Background: The mechanism by which BALB/c mice exhibit a propensity to induce T helper 2 (Th2) responses and allergic diseases is unknown.
Results: Prostaglandin endoperoxide E2 (PGE2) prevents activation-induced cell death in Th2 cells of BALB/c mice via the E-prostanoid 2 (EP2) receptor and is dependent on granzyme B.
Conclusion: Signaling of PGE2 through EP2 promotes Th2 immune responses.
Significance: EP2 can be targeted as a therapeutic modality for Th2-mediated diseases.
Keywords: Cell Death, Cytokine, Immunology, Prostaglandins, T Cell, T Cell Biology, Activation-induced Cell Death, E Prostanoid Receptor EP2
Abstract
T helper 2 (Th2) cells play a central role in the progression of many diseases such as allergic airway inflammation, autoimmune diseases, and infections caused by intracellular pathogens. Consequently, animals such as BALB/c mice, which exhibit a propensity for generating Th2 responses, are susceptible to allergic airway inflammation, type-II autoimmune diseases, and various infections induced by intracellular pathogens, namely, Leishmania. In contrast, C3H/OuJ mice have a tendency for generating T helper 1 (Th1) responses and show resistance to these diseases. Here, we show that prostaglandin endoperoxide E2 selectively inhibits activation-induced cell death of Th2 cells by signaling through its receptor E-prostanoid receptor 2 (EP2). Consequently, Th2 cells derived from BALB/c mice expressed very high levels of EP2. On the other hand, Th2 cells derived from C3H/OuJ mice expressed very low levels of EP2, which failed to support the survival of Th2 cells. Furthermore, we found that this effect of EP2 on Th2 cells from BALB/c mice was executed by a granzyme B-mediated mechanism. EP2 belongs to a group of G-protein-coupled receptors that are amenable to therapeutic targeting. Our findings therefore identify EP2 as a promising target for small molecule-directed immunomodulation.
Introduction
Animal models of many human diseases have become fundamental experimental tools in biology. Inbred strains of mice have been extensively used for studying the immunological basis of susceptibility or resistance to various human diseases that a particular genetic constitution confers upon the host. Certain mouse strains such as BALB/c (haplotype H2d), which have a propensity for inducing T helper 2 (Th2)3-biased immune responses, are highly susceptible to allergic airway inflammation, humoral autoimmune inflammation, and infection by intracellular pathogens (1, 2). On the other hand, mouse strains such as C3H (haplotype H2k), which predominantly mount Th1-biased responses, are resistant to allergic airway inflammation (3) and successfully clear intracellular pathogens (1). Therefore, these strains are often employed as models to study various diseases. However, the mechanism that underlies the immunological divergence of these strains is not well understood. Identification of genetic differences that are responsible for the immunological differences in these two types of mouse strains might lead to the identification of novel targets for therapeutic intervention in allergic, autoimmune, and infectious diseases.
Polarization of Th cell responses is governed by two fundamental processes: (i) differentiation and (ii) maintenance of polarized effector cells. Considerable efforts have been made to correlate the differentiation process, and these studies have identified various factors involved in the differentiation of distinct Th cell subsets (4–7). However, the effector state of an immune response is determined by a dynamic balance between the generation of these effector cells and effector cell survival and death. Activated cells are eliminated from the system by an active process called activation-induced cell death (AICD). The role of AICD in the polarization of effector Th cell responses has not been well studied. In the last three decades, several Th cell subsets have been described in various circumstances, and the list is still growing. However, the biology of IFN-γ-producing Th1 cells and IL-4-producing Th2 cells has been well studied in multiple diseases (8–11). In a given polarized condition, Th1 and Th2 cells are in a dynamic balance (8–11), which in turn determines susceptibility or resistance in various immunological and infectious diseases such as allergic asthma (12) and leishmaniasis (13–15).
Here, we studied AICD of Th cells and the consequences of Th cell polarization in BALB/c and C3H/OuJ mice. We found that the C3H/OuJ strain does not support survival of Th2 cells in vivo. Prostaglandin endoperoxide E2 (PGE2) derived from macrophages inhibited AICD of Th2 cells derived from BALB/c mice but not those derived from C3H/OuJ mice. Finally, we showed that expression of the E-prostanoid receptor 2 (EP2) on Th2 cells is dramatically reduced in C3H/OuJ mice as compared with BALB/c mice.
EXPERIMENTAL PROCEDURES
Mice and Reagents
Female BALB/c mice were initially obtained from The Jackson Laboratory, Bar Harbor, ME. These mice were maintained in a specific pathogen-free animal facility at the International Center for Genetic Engineering and Biotechnology (ICGEB), New Delhi, India. Female mice at 6–8 weeks of age were used throughout the study following institutional ethical committee guidelines. Purified anti-IL4 (11.B.11), anti-IFN-γ (XMG1.2), and anti-IL12 (C17.8) antibodies, recombinant mouse IL-12p70, and purified mouse IL-4 were obtained from eBioscience, Inc. Fluorescently labeled anti-CD4, anti-TCR-β, and anti-granzyme B (GrB) antibodies and isotype controls and purified anti-CD3 (145.2C.11) and anti-CD28 (37.51) antibodies were purchased from BD Biosciences. Purified mouse IL-2 (R&D systems), ovalbumin (OVA), PGE2, and indomethacin were purchased from Sigma. Accell siRNA delivery reagents and EP2 small interfering RNA (siRNA) were purchased from Dharmacon. EP2 receptor antagonist AH6809 was purchased from Cayman Chemical. EP2 receptor and donkey anti-rabbit immunoglobulin G-conjugated horseradish peroxidase antibodies were purchased from Abcam Biochemicals.
Differentiation of T Helper Cell Subsets and Cytokine Detection
CD4+ T cells were purified from splenocytes by positive selection using a mouse CD4+ T cell isolation kit (Miltenyi Biotech). Differentiation of Th1 and Th2 cells was performed as described elsewhere (16). Briefly, CD4+ T cells at 1 × 106 cells/ml were activated with plate-bound anti-CD3 (1 μg/ml) and anti-CD28 (2 μg/ml) antibodies for 96 h. Cultures were supplemented with IL-2 (100 units/ml), IL-12 (10 ng/ml), and anti-IL-4 antibodies (10 μg/ml) for Th1 cell differentiation, whereas IL-4 (10 ng/ml), anti-IL-12 (10 μg/ml), and anti-IFN-γ (10 μg/ml) antibodies were used for Th2 cell differentiation. Cells were rested under the above cytokine conditions in the absence of anti-CD3 and anti-CD28 antibodies for an additional 48 h. These cells (1 × 106/ml) were then restimulated with plate-bound anti-CD3 and anti-CD28 antibodies, and supernatant was collected after 48 h for phenotype detection using X-map Luminex cytokine detection technology. AICD experiments of Th1 and Th2 cells were performed by stimulating cells (1 × 106 cells/well/ml) with plate-bound anti-CD3 antibodies (1 μg/ml) for 16 h. Cell death was measured by staining with propidium iodide (PI) (16). Briefly, cells were washed in phosphate-buffered saline (PBS) and resuspended in DNA staining buffer consisting of 0.5% saponin, 50 μg/ml PI, and 0.1 mg/ml RNase A in PBS and analyzed by flow cytometry for the percentage of cells in the hypodiploid region. Supernatants from lipopolysaccharide (LPS)-activated macrophages were generated by activating RAW 264.7 cells with LPS (100 ng/ml) for 24 h. Some of the cultures were supplemented with indomethacin (10 μm) to inhibit prostaglandin production. These supernatants were used at a ratio of 1:1 to assess their effects on AICD of differentiated Th1 and Th2 cells. PGE2 (100 nm–1 μm) was used to assess its effect on AICD in terminally differentiated Th1 and Th2 cells of BALB/c and C3H/OuJ mice. All cell cultures were maintained in RPMI 1640 medium supplemented with 2 mm l-glutamine, 50 μm 2-mercaptoethanol, heat-inactivated FBS (10%), and 10 mm gentamycin. The protein level of GrB was measured in Th2 cells in the presence or absence of PGE2 after activation with plate-bound anti-CD3. These experiments are representative of three independent experiments.
FACS Staining and Analysis
Th2-differentiated cells from BALB/c and C3H/OuJ mice were surface-stained with anti-CD4 antibody (eBioscience), and then GrB was detected by intracellular staining using phycoerythrin-labeled anti-GrB antibody (clone:16G6, eBioscience).
RNA Interference
Cells were transfected with siRNA for EP2 using the Accell delivery medium (Dharmacon) according to the manufacturer's protocol. EP2-specific and nontargeting siRNA were used at 1 μm concentration and incubated with cells for 96 h before cells were used for further processing.
Antigen-specific T Cell Proliferation Assay
For determination of antigen-specific T cells, 6–8-week-old female BALB/c and C3H/OuJ mice were immunized intraperitoneally with 100 μg of OVA plus alum (Pierce) in 0.2 ml of PBS on days 0 and 7 and sacrificed on day 9 to harvest the spleen and draining lymph nodes. OVA-specific T cells were expanded in culture in the presence of OVA (50 μg/ml) for 72 h followed by resting for 48 h. Cells were then restimulated with various OVA concentrations for 72 h. One μCi/well [3H]thymidine was added to the cultures for the last 12 h. Cells were harvested using the FilterMate cell harvester, and radioactivity counts were obtained using MicroBeta TriLux (PerkinElmer Life Sciences). Culture supernatants were collected at 48 h of in vitro stimulation with OVA, and cytokines were quantified using the Luminex xMAP technology.
T Cell Adoptive Transfer
OVA-specific Th cells from BALB/c and C3H/OuJ mice were expanded in vitro by restimulating draining lymph node cells derived from OVA-immunized animals. Cells were labeled with 5 μm carboxyfluorescein diacetate-succinimidyl ester (CFDA-SE) per 5 × 106 cells, and ∼10 × 106 cells were adoptively transferred into syngeneic BALB/c or C3H/OuJ mice, respectively, via tail vein in a total volume of 100 μl PBS. These mice were immunized with OVA and alum mixture 24 h prior to adoptive transfer and also at the time of transfer. Five days later, cells were harvested from spleens and assessed for the presence of CFDA-SE-labeled cells.
Quantitative Real-time PCR
Total RNA was isolated with an RNeasy mini kit (Qiagen). Genomic DNA was removed with RNase-free DNase prior to cDNA synthesis. First-strand cDNA synthesis was performed for each RNA sample with the Sensiscript RT kit (Qiagen). Reverse-transcribed cDNA was subjected to real-time PCR with the SYBR Green master mix (Qiagen). EP1, EP2, EP3, EP4, and β-actin real-time PCR primer sequences were: EP1 forward, 5′-CTCCTTGCGGCATTAGTGTG-3′, reverse, 5′-TGCGGTCTTTCGGAATCGT-3′; EP2 forward, 5′-CGTTATCCTCAACCTCATTCGC-3′, reverse, 5′-TCCGTCTCCTCTGCCATCG-3′; EP3 forward, 5′-TTGCTGGCTCTGGTGGTGAC-3′, reverse, 5-GCTGGACTGCGAGACGGC-3′; EP4 forward 5′-TGACCCAAGCAGACACCACCT-3′, reverse, 5′-TCCCACTAACCTCATCCACCAA-3′. The relative expression level of mRNAs was normalized to that of internal control β-actin by using the 2−ΔΔCt cycle threshold method.
Western Blot Analysis
Whole cell extract was prepared after restimulation of Th1- or Th2-differentiated cells from BALB/c and C3H/OuJ mice with Triton X-100 buffer (50 mmol/liter Tris (pH 7.5), 150 mmol/liter NaCl, 0.5% Triton X-100). Samples were electrophoresed on a 12% SDS-polyacrylamide gel and electroblotted onto polyvinylidene difluoride (PVDF) membranes. Blots were blocked for 1 h in 5% BSA in PBS. EP2 (40-kDa) protein was detected with anti-EP2 polyclonal antibody as recommended by the manufacturer (Abcam) and diluted at 1:500; in addition, donkey anti-rabbit-immunoglobulin G-conjugated horseradish peroxidase (diluted 1:5000) was used as a secondary antibody. Immunoblotting for β-actin was carried out to confirm equal loading.
Statistical Analysis
Differences in treatment groups were assessed by paired two-tailed Student's t test using Prism software (GraphPad).
RESULTS AND DISCUSSION
C3H/OuJ Mice Fail to Support Th2 Cell Survival in Vivo
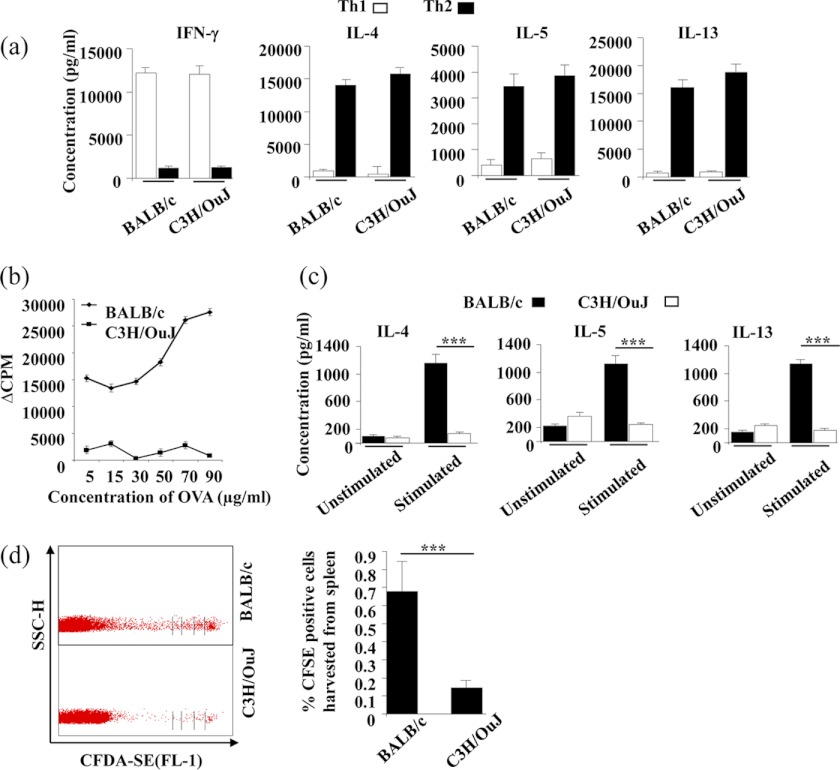
It is well established that BALB/c mice tend to mount Th2 responses, whereas C3H/OuJ mice have a propensity to induce Th1 responses (17). However, the mechanisms involved in this differential response are not clear. Therefore, we revisited this issue. First, we determined whether C3H/OuJ mice have an inherent deficiency in the differentiation of naive CD4+ T cells toward the Th2 cell lineage. Purified CD4+ T cells from C3H/OuJ and BALB/c mice were subjected to Th1 and Th2 cell differentiation and analyzed for cytokine production. We did not observe any noticeable differences in the differentiation of Th1 or Th2 cells between the two strains (Fig. 1a). Second, to determine whether differential responses are due to poor maintenance of the Th1 or Th2 cell subset, we tested whether there is any difference in Th2-biased immune responses between C3H/OuJ and BALB/c mice. We immunized mice with OVA antigens emulsified in alum and measured antigen-specific proliferation and cytokine responses. We found that as compared with BALB/c mice, C3H/OuJ mice generated significantly lower proliferative and Th2 cytokine responses to OVA antigens (Fig. 1, b and c). Using this immunization protocol, we were unable to detect IFN-γ production by T cells from either of these strains (data not shown). Finally, we tested the possibility that C3H/OuJ mice have a defect in the maintenance of Th2 cells. For this purpose, we expanded OVA antigen-specific Th2 cells of both BALB/c and C3H/OuJ mice in vitro. These cells were labeled with the dye CFDA-SE and were adoptively transferred into syngeneic C3H/OuJ and BALB/c mice, respectively. These mice were then immunized with OVA and alum mixture. Five days later, cells were harvested from spleens and lymph nodes and assessed for the presence and cellular division of adoptively transferred Th2 cells. We recovered fewer Th2 cells from C3H/OuJ mice, and these cells had divided fewer times than the Th2 cells from BALB/c mice (Fig. 1d). Therefore, C3H/OuJ mice fail to support the maintenance of Th2 cells.
FIGURE 1.
BALB/c mice support survival of Th2 cells in vivo, whereas C3H/OuJ mice do not. a, BALB/c and C3H/OuJ mice generate comparable Th1 and Th2 cell responses in vitro. CD4+ T cells from BALB/c and C3H/OuJ strains were stimulated with plate-bound anti-CD3 (1 μg/ml) and anti-CD28 (2 μg/ml) antibodies under Th1- or Th2-priming conditions. Terminally differentiated cells were restimulated with plate-bound anti-CD3 and anti-CD28 antibodies, and supernatant was collected after 48 h for cytokine measurements. b, C3H/OuJ mice generate attenuated antigen-specific T cell responses to OVA immunization. Draining lymph node cells were harvested from mice immunized with OVA + alum and in vitro challenged with OVA at various concentrations. Proliferation was assessed following culture for 72 h in vitro and pulsing with [3H]thymidine for the last 8–10 h of incubation time. c, cytokine production was determined from the culture supernatants of the cells stimulated with 70 μg/ml OVA from the above culture plates after 48 h of incubation. d, OVA antigen-specific Th cells of both BALB/c and C3H/OuJ mice were expanded in vitro, CFDA-SE-labeled, and adoptively transferred in syngeneic BALB/c and C3H/OuJ mice (immunized with OVA + alum), respectively. After 5 days, spleens and lymph node cells were harvested and assessed for the presence and proliferation of the transferred cells using FACS. SSC-H, side scatter pulse height; CFSE, carboxyfluorescein succinimidyl ester. Representative data (mean ± S.E.) in triplicate wells from at least three independent experiments are shown. ***, p < 0.001 versus untreated.
Prostaglandin E2 Mediates the Differential Susceptibility of Th2 Cells from BALB/c and C3H/OuJ Mice to AICD
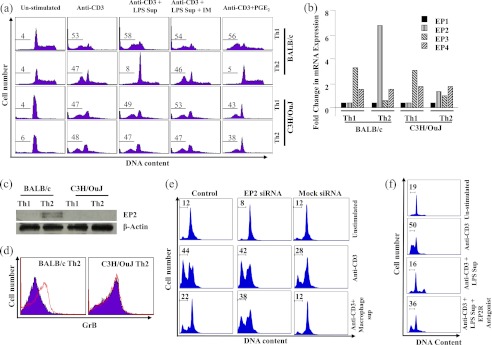
Polarization and maintenance of an effective immune response is a dynamic process (18). Some of the effector cells are continuously eliminated by an active process called AICD (18). Therefore, we tested AICD in Th1 and Th2 cells from BALB/c and C3H/He mice in vitro. Terminally differentiated Th1 and Th2 cells were activated overnight with plate-bound anti-CD3 antibodies. We found that AICD was induced in Th1 and Th2 cells from each of these strains at comparable levels (Fig. 2a, left panels). Therefore, we concluded that intrinsic susceptibility to effector T cell death is not responsible for the observed differences in immune responses between the two mouse strains. Because AICD is controlled in vivo by active mechanisms (19), we tested the role of antigen-presenting cells in the differential death of differentiated Th cells from BALB/c and C3H/OuJ mice. We first prepared supernatants from LPS-activated macrophages by treating RAW 264.7 cells with 100 ng/ml LPS for 24 h. We found that supernatant from LPS-activated macrophages profoundly inhibited AICD in Th2 cells from BALB/c but not C3H/OuJ mice (Fig. 2a, middle panels). Therefore, we concluded that the LPS-activated supernatant contains AICD inhibitory factors.
FIGURE 2.
PGE2 inhibits AICD of Th2 cells derived from BALB/c but not C3H/OuJ mice. a, terminally differentiated Th1 and Th2 cells were activated with plate-bound anti-CD3 antibodies overnight. DNA fragmentation was measured by PI staining. Th1 and Th2 cells of these strains induced comparable AICD (left two sets of panels). Supernatant (Sup) from LPS-activated macrophages drastically inhibited AICD in Th2 cells of BALB/c but not C3H/OuJ origin (middle panels). Supernatant from LPS-activated macrophages generated in the presence of indomethacin (IM; 10 μm) did not alter the inhibition of AICD in Th2 cells of either strain (second set of panels from the right). The addition of PGE2 to the culture medium (1 μm) dramatically inhibited AICD of Th2 cells of BALB/c but not C3H/OuJ origin (right panels). Numbers indicated in the figures are the percentages of apoptotic cells. b, expression of PGE2 receptor EP2 in Th2 cells from BALB/c and C3H/OuJ mice. Th1 and Th2 cells from BALB/c and C3H/OuJ mice were activated with plate-bound anti-CD3 + anti-CD28 antibodies, and total mRNA was isolated. Quantitative real-time PCR was performed for quantifying expression of PGE2 receptors. Relative expression was calculated using β-actin as an internal control. c, Western blot analysis of EP2 receptor (40 kDa) levels in Th1 and Th2 cells of BALB/c and C3H/OuJ mice. Terminally differentiated Th1 and Th2 cells were activated with plate-bound anti-CD3 + anti-CD28 antibodies. β-Actin (47 kDa) was used as an internal control. d, expression of GrB was measured in Th2 cells in the presence or absence of PGE2 after activation with plate-bound anti-CD3 antibodies. The red line indicates GrB staining, whereas the blue area indicates isotype control. e, selective inhibition of EP2 by siRNA abrogates the AICD inhibitory activity of PGE2. Terminally differentiated Th2 cells were activated on plate-bound anti-CD3 overnight. Supernatant from LPS-activated macrophages inhibited AICD in control and mock-treated Th2 cells but not in EP2-siRNA (1 μm)-treated cells. DNA fragmentation was measured by PI staining. f, PGE2 is unable to inhibit AICD of Th2 cells derived from BALB/c mice in the presence of EP2 receptor antagonist AH6809 (100 μm). Numbers indicated in the figures are the percentage of apoptotic cells. Data presented here are representative of at least three independent experiments.
Previously, it has been shown that apoptosis in B cells, immature thymocytes, and tumor cell lines is controlled by PGE2 (20). In fact, PGE2 promotes the survival of cancer cells (21). Therefore, we tested whether prostaglandins are responsible for the inhibitory effect of supernatant from LPS-activated macrophages on AICD in Th2 cells from BALB/c mice. We prepared supernatants from LPS-activated macrophages in the presence of indomethacin, an inhibitor of prostaglandin synthase. Although indomethacin did not affect cytokine production (supplemental Fig. 1), these supernatants were unable to inhibit AICD in Th2 cells from BALB/c mice (Fig. 2a, second set of panels from the right). These findings suggested that prostaglandins play a role in protecting Th2 cells from AICD in BALB/c but not C3H/OuJ mice. It is well known that PGE2 contributes to the polarization of Th2 cell responses and has been implicated in the generation of allergic immune responses (22). Consistent with our studies using supernatants from LPS-activated macrophages, the addition of commercially available PGE2 to the culture medium dramatically inhibited AICD in Th2 cells from BALB/c but not C3H/OuJ mice (Fig. 2a, right panels). These findings suggested that Th2 cells from C3H/OuJ mice are defective in PGE2-mediated inhibition of AICD.
PGE2 Receptor EP2 Is Differentially Regulated in Th2 Cells of BALB/c and C3H/OuJ Mice
We examined the expression of PGE2 signaling components in Th1 and Th2 cells of BALB/c and C3H/OuJ mice. First, we analyzed the expression of PGE2 receptors. There are four receptors (EP1–EP4) for PGE2 with wide expression profiles in tissues and cells (23). To determine the expression of these receptors, we generated Th1 and Th2 cells from purified CD4+ T cells of either of the stains and activated the cells with plate-bound anti-CD3 and anti-CD28 antibodies. Total RNA was extracted and subjected to quantitative PCR for EP1, EP2, EP3, and EP4 using specific primer probes (Fig. 2b). We were unable to detect expression of EP1 in Th1 or Th2 cells of either mouse strain. In contrast, EP2 was expressed significantly higher in Th2 cells of BALB/c origin than those of C3H/OuJ origin. Th1 cells of either of these strains lacked EP2 expression. EP3 was expressed in Th1 cells of both strains, but was not expressed in Th2 cells of either strain. In contrast, expression of EP4 was detected in both Th1 and Th2 cells of both strains. Based on these findings, we predicted that EP2-mediated signaling may play a role in the inhibition of AICD in Th2 cells of BALB/c mice and that the absence of EP2 mediates the resistance of Th2 cells from C3H/OuJ mice to AICD.
To further confirm that BALB/c mice and C3H mice differ in EP2 expression, we performed Western blot analysis of EP2 in terminally differentiated and reactivated Th1 and Th2 cells derived from BALB/c and C3H/OuJ mice. Interestingly, we observed that Th2 cells derived from BALB/c but not C3H/OuJ mice expressed EP2 receptor (Fig. 2c). As expected, Th1 cells from neither of these strains expressed EP2 (Fig. 2c).
PGE2 Mediates AICD in Th2 Cells from BALB/c Mice via GrB
Previously, we have shown that AICD of Th2 cells is mediated by cytosolic GrB (16). Therefore, we tested GrB expression in Th2 cells treated with PGE2. We found that PGE2 dramatically inhibits GrB expression in Th2 cells of BALB/c but not C3H/OuJ origin (Fig. 2d). These findings suggested that activation ofEP2 in Th2 cells by PGE2 prevents AICD by inhibiting GrB production.
To further confirm that EP2 plays a role in the observed inhibition of AICD mediated by PGE2 in BALB/c mice, we performed knockdown experiments of EP2 by siRNA in Th2 cells of BALB/c mice. Transfection efficiency was assessed by using green nontargeting siRNA (supplemental Fig. 2). As expected, EP2 knockdown Th2 cells remained susceptible to AICD even in the presence of PGE2 (Fig. 2e). To provide further evidence for the role of EP2 for inhibiting AICD of Th2 cells, we performed receptor competition assays. We pretreated Th2 cells with EP2 receptor antagonist AH6809 and then performed AICD assays in the presence or absence of PGE2. As shown in Fig. 2f, the presence of the EP2 antagonist dramatically abrogated PGE2-mediated inhibition of AICD. Taken together, these data provide strong evidence that in the absence of EP2 receptor activity, PGE2 is unable to exhibit its inhibitory effect on AICD.
PGE2 is widely known for its immunoregulatory functions, promotion of tumor growth, and inhibition of thymocyte apoptosis (24). It is also known that PGE2 plays critical roles in the development of Th2 cell responses (25). However, a role of PGE2 in susceptibility of BALB/c mice to various diseases has not been fully established. Until now, the relevant receptor that is responsible for the Th2 cell-enhancing activities of PGE2 has remained unknown. Our study provides strong evidence that signaling of PGE2 through EP2 promotes Th2 immune responses. Therefore, EP2 is an attractive target for modulating Th2 cell-mediated inflammatory and infectious diseases. EP2 is a G-protein-coupled receptor, and thus, pharmacological antagonists can be designed to inhibit its functions (26, 27). All commercially available EP2 antagonists cross-react with EP4, which has an opposing function to EP2. Specific antagonists to EP2 will need to be designed to selectively inhibit the Th2-promoting activities of PGE2 during therapy.
In summary, we have shown that EP2 plays a role in the polarization of Th2 cells by inhibiting AICD in these cells. Thus, EP2 is an attractive new target for therapeutic intervention in allergic and infectious diseases.
Supplementary Material
Acknowledgment
C3H/OuJ mice were a kind gift from Dr. Anna George, National Institute of Immunology, New Delhi, India.
This work was supported in part by a Wellcome Trust International senior fellowship, a Ramaligaswami fellowship (Government of India), and a grant from the Department of Biotechnology, Government of India.

This article contains supplemental Figs. 1 and 2.
- Th2
- T helper 2
- Th1
- T helper 1
- PGE2
- prostaglandin endoperoxide E2
- EP
- E-prostanoid
- AICD
- activation-induced cell death
- PI
- propidium iodide
- GrB
- granzyme B
- OVA
- ovalbumin
- CFDA-SE
- carboxyfluorescein diacetate-succinimidyl ester.
REFERENCES
- 1. Gorczynski R. M. (1982) Nature of resistance to leishmaniasis in experimental rodents. Dev. Comp. Immunol. 6, 199–207 [DOI] [PubMed] [Google Scholar]
- 2. Launois P., Himmelrich H., Tacchini-Cottier F., Milon G., Louis J. A. (1999) New insight into the mechanisms underlying Th2 cell development and susceptibility to Leishmania major in BALB/c mice. Microbes Infect. 1, 59–64 [DOI] [PubMed] [Google Scholar]
- 3. Brewer J. P., Kisselgof A. B., Martin T. R. (1999) Genetic variability in pulmonary physiological, cellular, and antibody responses to antigen in mice. Am. J. Respir. Crit. Care Med. 160, 1150–1156 [DOI] [PubMed] [Google Scholar]
- 4. Bajénoff M., Wurtz O., Guerder S. (2002) Repeated antigen exposure is necessary for the differentiation, but not the initial proliferation, of naive CD4+ T cells. J. Immunol. 168, 1723–1729 [DOI] [PubMed] [Google Scholar]
- 5. Bird J. J., Brown D. R., Mullen A. C., Moskowitz N. H., Mahowald M. A., Sider J. R., Gajewski T. F., Wang C. R., Reiner S. L. (1998) Helper T cell differentiation is controlled by the cell cycle. Immunity 9, 229–237 [DOI] [PubMed] [Google Scholar]
- 6. Gett A. V., Hodgkin P. D. (1998) Cell division regulates the T cell cytokine repertoire, revealing a mechanism underlying immune class regulation. Proc. Natl. Acad. Sci. U.S.A. 95, 9488–9493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grogan J. L., Mohrs M., Harmon B., Lacy D. A., Sedat J. W., Locksley R. M. (2001) Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity 14, 205–215 [DOI] [PubMed] [Google Scholar]
- 8. Azar S. T., Tamim H., Beyhum H. N., Habbal M. Z., Almawi W. Y. (1999) Type I (insulin-dependent) diabetes is a Th1- and Th2-mediated autoimmune disease. Clin. Diagn. Lab. Immunol. 6, 306–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Biedermann T., Röcken M., Carballido J. M. (2004) TH1 and TH2 lymphocyte development and regulation of TH cell-mediated immune responses of the skin. J. Investig. Dermatol. Symp. Proc. 9, 5–14 [DOI] [PubMed] [Google Scholar]
- 10. Mazzarella G., Bianco A., Catena E., De Palma R., Abbate G. F. (2000) Th1/Th2 lymphocyte polarization in asthma. Allergy 55, Suppl. 61, 6–9 [DOI] [PubMed] [Google Scholar]
- 11. Wangoo A., Sparer T., Brown I. N., Snewin V. A., Janssen R., Thole J., Cook H. T., Shaw R. J., Young D. B. (2001) Contribution of Th1 and Th2 cells to protection and pathology in experimental models of granulomatous lung disease. J. Immunol. 166, 3432–3439 [DOI] [PubMed] [Google Scholar]
- 12. Das J., Eynott P., Jupp R., Bothwell A., Van Kaer L., Shi Y., Das G. (2006) Natural killer T cells and CD8+ T cells are dispensable for T cell-dependent allergic airway inflammation. Nat. Med. 12, 1345–1346; author reply 1347 [DOI] [PubMed] [Google Scholar]
- 13. Chatelain R., Varkila K., Coffman R. L. (1992) IL-4 induces a Th2 response in Leishmania major-infected mice. J. Immunol. 148, 1182–1187 [PubMed] [Google Scholar]
- 14. Radwanska M., Cutler A. J., Hoving J. C., Magez S., Holscher C., Bohms A., Arendse B., Kirsch R., Hunig T., Alexander J., Kaye P., Brombacher F. (2007) Deletion of IL-4Rα on CD4 T cells renders BALB/c mice resistant to Leishmania major infection. PLoS Pathog. 3, e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scott P. (1991) IFN-γ modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J. Immunol. 147, 3149–3155 [PubMed] [Google Scholar]
- 16. Devadas S., Das J., Liu C., Zhang L., Roberts A. I., Pan Z., Moore P. A., Das G., Shi Y. (2006) Granzyme B is critical for T cell receptor-induced cell death of type 2 helper T cells. Immunity 25, 237–247 [DOI] [PubMed] [Google Scholar]
- 17. Spellberg B., Edwards J. E., Jr. (2001) Type 1/Type 2 immunity in infectious diseases. Clin. Infect. Dis. 32, 76–102 [DOI] [PubMed] [Google Scholar]
- 18. Lanzavecchia A., Sallusto F. (2000) Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science 290, 92–97 [DOI] [PubMed] [Google Scholar]
- 19. Green D. R., Droin N., Pinkoski M. (2003) Activation-induced cell death in T cells. Immunol. Rev. 193, 70–81 [DOI] [PubMed] [Google Scholar]
- 20. Kim R., Emi M., Tanabe K. (2006) Cancer immunosuppression and autoimmune disease: beyond immunosuppressive networks for tumor immunity. Immunology 119, 254–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dempke W., Rie C., Grothey A., Schmoll H. J. (2001) Cyclooxygenase-2: a novel target for cancer chemotherapy? J. Cancer Res. Clin. Oncol. 127, 411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fedyk E. R., Phipps R. P. (1996) Prostaglandin E2 receptors of the EP2 and EP4 subtypes regulate activation and differentiation of mouse B lymphocytes to IgE-secreting cells. Proc. Natl. Acad. Sci. U.S.A. 93, 10978–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hata A. N., Breyer R. M. (2004) Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol. Ther. 103, 147–166 [DOI] [PubMed] [Google Scholar]
- 24. Goetzl E. J., An S., Zeng L. (1995) Specific suppression by prostaglandin E2 of activation-induced apoptosis of human CD4+CD8+ T lymphoblasts. J. Immunol. 154, 1041–1047 [PubMed] [Google Scholar]
- 25. Kapsenberg M. L., Hilkens C. M., Wierenga E. A., Kalinski P. (1999) The paradigm of type 1 and type 2 antigen-presenting cells: implications for atopic allergy. Clin. Exp. Allergy 29, Suppl. 2, 33–36 [PubMed] [Google Scholar]
- 26. Doré S. (2006) GPCR antagonists as an alternative to COX-2 inhibitors: a case for the PGE2 EP1 receptor. Trends Pharmacol. Sci. 27, 458–460 [DOI] [PubMed] [Google Scholar]
- 27. Wilson R. J., Rhodes S. A., Wood R. L., Shield V. J., Noel L. S., Gray D. W., Giles H. (2004) Functional pharmacology of human prostanoid EP2 and EP4 receptors. Eur. J. Pharmacol. 501, 49–58 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.