During my residence in the Amazon district I took every opportunity of determining the limits of species, and I soon found that the Amazon, the Rio Negro and the Madeira formed the limits beyond which certain species never passed. The native hunters are perfectly acquainted with this fact, and always cross over the river when they want to procure particular animals, which are found even on the river's bank on one side, but never by any chance on the other. On approaching the sources of the rivers they cease to be a boundary, and most of the species are found on both sides of them. Alfred Russel Wallace (1)
In December of 1852, Wallace read his paper “On the Monkeys of the Amazon” (1) at a meeting of the Zoological Society of London, just months after scarcely surviving a fiery shipwreck in the mid-Atlantic that cost him every Amazonian specimen he had brought on board. Within 14 mo he would begin his 8-yr odyssey in the Malay Archipelago [punctuated famously by a certain letter to Darwin in 1858 (2)]. Wallace's writings based on that second journey (3), together with his later works (e.g., ref. 4), would lay the broad foundations for modern evolutionary biogeography, a discipline lately in the midst of rebirth and redefinition.
The astonishing richness of species in Amazonian forests remains as intriguing to us as it was to Humboldt, Spruce, Bates, and Wallace. Among competing hypotheses for the evolutionary origin of this richness (5), the oldest is the “riverine barrier hypothesis,” which had its own origin in Wallace's 1852 paper (1). Briefly stated in modern terms, the hypothesis is that the major rivers of Amazonia represent barriers to gene flow, promoting genetic divergence of populations, either initiated by population subdivision (vicariance) or after colonization by a few founders. Although he did not state so publicly (1), it is arguable (6) that Wallace already saw rivers as causes of speciation in 1852, given his intention to gather data in the Amazon “toward solving the problem of [the] origin of species” (7). Or perhaps he saw rivers simply as locations of coincident range boundaries. But the Amazonian example reappears in his later work (ref. 4, pp. 12–13), along with other examples of barriers to dispersal, in the context of a remarkably anticipatory model of vicariance cladogenesis (ref. 4, pp. 46–49).
Whether the great rivers of Amazonia have something to do with species origins or are simply biogeographic sutures, the biotas of opposite banks ought to differ if the riverine barrier hypothesis is correct. Characteristically, in Wallace's monkey paper (1), he not only presented his data on primate distributions in relation to major rivers in the Amazon basin, but also suggested a testable, quantitative hypothesis: that the composition of species assemblages would differ in relation to the width of the river, the difference thus increasing from headwaters toward the mouth.
In this issue, Claude Gascon and a host of collaborators take up the gauntlet (8), testing Wallace's hypothesis with a large, field-collected dataset for frogs and small mammals (rodents and marsupials) from the banks of the Juruá River, a major tributary of the Amazon. Like other large Amazonian rivers, the Juruá floods annually, inundating floodplain (várzea) forest nearest the banks while sparing the upland, terra firme forest. In addition to Wallace's original hypothesis, Gascon et al. test three related ones. They propose, first, that, if Wallace was right, paired species assemblages in terra firme forest on opposite banks should differ more than paired assemblages in várzea forest on opposite banks, because the effective width of the barrier should be greater for terra firme species. Second, recognizing that assemblages may differ for many reasons (including sampling error and random historical causes), they add a powerful control to Wallace's hypothesis. For the mammal data, they compare the similarity between assemblage pairs on the same bank to the similarity between those same assemblages and their counterparts on the opposite bank of the Juruá. (The frog data were considered too prone to seasonal effects.) Finally, they test whether small mammals, species by species, tend to occur on both banks less frequently than the binomial expectation, a test that depends on the (rather dubious) assumption that a species' presence at several sampling sites can be counted as independent events.
Gascon et al. (8) find no evidence in support of the riverine barrier hypothesis, either for Wallace's specific prediction of increasing up-river faunal similarity between the banks of the Juruá, or for any of their related hypotheses. (Opposite-bank terra firme faunas are no less similar than opposite-bank várzea faunas, opposite-bank mammal assemblages differ just as much as same-bank assemblages, and mammal species are actually more likely to occur on both banks than expected.) In short, the data at hand fail to reject the null hypothesis that the Juruá does not restrict movement of small mammals and frogs. Inevitably, this result raises the question of statistical power. Would more or better data, a more quantitative and probabilistic measure of similarity (9), or a more powerful statistical design have yielded evidence in support of riverine barriers? A critical look at the quantitative results suggests that they are most likely robust to any such improvements: the Juruá is apparently not much of a barrier to frogs and small mammals, a finding supported by phylogeographic (genetic) studies (10).
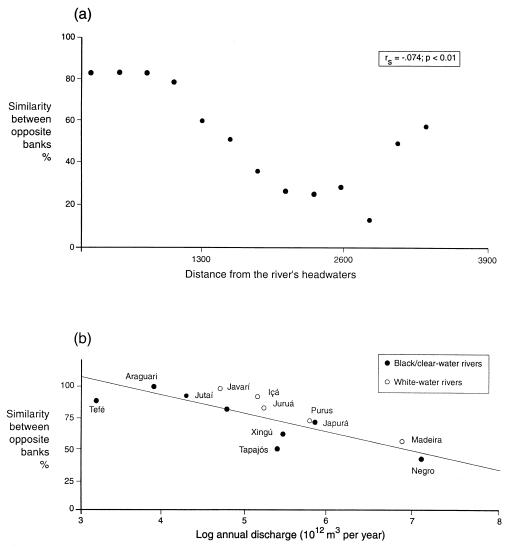
But what about other taxa and other rivers? Wallace's account (1) focused on primates and did not mention the Juruá. In fact, Ayers and Clutton-Brock (11) produced strong support for Wallace's hypothesis, for primates. They showed that similarity between the primate faunas of opposite banks of the Amazon itself declines with distance from headwaters, although similarity rebounds somewhat near the mouth of the river, where numerous islands, they suggest, may provide stepping-stones for dispersal (Fig. 1a). More persuasively, with data for 12 major tributaries of the Amazon (including the Juruá), Ayers and Clutton-Brock showed that opposite-bank similarity of primate assemblages declines significantly (and independently) with both increasing width and increasing annual discharge (Fig. 1b). Interestingly, the Juruá and other white-water rivers tend to lie above the regression line, a result that Ayers and Clutton-Brock attribute to the slower flow and more meandering course of white-water rivers compared with black and clear-water rivers. Genetic studies provide mixed support for the riverine barrier hypothesis, but those that support it tend to focus on the largest rivers, as Gascon et al. (8) acknowledge.
Figure 1.
(a) Similarity of primate faunas on opposite banks of the Amazon River, plotted as a function of distance from its headwaters. The secondary increase in similarity near the river's mouth is probably a consequence of the historical instability of its course and of the existence of islands (11). (b) Similarity of primate faunas on opposite banks of Amazonian rivers, plotted as a function of the annual discharge of each river. White-water rivers tend to flow more slowly than black and clear-water rivers. Redrawn from ref. 11 (© 1992 by American Naturalist).
In short, the answer to the riverine barrier question, like so many in evolution, ecology, and biogeography, turns out to be, “It depends.” The great rivers of the Amazon basin cannot simply be assumed to set limits to dispersal of forest animals, nor to have enforced the level and duration of genetic isolation required for population differentiation to cross the threshold to speciation. Likewise, the hypothesis of species origination and divergence in Pleistocene forest refugia (12), initially welcomed as a key engine of Amazonian richness (e.g., ref. 13), has become increasingly circumscribed in its application. Instead of forest islands in a sea of savanna during glacial maxima, it now appears that the “sea” was more likely seasonal forest (5), a less daunting barrier to many forest species. Despite the undoubted effectiveness of island archipelagos as nurseries of cladogenesis, the search for mainland counterparts has been frustrating, although not fruitless.
Not the least of many impediments to the pursuit of evidence of the process of speciation in biogeographical patterns is our continuing ignorance of geographical distributions for most groups of organisms, especially in the tropics. Although Wallace's complaint that “there is scarcely an animal whose exact geographical limits we can mark out on the map” (1) no longer applies to most terrestrial vertebrates and higher plants, terra incognita still marks the map for many tropical taxa even within these groups, not to mention for hyperdiverse taxa such as insects, mites, nematodes, and fungi. Ongoing, industrial-scale biotic inventories promise to advance geographic knowledge for some of these groups, albeit slowly (e.g., refs. 14 and 15), while massive efforts are underway to digitize and georeference label data from historical specimens (e.g., ref. 16) for all taxa.
Whereas assembling good datasets remains the limiting step for most taxa, new tools are revolutionizing the way data are used in biogeography. “Marking out on maps” what we do know about distributions has been powerfully facilitated by geographical information system (GIS) and biodiversity database software (16, 17), enabling complex modeling and analysis that was, until recently, simply beyond our grasp (e.g., refs. 18 and 19). On another front, remotely sensed information such as multiband satellite imagery and synthetic aperture radar, mapped statistically to known plant and animal distributions, is being used to predict biotic characteristics of unexplored areas. (20, 21).
These advances and others have allowed evolutionary biogeographers to exploit the ongoing revolution in molecular systematics to answer longstanding questions about the origin of species. Among these is another query (of which the modern relevance may be foresightful or merely fortuitous) posed by Wallace in his study of Amazonian primates (1). He asked, “Are very closely allied species ever separated by a wide interval of country?” Combining species-level, molecular phylogenies with geographical distributions, Barraclough and Vogler (22) recently showed that sister species (the most recent nodes in each cladogram) rarely occur in the same place. In contrast, sister clades linked at increasingly more ancient nodes show increasing geographic overlap—striking confirmation of the role of geographic isolation (allopatry) in speciation, for the taxa examined. At the intraspecific level, where genetic divergence must begin, molecular genetic “gene genealogies,” mapped on biogeographic patterns, aim to bridge the gap between microevolutionary processes and speciation in a spatial context (e.g., ref. 10), sometimes revealing surprising but informative geographic patterns of relationship (23).
Many of the hypotheses and questions posed by Wallace and other early biogeographers remain fresh because they still await answers. Ambitious collaborations like the Juruá River study (8) reported in this issue promise, at least, to refine the questions and to point the way toward specifying just what the “it” is in the inevitable answer, “it depends.”
Acknowledgments
I thank Robert Dunn, Sacha Spector, Nigel Stork, David Wake, and Kentwood Wells for their comments and bibliographic assistance.
Footnotes
See companion article on page 13672.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250497697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250497697
References
- 1.Wallace A R. Proc Zool Soc London. 1852;20:107–110. [Google Scholar]
- 2.Wallace A R. J Linn Soc. 1858;3:48–50. [Google Scholar]
- 3.Wallace A R. The Malay Archipelago. London: Macmillan; 1869. [Google Scholar]
- 4.Wallace A R. The Geographical Distribution of Animals. Vol. 1. New York: Harper; 1876. [Google Scholar]
- 5.Haffer J. Biodiversity Conservation. 1997;6:451–476. [Google Scholar]
- 6.McKinney H L. Wallace and Natural Selection. New Haven: Yale Univ. Press; 1972. [Google Scholar]
- 7.Knapp S. Footsteps in the Forest: Alfred Russel Wallace in the Amazon. London: The Natural History Museum; 1999. [Google Scholar]
- 8.Gascon C, Malcolm J R, Patton J L, da Silva M N F, Bogart J P, Lougheed S C, Peres C A, Neckel S, Boag P T. Proc Natl Acad Sci USA. 2000;97:13672–13677. doi: 10.1073/pnas.230136397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chao A, Hwang W-H, Chen Y-C, Kuo C-Y. Statistica Sinica. 2000;10:227–246. [Google Scholar]
- 10.Patton J L, da Silva M N F, Malcolm J R. Evolution. 1994;48:1314–1323. doi: 10.1111/j.1558-5646.1994.tb05315.x. [DOI] [PubMed] [Google Scholar]
- 11.Ayers J M, Clutton-Brock T H. Am Nat. 1992;140:531–537. doi: 10.1086/285427. [DOI] [PubMed] [Google Scholar]
- 12.Haffer J. Science. 1969;165:131–137. doi: 10.1126/science.165.3889.131. [DOI] [PubMed] [Google Scholar]
- 13.Prance G T, editor. Biological Diversification in the Tropics. New York: Columbia Univ. Press; 1982. [Google Scholar]
- 14.Gamez R. Trends Ecol Evol. 1991;6:377–378. doi: 10.1016/0169-5347(91)90154-P. [DOI] [PubMed] [Google Scholar]
- 15.Longino J T, Colwell R K. Ecol Appl. 1997;7:1263–1277. [Google Scholar]
- 16.Soberon J, Koleff P. In: Nature and Human Society: The Quest for a Sustainable World. Raven P H, editor. Washington, DC: Natl. Acad. Press; 1997. pp. 586–595. [Google Scholar]
- 17.Iverson L R, Prasad A. Diversity Distributions. 1998;4:49–61. [Google Scholar]
- 18.Lees D C, Kremen C, Andriamampianina L. Biol J Linn Soc. 1999;67:529–584. [Google Scholar]
- 19.Rahbek C. Am Nat. 1997;149:875–902. doi: 10.1086/286028. [DOI] [PubMed] [Google Scholar]
- 20.Tuomisto H. Ann Mo Bot Gard. 1998;85:48–62. [Google Scholar]
- 21.Imhoff M L, Sisk T D, Milne A, Morgan G, Orr T. Remote Sensing Environ. 1997;60:217–227. [Google Scholar]
- 22.Barraclough T G, Vogler A P. Am Nat. 2000;155:419–434. doi: 10.1086/303332. [DOI] [PubMed] [Google Scholar]
- 23.Moritz C, Joseph L, Cunningham M, Schneider C. In: Tropical Forest Remnants. Laurence W F, Bierregaard R O, editors. Chicago: Univ. Chicago Press; 1997. pp. 442–454. [Google Scholar]