Background: Pellino proteins are involved in polyubiquitination and degradation of IRAK1, a key molecule in TLR/IL-1R-mediated signaling.
Results: Pellino 2 knockdown reduced TAK1-dependent NFκB activation and inflammatory gene mRNA stabilization.
Conclusion: Pellino 2 plays a critical role for TLR/IL-1R-mediated post-transcriptional control.
Significance: Pellino 2 is the critical signaling molecule controlling TAK1- and MEKK3-dependent pathways and also plays a critical role for TLR/IL-1R-mediated post-transcriptional control.
Keywords: E3 Ubiquitin Ligase, MAP Kinases (MAPKs), MRNA Decay, Toll-like Receptors (TLR), Ubiquitination, IL-1, IRAK1, Pellino 2, Post-transcriptional Control
Abstract
Interleukin 1 receptor-associated kinase 1(IRAK1), a key molecule in TLR/IL-1R-mediated signaling, is phosphorylated, ubiquitinated, and degraded upon ligand stimulation. We and others have recently identified Pellino proteins as novel RING E3 ubiquitin ligases involved in IRAK1 polyubiquitination and degradation. However, it remains unclear how each Pellino member distinctly regulates TLR/IL-1R signaling by modulating IRAK1 ubiquitination. In this study we examined the role of Pellino 2 in IL-1- and LPS-mediated signaling and gene expression by knocking down Pellino 2 in human 293-IL-1R cells and primary bone marrow macrophages. Pellino 2 (but not Pellino 1) knockdown abolished IL-1- and LPS-induced Lys-63-linked IRAK1 ubiquitination with reduced Lys-48-linked IRAK1 ubiquitination. Furthermore, Pellino 2 is required for TAK1-dependent NFκB activation. However, because of the retained TAK1-independent NFκB activation, the levels of IL-1- and LPS-induced NFκB activation were not substantially affected in Pellino 2 knockdown 293-IL-1R cells and primary macrophages, respectively. On the other hand, Pellino 2 knockdown reduced the IL-1- and LPS-induced inflammatory gene expression at late time points, which was accompanied by increased decay rates of the mRNAs of the inflammatory genes. Importantly, IL-1- and LPS-mediated JNK and ERK activation were substantially attenuated in Pellino 2 knock-down cells, implicating MAPK activation in TLR/IL-1R-induced mRNA stabilization. Taken together, this study demonstrated that Pellino 2 plays a critical role for TLR/IL-1R-mediated post-transcriptional control.
Introduction
The Toll-like receptor/interleukin-1 receptor (TLR/IL-1R)3 family plays a critical role in mounting inflammation, shaping innate as well as adaptive immunity (1–3). Upon binding to their cognate ligands, TLRs and IL-1R recruit the adaptor MyD88 through their TIR domain, thereby recruiting serine-threonine kinases IRAK4 (IL-1 receptor-associated kinase 4), IRAK1, and IRAK2, activating the MyD88-dependent pathway (4–9). Although IRAK4 functions as the upstream kinase promoting the phosphorylation of IRAK1 and IRAK2, the phosphorylated IRAK1 and IRAK2 then recruit TRAF6 to the receptor complex (10, 11). Subsequently, the IRAKs-TRAF6 complex dissociates from the receptor complex to activate cascades of downstream kinases, leading to the activation of NFκB and JNK (10, 12).
We recently uncovered two parallel IL-1β-mediated signaling pathways for NFκB activation: TAK1-dependent and MEKK3-dependent, respectively (13). The TAK1-dependent pathway leads to IKKα/β phosphorylation and IKKβ activation, resulting in classical NFκB activation through IκBα phosphorylation and degradation. The TAK1-independent MEKK3-dependent pathway involves IKKγ phosphorylation and IKKα activation, resulting in NFκB activation through IκBα phosphorylation (but without IκBα degradation) and subsequent dissociation from NFκB. These two pathways bifurcate at the level of IRAK1 phosphorylation and ubiquitination. Consistently, we reported that IRAK4 kinase activity is required for TAK1-dependent but not MEKK3-dependent IL-1β signaling, revealing that IRAK4-mediated IRAK1 phosphorylation is essential for TAK1 activation (14). However, the signaling molecules critically controlling TAK1- and MEKK3-mediated pathways, in particular the functions of E3 ligases regulating IRAK1 ubiquitination, have not yet been clearly elucidated. Interestingly, we and others recently reported that Pellino proteins can act as E3 ligases promoting IRAK1 ubiquitination in vitro (15–18), implicating the possible role for Pellino proteins in dictating TAK1- versus MEKK3-dependent IL-1β-mediated signaling.
The Pellino family is composed of four members, Pellino 1, 2, and 3a and splicing variant Pellino 3b. Through overexpression and in vitro kinase assay, we and others have recently reported that all the Pellino proteins can function as novel RING E3 ubiquitin ligases, mediating Lys-63-type polyubiquitination of IRAK (15, 17, 19, 20). Furthermore, the ubiquitination ligase activity of Pellino proteins can be greatly enhanced by phosphorylation promoted by IRAK4 and/or IRAK1 (18, 20, 21). The C-terminal portion of Pellino is reminiscent of the structure of the C3HC4 RING finger subfamily of Zinc-finger domain (22), and mutation of the key residues within this domain abolishes the E3 activity of Pellino proteins (15, 20). Despite all the Pellino proteins carry E3 ligase activity, Pellino 1 and 2 appear to function as positive regulators for NFκB activation (23–25), whereas Pellino 3b plays a negative role in IL-1β-induced TAK1-dependent NFκB activation (19). The molecular basis underlying their distinct roles is still elusive.
In this study we examined the role of Pellino 2 in IL-1- and LPS-mediated signaling and gene expression by knocking down Pellino 2 in human 293-IL-1R cells and primary bone marrow macrophages. Pellino 2 knockdown abolished IL-1- and LPS-induced Lys-63-linked IRAK1 ubiquitination with reduced Lys-48-linked IRAK1 ubiquitination. Furthermore, IL-1β- and LPS-induced IRAK1 ubiquitination is required for the formation of IRAK1-TAK1 complex and TAK1 activation. However, Pellino 2 is required for TAK1-dependent but not TAK1-independent NFκB activation; the levels of IL-1- and LPS-induced NFκB activation were not substantially affected in Pellino 2 knockdown 293-IL-1R cells and primary macrophages, respectively. On the other hand, IL-1- and LPS-mediated JNK and extracellular signal regulated kinase (ERK) activation were greatly reduced in Pellino 2 knockdown cells. Consistently, although mitogen-activated protein kinase (MAPK) activation has been implicated in TLR/IL-1R-induced mRNA stabilization, Pellino 2 knockdown increased the decay rates of IL-1- and LPS-induced mRNAs of inflammatory genes.
EXPERIMENT PROCEDURES
Cells and Reagents
C6 (HEK293/IL-1RI) cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), and penicillin/streptomycin. Bone marrow-derived macrophages (BMMs) were obtained from bone marrow of tibia and femur and cultured by DMEM with 20% FBS and 30% L929 cell-conditioned medium and penicillin/streptomycin for differentiation and proliferation of BMMs.
Oligonucleotides encoding either scrambled or Pellino 1- and Pellino 2-specific small hairpin RNAs were cloned into pSUPER to generate pSUPER-scrambled or pSUPER-Pellino 1 and pSUPER-Pellino 2, respectively. 1 μg of pSUPER-scrambled or pSuper-Pellino 1 and pSUPER-Pellino 2 was transfected into C6 cells along with 0.1 μg of pBabe-puromycin by FuGENE 6 (Roche Applied Science). Two days after transfection, puromycin (1 μg/ml) containing DMEM was added to the cells to select puromycin-resistant clones. After 10 days of puromycin selection, single clones were picked out and subjected to further analysis by human Pellino 1- and Pellino 2-specific quantitative real-time PCR.
Antibodies against phosphorylated IκBα (Ser-32/36), JNK, IKKα/β (Ser-176/180), p38, total IκBα, JNK, and IKKα/β were purchased from Cell Signaling. Antibody to FLAG (anti-FLAG) and β-actin were purchased from Sigma. Antibodies against IRAK1, TRAF6, Pellino, phosphorylated ERK, and GAPDH were purchased from Santa Cruz Biotechnology, Inc. Antibody to MEKK3 was purchased from BD Biosciences Pharmingen. A polyclonal anti-TAK1 antibody from rabbit was made by Eli Lilly. Recombinant interleukin-1β was from R &D Systems. LPS (Escherichia coli 055:B5 (macrophages) was purchased from Sigma.
Lentiviral Vectors and Transduction Procedure
Mission lentiviral shRNA vectors were purchased from Sigma and included control vector (pLKO.1 non-targeting, SHC002) and Pellino 2-specific shRNA (pLKO.1-Pellino 2, TRCN0000248150). HEK293T cells were transduced with mission lentiviral vectors and lentiviral packaging vectors to produce lentivirus. BMMs were infected with lentivirus collected from transfected HEK293T cells. Two days later, puromycin (3 μg/ml) was added to select for lentiviral-transduced BMMs.
Immunoprecipitation
Pellino 2 knockdown cells or control cells were stimulated with IL-1β (1 ng/ml) or LPS (1 μg/ml) for various time periods as indicated. After washing with cold PBS, cells were lysed in 0.5 ml of lysis buffer (50 mm Tris-HCL, pH 7.4, 150 mm NaCl, 1% Triton, 1 mm EDTA, 5 mm NaF, 2 mm NaVO3, 1 mm PMSF, 1× complete protease inhibitors). Cell lysates were cleared by centrifuging at 13,000 rpm for 10 min, and insoluble debris was discarded. Then supernatants were precleared by incubation with agarose beads containing normal human IgG for 1 h at 4 °C followed by centrifugation at 13,000 rpm. For co-immunoprecipitations, precleared supernatants were incubated with 20 μl of protein A-Sepharose beads with antibody at 4 °C overnight, and beads were washed with 1 ml of lysis buffer 5 times before dissolved in 40 μl of Laemmli buffer.
Immunoblotting
Whole cell lysates or immunoprecipitates were dissolved in Laemmli buffer and resolved by 10% SDS-PAGE. After electrophoresis, separated proteins were transferred onto polyvinylidene difluoride membrane (Millipore). For immunoblotting, the polyvinylidene difluoride membrane was blocked with 5% nonfat milk. After incubation with specific primary antibody, horseradish peroxidase-conjugated secondary antibody was applied. The positive immune reactive signal was detected by ECL (Amersham Biosciences).
Quantitative Real-time PCR
Total RNA was isolated using TRIzol reagent (Invitrogen). 3 μg of total RNA was then used for reverse transcription reaction using SuperScript reverse transcriptase (Invitrogen). Quantitative-PCR was performed in the AB 7300 Real Time PCR System, and the gene expression of human IL-8, mouse KC, mouse TNFα, mouse IL-6, and human/mouse β-actin was examined by SYBR® Green PCR Master Mix (Applied Biosystems). PCR amplification was performed in triplicate, and water was used to replace cDNA in each run as a negative control. The reaction protocol included preincubation at 95 °C to activate FastStart DNA polymerase for 10 min, amplification of 40 cycles that was set for 15 s at 95 °C, and annealing for 60 s at 60 °C. The results were normalized with the housekeeping gene human or mouse β-actin. Primer sequences were designed using online tools from GeneScript Corp.: human IL-8 forward (5′-AAGACATACTCCAAACCTTTCCA-3′), human IL-8 reverse (5′-CCAGACAGAGCTCTCTTCCA-3′), human β-actin forward (5′-CCTGGCACCCAGCACAAT-3′), human β-actin reverse (5′-GCCGATCCACACGGAGTACT-3′), mouse KC forward (5′-TAGGGTGAGGACATGTGTGG-3′), mouse KC reverse (5′-AAATGTCCAAGGGAAGCGT-3′), mouse TNFα forward (5′-CAAAGGGAGAGTGGTCAGGT-3′), mouse TNFα reverse (5′-ATTGCACCTCAGGGAAGAGT-3′), mouse IL-6 forward (5′-GGACCAAGACCATCCAATTC-3′), mouse IL-6 reverse (5′-ACCACAGTGAGGAATGTCCA-3′), mouse β-actin forward (5′-GGTCATCACTATTGGCAACG-3′), mouse β-actin reverse (5′-ACGGATGTCAACGTCACACT-3′), human Pellino 2 forward (5′-CGCGCGCGGATTTGACTCTT-3′), human Pellino 2 reverse (5′-CTGGGTGAAGCCCCCTCGTG-3′), human Pellino 1 forward (5′-TGGGGAAACAAAGAAGAACG-3′, human Pellino 1 reverse (5′-CATGAGGAAGTGGGATCTGG-3′), mouse Pellino 2 forward (5′-GGCATCTTCGTTCTGACC-3′), mouse Pellino 2 reverse (5′-GAGGACATCACAGCA-3′).
Electrophoretic Mobility Shift Assay
After stimulation with IL-1β (1 ng/ml) or LPS (1 μg/ml), cells were harvested, and an electrophoretic mobility shift assay was performed as described (14). The supershift assays were done with 1 μl of the antibody against p65 (c-20, sc-372, Santa Cruz), which was added to the binding reaction before the addition of the probe and incubated on ice for 30 min.
ELISA Assay
Supernatants from C6 (HEK293/IL-1RI) cell cultures were collected and measured for the level of human cytokines IL-8 using OptEIA ELISA kits II (BD Biosciences) according to the manufacturer's instructions. Supernatants from BMMs cultures were collected and measured for the levels of KC, TNFα, and IL-6 using Duo-set ELISA kits (R&D Systems) according to the manufacturer's instructions.
Statistical Analysis
The difference between two groups was statistically analyzed by Student's t test. A p value of <0.05 was considered significant.
RESULTS
Pellino 2, but Not Pellino 1, Is Critical for IL-1-induced IRAK1 Ubiquitination
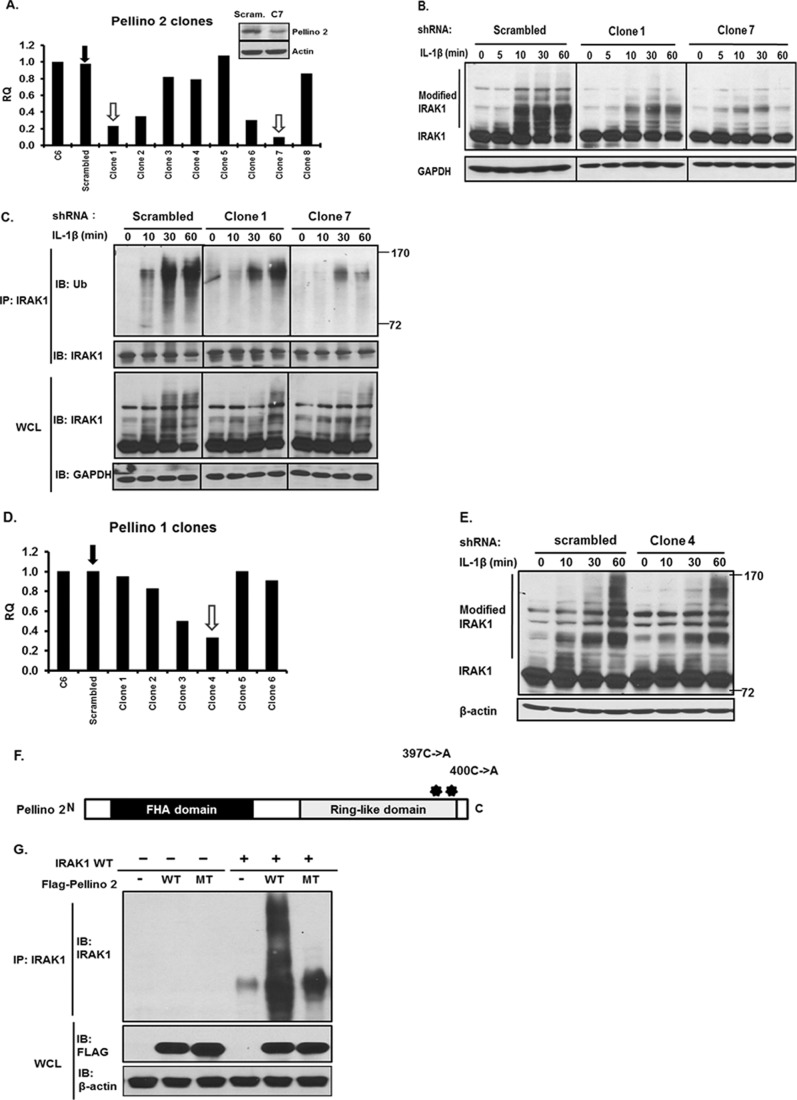
Pellino proteins have been demonstrated as RING E3-ubiquitin ligases and implicated in IRAK1 ubiquitination (7, 8). There are four Pellino family members, Pellino 1, 2, and 3a and splicing variant Pellino 3b. Pellino 2 and Pellino 3 (to a much lesser degree Pellino 1) carry the E3 ubiquitin ligase activity and promote polyubiquitination on IRAK1. We and others have previously demonstrated that Pellino 1 and 2 play an important role in IL-1R-TLR-mediated signaling (5, 9, 10). We now found that Pellino 2 shRNA (but not Pellino 1) reduced IL-1β-induced IRAK1 ubiquitination, indicating a critical role of Pellino 2 in IRAK1 ubiquitination. We employed an shRNA approach to specifically knock down Pellino 1 or 2 in 293-IL-1R cells (293 cells transfected with IL-1 receptor). By real-time PCR screening, several clones showed significantly reduced Pellino 1 or 2 expression compared with the clones transfected with scrambled shRNA. Clones with 80 and 90% reduction of Pellino 1 or 2 expression were chosen for further studies (Fig. 1, A and D). The knockdown was specific to the target molecule, as the mRNA levels of other Pellino family members were not affected in the knockdown clones (supplemental Fig. 1, A and B). IL-1β-induced polyubiquitination of IRAK1 was decreased in Pellino 2 knockdown clones (clones 1 and 7) compared with that in the control clone (Fig. 1, B and C), consistent with the function of Pellino 2 as an E3 ubiquitin ligase for IRAK1. It is important to note that Pellino 1 shRNA did not have substantial impact on IL-1β-induced IRAK1 ubiquitination (Fig. 1, D and E), indicating the specific role of Pellino 2 in IRAK1 ubiquitination. Furthermore, although overexpression of Pellino 2 induced IRAK1 ubiquitination, mutation of two key residues (C397A and C400A) in the Ring-like domain of Pellino 2 abolished the capability of Pellino 2 to promote the polyubiquitination of IRAK1, confirming that the E3 ubiquitin ligase activity of Pellino 2 depends on the highly conserved Ring-like domain (Fig. 1, F and G).
FIGURE 1.
Knockdown of Pellino 2 attenuates IL-1-mediated IRAK1 ubiquitination. A, 293-IL-1R cells were stably cotransfected with either Pellino 2-specific shRNA (pSuper-Pellino 2) or scrambled shRNA (pSuper-scrambled) with pBabe-Puro. Puromycin-resistant clones were subjected to analysis by human Pellino 2-specific quantitative real-time PCR. Cell lysates from scrambled (Scram.) and Peli2C7 (C7) were analyzed by Western analysis with anti-Pellino 2 and anti-β-actin. B, Pellino 2 knockdown clones (Peli2C1 and Peli2C7) and control cells (Scrambled) were stimulated with IL-1β (1 ng/ml) for the indicated times. Cell lysates were analyzed by Western analysis with anti-IRAK1 and anti-β-actin. Two different batches of cells were analyzed. C, cell lysates from Pellino 2 knockdown (Peli2C1 and Peli2C7) and control cells (Scrambled) stimulated with IL-1β (1 ng/ml) for the indicated times were immunoprecipitated (IP) with anti-IRAK1 followed by Western analysis (IB) with anti-ubiquitin (Ub) antibody and anti-IRAK1. WCL, whole cell lysates. D, 293-IL-1R cells were stably cotransfected with either Pellino 1-specific-shRNA (pSuper-Pellino 1) or scrambled shRNA (pSuper-scrambled) with pBabe-Puro. Puromycin-resistant clones were subjected to analysis by human Pellino 1-specific quantitative real-time PCR. E, Pellino 1 knockdown clones (Peli1C4) and control cells (scrambled) were stimulated with IL-1β (1 ng/ml) for the indicated times. F, shown is a schematic diagram of human Pellino 2 protein. Two point mutations on the Pellino 2 C terminus CHC2CHC2 domain are shown as asterisks. G, the FLAG-tagged Pellino 2 wild type or point mutant shown in F was co-transfected into IRAK1-deficient cells with or without IRAK1. Cell lysates were denatured and immunoprecipitated with anti-IRAK1 followed by analysis with anti-FLAG and anti-IRAK1. All results are representative of three independent experiments.
Pellino 2 Is Critical for IL-1- and LPS-induced Lys-63- and Lys-48-linked IRAK1 Ubiquitination
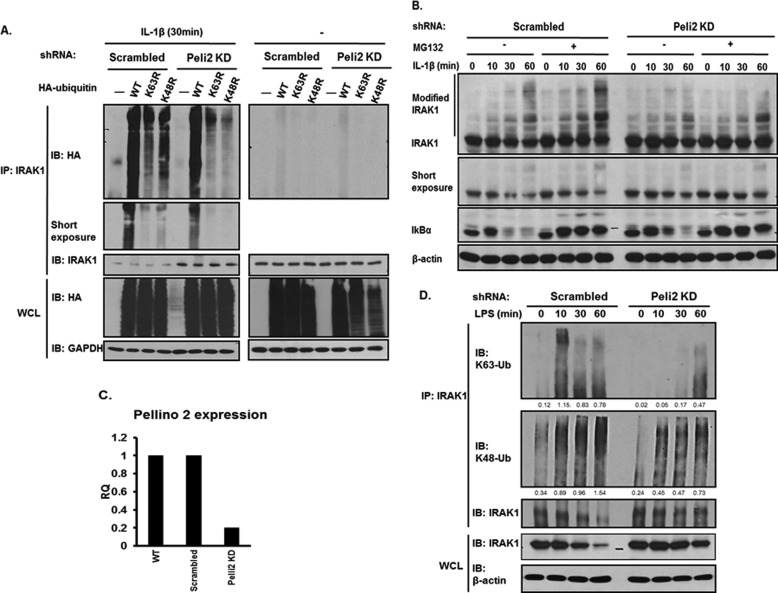
Previous studies have shown that IL-1β stimulation leads to both Lys-63- and Lys-48-linked polyubiquitination of IRAK1. To define whether Pellino 2 regulates Lys-63- or Lys-48-linked polyubiquitination on IRAK1, HA-tagged wild type, K63R (Lys-63 is replaced by arginine), or K48R, mutants of ubiquitin were transfected into Pellino 2 knockdown (clone 7) and control clones. Upon IL-1β induction, immunoprecipitated IRAK1 was probed by anti-HA to detect ubiquitinated IRAK1. IL-1β stimulation induced both Lys-48- and Lys-63-linked polyubiquitination on IRAK1, which was demonstrated by the incorporation of both K63R and K48R mutants of ubiquitin into IRAK1 in IL-1β-stimulated wild-type cells. It is evident that K48R-mediated IRAK1 ubiquitination was impaired in Pellino 2 knockdown clone (Fig. 2A), which is consistent with the fact that Pellino 2 mediates Lys-63-linked polyubiquitination in vitro.
FIGURE 2.
Pellino 2 mediates Lys-63-linked and Lys-48-linked IRAK1 polyubiquitination. A, Pellino 2 knockdown clones (Peli2C7) and control cells (Scrambled) were transfected with wild type or mutants of HA-ubiquitin plasmids. Two days after transfection cells were either treated with IL-1β (1 ng/ml) for 30 min or left untreated. Cell lysates were denatured and immunoprecipitated (IP) by anti-IRAK1 followed by Western (IB) analysis with anti-HA and anti-IRAK1. B. Pellino 2 knockdown clones (Peli2C7) and control cells (Scrambled) were untreated or treated with IL-1β with or without MG-132. C, BMMs were infected with either lentiviral Pellino 2 shRNA (pLKO.1-Pellino 2) or scrambled shRNA (pLKO.1 non-targeting). After puromycin selection, BMMs were subjected to analysis by mouse Pellino 2-specific quantitative real-time PCR. D, Pellino 2 knockdown BMMs and control BMMs (Scrambled) were stimulated with LPS (1 μg/ml) for the indicated times. Cell lysates were immunoprecipitated with anti-IRAK1 followed by Western analysis with anti-Lys-63 ubiquitin (Ub), anti-Lys-48 ubiquitin, and anti-IRAK1, respectively. The densitometric analysis of band density was done by ImageJ 1.43u and normalized to β-actin. All results are representative of three independent experiments. WCL, whole cell lysates.
It is intriguing that K63R-dependent IRAK1 ubiquitination was also reduced in Pellino 2 knockdown clones, suggesting the potential impact of Pellino 2 on IL-1β-induced Lys-48-linked IRAK1 polyubiquitination in vivo. Consistently, MG132 treatment indeed led to accumulation of ubiquitinated IRAK1 in 293-IL-1R cells, demonstrating IL-1β-induced Lys-48-linked IRAK1 polyubiquitination and degradation. It is important to note that the accumulation of ubiquitinated IRAK1 was reduced in Pellino 2 knockdown clones after MG132 treatment (Fig. 2B), suggesting that Pellino 2 is probably required for both Lys-63- and Lys-48-linked IRAK1 ubiquitination in IL-1β-treated cells.
To determine whether Pellino 2 is also important for the TLR-mediated signaling, we knocked down Pellino 2 in bone-marrow macrophages by infecting them with a lentiviral Pellino 2 shRNA construct (Fig. 2C). Macrophages infected with lentiviral Pellino 2 shRNA had about 80% knockdown of Pellino 2. Upon LPS stimulation, Lys-63-linked polyubiquitination of IRAK1 was greatly attenuated in Pellino 2 knockdown macrophages compared with that in control cells, whereas Lys-48-linked polyubiquitination of IRAK1 was also noticeably reduced (Fig. 2D). These data demonstrate the critical role of Pellino 2 in mediating the TLR-induced Lys-63- and Lys-48-linked IRAK1 ubiquitination.
Pellino 2 Is Required IL-1- and LPS-induced TAK1 Activation
Through the study of IRAK1 modification, we previously uncovered two parallel IL-1-mediated signaling pathways for NFκB activation, TAK1-dependent and -independent, respectively. These two pathways bifurcate at the level of IRAK1 modification. The TAK1-dependent pathway leads to IKKα/β phosphorylation and IKKβ activation, resulting in classical NFκB activation through IκBα phosphorylation and degradation. The TAK1-independent pathway involves activation of MEKK3 and IKKα, resulting in NFκB activation through IκBα phosphorylation and subsequent dissociation from NFκB in the absence of IκBα degradation.
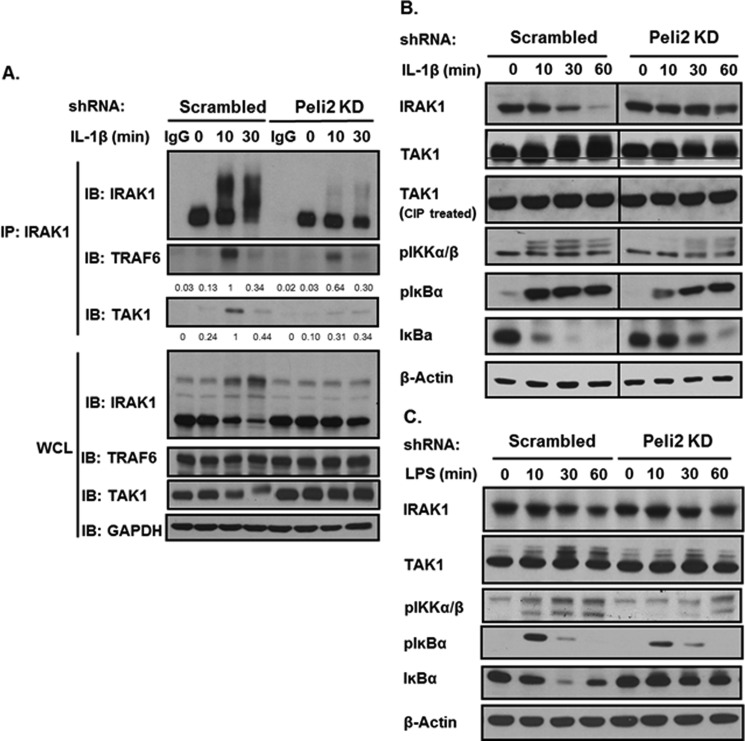
As Lys-63-linked polyubiquitin chain can recruit TAB2/3 and promote TAK1 activation (11, 12), we propose that Pellino 2-mediated Lys-63-linked IRAK1 ubiquitination might be required for the interaction of IRAK1 with TAK1 and consequent activation of TAK1. Importantly, through co-immunoprecipitation we found that IL-1-induced IRAK1-TAK1 complex was much reduced in Pellino 2 knockdown cells compared with that in control cells (Fig. 3A), indicating that Pellino 2-mediated IRAK1 ubiquitination is critical for the IL-1-induced formation of IRAK1-TAK1 complex. Consistently, TAK1 activation, shown as slower mobility shift bands, was greatly reduced in Pellino 2 knock-down clones (Fig. 3B). Phosphorylation of IKKα/β was also substantially decreased in the knockdown cells compared with the control cells. IL-1-induced TAK1-mediated IKK activation led to the phosphorylation and degradation of IκBα. Indeed, IL-1-induced IκBα degradation was attenuated in the Pellino 2 knockdown clones (Fig. 3B). Furthermore, Pellino 2 knockdown in primary macrophages also reduced LPS-induced phosphorylation of TAK1 and IKKα/β accompanied by attenuated IκBα degradation (Fig. 3C). These data suggest that Pellino 2 plays an important role for IL-1- and LPS-induced TAK1-dependent pathway, probably through its impact on IRAK1 ubiquitination.
FIGURE 3.
Knockdown of Pellino 2 attenuates IL-1- and LPS-induced IRAK1 modification and TAK1-dependent signaling. A, Pellino 2 knockdown clones (Peli2C7) and control cells (Scrambled) were stimulated by IL-1β (1 ng/ml) for the indicated times. Cell lysates were denatured and immunoprecipitated by anti-IRAK1. Immunoprecipitates (IP) were probed with anti-IRAK1, anti-TRAF6, and anti-TAK1. The protein levels were analyzed by ImageJ 1.43u and normalized to GAPDH. IB, immunoblot; WCL, whole cell lysates. B, Pellino 2 knockdown clones (Peli2C7) and control cells (Scrambled) were stimulated by IL-1β (1 ng/ml) for the indicated times. Cell lysates were analyzed by Western blotting with anti-IRAK1, anti-TAK1, anti-p-IKKα/β, anti-p-IκBα, anti-IκBα, and anti-β-actin. C, Pellino 2 knockdown BMMs and control BMMs (Scrambled) were stimulated by LPS (1 μg/ml) for the indicated times. Cell lysates were analyzed by Western blotting with anti-IRAK1, anti-TAK1, anti-p-IKKα/β, anti-p-IκBα, anti-IκBα, and anti-β-actin. All results are representative of three independent experiments.
IL-1- and LPS-induced NFκB Activation Was Retained in Pellino 2 Knock-down Cells
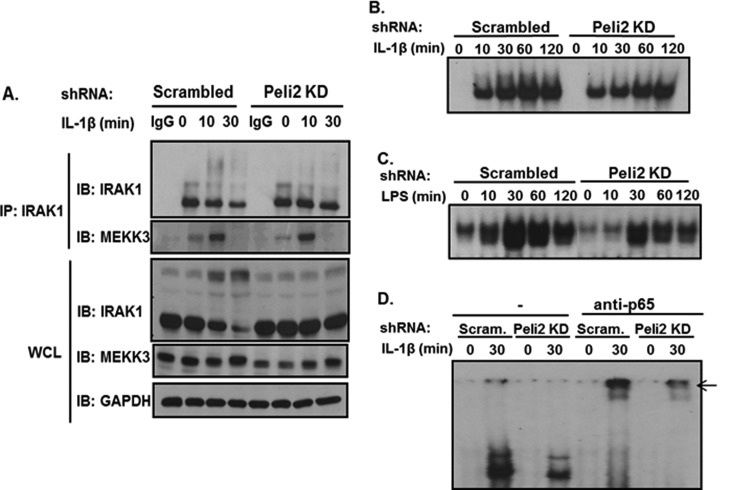
It is important to note that whereas IL-1- and LPS-induced TAK1 activation and TAK1-dependent downstream signaling events (phosphorylation of IKKα/β and IκBα degradation) were reduced by Pellino 2 knock-down in 293-IL-1R cells, ligand-induced IκBα phosphorylation was still intact in those cells, suggesting that Pellino 2 knockdown probably did not affect the TAK1-independent NFκB activation pathway. Because previous studies have shown that MEKK3 interacts with unmodified IRAK1 and plays a key role in mediating the TLR/IL-1R-induced TAK1-independent pathway, we examined the interaction of IRAK1 with MEKK3 in Pellino 2 knockdown cells. Importantly, through co-immunoprecipitation we indeed found that IL-1-induced IRAK1-MEKK3 complex was formed in Pellino 2 knockdown cells compared with that in control cells (Fig. 4A). Consistently, we detected IL-1-induced NFκB activation by electrophoretic mobility shift assay in Pellino 2 knockdown cells (Fig. 4B). Furthermore, LPS-induced NFκB activation was also intact in Pellino 2 knockdown primary macrophages (Fig. 4C). These results suggest that IL-1- and LPS-induced NFκB activation was retained in Pellino 2 knockdown cells.
FIGURE 4.
Pellino 2 knockdown cells showed retained IL-1- and LPS-mediated NFκB activation. A, Pellino 2 knockdown clones (Peli2C7) and control cells (Scrambled) were stimulated by IL-1β (1 ng/ml) for the indicated times. Cell lysates were denatured and immunoprecipitated (IP) by anti-IRAK1 followed by Western (IB) analysis with anti-IRAK1 and anti-MEKK3. WCL, whole cell lysates. B, cell lysates from Pellino 2 knockdown clones (Peli2C7) and control cells (Scrambled) stimulated by IL-1β (1 ng/ml) for the indicated times were analyzed by electrophoresis mobility shift assay with an NFκB-specific probe. C, cell lysates from Pellino 2 knockdown BMMs and control BMMs (Scrambled) treated with LPS (1 μg/ml) for indicated times were analyzed by electrophoresis mobility shift assay with an NFκB-specific probe. D, cell lysates from Pellino 2 knockdown clones (Peli2C7) and control cells (Scrambled) stimulated by IL-1β (1 ng/ml) for the indicated times were analyzed by electrophoresis mobility shift assay with an NFκB-specific probe and anti-p65 antibody for NFκB p65 supershift.
Pellino 2 Is Critical for TLR/IL-1R-mediated Post-transcriptional Control
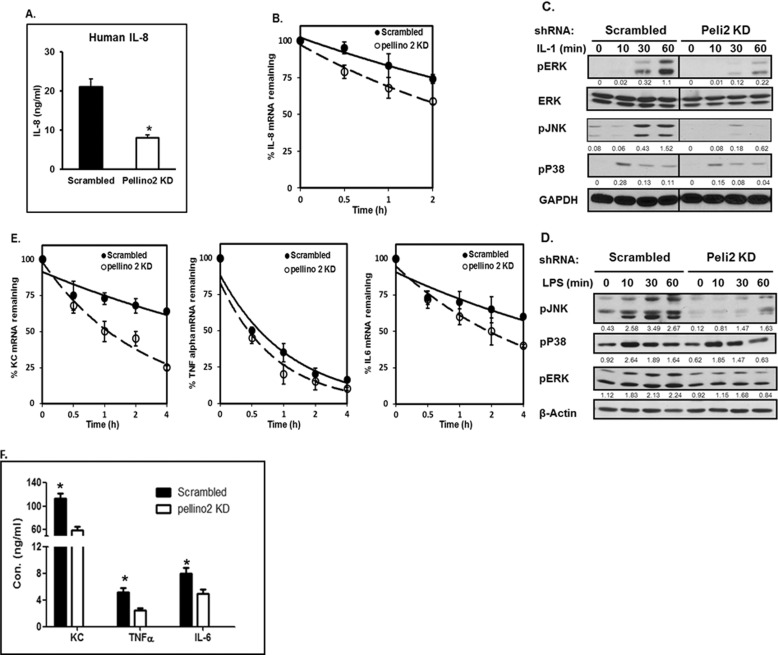
Because Pellino 2 is required for TAK1-dependent, but not TAK1-independent NFκB activation, the levels of IL-1- and LPS-induced NFκB activation were not substantially affected in Pellino 2 knockdown 293-IL-1R cells and primary macrophages, respectively. We then wondered whether Pellino 2 has any impact on TLR/IL-1R-mediated gene expression. Although the IL-1-induced IL-8 mRNAs were comparable at early time points (0.5–2 h), the IL-8 mRNA levels were substantially reduced in Pellino 2 knockdown 293-IL-1R cells compared with that in the control cells at late time points (Fig. 5A). Furthermore, IL-8 protein secretion was drastically decreased in Pellino 2 knockdown 293-IL-1R cells compared with that in the control clone (Fig. 6A), suggesting a possible role of Pellino 2 in regulating IL-1-mediated gene expression at post-transcriptional levels. To test this hypothesis, we measured the stability of IL-8 mRNA in Pellino 2 knockdown 293-IL-1R cells (Fig. 6B). Pellino 2 knockdown and control 293-IL-1R cells were pretreated with IL-1 for 1.5 h to induce IL-8 transcription and then treated with actinomycin D to block nascent transcription for indicated times. Although IL-8 mRNA was induced to similar levels in Pellino 2 knockdown and control cells in response to IL-1 stimulation, the decay rate of IL-8 mRNA was increased in Pellino 2 knockdown cells after actinomycin D treatment (Fig. 6B). Taken together, these results suggest that the Pellino 2 modulates IL-1-induced IL-8 production at least partially through its impact on IL-1-mediated mRNA stabilization.
FIGURE 5.
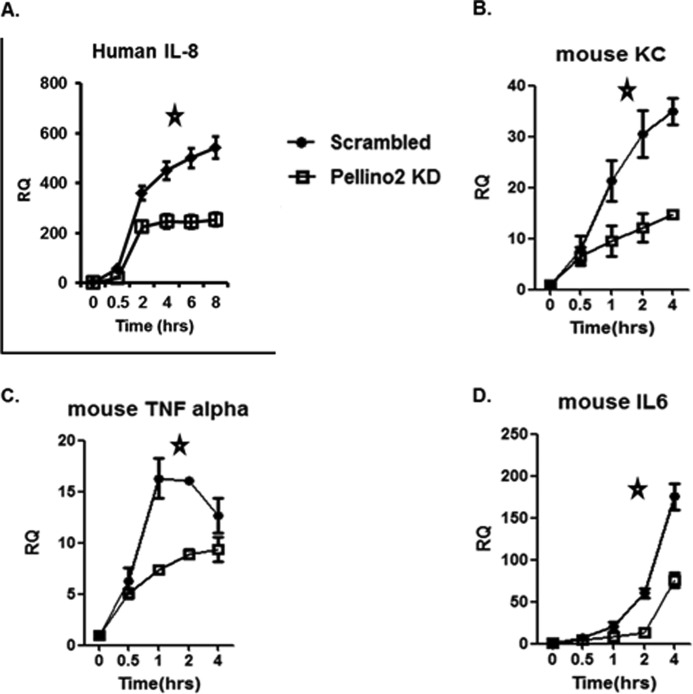
Pellino 2 knockdown showed dramatic reduction in IL-1- and LPS-mediated gene induction at late time points. A, Pellino 2 knockdown clones (Peli2C7) and control cells (Scrambled) were treated with IL-1β (1 ng/ml) for the indicated times. Total RNA was subjected to real-time PCR analysis for the levels of human IL-8 mRNA. Similar results were obtained in three separate experiments. Statistical analyses (*) were performed as described under “Experimental Procedures.” *, p < 0.05). B–D, Pellino 2 knockdown BMMs and control BMMs (Scrambled) were treated with LPS (1 μg/ml) for the indicated times. Total RNA was subjected to real-time PCR analysis for the levels of mouse KC (Cxcl1 chemokine (C-X-C motif) ligand 1) (B), mouse TNFα (C), and mouse IL-6 (D) mRNA. Similar results were obtained in three separate experiments. *, p < 0.05.
FIGURE 6.
Pellino 2 is critical for IL-1- and LPS-mediated post-transcriptional control. A, Pellino 2 knockdown clones (Peli2C7) and control cells (Scrambled) were stimulated by IL-1β (1 ng/ml) for 8 h or left untreated. Media were collected, and secreted IL-8 was measured by enzyme-linked immunosorbent assay. Results shown are the means (±S.D.) of duplicate determinations. Statistical analyses (*) were performed as described in “Experimental Procedures.” *, p < 0.05. B, Pellino 2 knockdown clones (Peli2C7) and control cells (Scrambled) were stimulated by IL-1β (1 ng/ml) for 90 min and then treated with actinomycin D (5 μg/ml) and IL-1 (1 ng/ml) for the indicated times. Total RNA was subjected to real-time PCR analysis for the levels of IL-8 mRNA. Similar results were obtained in three independent experiments. *, p < 0.05. C and D, Pellino 2 knockdown clones (Peli2C7) and control cells (Scrambled) were stimulated by IL-1β (1 ng/ml) for the indicated times (C). Pellino 2 knockdown BMMs and control BMMs (Scrambled) were stimulated by LPS (1 μg/ml) for the indicated times. D, cell lysates were analyzed by Western blotting with anti-pERK, anti-ERK, anti-pJNK, anti-pP38, and anti-GAPDH. The protein levels were analyzed by ImageJ 1.43u and normalized to actin. All results are representative of three independent experiments. E, Pellino 2 knockdown BMMs and control BMMs (Scrambled) were stimulated by LPS (1 μg/ml) for 90 min and then treated with actinomycin D (5 μg/ml) and LPS (1 μg/ml) for the indicated times. Total RNA was subjected to real-time PCR analysis for the levels of mouse KC (Cxcl1 chemokine (C-X-C motif) ligand 1), TNFα, and IL-6 mRNA. Similar results were obtained in three separate experiments. *, p < 0.05. F, Pellino 2 knockdown BMMs and control BMMs (Scrambled) were stimulated by LPS (1 μg/ml) for 8 h or left untreated. Media were collected, and secreted KC, IL-6, and TNFα were measured by enzyme-linked immunosorbent assay. Results shown are the means (±S.D.) of duplicate determinations. Statistical analyses (*) were performed as described under “Experimental Procedures.” *, p < 0.05.
Furthermore, we found that LPS-induced KC, IL-6, and TNFα mRNAs were substantially reduced in Peillino 2 knockdown macrophages, especially at late time points (Fig. 5, B–D). Consistently, the production of KC, IL-6, and TNFα was also substantially decreased in Pellino 2 knockdown cells (Fig. 6F). We also measured the stability of KC, IL-6, and TNFα mRNAs in Pellino 2 knockdown macrophages (Fig. 6E). Pellino 2 knockdown and control macrophages were pretreated with LPS for 1.5 h to induce gene transcription and then treated with actinomycin D to block nascent transcription for the indicated times. We found that KC and IL-6 (but not TNFα) mRNAs decayed much faster in Pellino 2 knockdown cells compared with control cells after actinomycin D treatment (Fig. 6E), demonstrating the importance of Pellino 2 in LPS-mediated KC and IL-6 mRNA stabilization (Table 1).
TABLE 1.
Pellino 2 is critical for IL-1- and LPS-mediated mRNA stabilization.
mRNA decay rates and half-lives of human IL-8, mouse KC, TNFα, and IL-6 mRNA were measured from the experiments done in Fig. 6, B and E.
| mRNA | shRNA | Ki | t½ | P-valuea |
|---|---|---|---|---|
| h−1 | min | |||
| Human IL-8 | Scrambled | −0.104 ± 0.09 | 472 | <0.01 |
| Peli2 KD | −0.173 ± 0.06 | 291 | ||
| Mouse KC | Scrambled | −0.104 ± 0.20 | 426 | <0.01 |
| Peli2 KD | −0.319 ± 0.11 | 187 | ||
| Mouse TNFα | Scrambled | −0.458 ± 0.08 | 135 | 0.08 |
| Peli2 KD | −0.57 ± 0.13 | 113 | ||
| Mouse IL-6 | Scrambled | −0.114 ± 0.23 | 375 | <0.05 |
| Peli2 KD | −0.22 ± 0.14 | 235 |
a Two-way ANOVA analysis demostrated a statistically difference (P-value) between the curves (shown in Fig. 6, B and E) for Scrambled and Peli2 KD cells.
Because previous studies implicated MAPKs in posttranscriptional control of TLR/IL-1R-mediated gene expression, we examined whether Pellino 2 has any effect on IL-1- and LPS-induced activation MAPKs in 293 cells and primary macrophages, respectively. Interestingly, IL-1- and LPS-induced JNK and ERK phosphorylation were dramatically reduced in Pellino 2 knockdown 293 cells (Fig. 6C) and macrophages (Fig. 6D), whereas Pellino 2 did not affect IL-1- and LPS-mediated p38 phosphorylation, implicating MAPK activation in TLR/IL-1R-induced mRNA stabilization.
DISCUSSION
In this study we demonstrate that Pellino 2 is required for IL-1- and LPS-induced IRAK1 polyubiquitination. In Pellino 2 knockdown cells, the Lys-63-linked polyubiquitination of IRAK1 was greatly reduced (Fig. 2, A and D), which correlated with reduced IRAK1-TAK1 complex formation (Fig. 3A), suggesting that that Pellino 2-mediated Lys-63-linked polyubiquitination of IRAK1 positively impacts on TLR/IL-1R signaling. Furthermore, when Pellino 2 was ablated, TLR/IL-1R-induced TAK1-dependent NFκB activation was compromised. We detected attenuated IL-1- and LPS-induced TAK1 activation, IKKα/β phosphorylation, and IκBα degradation (Fig. 3, B and C). It is important to note that although Pellino 2 is required for the TAK1-dependent NFκB activation, the TAK1-independent MEKK3-dependent pathway was retained in Pellino 2 knockdown cells (Fig. 3).
This study demonstrated that Pellino 2 plays a critical role for TLR/IL-1R-mediated post-transcriptional control. Pellino 2 knockdown reduced the IL-1- and LPS-induced inflammatory gene expression at late time points (Fig. 5), which was accompanied by increased decay rates of the mRNAs of the inflammatory genes (Fig. 6, B and E). Many of the mRNAs of cytokines and chemokines have very short half-lives because of AU-rich sequence elements located within their 3′-untranslated regions (26). Binding proteins that interact with AU-rich sequence element in mRNAs play essential roles in directing mRNA decay (26). Several signaling pathways modulate the function of these AU-rich sequence element binding proteins, including the p38 MAPK, ERK, and JNK pathways (26). Importantly, IL-1- and LPS-mediated JNK and ERK activation were substantially attenuated in Pellino 2 knockdown cells (Fig. 6, C and D), implicating MAPK activation in TLR/IL-1R-induced mRNA stabilization.
We and others have found that IL-1β signaling triggers both Lys-63- and Lys-48-linked IRAK1 polyubiquitination (20, 27). IL-1β stimulation first induced Lys-63-linked IRAK1 polyubiquitination, and then the Lys-63-linked polyubiquitin chains were replaced with Lys-48-linked polyubiquitin chains which targeted IRAK1 for proteasome-dependent degradation (28). Interestingly, we observed that both LPS- and IL-1-induced Lys-63- and Lys-48-linked IRAK1 polyubiquitination were reduced in Pellino 2 knockdown cells (Fig. 2, A and D). One possibility is that IRAK1 first undergoes Lys-63-linked polyubiquitination upon ligand stimulation and then was de-ubiquitinated by an unknown mechanism followed by Lys-48-linked polyubiquitination through an unidentified E3 ligase. The impairment of Lys-63-linked IRAK1 polyubiquitination could lead to the blockade in Lys-48-linked IRAK1 polyubiquitination. In our previous study we found that IL-1β-induced Lys-48-linked IRAK1 polyubiquitination and subsequent degradation might be a necessary step in the activation of the TAK1-dependent pathway by promoting the release of TAK1 complex from the membrane to the cytosol. Future studies are required to determine which E3 ubiquitin ligase is responsible for IL-1/TLRs-induced Lys-48-linked IRAK1 polyubiquitination.
It is intriguing that Pellino 2, but not Pellino 1, knockdown abolished IL-1- and LPS-induced Lys-63-linked IRAK1 ubiquitination with reduced Lys-48-linked IRAK1 ubiquitination. It is unclear what the difference is between Pellino 1 and Pellino 2 that could account for their distinct functions. Pellino 1 and Pellino 2 share about 80% identity and 90% homology. Biochemically, although purified Pellino 2 can efficiently mediate polyubiquitination in vitro, purified Pellino 1 has to be phosphorylated to exert its E3 activity, indicating intrinsic difference of these two E3 ligases (20). Recent studies have shown that although IRAK1 kinase activity is required for the activation of Pellino 1 in IL-1 signaling in MEFs, IKKi-TBK1 are required for TLR-induced Pellino 1 activation in macrophages (28). The activation of Pellino 1 seems to be receptor and/or cell type-specific. However, it is still unclear how Pellino 2 is activated in response to TLR/IL-1R stimulation. Based on our results, it is possible that although Pellino 2 directly targets IRAK1, Pellino 1 might have a different substrate(s) in vivo. As a matter of fact, Pellino 1 was shown to ubiquitinate RIP1 to mediate TIR-domain-containing adapter-inducing interferon-β (TRIF)-dependent NFκB activation (23). Furthermore, through overexpression of tagged-Pellino 1, we previously found that Pellino 1 binds constitutively with IL-1R and forms an lL-1-induced complex with IRAK4-IRAK1-TRAF6 (15). Therefore, we previously proposed that Pellino 1 functions to dissociate IRAK4-IRAK1 complex from IL-1R. Because of the overexpression system, these data have to be interpreted with caution. Future studies are required to elucidate the distinct functions of Pellino 1 and Pellino 2.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants 2PO1 HL 029582-26A1 and 2PO1CA062220-16A1.

This article contains supplemental Fig. 1.
- TLR/IL-1R
- Toll-like receptor/interleukin-1 receptor
- IRAK1
- Interleukin 1 receptor-associated kinase 1
- BMM
- bone marrow-derived macrophage
- KD
- knockdown
- IKK
- IκB kinase.
REFERENCES
- 1. Medzhitov R., Preston-Hurlburt P., Janeway C. A., Jr. (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 [DOI] [PubMed] [Google Scholar]
- 2. Zhang D., Zhang G., Hayden M. S., Greenblatt M. B., Bussey C., Flavell R. A., Ghosh S. (2004) A toll-like receptor that prevents infection by uropathogenic bacteria. Science 303, 1522–1526 [DOI] [PubMed] [Google Scholar]
- 3. Takeda K., Akira S. (2005) Toll-like receptors in innate immunity. Int Immunol. 17, 1–14 [DOI] [PubMed] [Google Scholar]
- 4. Akira S., Takeda K., Kaisho T. (2001) Toll-like receptors. Critical proteins linking innate and acquired immunity. Nat Immunol. 2, 675–680 [DOI] [PubMed] [Google Scholar]
- 5. Li S., Strelow A., Fontana E. J., Wesche H. (2002) IRAK-4. A novel member of the IRAK family with the properties of an IRAK-kinase. Proc. Natl. Acad. Sci. U.S.A. 99, 5567–5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suzuki N., Suzuki S., Duncan G. S., Millar D. G., Wada T., Mirtsos C., Takada H., Wakeham A., Itie A., Li S., Penninger J. M., Wesche H., Ohashi P. S., Mak T. W., Yeh W. C. (2002) Severe impairment of interleukin-1 and Toll-like receptor signaling in mice lacking IRAK-4. Nature 416, 750–756 [DOI] [PubMed] [Google Scholar]
- 7. Picard C., Puel A., Bonnet M., Ku C. L., Bustamante J., Yang K., Soudais C., Dupuis S., Feinberg J., Fieschi C., Elbim C., Hitchcock R., Lammas D., Davies G., Al-Ghonaium A., Al-Rayes H., Al-Jumaah S., Al-Hajjar S., Al-Mohsen I. Z., Frayha H. H., Rucker R., Hawn T. R., Aderem A., Tufenkeji H., Haraguchi S., Day N. K., Good R. A., Gougerot-Pocidalo M. A., Ozinsky A., Casanova J. L. (2003) Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 299, 2076–2079 [DOI] [PubMed] [Google Scholar]
- 8. Li X., Commane M., Burns C., Vithalani K., Cao Z., Stark G. R. (1999) Mutant cells that do not respond to interleukin-1 (IL-1) reveal a novel role for IL-1 receptor-associated kinase. Mol. Cell. Biol. 19, 4643–4652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitcham J. L., Parnet P., Bonnert T. P., Garka K. E., Gerhart M. J., Slack J. L., Gayle M. A., Dower S. K., Sims J. E. (1996) T1/ST2 signaling establishes it as a member of an expanding interleukin-1 receptor family. J. Biol. Chem. 271, 5777–5783 [DOI] [PubMed] [Google Scholar]
- 10. Jiang Z., Ninomiya-Tsuji J., Qian Y., Matsumoto K., Li X. (2002) Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol. Cell. Biol. 22, 7158–7167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao Z, Henzel W. J., Gao X. (1996) IRAK. A kinase associated with the interleukin-1 receptor. Science 271, 1128–1131 [DOI] [PubMed] [Google Scholar]
- 12. Qian Y., Commane M., Ninomiya-Tsuji J., Matsumoto K., Li X. (2001) IRAK-mediated translocation of TRAF6 and TAB2 in the interleukin-1-induced activation of NFκB. J. Biol. Chem. 276, 41661–41667 [DOI] [PubMed] [Google Scholar]
- 13. Yao J., Kim T. W., Qin J., Jiang Z., Qian Y., Xiao H., Lu Y., Qian W., Gulen M. F., Sizemore N., DiDonato J., Sato S., Akira S., Su B., Li X. (2007) Interleukin-1 (IL-1)-induced TAK1-dependent versus MEKK3-dependent NFκB activation pathways bifurcate at IL-1 receptor-associated kinase modification. J. Biol. Chem. 282, 6075–6089 [DOI] [PubMed] [Google Scholar]
- 14. Kim T. W., Staschke K., Bulek K., Yao J., Peters K., Oh K. H., Vandenburg Y., Xiao H., Qian W., Hamilton T., Min B., Sen G., Gilmour R., Li X. (2007) A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J. Exp. Med. 204, 1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang Z. (2003) Pellino 1 is required for interleukin-1 (IL-1)-mediated signaling through its interaction with the IL-1 receptor-associated kinase 4 (IRAK4)-IRAK-tumor necrosis factor receptor-associated factor 6 (TRAF6) complex. J. Biol. Chem. 278, 10952–10956 [DOI] [PubMed] [Google Scholar]
- 16. Yu K. Y., Kwon H. J., Norman D. A., Vig E., Goebl M. G., Harrington M. A. (2002) Cutting edge. Mouse Pellino-2 modulates IL-1 and lipopolysaccharide signaling. J. Immunol. 169, 4075–4078 [DOI] [PubMed] [Google Scholar]
- 17. Schauvliege R., Janssens S., Beyaert R. (2006) Pellino proteins are more than scaffold proteins in TLR/IL-1R signaling. A role as novel RING E3-ubiquitin ligases. FEBS Lett. 580, 4697–4702 [DOI] [PubMed] [Google Scholar]
- 18. Butler M. P., Hanly J. A., Moynagh P. N. (2007) Kinase-active interleukin-1 receptor-associated kinases promote polyubiquitination and degradation of the Pellino family. Direct evidence for Pellino proteins being ubiquitin-protein isopeptide ligases. J. Biol. Chem. 282, 29729–29737 [DOI] [PubMed] [Google Scholar]
- 19. Ordureau A., Smith H., Windheim M., Peggie M., Carrick E., Morrice N., Cohen P. (2008) The IRAK-catalyzed activation of the E3 ligase function of Pellino isoforms induces the Lys-3-linked polyubiquitination of IRAK1. Biochem. J. 409, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiao H., Qian W., Staschke K., Qian Y., Cui G., Deng L., Ehsani M., Wang X., Qian Y.W., Chen Z. J., Gilmour R., Jiang Z., Li X. (2008) Pellino 3b negatively regulates interleukin-1-induced TAK1-dependent NFκB activation. J. Biol. Chem. 283, 14654–14664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith H., Peggie M., Campbell D. G., Vandermoere F., Carrick E., Cohen P. (2009) Identification of the phosphorylation sites on the E3 ubiquitin ligase Pellino that are critical for activation by IRAK1 and IRAK4. Proc. Natl. Acad. Sci. U.S.A. 106, 4584–4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rich T., Allen R. L., Lucas A. M., Stewart A., Trowsdale J. (2000) Pellino-related sequences from Caenorhabditis elegans and Homo sapiens. Immunogenetics 52, 145–149 [DOI] [PubMed] [Google Scholar]
- 23. Chang M., Jin W., Sun S. C. (2009) Peli1 facilitates TRIF-dependent Toll-like receptor signaling and proinflammatory cytokine production. Nat Immunol. 10, 1089–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kanayama A., Seth R. B., Sun L., Ea C. K., Hong M., Shaito A., Chiu Y. H., Deng L., Chen Z. J. (2004) TAB2 and TAB3 activate the NF-κB pathway through binding to polyubiquitin chains. Mol. Cell 15, 535–548 [DOI] [PubMed] [Google Scholar]
- 25. Wang C., Deng L., Hong M., Akkaraju G. R., Inoue J., Chen Z. J. (2001) TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412, 346–351 [DOI] [PubMed] [Google Scholar]
- 26. Anderson P. (2008) Post-transcriptional control of cytokine production. Nat Immunol. 9, 353–359 [DOI] [PubMed] [Google Scholar]
- 27. Newton K., Matsumoto M. L., Wertz I. E., Kirkpatrick D. S., Lill J. R., Tan J., Dugger D., Gordon N., Sidhu S. S., Fellouse F. A., Komuves L., French D. M., Ferrando R. E., Lam C., Compaan D., Yu C., Bosanac I., Hymowitz S. G., Kelley R. F., Dixit V. M. (2008) Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell 134, 668–678 [DOI] [PubMed] [Google Scholar]
- 28. Goh E. T., Arthur J. S., Cheung P. C., Akira S., Toth R., Cohen P. (2012) Identification of the protein kinases that activate the E3 ubiquitin ligase Pellino 1 in the innate immune system. Biochem. J. 441, 339–346 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.