Background: Epithelial-mesenchymal transition (EMT) is an important program in tumor metastasis.
Results: Nickel chloride (NiCl2) induced EMT in human bronchial epithelial cells, including down-regulation of epithelial-cadherin (E-cadherin) and up-regulation of fibronectin.
Conclusion: ROS generation and promoter hypermethylation of E-cadherin are involved in NiCl2-mediated EMT.
Significance: The results of this study may shed new light on the role of nickel in carcinogenesis.
Keywords: E-cadherin, Epigenetics, Epithelial Mesenchymal Transition, Hypoxia-inducible Factor (HIF), Nickel, Reactive Oxygen Species (ROS), Promoter Hypermethylation
Abstract
Epithelial-mesenchymal transition (EMT) is considered a critical event in the pathogenesis of lung fibrosis and tumor metastasis. During EMT, the expression of differentiation markers switches from cell-cell junction proteins such as E-cadherin to mesenchymal markers such as fibronectin. Although nickel-containing compounds have been shown to be associated with lung carcinogenesis, the role of nickel in the EMT process in bronchial epithelial cells is not clear. The aim of this study was to examine whether nickel contributes to EMT in human bronchial epithelial cells. We also attempted to clarify the mechanisms involved in NiCl2-induced EMT. Our results showed that NiCl2 induced EMT phenotype marker alterations such as up-regulation of fibronectin and down-regulation of E-cadherin. In addition, the potent antioxidant N-acetylcysteine blocked EMT and expression of HIF-1α induced by NiCl2, whereas the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine restored the down-regulation of E-cadherin induced by NiCl2. Promoter hypermethylation of E-cadherin, determined by quantitative real time methyl-specific PCR and bisulfate sequencing, was also induced by NiCl2. These results shed new light on the contribution of NiCl2 to carcinogenesis. Specifically, NiCl2 induces down-regulation of E-cadherin by reactive oxygen species generation and promoter hypermethylation. This study demonstrates for the first time that nickel induces EMT in bronchial epithelial cells.
Introduction
Epithelial mesenchymal transition (EMT)2 is the biological process by which cells switch from polarized immotile epithelial type to motile mesenchymal type. The processes of EMT include reorganization of the actin cytoskeleton, remodeling of epithelial cell-cell and cell-matrix adhesion contacts, repression of epithelial markers (e.g. epithelial-cadherin and E-cadherin), induction of mesenchymal markers (e.g. fibronectin), and acquisition of motile capacity. EMT has been primarily described in embryonic development and organ formation. Nevertheless, recent studies have demonstrated an association between the EMT process and cancer progression. This process is considered a transient and reversible event that can lead to tumor progression (1, 2). Inhibition of the EMT process in cancer cells results in reduced tumor invasion and metastatic spread, indicating that this is an important therapeutic target for cancer therapy (3–5).
E-cadherin is a cell surface adhesion glycoprotein that plays an important role in EMT-mediated cancer cell progression (6). DNA deletion, mutational inactivation, gene silencing, and proteolytic degradation contribute to the loss of E-cadherin expression during tumor progression (4, 7). Accumulated hypoxia-inducible factor-1α (HIF-1α), a key mediator of cellular adaptation to hypoxia, and up-regulated Snail and Slug repress E-cadherin expression and E-cadherin tumor malignancy (5, 8). Moreover, epigenetic inhibition of E-cadherin by promoter hypermethylation following chronic exposure to low doses of cigarette smoke condensate has been reported in vitro (9). Various pathways in the regulation of E-cadherin might inhibit the EMT process. We attempted to determine the key pathway for inhibiting the EMT process in our tumor model.
DNA damage induced by metal compounds (e.g. nickel, arsenic, lead, chromium, manganese, and cadmium) promotes ROS production in carcinogenesis (10). Nickel and several of its compounds are widely used in modern industry. The International Agency for Research on Cancer classifies metallic nickel and nickel compounds as possibly carcinogenic and carcinogenic to humans, respectively (10). High consumption of nickel products leads to exposure to nickel via air, soil, food, water, tobacco smoke, and occupational and environmental pollution (11). Significantly higher nickel concentrations in lung tissues of lung cancer patients, in comparison with normal controls, suggest that nickel contributes to lung carcinogenesis in vivo. Recently, it has been found that soluble nickel ions interact with cell surface receptors and disrupt active cell signaling, resulting in induction of genes, such as HIF-1α (12). This indicates that nickel contributes to the EMT process in lung cancer. We hypothesized that nickel compounds induce EMT and increase malignancy during lung carcinogenesis. To test this hypothesis, we treated bronchial epithelial BEAS-2B cells with nickel chloride (NiCl2) and found that NiCl2 induces EMT. In addition, we demonstrated that repression of E-cadherin by NiCl2 is involved in ROS induction and aberrant methylation of E-cadherin promoter.
EXPERIMENTAL PROCEDURES
Cell Lines and Chemicals
Human bronchial epithelial cell lines (BEAS-2B cells) immortalized with SV40 (American Type Culture Collection, Manassas, VA) were maintained in serum-free LHC-9 medium (BioSource International Inc., Nivelles, Belgium) in an incubator at 37 °C in a humidified atmosphere of 5% CO2. NiCl2 (Sigma, N-6136), nickel sulfate (NiSO4, Sigma, N-4882), sodium arsenite (NaAsO2, Sigma, S-7400), N-acetylcysteine (NAC, Sigma, A-7250), deferoxamine mesylate (Sigma, D9533), and 5-aza-2′-deoxycytidine (Sigma, A-3656) were obtained from Sigma.
Immunofluorescence
BEAS-2B cells were seeded onto 24-well plates (2 × 105 cells/well) with coverslips and treated with NiCl2 (0, 0.25, and 0.5 mm) for 72 h. Next, the cells were fixed and incubated with primary antibody against human E-cadherin (BD Biosciences, 610182) at 4 °C overnight. This was followed by incubation with secondary antibody goat anti-mouse FITC (A11011, Alexa Fluor® 488) for 60 min after washing with PBS. Thereafter, cells were stained with DAPI (1:1000) for 45 min. E-cadherin was detected on a confocal laser scanning microscope (Zeiss LSM 510 META) at 630× magnification.
Western Blot Analysis
Anti-fibronectin (BD Biosciences, 610077), anti-E-cadherin (BD Biosciences, 610182), anti-Snail (Cell Signaling, 3879S), anti-Slug (Cell Signaling, 9585S), anti-HIF-1α (BD Biosciences, 610959), anti-NADPH oxidase 1 (NOX1) (GeneTex Inc., GTX103888), anti-SOD2 (Santa Cruz Biotechnology, sc-18503), anti-catalase (GeneTex Inc., GTX110704), anti-glutathione peroxidase-1/2 (GPX-1/2) (Santa Cruz Biotechnology, sc-133160), and anti-β-actin (Sigma, A5441) antibodies were used to detect fibronectin, E-cadherin, Snail, Slug, HIF-1α, SOD2, catalase, GPX-1/2, and β-actin, respectively. The complete protocol for Western blot analysis has been described previously (13).
Isolation of RNA, Reverse Transcriptase-PCR, and Quantitative Real Time-PCR Analysis
Total RNA was isolated using TRIzol reagent (Invitrogen). The combinational DNA was then reverse-transcribed from 2 μg of total RNA using random hexamer primers and Moloney murine leukemia virus reverse transcriptase, RNase H minus (Promega). For fibronectin, the primers were 5′-CCGTGGGCAACTCTGTC-3′ (forward) and 5′-TGCGGCAGTTGTCACAG-3′ (reverse); for E-cadherin, the primers were 5′-TGGAGAGACACTGCCAACTG-3′ (forward) and 5′-AGGCTGTGCCTTCCTACAGA3′ (reverse); and for β-actin, the primers were 5′-TCATCACCATTGGCAATGAG-3′ (forward) and 5′-CACTGTGTTGGCGTACAGGT-3′ (reverse). Real time-PCR was performed using an ABI PRISM 7000 real time PCR system with gene-specific primers and Taqman Universal PCR Master Mix, No AmpErase UNG (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase served as the internal control.
Detection of ROS by Flow Cytometry and Fluorescence Spectroscopy
BEAS-2B cells were pretreated with or without 10 mm NAC for 1 h. This was followed by treatment with NiCl2 and staining with 10 μm 2′,7′-dichlorodihydrofluorescein diacetate (D399), 10 μm 3′-(p-aminophenyl) fluorescein (Invitrogen, A36003), 10 μm 3′-(p-hydroxyphenyl) fluorescein (HPF, Invitrogen, H36004), or 10 μm dihydroethidium (Invitrogen, D1168) for 30 min. The cells were harvested and washed twice with PBS, resuspended in PBS, and analyzed by flow cytometry. Ethidium fluorescence was measured using fluorescence spectroscopy (excitation 485 nm and emission 590 nm).
Mitochondrial Membrane Potential Measurement
Mitochondrial membrane potential levels were measured using lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide 1 (JC-1) fluorescent dye (Invitrogen, T3168). The cells were plated and treated as indicated, and 1 μm JC-1 was added 30 min prior to harvest. Cells were collected by trypsinization and washed with PBS. The red (aggregated JC-1; R2 region) and green (monomeric JC-1; R1 region) fluorescence signals were analyzed by flow cytometry (BD Biosciences) and Cell Quest software (BD Biosciences).
RNA Interference
RNAi reagents were obtained from the National RNAi Core Facility of the Institute of Molecular Biology/Genomic Research Center, Academia Sinica. Individual clones were identified by their unique TRC number, e.g. shLuc TRCN0000072246 for vector control targeted to luciferase and shHIF-1α (810) and TRCN0000003810 (responding sequence, GTGATGAAAGAATTACCGAAT) for vector targeted to HIF-1α. The cells were selected using 2 μg/ml puromycin (Sigma, P8833).
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assay was carried out according to the manufacturer's protocol (chromatin immunoprecipitation assay kit, catalog no. 17-295, Upstate Biotechnology Inc., Lake Placid, NY). Immune complexes were prepared using anti-HIF-1α (BD Biosciences, 610959) or anti-Snail (Cell Signaling, 3879S) antibody. The supernatant of immunoprecipitation reaction carried out in the absence of antibody served as the total input DNA control. PCR was carried out with 10 μl of each sample using the following primers: E-cadherin promoter-S, 5′-CTGGTACCTCCAGGCTAGAGGGTCACCG-3′; E-cadherin promoter-AS, 5′-TTAAAGCTTCCGGGTGCGGTCGGGTCGGG-3′. This was followed by analysis on 2% agarose gels. Primers from the E-cadherin open reading frame for amplification of a 270-bp fragment served as the PCR control.
Methylation-specific PCR
Genomic DNA was isolated using the genomic DNA isolation kit (Qiagen, Inc., Hilden, Germany). Bisulfite treatment was carried out as described previously (14). Methylation patterns within the E-cadherin CpG island of exon 1 (sequence −126 bp to +144 bp relative to transcription start site, GenBankTM accession number D49685) were determined using a previously described nested PCR approach (15). The sequencing primers were 5′-GTTTAGTTTTGGGGAGGGGTT-3′ (sense) and 5′-ACTACTACTCCAAAAACCCATAACTAA-3′ (antisense). The cycling conditions consisted of an initial denaturation step at 95 °C for 5 min, followed by the addition of 1 unit of Taq polymerase and 30 cycles at 95 °C for 30 s, 50 °C for 30 s, and 72 °C for 30 s. Nested primer sequences for E-cadherin for the methylated reaction were 5′-TGTAGTTACGTATTTATTTTTAGTGGCGTC-3′ (sense) and 5′-CGAATACGATCGAATCGAACCG-3′ (antisense). The primer sequences for the unmethylated reaction were 5′-TGGTTGTAGTTATGTATTTATTTTTAGTGGTGTT-3′ (sense) and 5′-ACACCAAATACAATCAAATCAAACCAAA-3′ (antisense). PCR parameters were the same as those listed above, except that the annealing temperatures for the methylated and unmethylated reactions were 64 and 62 °C, respectively. The product sizes of the methylated and unmethylated reactions were 112 and 120 bp, respectively.
Quantitative Real Time Methylation-specific PCR
Semi-quantitative methylation specific PCR (QMSP) was carried out with Smart Quant Green Master Mix kit (Protech Technology, Taiwan) and primers specific for fully methylated E-cadherin promoter sequences (16). Real time-PCR conditions were 95 °C for 15 min followed by 45 cycles at 94 °C for 15 s and 60 °C for 1 min with data acquisition after each cycle. Triple replicates of each sample were obtained by laser detector of ABI Prism 7000 sequence detection system. CT values of each sample were determined.
Bisulfate Genomic Sequencing
Modified DNA was amplified with two rounds of PCR using two pairs of nested primers. First round PCR was performed using primer S1 5′-TTTTGATTTTAGGTTTTAGTGAGTTAT-3′ (upstream, sequence position −277 to −250) and primer S2 5′-AATACCTACAACAACAACAACAA-3′ (downstream, +177 to +154). Amplification for PCR was performed with ProTaq polymerase (Protech Technology, Taiwan) under the following PCR conditions (94 °C for 2 min and 35 cycles at 94 °C for 20 s, 52 °C for 20 s, and 72 °C for 30 s, with a final extension at 72 °C for 5 min). For the second round of PCR, nested primers S3 5′-TGTAGGTTTTATAATTTATTTAGATTT-3′ (upstream, −211 to −184) and S4 5′-ACTCCAAAAACCCATAACTAAC-3′ (downstream, +138 to +116) were used. The PCR conditions were 94 °C for 2 min with 30 cycles at 94 °C for 30 s, 54 °C for 30 s, and 72 °C for 45 s, followed by an extension at 72 °C for 5 min. The PCR products were purified and inserted into vector pGEM-T Easy Vector System (A3610; Promega) using Fast DNA Ligation System (Promega). Five clones from each cell line or tissue were sequenced to assess the level of methylation at each CpG site, as described previously (17).
RESULTS
Metal Compounds Induce EMT in BEAS-2B Cells
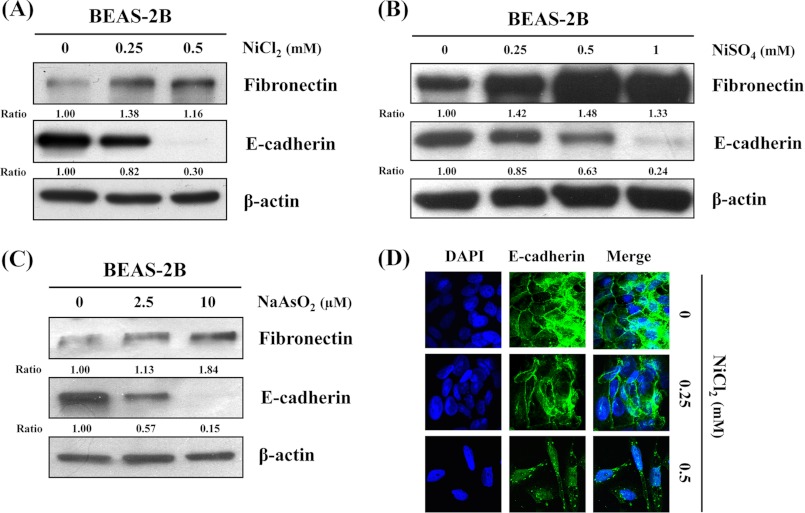
To investigate whether metal compounds induce EMT in lung carcinogenesis and fibrosis, we analyzed the effects of NiCl2, NiSO4, and NaAsO2 on E-cadherin and fibronectin expressions in BEAS-2B cells. After exposure to these compounds for 72 h, the expressions of epithelial marker E-cadherin decreased 70, 76, and 85%, respectively. In contrast, the expressions of mesenchymal marker fibronectin increased 1.16-, 1.33-, and 1.84-fold at the highest doses, respectively (Fig. 1, A–C). The EMT process for the E-cadherin complex on the lateral plasma membrane in untreated BEAS-2B cells is described on immunofluorescence assay in Fig. 1D. Expression of E-cadherin was significantly reduced, and cells underwent morphological changes following NiCl2 treatment (Fig. 1D). To better understand the role of nickel compounds in the EMT process, we selected NiCl2 for further validation experiments.
FIGURE 1.
Protein levels of EMT marker in metal compound-treated human BEAS-2B cells. BEAS-2B cells (1 × 106 cells/6-cm dish) were treated with the nickel chloride (NiCl2) 0, 0.25, and 0.5 mm (A); nickel sulfate (NiSO4) 0, 0.25, 0.5, and 1 mm (B); or sodium arsenite (NaAsO2) 0, 2.5, 10 μm for 72 h (C); and protein levels of fibronectin and E-cadherin were detected by Western blot. β-Actin was used as the internal control. The relative ratios of fibronectin/β-actin and E-cadherin/β-actin are shown. D, NiCl2 (0, 0.25, and 0.5 mm) for 72 h and immunofluorescence staining for E-cadherin (green) plus DAPI counterstaining for DNA (blue). Original magnification, ×630.
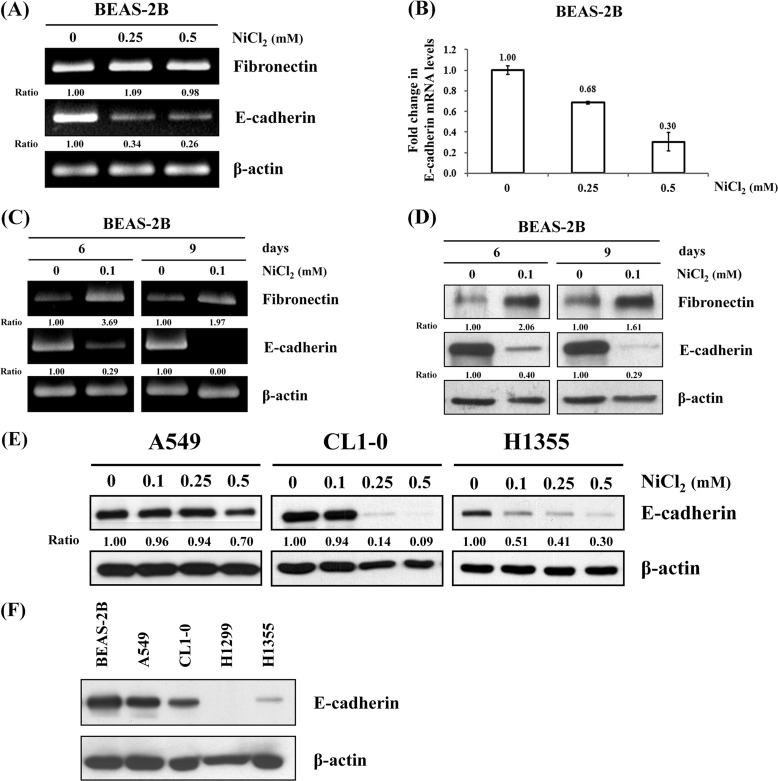
NiCl2 Induces Dose- and Time-dependent EMT in BEAS-2B Cells
To assess the mRNA expressions of EMT markers in NiCl2-treated BEAS-2B cells, cells were treated with 0, 0.25, or 0.5 mm NiCl2 for 48 h and analyzed by RT-PCR. As shown in Fig. 2A, E-cadherin mRNA levels were down-regulated 66 and 74% in BEAS-2B cells treated with NiCl2 at 0.25 and 0.5 mm, respectively, when compared with untreated cells. However, fibronectin mRNA was not up-regulated by NiCl2. The same results were obtained using real time RT-PCR for the detection of mRNA expression of E-cadherin in BEAS cells treated with NiCl2 (Fig. 2B). The results revealed that E-cadherin is repressed by NiCl2 at transcriptional levels.
FIGURE 2.
Effects of NiCl2 on EMT in BEAS-2B cells. A, BEAS-2B cells (1 × 106 cells/6-cm dish) were treated with NiCl2 (0, 0.25, and 0.5 mm) for 48 h, and mRNA levels of fibronectin and E-cadherin were determined on RT-PCR. β-Actin was used as the internal control. The relative ratios of fibronectin/β-actin and E-cadherin/β-actin are shown. B, quantitative real time PCR analysis was used to detect E-cadherin expression with total RNA extracted from cells treated with NiCl2 (0, 0.25, and 0.5 mm) for 48 h. All values have been normalized to the level of GAPDH and are the averages of three independent readings. C, during the time course study, BEAS-2B cells were exposed to NiCl2 (0 and 0.1 mm) for the indicated times. The EMT marker expressions were analyzed on RT-PCR (D) and Western blot. β-Actin was used as the internal control. The relative ratios of fibronectin/β-actin and E-cadherin/β-actin are shown. E, A549, CL1-0, and H1355 lung cancer cells (5 × 105 cells/6-cm dish) were treated with NiCl2 (0, 0.1, 0.25, and 0.5 mm) for 72 h, and the protein levels of E-cadherin were detected on Western blot. β-Actin was used as the loading control. The relative ratios of E-cadherin/β-actin are shown. F, protein levels of E-cadherin in BEAS-2B, A549, CL1-0, H1299, and H1355 cells. Thirty μg of total proteins were loaded onto each lane for Western blot analysis. β-Actin was used as the internal control.
In general, humans are exposed to low doses of nickel compounds via air pollution. To explore the effect of long term low dose exposure to NiCl2 on human lung cells, BEAS-2B cells were treated with low dose (0.1 mm) NiCl2 for various times (6 and 9 days). The expression of E-cadherin decreased and that of fibronectin increased (Fig. 2, C and D). These results demonstrated that long term low dose exposure to NiCl2 promotes the EMT process in bronchial epithelial cells. In addition, to assess whether NiCl2 represses expression of E-cadherin in lung cancer cell lines, A549, CL1-0, and H1355 cells were treated with various concentrations of NiCl2. As shown in Fig. 2E, the expression of E-cadherin slightly decreased in A549 cells but dramatically decreased in CL1-0 and H1355 cells. These results further support the role of NiCl2 in promoting lung cancer EMT. We also found that the protein levels of E-cadherin in BEAS-2B, A549, and CL1-0 cells were higher than in H1299 and H1355 cells in the untreated state (Fig. 2F).
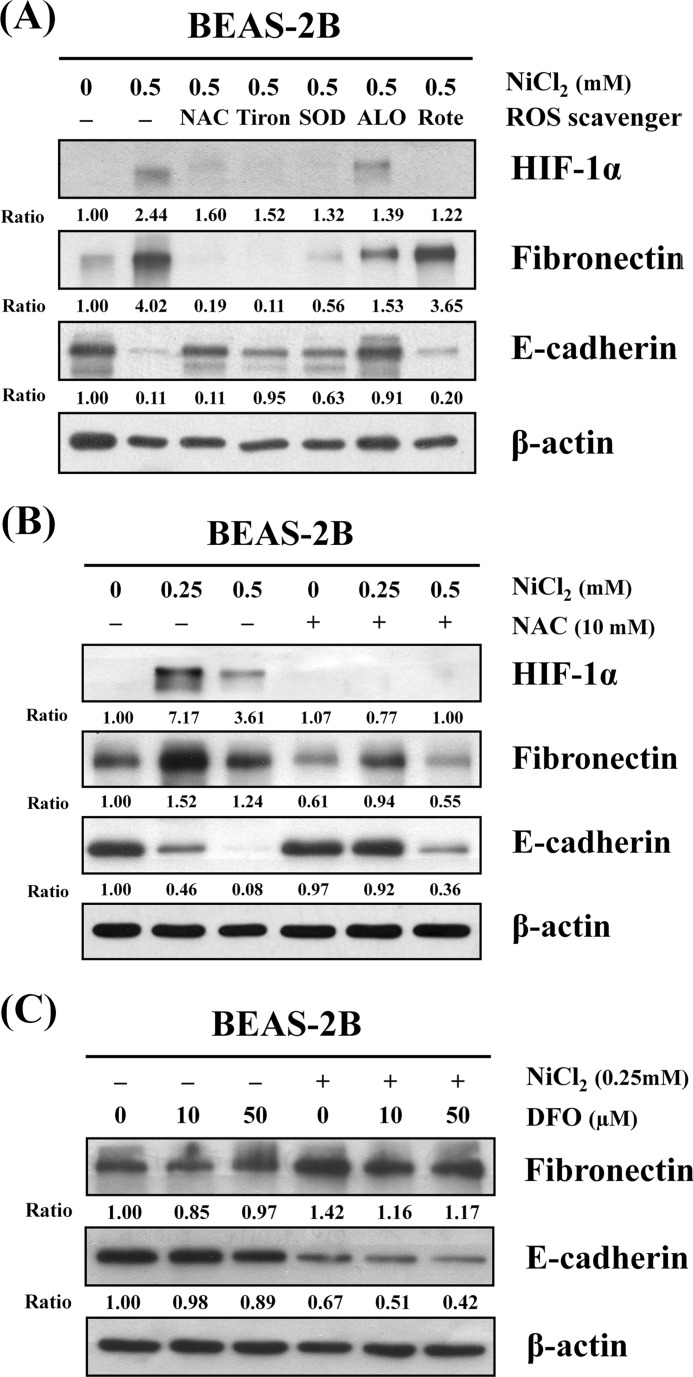
ROS Mediates NiCl2-induced EMT
It has been well documented that nickel exposure leads to generation of ROS and activation of signaling pathways by activating transcription factor (18). BEAS-2B cells were pretreated with different antioxidants or ROS inhibitors/scavengers, including NAC (antioxidant), Tiron (superoxide anion scavenger), superoxide dismutase (SOD), allopurinol (xanthine oxidase inhibitor), and rotenone (mitochondria respiratory chain inhibitor). As shown in Fig. 3A, NAC, Tiron, and SOD all abolished NiCl2-induced EMT. NAC was able to simultaneously diminish up-regulation of fibronectin and restore E-cadherin expression in the presence of NiCl2.
FIGURE 3.
ROS are involved in NiCl2-induced EMT. A, BEAS-2B cells (1 × 106 cells/6-cm dish) were preincubated with 10 mm NAC, 10 mm Tiron, 500 units/ml SOD, 200 μm allopurinol (ALO), or 1 μm rotenone (Rote) for 1 h followed by culture with or without 0.5 mm NiCl2 for 72 h. B, BEAS-2B cells were pretreated with 10 mm NAC for 1 h followed by exposure to NiCl2 (0, 0.25, and 0.5 mm) for 72 h. C, BEAS-2B cells were treated with NiCl2 (0 and 0.25 mm, respectively) with or without desferrioxamine (DFO) (0, 10, and 50 μm) for 72 h. The protein levels were determined on Western blot analysis. β-Actin was used as the internal control. The relative ratios of HIF-1α/β-actin, fibronectin/β-actin, and E-cadherin/β-actin are shown.
To further confirm this finding, cells were pretreated with 10 mm NAC and then treated with various concentrations of NiCl2. This resulted in the mitigation of NiCl2-mediated down-regulation of E-cadherin and up-regulation of fibronectin and HIF-1α (Fig. 3B). Intracellular nickel competes with iron for iron- binding sites on enzymes and proteins (19). Free iron can increase ROS formation, which may be important during ROS-associated EMT (20). To investigate whether free iron promotes NiCl2-induced EMT, we co-treated cells with NiCl2 and deferoxamine mesylate, an iron chelator. Results showed that NiCl2 reduces E-cadherin and up-regulates fibronectin after deferoxamine mesylate co-treatment, indicating that intracellular free iron ions are not involved in NiCl2-induced EMT (Fig. 3C).
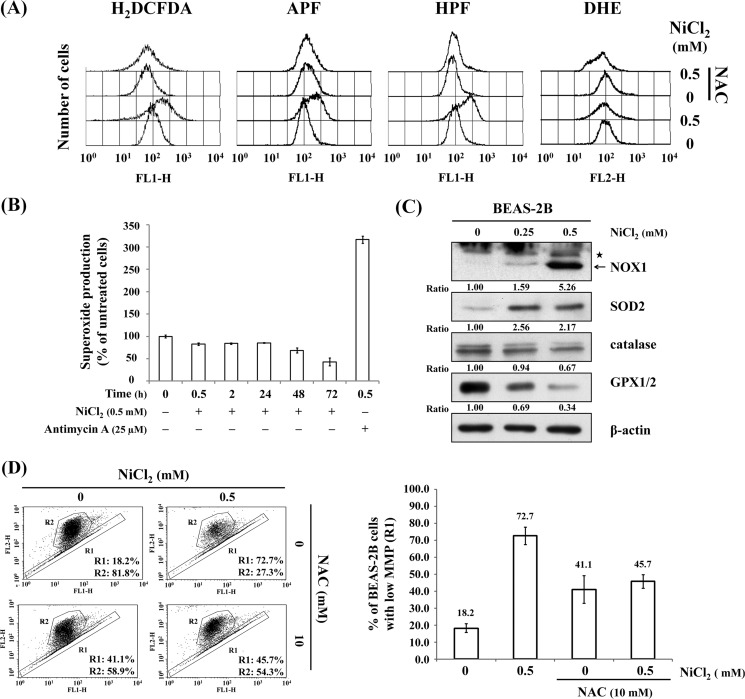
Metals can induce DNA damage either directly or indirectly through the formation of ROS (21). To determine whether NiCl2 induces specific ROS production, cells were treated with various ROS-sensitive probes using flow cytometry. The generation of ROS by NiCl2 was detected by photocatalysis using 2′,7′-dichlorodihydrofluorescein diacetate, 3′-(p-aminophenyl) fluorescein, and HPF. Pretreatment of cells with 10 mm NAC for 1 h significantly blunted the ROS production after NiCl2 treatment (Fig. 4A and supplemental Fig. S1A). However, there was no apparent alteration in superoxide anion generation. To confirm these results, antimycin A was used as a positive control to induce superoxide production. Superoxide levels increased in comparison with untreated cells after antimycin A treatment (Fig. 4B and supplemental Fig. S1B). However, NiCl2 did not induce superoxide generation in a time course study. Rather, the levels of superoxide decreased following NiCl2 treatment for 72 h (Fig. 4B). Next, we assessed the effects of antioxidant enzymes on Ni-induced EMT. Catalase and GPX1/2 were down-regulated, whereas NOX1 and SOD2 were up-regulated by NiCl2 (Fig. 4C). In addition, we measured the changes in mitochondrial membrane potential in NiCl2-treated BEAS-2B cells. Treatment with 0.5 mm NiCl2 converted JC-1 from aggregate form (R2) to monomer form (R1), indicating a disruption in mitochondrial function (18.2% versus 72.7%). NiCl2-mediated mitochondrial apoptotic pathway was restrained in NAC-pretreated cells (Fig. 4D).
FIGURE 4.
NiCl2 induces ROS generation. A, BEAS-2B cells (1 × 106 cells) were pretreated with 10 mm NAC for 1 h followed by exposure to 0.5 mm NiCl2 for 72 h. The cells were stained with 10 μm 2′,7′-dichlorodihydrofluorescein diacetate (ROS indicator), 10 μm 3′-(p-aminophenyl) fluorescein (hypochlorite anion indicator), 10 μm (HPF hydroxyl radical indicator), or 10 μm dihydroethidium (superoxide anion indicator) dye and analyzed by flow cytometry. B, cells were treated with 0.5 mm NiCl2 for 0.5, 2, 24, 48, and 72 h or 25 μm antimycin A for 30 min (positive control). Superoxide anion production was measured by ethidium fluorescence generated from dihydroethidium (10 μm) by fluorescence spectroscopy and expressed as the percentage of untreated cells. C, cells were treated with 0, 0.25, and 0.5 mm NiCl2 for 72 h, and protein levels of NADPH oxidase 1 (NOX1), SOD2, catalase, and glutathione peroxidase (GPX1/2) were detected on Western blot. β-Actin was used as the internal control. Asterisk represents a nonspecific band. The relative ratios of NOX1/β-actin, SOD2/β-actin, catalase/β-actin, and GPX1/2/β-actin are shown. D, BEAS-2B cells were untreated/treated with 0.5 mm NiCl2 as well as 0 or 10 mm NAC for 72 h. The changes in mitochondrial membrane potential (MMP) were assessed by the intensity of green fluorescence (R1) and red fluorescence (R2) of JC-1. Bar graph shows the quantification changes in JC-1 fluorescence in the presence of NiCl2 with/without NAC co-treatment as detected on flow cytometry assay. There was interaction between NiCl2 and NAC with NiCl2 decreasing JC-1 fluorescence in the presence of NAC. Data are presented as mean ± S.E.
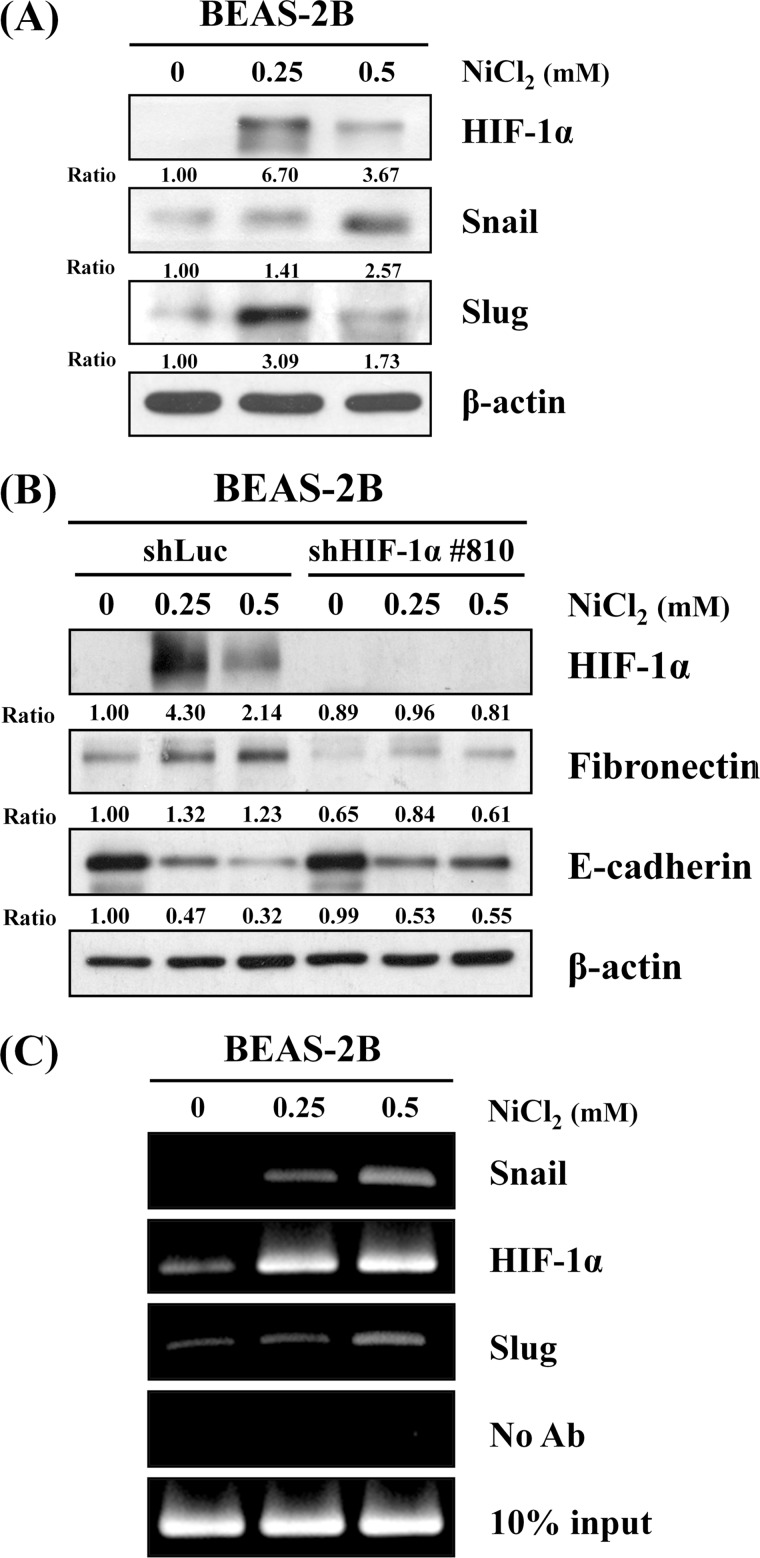
HIF-1α, Snail, and Slug Are Involved in NiCl2-mediated Inhibition of E-cadherin
HIF-1α is produced during exposure to arsenite through the generation of ROS in arsenite-induced carcinogenesis (22). Therefore, we hypothesized that NiCl2 induces EMT by aberrant activation of HIF-1α signaling. HIF-1α was markedly up-regulated after NiCl2 stimulation (Fig. 5A), indicating that NiCl2 down-regulation of E-cadherin is mediated by HIF-1α. To clarify the role of HIF-1α in NiCl2-diminished expression of E-cadherin, HIF-1α silencing experiment was carried out with VZV-G pseudotyped lentivirus-shRNA system. The responses of BEAS-2B shLuc cells to NiCl2 treatment were similar to those of parental BEAS-2B cells. E-cadherin was partially restored, and fibronectin did not increase following exposure to NiCl2 in BEAS-2B shHIF1-α cells (Fig. 5B), suggesting a partial role for HIF-1α in NiCl2-mediated E-cadherin and fibronectin regulation.
FIGURE 5.
HIF-1α, Snail, and Slug are involved in nickel-induced EMT. A, Western blot analysis shows HIF-1α, Snail, and Slug expressions in protein lysates from BEAS-2B cells (1 × 106 cells) treated with NiCl2 at the indicated dosages for 72 h. β-Actin was used as the internal control. The relative ratios of HIF-1α/β-actin, Snail/β-actin, and Slug/β-actin are shown. B, HIF-1α, fibronectin, and E-cadherin protein levels were determined on Western blot using protein lysates from cells treated with NiCl2 for 72 h after infection with lentivirus carrying shHIF-1− or vector control. β-Actin was used as the internal control. The relative ratios of HIF-1α/β-actin, fibronectin/β-actin, and E-cadherin/β-actin are shown. C, ChIP assay was performed on BEAS-2B cells treated with NiCl2 for 48 h. The precipitated chromatin was PCR-amplified with the use of specific primers with E-boxes in the E-cadherin promoter. In vivo identification of reciprocal E-box occupancy by Snail or HIF-1α at the E-cadherin promoter in BEAS-2B cells was carried out. No antibodies were used as negative control. Input, PCRs performed on total chromatin from BEAS-2B cells. Ab, antibody.
Several transcriptional repressors, including Snail and Slug, are involved in the repression of E-cadherin expression (23). To assess whether NiCl2 inhibits E-cadherin expression through these transcription factors, we evaluated the effect of NiCl2 treatment on Snail and Slug expressions. As shown in Fig. 5A, cells exposed to NiCl2 had higher Snail and Slug protein levels than untreated cells. Next, we performed ChIP assay to examine whether HIF-1α, Snail, and Slug transcription factors are responsible for E-cadherin promoter activity (−178 to +92). As shown in Fig. 5C, HIF-1α, Snail, and Slug were present on the E-cadherin promoter following NiCl2 treatment, indicating that HIF-1α, Snail, and Slug are associated with NiCl2-regulated E-cadherin reduction.
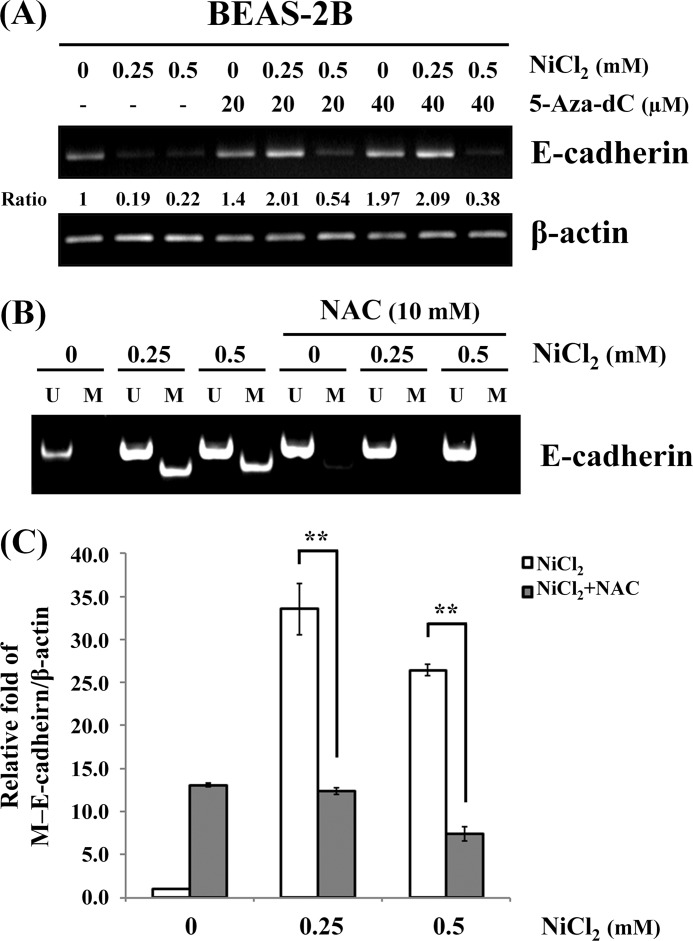
NiCl2 Inhibits E-cadherin Expression Partly by Promoter Hypermethylation via ROS Generation
The regulation of E-cadherin gene expression in many biological processes involves epigenetic mechanisms, and aberrant methylation of E-cadherin correlates with transcriptional silencing of the gene in non-small cell lung cancer cells (24, 25). To examine whether NiCl2 repression of E-cadherin is mediated by epigenetic effects, cells were pre(co)treated with 5-aza-2′-deoxycytidine (20 and 40 μm), a DNA methyltransferase inhibitor, and NiCl2 at various concentrations for 48 h. It was found that 5-aza-2′-deoxycytidine restores E-cadherin expression following treatment with 0.25 mm NiCl2 (Fig. 6A).
FIGURE 6.
Epigenetic effects of NiCl2 down-regulate E-cadherin expression. A, BEAS-2B cells were treated with NiCl2 (0, 0.25, and 0.5 mm) and 5-Aza-dc (0, 20, and 40 μm) for 48 h. The mRNA levels of fibronectin and E-cadherin were determined by RT-PCR. β-Actin was used as the internal control. The relative ratios of E-cadherin/β-actin are shown. B, methylation-specific PCR assays were performed after BEAS-2B cells were pretreated with/without 10 mm NAC and then treated with NiCl2 for 48 h. Visible PCR product in lanes U indicates the presence of unmethylated alleles; visible PCR product in lanes M indicates the presence of methylated alleles. C, QMSP analysis confirmed the same sample. DNA methylation of E-cadherin was calculated as the relative expression compared with β-actin. Each bar represents the mean ± S.D. of triplicate experiments. **, p < 0.01.
In an effort to facilitate the analysis of DNA samples isolated from BEAS-2B cells treated with NiCl2, we used nested-PCR approach to study the methylation of the E-cadherin promoter. As shown in Fig. 6B, methylation of E-cadherin promoter increased following treatment with NiCl2. To elucidate a link between ROS and DNA methylation, we examined whether the E-cadherin promoter methylation is altered by ROS. Nickel-induced methylation was blocked following treatment with NAC (Fig. 6B). The same results were obtained on QMSP assay of DNA methylation of E-cadherin. Treatment with 0.25 and 0.5 mm NiCl2 caused 33.5-fold (p < 0.01) and 26.5-fold (p < 0.01) increases in E-cadherin methylation, respectively. Pretreatment with NAC significantly abolished nickel-induced E-cadherin hypermethylation (Fig. 6C). These data support the suppression of E-cadherin expression by NiCl2-induced aberrant promoter methylation via ROS generation.
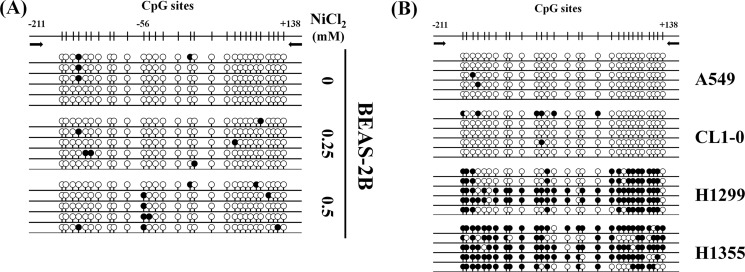
We also analyzed the methylation status of CpG islands at the E-cadherin promoter, including 29 CpG sites within a 349-bp fragment from sequence positions −211 to +138. Bisulfate sequencing analysis of five individual clones of each sample was carried out using PCR products from NiCl2-treated BEAS-2B cells and lung cancer cell lines (A549, CL1-0, H1299, and H1355 cells). As shown in Fig. 7A, methylated CpG sites were more frequent in NiCl2-treated BEAS-2B cells, especially at CpG site −56, when compared with untreated cells. Densely methylated clones were also detected in H1299 and H1355 cells (Fig. 7B). These results are consistent with the findings of our previous study that protein levels of E-cadherin are low in H1299 and H1355 cells (Fig. 2F). NiCl2 contributes to the methylation of CpG islands on the E-cadherin promoter, resulting in decreased E-cadherin expression.
FIGURE 7.
Bisulfite genomic sequencing of E-cadherin promoter (−211 to +138). Methylation status of CpG sites around the E-cadherin promoter region was analyzed in BEAS-2B cell line following treatment with NiCl2 (0, 0.25, and 0.5 mm) (A) and lung cancer cell lines (A549, CL1-0, H1299, and H1355 cells) (B). Region spans −211 to +138 including 29 CpG sites. Each row represents an individual subclone. White circles represent unmethylated CpGs. Black circles represent methylated CpGs.
DISCUSSION
It has been well documented that nickel exposure leads to generation of ROS. Incremental levels of intracellular ROS, lipid peroxidation, hydroxyl radicals (OH·), and DNA damage are induced in human lymphocytes after acute exposure to NiCl2 (26). ROS are also enhanced in signal transduction events required for apoptosis under nickel exposure (27). Moreover, ROS are crucial conspirators in EMT engagement and tumor aggressiveness (28).
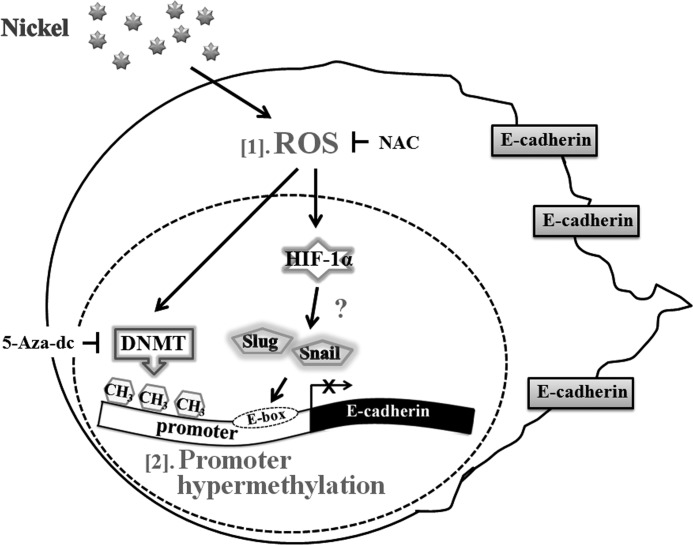
Nickel compounds are carcinogens that enhance invasion of human lung cancer cells (29, 30). In this study, NiCl2 induced EMT phenotype marker alterations such as up-regulation of fibronectin and down-regulation of E-cadherin. Prolonged ROS stress by nickel stimulation down-regulated expression of E-cadherin via the HIF-1α-dependent pathway and DNA methylation. We addressed a novel aspect of lung carcinogenesis, specifically the transition to a stage of higher motility and thus progression of carcinogenesis. Nickel is potentially implicated in carcinogenesis via the production of ROS. Its potential to promote a migratory phenotype was investigated at the levels of gene and epigenetic modifications in EMT (Fig. 8).
FIGURE 8.
Model depicting the mechanisms of nickel-repressed E-cadherin in BEAS-2B cells. [1] nickel induces ROS generation and up-regulation of HIF-1α. [2] transcription factors, such as Snail and Slug, may be activated and inhibit expression of E-cadherin. Nickel represses E-cadherin by promoter hypermethylation via ROS generation.
We used NAC, Tiron, SOD, allopurinol, and rotenone to test our hypothesis that some ROS antioxidants or specific scavengers alleviate the EMT phenotype induced by NiCl2. Both Tiron and SOD eliminated the superoxide anion and partially altered nickel-induced EMT signaling (Fig. 3A). Moreover, NOX1 increased in response to treatment with NiCl2 (Fig. 4C). Knockdown of superoxide dismutase 2 genes did not restore the expression of E-cadherin (supplemental Fig. S2B), suggesting that superoxide is at least partly associated with nickel-induced EMT. The half-life of superoxide anion is short, and it rapidly dismutates either nonenzymically or via SOD to H2O2, which is relatively stable. NAC was found to be the best antioxidant for alleviating the EMT phenotype induced by NiCl2 (Fig. 3A).
Hypochlorite anion (OCl−) is a natural compound that forms from the reaction catalyzed by myeloperoxidase, an enzyme present only in innate immune cells (31). Both OCl− and OH· can be detected in photocatalytic ROS production using 3′-(p-aminophenyl) fluorescein. Monitoring of OH· production using another fluorochrome called HPF suggested that nickel mainly induces OH· generation (Fig. 4A). OH· is produced with superoxide or H2O2. However, nickel did not affect the generation of superoxide anion (Fig. 4, A and B), which can also occur in the Haber-Weiss reaction by oxidation of the superoxide anion radical. In addition, we found that expressions of catalase and GPX1/2 decreased, whereas expression of SOD2 increased following nickel treatment (Fig. 4C), indicating that superoxide anions are barely detectable following nickel treatment. Moreover, in the presence of divalent metal ions such as Ni2+ and Fe2+, H2O2 can undergo the Fenton reaction to produce OH·. The responses of BEAS-2B shSOD2 cells to nickel treatment were similar to those of BEAS-2B shLuc cells with EMT phenotype and superoxide generation (supplemental Fig. S2). H2O2 generation partly involves oxidases, SOD, and cytochrome P450 family of enzymes that are found in several subcellular compartments such as mitochondria, peroxisomes, and microsomes (32). Thus, the source of H2O2 by nickel production may be mediated through various pathways.
The most widely implemented application of JC-1 is for the detection of mitochondrial depolarization in the early stages of apoptosis. The effect of NAC on JC-1 monomer formation was highly unspecific as the readout doubled in the control and did not change regardless of nickel exposure (Fig. 4D). Similarly, the use of antioxidants, including NAC and penicillamine, induces apoptosis in multiple types of human cancer cells (33). The limitation of this study involves selection of optimal concentration of NAC to balance prevention of cytotoxicity and alleviation of EMT. EMT may be involved in multiple processes, and it is difficult to abolish the EMT phenotype by blockage of repressors for E-cadherin. Thus, multiple inhibitors might be helpful in lung cancer treatment.
Mutant P53 (R175H) induces Twist1 expression that is involved in the alleviation of epigenetic repression and EMT (34). Nickel may induce p53 gene mutation and expression (35). Calcium signaling is required for EMT and E-cadherin silencing. In this study, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM) restored nickel-induced ROS generation (supplemental Fig. S3). Moreover, intracellular calcium may be involved in nickel-induced EMT. In this study, we used BEAS-2B cells, an immortalized lung bronchial epithelial cell line, to investigate metal compound-induced EMT. The data showed that metal compounds promote EMT, which may induce metastasis in early stage lung cancer.
HIF-1α has been shown to contribute to EMT through direct regulation of Twist expression, which inhibits E-cadherin transcription (36). Overexpression of HIF-1α induces EMT and promotes migration, invasion, and metastasis in vitro and in vivo (37), suggesting that HIF-1α is an important positive regulator of EMT. We observed induction of EMT by up-regulation of HIF-α following NiCl2 treatment. In addition, knockdown of HIF-1α abolished EMT phenotype, providing further support for the role of HIF-1α in NiCl2-induced EMT in BEAS-2B cells.
As shown in Figs. 5C and 6C, the results of ChIP and QMSP demonstrated that HIF-1α- and Snail-bound E-cadherin promoter regions contain methylated CpG sites in NiCl2-treated cells. HIF-1α and Snail not only bind to but also induce DNA methylation of E-cadherin promoter, causing repression of transcription. Acute exposure to carcinogenic compounds of Ni(II) activates the transcription factor HIF-1α, which is associated with carcinogenesis, invasion, and angiogenesis (7, 36). A previous study has demonstrated that HIF-1α directly regulates the transcriptional repression of equilibrative nucleoside transporter during hypoxia (38). It has also been reported that HIF-1α acts in cooperation with other transcription factors and regulates target genes (39). Therefore, HIF-1α might directly mediate down-regulation of E-cadherin or cooperate with transcription repressors, such as Snail and Slug, to inhibit E-cadherin expression in NiCl2-treated BEAS-2B cells. Further experiments are needed to prove our hypothesis.
Epigenetic alteration mechanisms, which include DNA hypermethylation and histone modifications, are involved in the regulation of gene expression. In addition, water-soluble nickel compounds can signal hypoxia (40). Silencing of seripina3g gene by nickel is partially reversed with trichostatin A (TSA) pretreatment (41). It is interesting to note that co-treatment with TSA does not affect down-regulation of E-cadherin by NiCl2, suggesting that histone deacetylation is not involved in NiCl2-repressed expression of E-cadherin (data not shown). Hypoxia and nickel exposure increase the level of H3K9me2 at the Spry2 (Sprouty homolog 2) promoter by inhibiting histone demethylase JMJD1A, resulting in repression of Spry2 in BEAS-2B cells (42). In addition to histone methylation, nickel can cause DNA hypermethylation (29). It has also been reported that inactivation of E-cadherin is the hallmark of tissue invasion and metastasis (43). Snail transcription factor represses the expression of E-cadherin by influencing transcription level and promoter hypermethylation. In hepatocellular carcinoma cells, ROS induces the hypermethylation of E-cadherin promoter via increasing Snail expression (44). Although down-regulation of E-cadherin by Snail through transcriptional inhibition could not be excluded, Snail might play an important role in NiCl2-induced E-cadherin hypermethylation.
In conclusion, our data provided evidence that NiCl2 promotes EMT. Persistently high levels of ROS stress trigger epigenetic changes in NiCl2-treated cells. The results of this study showed that NiCl2 induces EMT through a variety of mechanisms and provide novel insights into tumor progression by illustrating the association between NiCl2 exposure and early lung cancer metastasis.
Acknowledgments
RNAi reagents were obtained from the National RNAi Core Facility (Institute of Molecular Biology/Genomic Research Center, Academia Sinica), which is supported by National Research Program for Genomic Medicine, National Science Council Grant NSC-97-3112-B-001-016. Confocal microscopy was performed by the Instrument Center of Chung Shan Medical University, which is supported in part by the National Science Council, Ministry of Education, and Chung Shan Medical University.
This work was supported by National Science Council, Taiwan, Grant NSC-99-2314-B-040-012-MY3.

This article contains supplemental Figs. S1–S3.
- EMT
- epithelial-mesenchymal transition
- ROS
- reactive oxygen species
- SOD
- superoxide dismutase
- NAC
- N-acetylcysteine
- QMSP
- quantitative real time methyl-specific PCR
- HPF
- 3′-(p-hydroxyphenyl) fluorescein.
REFERENCES
- 1. Thiery J. P. (2002) Epithelial-mesenchymal transitions in tumor progression. Nat. Rev. Cancer 2, 442–454 [DOI] [PubMed] [Google Scholar]
- 2. Thiery J. P., Acloque H., Huang R. Y., Nieto M. A. (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 [DOI] [PubMed] [Google Scholar]
- 3. Christiansen J. J., Rajasekaran A. K. (2006) Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 66, 8319–8326 [DOI] [PubMed] [Google Scholar]
- 4. Kalluri R. (2009) EMT. When epithelial cells decide to become mesenchymal like cells. J. Clin. Invest. 119, 1417–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thiery J. P., Sleeman J. P. (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7, 131–142 [DOI] [PubMed] [Google Scholar]
- 6. Christofori G., Semb H. (1999) The role of the cell-adhesion molecule E-cadherin as a tumor-suppressor gene. Trends Biochem. Sci. 24, 73–76 [DOI] [PubMed] [Google Scholar]
- 7. Strathdee G. (2002) Epigenetic versus genetic alterations in the inactivation of E-cadherin. Semin. Cancer Biol. 12, 373–379 [DOI] [PubMed] [Google Scholar]
- 8. Chen J., Imanaka N., Chen J., Griffin J. D. (2010) Hypoxia potentiates Notch signaling in breast cancer leading to decreased E-cadherin expression and increased cell migration and invasion. Br. J. Cancer 102, 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Veljkovic E., Jiricny J., Menigatti M., Rehrauer H., Han W. (2011) Chronic exposure to cigarette smoke condensate in vitro induces epithelial to mesenchymal transition-like changes in human bronchial epithelial cells, BEAS-2B. Toxicol. In Vitro 25, 446–453 [DOI] [PubMed] [Google Scholar]
- 10. Galanis A., Karapetsas A., Sandaltzopoulos R. (2009) Metal-induced carcinogenesis, oxidative stress and hypoxia signaling. Mutat. Res. 674, 31–35 [DOI] [PubMed] [Google Scholar]
- 11. Edelman D. A., Roggli V. L. (1989) The accumulation of nickel in human lungs. Environ. Health Perspect. 81, 221–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Costa M., Yan Y., Zhao D., Salnikow K. (2003) Molecular mechanisms of nickel carcinogenesis. Gene silencing by nickel delivery to the nucleus and gene activation/inactivation by nickel-induced cell signaling. J. Environ. Monit. 5, 222–223 [DOI] [PubMed] [Google Scholar]
- 13. Hsin I. L., Sheu G. T., Chen H. H., Chiu L. Y., Wang H. D., Chan H. W., Hsu C. P., Ko J. L. (2010) N-acetylcysteine mitigates curcumin-mediated telomerase inhibition through rescuing of Sp1 reduction in A549 cells. Mutat. Res. 688, 72–77 [DOI] [PubMed] [Google Scholar]
- 14. Law A. Y., Lai K. P., Ip C. K., Wong A. S., Wagner G. F., Wong C. K. (2008) Epigenetic and HIF-1 regulation of stanniocalcin-2 expression in human cancer cells. Exp. Cell Res. 314, 1823–1830 [DOI] [PubMed] [Google Scholar]
- 15. Corn P. G., Smith B. D., Ruckdeschel E. S., Douglas D., Baylin S. B., Herman J. G. (2000) E-cadherin expression is silenced by 5′ CpG island methylation in acute leukemia. Clin. Cancer Res. 6, 4243–4248 [PubMed] [Google Scholar]
- 16. Hiraki M., Kitajima Y., Sato S., Mitsuno M., Koga Y., Nakamura J., Hashiguchi K., Noshiro H., Miyazaki K. (2010) Aberrant gene methylation in the lymph nodes provides a possible marker for diagnosing micrometastasis in gastric cancer. Ann. Surg. Oncol. 17, 1177–1186 [DOI] [PubMed] [Google Scholar]
- 17. Nojima D., Nakajima K., Li L. C., Franks J., Ribeiro-Filho L., Ishii N., Dahiya R. (2001) CpG methylation of promoter region inactivates E-cadherin gene in renal cell carcinoma. Mol. Carcinog. 32, 19–27 [DOI] [PubMed] [Google Scholar]
- 18. Salnikow K., Costa M. (2000) Epigenetic mechanisms of nickel carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 19, 307–318 [PubMed] [Google Scholar]
- 19. Davidson T., Chen H., Garrick M. D., D'Angelo G., Costa M. (2005) Soluble nickel interferes with cellular iron homeostasis. Mol. Cell Biochem. 279, 157–162 [DOI] [PubMed] [Google Scholar]
- 20. Zhang K. H., Tian H. Y., Gao X., Lei W. W., Hu Y., Wang D. M., Pan X. C., Yu M. L., Xu G. J., Zhao F. K., Song J. G. (2009) Ferritin heavy chain-mediated iron homeostasis and subsequent increased reactive oxygen species production are essential for epithelial-mesenchymal transition. Cancer Res. 69, 5340–5348 [DOI] [PubMed] [Google Scholar]
- 21. Galaris D., Evangelou A. (2002) The role of oxidative stress in mechanisms of metal-induced carcinogenesis. Crit. Rev. Oncol. Hematol. 42, 93–103 [DOI] [PubMed] [Google Scholar]
- 22. Gao N., Shen L., Zhang Z., Leonard S. S., He H., Zhang X. G., Shi X., Jiang B. H. (2004) Arsenite induces HIF-1α and VEGF through PI3K, Akt, and reactive oxygen species in DU145 human prostate carcinoma cells. Mol. Cell Biochem. 255, 33–45 [DOI] [PubMed] [Google Scholar]
- 23. de Herreros A. G., Peiró S., Nassour M., Savagner P. (2010) Snail family regulation and epithelial mesenchymal transitions in breast cancer progression. J. Mammary Gland Biol. Neoplasia 15, 135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maeda G., Chiba T., Aoba T., Imai K. (2007) Epigenetic inactivation of E-cadherin by promoter hypermethylation in oral carcinoma cells. Odontology 95, 24–29 [DOI] [PubMed] [Google Scholar]
- 25. Kim D. S., Kim M. J., Lee J. Y., Kim Y. Z., Kim E. J., Park J. Y. (2007) Aberrant methylation of E-cadherin and H-cadherin genes in non-small cell lung cancer and its relation to clinicopathologic features. Cancer 110, 2785–2792 [DOI] [PubMed] [Google Scholar]
- 26. Chen C. Y., Wang Y. F., Huang W. R., Huang Y. T. (2003) Nickel induces oxidative stress and genotoxicity in human lymphocytes. Toxicol. Appl. Pharmacol. 189, 153–159 [DOI] [PubMed] [Google Scholar]
- 27. Patel E., Lynch C., Ruff V., Reynolds M. (2012) Co-exposure to nickel and cobalt chloride enhances cytotoxicity and oxidative stress in human lung epithelial cells. Toxicol. Appl. Pharmacol. 258, 367–375 [DOI] [PubMed] [Google Scholar]
- 28. Giannoni E., Parri M., Chiarugi P. (2012) EMT and oxidative stress. A bidirectional interplay affecting tumor malignancy. Antioxid. Redox Signal 16, 1248–1263 [DOI] [PubMed] [Google Scholar]
- 29. Kasprzak K. S., Sunderman F. W., Jr., Salnikow K. (2003) Nickel carcinogenesis. Mutat. Res. 533, 67–97 [DOI] [PubMed] [Google Scholar]
- 30. Xu Z., Ren T., Xiao C., Li H., Wu T. (2011) Nickel promotes the invasive potential of human lung cancer cells via TLR4/MyD88 signaling. Toxicology 285, 25–30 [DOI] [PubMed] [Google Scholar]
- 31. Vladimirov Y. A., Proskurnina E. V. (2009) Free radicals and cell chemiluminescence. Biochemistry (Mosc.) 74, 1545–1566 [DOI] [PubMed] [Google Scholar]
- 32. Yeldandi A. V., Rao M. S., Reddy J. K. (2000) Hydrogen peroxide generation in peroxisome proliferator-induced oncogenesis. Mutat. Res. 448, 159–177 [DOI] [PubMed] [Google Scholar]
- 33. Guan D., Xu Y., Yang M., Wang H., Wang X., Shen Z. (2010) N-Acetylcysteine and penicillamine induce apoptosis via the ER stress response-signaling pathway. Mol. Carcinog. 49, 68–74 [DOI] [PubMed] [Google Scholar]
- 34. Kogan-Sakin I., Tabach Y., Buganim Y., Molchadsky A., Solomon H., Madar S., Kamer I., Stambolsky P., Shelly A., Goldfinger N., Valsesia-Wittmann S., Puisieux A., Zundelevich A., Gal-Yam E. N., Avivi C., Barshack I., Brait M., Sidransky D., Domany E., Rotter V. (2010) Mutant p53(R175H) up-regulates Twist1 expression and promotes epithelial-mesenchymal transition in immortalized prostate cells. Cell Death Differ. 18, 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cameron K. S., Buchner V., Tchounwou P. B. (2011) Exploring the molecular mechanisms of nickel-induced genotoxicity and carcinogenicity. A literature review. Rev. Environ. Health 26, 81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang M. H., Wu K. J. (2008) TWIST activation by hypoxia inducible factor-1 (HIF-1). Implications in metastasis and development. Cell Cycle 7, 2090–2096 [DOI] [PubMed] [Google Scholar]
- 37. Yang M. H., Wu M. Z., Chiou S. H., Chen P. M., Chang S. Y., Liu C. J., Teng S. C., Wu K. J. (2008) Direct regulation of TWIST by HIF-1α promotes metastasis. Nat. Cell Biol. 10, 295–305 [DOI] [PubMed] [Google Scholar]
- 38. Eltzschig H. K., Abdulla P., Hoffman E., Hamilton K. E., Daniels D., Schönfeld C., Loffler M., Reyes G., Duszenko M., Karhausen J., Robinson A., Westerman K. A., Coe I. R., Colgan S. P. (2005) HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J. Exp. Med. 202, 1493–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Warnecke C., Zaborowska Z., Kurreck J., Erdmann V. A., Frei U., Wiesener M., Eckardt K. U. (2004) Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2α (EPAS-1) by the use of RNA interference. erythropoietin is an HIF-2α target gene in Hep3B and Kelly cells. FASEB J. 18, 1462–1464 [DOI] [PubMed] [Google Scholar]
- 40. Costa M., Davidson T. L., Chen H., Ke Q., Zhang P., Yan Y., Huang C., Kluz T. (2005) Nickel carcinogenesis. Epigenetics and hypoxia signaling. Mutat. Res. 592, 79–88 [DOI] [PubMed] [Google Scholar]
- 41. Zhao J., Yan Y., Salnikow K., Kluz T., Costa M. (2004) Nickel-induced down-regulation of serpin by hypoxic signaling. Toxicol. Appl. Pharmacol. 194, 60–68 [DOI] [PubMed] [Google Scholar]
- 42. Chen H., Kluz T., Zhang R., Costa M. (2010) Hypoxia and nickel inhibit histone demethylase JMJD1A and repress Spry2 expression in human bronchial epithelial BEAS-2B cells. Carcinogenesis 31, 2136–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hanahan D., Weinberg R. A. (2000) The hallmarks of cancer. Cell 100, 57–70 [DOI] [PubMed] [Google Scholar]
- 44. Lim S. O., Gu J. M., Kim M. S., Kim H. S., Park Y. N., Park C. K., Cho J. W., Park Y. M., Jung G. (2008) Epigenetic changes induced by reactive oxygen species in hepatocellular carcinoma. Methylation of the E-cadherin promoter. Gastroenterology 135, 2128–2140 [DOI] [PubMed] [Google Scholar]