact with the helical domain of Gα13 in our model (Fig. 6A). Q-SiteFinder, a ligand-binding site prediction program (28), identifies a hydrophobic pocket between the αB and αC helices of Gα13 in the helical domain (Fig. 6, B–D) as a possible site for protein-protein interaction. The shape of this pocket appears roughly complementary to the protruding bulge formed by Trp507 from DH. These surfaces are in close proximity in the low resolution model of the p115-Gα13 complex derived from SAXS (Fig. 4C). Part of the α3b helix from DH, including residues 502–510, can be docked into this hydrophobic pocket in the αB-αC loop on Gα13 using the program PatchDock (29). In this model, Trp507 from DH docks into the hydrophobic pocket on Gα13 formed by the side chains of Phe125, Ala129, Pro130, Met131, Ala132, Val137, Val141, and Tyr145 (Fig. 6D). Glu504 from DH also makes van der Waals contacts with residues from this region. Single point mutations of these hydrophobic residues in the αB-αC region had varied effects on the stimulation of GEF activity of p115 by Gα13 in vitro (Fig. 6E). Mutation of Ala129 to lysine or Ala132 to leucine resulted in a 40% reduction in stimulation of GEF activity of p115 by Gα13. Similar reduction of stimulation of p115 was observed with a double mutation of Phe125 and Tyr145 to alanines. Such a reduction in stimulation of p115 correlated with a lowered efficacy of the mutant when compared with the wild-type Gα13 (Fig. 6F). These mutations had no effect on the intrinsic GTPase activity of Gα13 or its ability to bind guanine nucleotide (supplemental Fig. S5).
FIGURE 6.
A binding pocket for DH within the helical domain of Gα13. A, ribbon diagram of the DH domain of p115. The molecule is positioned such that the proposed Gα13-binding site on DH (yellow) is facing the reader. The side chain of Trp507 is shown as sticks. The surface of DH is shown and colored gray. B, ribbon diagram of Gα13. The molecule is positioned such that the proposed DH binding site (green) is facing the reader. The side chain of Phe125 is shown as sticks. The surface of Gα13 is shown and colored gray. C, structural based sequence alignment of Gα13 and Gα12 helical domains. Helices are represented with bars on top of the amino acid sequences. The sequence alignment is carried out by the program Clustal W (35). Residues targeted for mutagenesis from the hydrophobic pocket are colored red. D, ribbon diagram depicting a modeled interface between α3b and the hydrophobic pocket in the helical domain of Gα13 using the same color scheme as in A and B. Side chains of residues involved are depicted as stick models. Residues from the helical domain of Gα13 that impact the stimulation of p115 when mutated are marked with asterisks. E, stimulation of the GEF activity of full-length p115 by activated Gα13. Reactions contained 2 μm RhoA, 30 nm p115, and 300 nm Gα13 (wild type or mutant). The effect of mutations in Gα13 on the initial rate of the GEF activity of p115 is compared against wild-type Gα13 (100%). Each experiment was repeated at least twice. F, stimulation of p115 with different concentrations of Gα13. The initial rate of the GEF activity of p115 (approximated by linear regression with the first 4 min of a 6-min reaction) was assessed in the presence of increasing concentrations of activated Gα13 (wild type or mutant). The reactions contained 2 μm RhoA and 30 nm p115: WT (closed circles), F125A (closed triangles), F125A/Y145A (open circles).
DISCUSSION
Earlier studies on interactions between RGS-RhoGEFs and activated Gα13 have been primarily focused on RH and its interaction with Gα13. The residues in the α3b helix of DH described here are the first outside of the RH domain to be implicated as direct mediators for activation by G proteins. The binding affinity between DH and activated Gα13 is low; hence DH alone may not be sufficient to associate with the α subunit. An important function of the RH domain, as suggested by data presented in this report, might be to hold activated Gα13 in a position for optimized interaction with the α3b helix in the DH domain of p115.
The DH and RH domains of RGS-RhoGEFs are separated by long linker regions. Interpretation of the size exclusion chromatography data (Fig. 2) and the low resolution molecular envelopes of Gα13 with p115 (Fig. 4) is most consistent with a model in which Gα13 interacts simultaneously with the RH and the DH domains of p115. Because the 150-residue linker region in p115 intervenes between RH and DH, it must therefore adopt a hairpin conformation, in which the N and C termini of the linker are nearly juxtaposed. The linker region from p115 would occupy the portion of the molecular envelope distal to the PH domain and beneath the RH domain (Fig. 4). The linker region in p115 appears to be rigid, because molecular envelopes of p115 alone and its complex with Gα13 are of the same length. Our results differ from a recent study of PRG by SAXS (30). Like that of p115, the RH-L-DH/PH fragment of PRG is also an elongated structure. However, the RH and DH modules of PRG are well separated in the SAXS model by the linker region. The linker connecting RH and DH in PRG appears to be very flexible and changes both in length and in overall shape in the presence of the N-terminal PDZ domain (not present in p115) or when bound to RhoA. It is not clear how Gα13 would affect the overall shape of PRG in solution, because PRG cannot be stimulated by Gα13 in vitro.
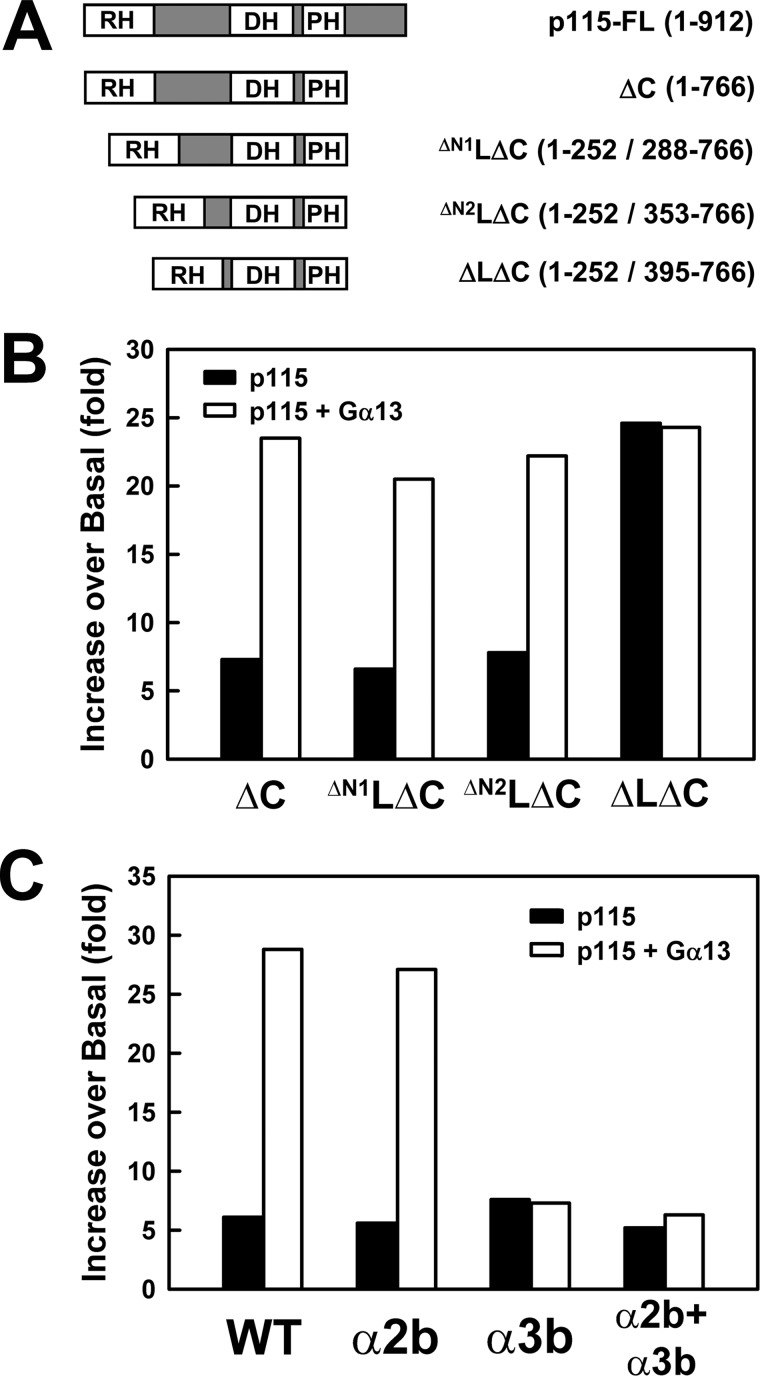
The hairpin conformation of the p115 linker in our model allows the C terminus of the linker to contact the RH domain as the polypeptide chain returns from its excursion to the hairpin back toward the DH domain. This is supported by the observation that C-terminally extended RH constructs (RH-L) are not stably expressed unless the full-length linker is included in the construct (data not shown). RH-L constructs missing the C-terminal 40 residues in the linker region did not express well as soluble protein. Furthermore, removal of the N-terminal ∼100 residues of the linker region (RH-ΔN2l-DH/PH; Fig. 7A) did not affect stimulation by Gα13 (Fig. 7B) or the apparent interaction between DH and Gα13 (Fig. 7C). Complete removal of the linker region, however, led to a significant increase in the basal exchange activity of p115 and diminished stimulation by Gα13 (Fig. 7B). This is consistent with recent reports in which the linker region in RGS-RhoGEFs has been shown to play an autoinhibitory role (20, 31). The presence of the 40 amino acids in the linker immediately preceding the start of DH lowers the basal exchange activity of these RhoGEFs. A recent study suggests that this segment from the linker perturbs the folding of the GEF switch at the N terminus of DH in p115 (20). The same segment in the linker would interact with RH in our model. A possible scenario for Gα13-stimulated GEF activity is that the binding of Gα13 to RH and DH leads to conformational changes at the RH-L interface, which leads to formation of a productive interface between the GEF switch and RhoA that is required for enhanced GEF activity. Thus, binding of activated Gα13 to RH, and to a less extent to DH, relieves the autoinhibition imposed by the linker region of p115.
FIGURE 7.
Removal of part of the linker region does not affect stimulation of p115 by Gα13. A, schematic representation of the truncated forms of p115 used; the included amino acids are listed in parentheses. B, stimulation of the GEF activity of RH-ΔL-DH/PH by activated Gα13. Binding of mant-GTP to RhoA was measured as shown in Fig. 1. Removal of the first 100 amino acids in the linker region had no effect on stimulation by Gα13. The last 40 residues within the linker region immediately preceding DH is important for activation by Gα13. C, stimulation of the GEF activity of mutated RH-ΔN2l-DH/PH by activated Gα13. The constructs are: WT, wild-type p115-ΔN2LΔC; α2b, mutant p115-ΔN2LΔC where residues 475–483 were substituted with corresponding residues from PRG; α3b, mutant p115-ΔN2LΔC where residues 503–507 were substituted with corresponding residues from PRG; and α2b+α3b, mutant p115-ΔN2LΔC where both α2b and α3b regions were substituted with corresponding regions from PRG.
Interaction between the DH domain and the α subunit of a G protein has been reported before. p63-RhoGEF, a GEF for the small GTPase Rho, can be activated by Gαq. In the crystal structure of the Gαq-p63-RhoGEF complex, parts of the α2 and α3 helices from the DH domain also make contact with the Gα subunit (32), but in this case, the segments in Gαq that interact with DH come from the Ras-like domain of Gαq, rather than its helical domain. Nevertheless, interactions between the DH domains of RhoGEFs and their regulatory proteins might be a common mechanism for regulation.
The proposed binding of the p115 DH domain to the helical domain of Gα13 defines a new effector interface for heterotrimeric G proteins and the potential to define the contribution of direct activation of the GEF activity of p115 in physiological function. All heterotrimeric G protein α subunits possess two domains: a Ras-like domain that makes direct contacts with regulators and effectors and a less well functionally characterized helical domain. Thus far, the only protein-protein interactions that have been ascribed to the helical domain are between Gαi and the GoLoco motif of RGS14 (33) and between Gα13 and the RH domain of RGS-RhoGEFs (13, 14). The α3b helix in the DH domain of p115, which includes Trp507, forms a protruding bulge on the surface of DH that is predicted to interact with the helical domain of Gα13 in our models (Fig. 4). One of the potential binding sites for the DH domain of p115 within the helical domain of Gα13 includes the αB-αC helices, because these surfaces are in close proximity in the low resolution model of the p115-Gα13 complex derived from SAXS (Figs. 4C and 6). The hydrophobic pocket in the helical domain is unique to Gα13. As described in previous studies (14), a notable feature of the helical domain of Gα13 that is not present in other known Gα structures is the “helical insert” (αB1) between the αB and αC helices, which forms part of this hydrophobic pocket (Fig. 6, B–D). In Gα12, the other G protein that interacts with the RGS-RhoGEFs, the loop connecting the αB and αC helices is shortened by four amino acids (Fig. 6C). Comparison of the structures of Gα13 and Gα12 (34) reveals that αB1 is not present in Gα12. The GEF activity of p115 can be stimulated by activated Gα13, but not Gα12, in vitro (7). The lack of the αB1 helix or an intact hydrophobic pocket in the helical domain of Gα12 might explain this observation. The GEF activity of p115 can be stimulated by activation of Gα12 in vivo. However, this activation is likely due to the localization of p115 to the plasma membrane where free RhoA is located. The conformation of the helical domain is not significantly different between the GDP- and the GTP-bound forms of G protein α subunits. Therefore it is anticipated that any specific regulation of effectors by this region would require coincident interactions of effector molecules with the conformationally flexible switch regions within the Ras-like domain as observed here. This could represent a common mechanism by which RGS domains in effector proteins could both regulate turnover of the G proteins and provide assistance to other sites that regulate effector activities.
Supplementary Material
Acknowledgments
We thank the staff of the Advanced Photon Source for assistance with data collection.
This work was supported, in whole or in part, by National Institutes of Health Grants GM31954 (to P. C. S.) and DK46371 (to S. R. S.). This work was also supported in part by U.S. Department of Energy, Basic Energy Sciences, Office of Science Contract W-31-109-ENG-38; National Institutes of Health Grant RR-08630; and the Alfred and Mabel Gilman Chair in Molecular Pharmacology (to P. C. S.).

This article contains supplemental Figs. S1–S5.
Z. Chen, L. Guo, J. Hadas, S. Gutowski, S. R. Sprang, and P. C. Sternweis, unpublished data.
- GEF
- guanine nucleotide exchange factor
- RGS
- regulators for G protein signaling
- RH
- RGS homology domain
- GAP
- GTPase-activating protein
- DH
- Dbl homology domain
- PH
- pleckstrin homology domain
- PDZ
- post synaptic density protein (PSD95), Drosophila disc large tumor suppressor (DlgA), and zonula occludens-1 protein (zo-1)
- mant-GTP
- N-methylanthraniloyl-GTP
- SAXS
- small angle x-ray scattering
- p115
- p115-RhoGEF
- PRG
- PDZ-RhoGEF
- LARG
- leukemia-associated RhoGEF
- GTPγS
- guanosine 5′-O-(thiotriphosphate).
REFERENCES
- 1. Sternweis P. C., Carter A. M., Chen Z., Danesh S. M., Hsiung Y. F., Singer W. D. (2007) Regulation of Rho guanine nucleotide exchange factors by G proteins. Adv. Protein Chem. 74, 189–228 [DOI] [PubMed] [Google Scholar]
- 2. Aittaleb M., Boguth C. A., Tesmer J. J. (2010) Structure and function of heterotrimeric G protein-regulated Rho guanine nucleotide exchange factors. Mol. Pharmacol. 77, 111–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Etienne-Manneville S., Hall A. (2002) Rho GTPases in cell biology. Nature 420, 629–635 [DOI] [PubMed] [Google Scholar]
- 4. Rossman K. L., Der C. J., Sondek J. (2005) GEF means go. Turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6, 167–180 [DOI] [PubMed] [Google Scholar]
- 5. Schmidt A., Hall A. (2002) Guanine nucleotide exchange factors for Rho GTPases. Turning on the switch. Genes Dev. 16, 1587–1609 [DOI] [PubMed] [Google Scholar]
- 6. Kozasa T., Jiang X., Hart M. J., Sternweis P. M., Singer W. D., Gilman A. G., Bollag G., Sternweis P. C. (1998) p115 RhoGEF, a GTPase activating protein for Ga12 and Ga13. Science 280, 2109–2111 [DOI] [PubMed] [Google Scholar]
- 7. Hart M. J., Jiang X., Kozasa T., Roscoe W., Singer W. D., Gilman A. G., Sternweis P. C., Bollag G. (1998) Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Ga13. Science 280, 2112–2114 [DOI] [PubMed] [Google Scholar]
- 8. Suzuki N., Nakamura S., Mano H., Kozasa T. (2003) Gα12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF. Proc. Natl. Acad. Sci. U.S.A. 100, 733–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suzuki N., Tsumoto K., Hajicek N., Daigo K., Tokita R., Minami S., Kodama T., Hamakubo T., Kozasa T. (2009) Activation of leukemia-associated RhoGEF by Gα13 with significant conformational rearrangements in the interface. J. Biol. Chem. 284, 5000–5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taya S., Inagaki N., Sengiku H., Makino H., Iwamatsu A., Urakawa I., Nagao K., Kataoka S., Kaibuchi K. (2001) Direct interaction of insulin-like growth factor-1 receptor with leukemia-associated RhoGEF. J. Cell Biol. 155, 809–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aurandt J., Vikis H. G., Gutkind J. S., Ahn N., Guan K. L. (2002) The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG. Proc. Natl. Acad. Sci. U.S.A. 99, 12085–12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swiercz J. M., Kuner R., Behrens J., Offermanns S. (2002) Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron 35, 51–63 [DOI] [PubMed] [Google Scholar]
- 13. Chen Z., Singer W. D., Danesh S. M., Sternweis P. C., Sprang S. R. (2008) Recognition of the activated states of Gα13 by the rgRGS domain of PDZRhoGEF. Structure 16, 1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen Z., Singer W. D., Sternweis P. C., Sprang S. R. (2005) Structure of the p115RhoGEF rgRGS domain-Gα13/i1 chimera complex suggests convergent evolution of a GTPase activator. Nat. Struct. Mol. Biol. 12, 191–197 [DOI] [PubMed] [Google Scholar]
- 15. Chen Z., Wells C. D., Sternweis P. C., Sprang S. R. (2001) Structure of the rgRGS domain of p115RhoGEF. Nat. Struct. Biol. 8, 805–809 [DOI] [PubMed] [Google Scholar]
- 16. Longenecker K. L., Lewis M. E., Chikumi H., Gutkind J. S., Derewenda Z. S. (2001) Structure of the RGS-like domain from PDZ-RhoGEF. Linking heterotrimeric G protein-coupled signaling to Rho GTPases. Structure 9, 559–569 [DOI] [PubMed] [Google Scholar]
- 17. Wells C. D., Liu M. Y., Jackson M., Gutowski S., Sternweis P. M., Rothstein J. D., Kozasa T., Sternweis P. C. (2002) Mechanisms for reversible regulation between p115 RhoGEF and GTRAP48 and the G12 class of heterotrimeric G proteins. J. Biol. Chem. 277, 1174–1181 [DOI] [PubMed] [Google Scholar]
- 18. Chen Z., Singer W. D., Wells C. D., Sprang S. R., Sternweis P. C. (2003) Mapping the Gα13 binding interface of the rgRGS domain of p115RhoGEF. J. Biol. Chem. 278, 9912–9919 [DOI] [PubMed] [Google Scholar]
- 19. Chen Z., Medina F., Liu M. Y., Thomas C., Sprang S. R., Sternweis P. C. (2010) Activated RhoA Binds to the pleckstrin homology (PH) domain of PDZ-RhoGEF, a potential site for autoregulation. J. Biol. Chem. 285, 21070–21081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen Z., Guo L., Sprang S. R., Sternweis P. C. (2011) Modulation of a GEF switch. Autoinhibition of the intrinsic guanine nucleotide exchange activity of p115-RhoGEF. Protein Sci. 20, 107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Semenyuk A. V., Svergun D. I. (1991) Gnom. A program package for small-angle scattering data-processing. J. Appl. Crystallogr. 24, 537–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Svergun D. I., Petoukhov M. V., Koch M. H. (2001) Determination of domain structure of proteins from x-ray solution scattering. Biophys. J. 80, 2946–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Volkov V. V., Svergun D. I. (2003) Uniqueness of ab initio shape determination in small-angle scattering. J. Appl. Crystallogr. 36, 860–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wriggers W. (2010) Using Situs for the integration of multi-resolution structures. Biophys. Rev. 2, 21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. (2004) UCSF chimera. A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 [DOI] [PubMed] [Google Scholar]
- 26. Derewenda U., Oleksy A., Stevenson A. S., Korczynska J., Dauter Z., Somlyo A. P., Otlewski J., Somlyo A. V., Derewenda Z. S. (2004) The crystal structure of RhoA in complex with the DH/PH fragment of PDZRhoGEF, an activator of the Ca2+ sensitization pathway in smooth muscle. Structure 12, 1955–1965 [DOI] [PubMed] [Google Scholar]
- 27. Kristelly R., Gao G., Tesmer J. J. (2004) Structural determinants of RhoA binding and nucleotide exchange in leukemia-associated Rho guanine-nucleotide exchange factor. J. Biol. Chem. 279, 47352–47362 [DOI] [PubMed] [Google Scholar]
- 28. Laurie A. T., Jackson R. M. (2005) Q-SiteFinder. An energy-based method for the prediction of protein-ligand binding sites. Bioinformatics 21, 1908–1916 [DOI] [PubMed] [Google Scholar]
- 29. Schneidman-Duhovny D., Inbar Y., Nussinov R., Wolfson H. J. (2005) PatchDock and SymmDock. Servers for rigid and symmetric docking. Nucleic Acids Res. 33, W363–W367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bielnicki J. A., Shkumatov A. V., Derewenda U., Somlyo A. V., Svergun D. I., Derewenda Z. S. (2011) Insights into the molecular activation mechanism of the RhoA-specific guanine nucleotide exchange factor, PDZRhoGEF. J. Biol. Chem. 286, 35163–35175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zheng M., Cierpicki T., Momotani K., Artamonov M. V., Derewenda U., Bushweller J. H., Somlyo A. V., Derewenda Z. S. (2009) On the mechanism of autoinhibition of the RhoA-specific nucleotide exchange factor PDZRhoGEF. BMC Struct. Biol. 9, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lutz S., Shankaranarayanan A., Coco C., Ridilla M., Nance M. R., Vettel C., Baltus D., Evelyn C. R., Neubig R. R., Wieland T., Tesmer J. J. (2007) Structure of Gαq-p63RhoGEF-RhoA complex reveals a pathway for the activation of RhoA by GPCRs. Science 318, 1923–1927 [DOI] [PubMed] [Google Scholar]
- 33. Kimple R. J., Kimple M. E., Betts L., Sondek J., Siderovski D. P. (2002) Structural determinants for GoLoco-induced inhibition of nucleotide release by Gα subunits. Nature 416, 878–881 [DOI] [PubMed] [Google Scholar]
- 34. Kreutz B., Yau D. M., Nance M. R., Tanabe S., Tesmer J. J., Kozasa T. (2006) A new approach to producing functional Gα subunits yields the activated and deactivated structures of Gα12/13 proteins. Biochemistry 45, 167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chenna R., Sugawara H., Koike T., Lopez R., Gibson T. J., Higgins D. G., Thompson J. D. (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31, 3497–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.