Background: ExoY induces inter-endothelial gaps, although the mechanisms by which this occurs are poorly understood.
Results: ExoY synthesized cAMP and cGMP, which caused endothelial Tau hyperphosphorylation, accumulation of insoluble Tau, inter-endothelial cell gaps, and increased permeability.
Conclusion: ExoY is a promiscuous cyclase and an edema factor.
Significance: Acute Pseudomonas infections cause a pathophysiological sequela in endothelium previously recognized only in chronic neurodegenerative diseases.
Keywords: Cyclic AMP (cAMP), Cyclic GMP (cGMP), Endothelium, Lung Injury, Permeability, Alzheimer Disease, Pseudomonas, Tau, Tauopathy, Vascular Leak
Abstract
Exotoxin Y (ExoY) is a type III secretion system effector found in ∼ 90% of the Pseudomonas aeruginosa isolates. Although it is known that ExoY causes inter-endothelial gaps and vascular leak, the mechanisms by which this occurs are poorly understood. Using both a bacteria-delivered and a codon-optimized conditionally expressed ExoY, we report that this toxin is a dual soluble adenylyl and guanylyl cyclase that results in intracellular cAMP and cGMP accumulation. The enzymatic activity of ExoY caused phosphorylation of endothelial Tau serine 214, accumulation of insoluble Tau, inter-endothelial cell gap formation, and increased macromolecular permeability. To discern whether the cAMP or cGMP signal was responsible for Tau phosphorylation and barrier disruption, pulmonary microvascular endothelial cells were engineered for the conditional expression of either wild-type guanylyl cyclase, which synthesizes cGMP, or a mutated guanylyl cyclase, which synthesizes cAMP. Sodium nitroprusside stimulation of the cGMP-generating cyclase resulted in transient Tau serine 214 phosphorylation and gap formation, whereas stimulation of the cAMP-generating cyclase induced a robust increase in Tau serine 214 phosphorylation, gap formation, and macromolecular permeability. These results indicate that the cAMP signal is the dominant stimulus for Tau phosphorylation. Hence, ExoY is a promiscuous cyclase and edema factor that uses cAMP and, to some extent, cGMP to induce the hyperphosphorylation and insolubility of endothelial Tau. Because hyperphosphorylated and insoluble Tau are hallmarks in neurodegenerative tauopathies such as Alzheimer disease, acute Pseudomonas infections cause a pathophysiological sequela in endothelium previously recognized only in chronic neurodegenerative diseases.
Introduction
Pulmonary microvascular endothelium forms a highly restrictive barrier to allow for proper gas exchange (1–6). Inflammatory mediators and vascular permeability-increasing compounds cause retraction of cell borders and inter-endothelial gaps by reorganizing the endothelial cytoskeleton, cell-cell, and cell matrix interactions (reviewed in Ref. 7). When this barrier is disrupted, exudative alveolar edema occurs, and gas exchange is compromised.
In endothelial cells, receptors coupled to adenylyl and guanylyl cyclases generate membrane-limited 3′-5′ cyclic adenosine monophosphate (cAMP) and 3′-5′ cyclic guanosine monophosphate (cGMP), respectively, which activate effectors to stabilize the cortical actin rim, insert adherens junction proteins into the plasma membrane, and strengthen overall inter-endothelial cell contacts (8–13). Therefore, compounds that generate subplasmalemmal cAMP and cGMP contribute to the prevention and reduction of inflammation by averting inter-endothelial gap formation and the subsequent increase in permeability (14–16). In contrast, soluble adenylyl and guanylyl cyclases that generate cytosolic cAMP and cGMP signals are endothelial barrier disruptive and thus contribute to inflammatory edema (17–19). Of note, bacteria like Bacillus anthracis and Pseudomonas aeruginosa promote edema formation by introducing soluble cyclases into host cells (20, 21).
P. aeruginosa is a leading cause of lung injury, particularly in critically ill patients that need mechanical ventilation (22). Importantly, exotoxin Y (ExoY)2 is found in ∼90% of P. aeruginosa clinical isolates (23). ExoY is a soluble adenylyl cyclase that is introduced into host cells via the type III secretion system and increases the cytoplasmic levels of cAMP (24), mediates the hyperphosphorylation of endothelial Tau protein (25), impairs microtubule and microfilament stability3 (26), induces inter-endothelial gap formation, and increases vascular permeability (27, 28).
Recently, Göttle et al. (29) reported that bacterial soluble cyclases similar to ExoY are capable of synthesizing more than one cyclic nucleotide simultaneously (i.e. cAMP, cIMP, and cUMP). This suggested to us that ExoY could also synthesize other cyclic nucleotides in addition to cAMP and that, if so, this would have important implications for the understanding of lung endothelial permeability and the pathophysiology of P. aeruginosa-mediated acute lung injury. Here, we report that P. aeruginosa ExoY is sufficient to increase intracellular levels of both cAMP and cGMP in endothelial cells. We show that both cytosolic cAMP and, to a lesser degree, cGMP mediate the hyperphosphorylation of endothelial Tau Ser-214. We also show that P. aeruginosa ExoY intoxication leads to accumulation of insoluble Tau. Finally, we demonstrate that accumulation of cytosolic cAMP, and not cGMP, leads to large inter-endothelial gaps and increased permeability in pulmonary microvascular endothelial cells. Because hyperphosphorylated and insoluble Tau are hallmarks of neurodegenerative tauopathies such as Alzheimer disease (30, 31), these findings suggest that acute P. aeruginosa infections and chronic neurodegenerative diseases share Tau hyperphosphorylation and insolubility as a common pathophysiological mechanism.
EXPERIMENTAL PROCEDURES
Cell Culture
Pulmonary microvascular endothelial cells (internal identification: PMVECR1) were obtained from the cell culture core at the University of South Alabama Center for Lung Biology. The isolation and characterization of these cells has been described previously in detail (1, 32, 33). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% heat-inactivated fetal bovine serum (catalogue No. 10082, Invitrogen) and 1% penicillin/streptomycin (catalogue No. 15140, Invitrogen) at 37 °C in 21% oxygen and 5% carbon dioxide. Pulmonary artery smooth muscle cells were the kind gift of Dr. Celina Gairhe (Department of Pharmacology, University of South Alabama) and were cultured as described previously (34).
DNA and Viral Constructs
Retrovirus 2641 (rv2641) and pulmonary microvascular endothelial cells infected with this virus (MV/2641) were described previously (35). Construction of the lentiviral vector for doxycycline-inducible C-terminal fusions to a destabilizing variant of FKBP12 (F36V, E31G, R71G, and K105E), pMA3174, will be described separately.4 The full-length synthetic gene for P. aeruginosa, exoY, was codon-optimized commercially for expression in rat cells (rat codon adaptation index, 0.99) (see supplemental material for sequence). The termination codon was removed from the full-length codon-optimized exoY gene by PCR using primers ExoYRIf (GCGAATTCGCCACCATGAGGATCGACGGCCACAG) and ExoYdTerHpa (RGCGTTAACCAGCTCCACCTTCCTCTGGA). pMA3200 was constructed by inserting the full-length wild-type codon-optimized exoY gene without a termination codon into pMA3174 in-frame with a destabilizing variant of FKBP-12 (36–38). To codon-optimize for expression in mammalian cells, the mutant (exoYK81M) catalytic domain of exoY (amino acids 1–207; codon adaptation index = 0.94), was also generated commercially (BioBasic, Markham, Ontario, Canada). pMA3228 was generated by replacing the 5′-terminal EcoRI-BsrGI fragment of the WT exoY gene in pMA3200 with the corresponding fragment of the gene encoding the codon-optimized mutant catalytic domain.
cDNAs encoding rat sGC1α3 and sGC1β3 were purchased from Open Biosystems (Huntsville, AL; catalogue No. 7104600 and 7190419). sGC1α3 was modified to introduce a Kozak sequence at the 5′-end and a Myc tag plus XhoI site at the 3′-end by PCR using primers GC1a3-A-Mlu (FGTCAACGCGTGCCACCATGTTCTGCAGGAAGTTCAA) and GC1a3-B-mycXho (CCTCGAGTCACAGGTCCTCCTCGCTGATGAGTTTCTGCTCATCTACCCCTGATGCTTTGC). In the PCR product, 6 bp at the 5′-terminus were lost due to a PCR/cloning incident, thus destroying the MluI site. To generate pMA3379, the cDNA for sGC1α3 was inserted into pMA3211, a lentiviral vector for doxycycline-inducible expression derived from pMA2780 (35), from which an extra SalI site near the 3′-LTR was removed. The cDNA for sGC1β3 was modified by PCR to introduce an EcoRI site plus a Kozak sequence at the 5′-end and to truncate 3′-UTR and introduce a XbaI site at the 3′-end using primers GC1b3-A-Rif (GCGAATTCGCCACCATGTACGGTTTTGTGAACCA) and GC1b3-B-XbaR (GCTCTAGATTCAGTTTTCATCCTGGTTTG). To generate pMA3383 this modified cDNA was inserted into hygromycin resistance-encoding retroviral vector pMA1662. The mutation R592Q was introduced into sGC1α3 by overlap extension PCR (39) using primers GC1a3592QF (GAGTGAAGATGCCCCAGTATTGCCTGTTTG) and GC1a3592QR (CAAACAGGCAATACTGGGGCATCTTCACTC). The resulting mutant cDNA was inserted into pMA3211, thus generating pMA3431. Mutations E473K and C541D were introduced into sGC1β3 the same way using primers GC1b3D541F (GGATGCCACGGTATGATCTCTTCGGAAATA), GC1b3D541R (TATTTCCGAAGAGATCATACCGTGGCATCC), GC1b3K473F (CATTTGTTTACAAGGTGAAAACAGTTGGTG), and GC1b3K473R (CACCAACTGTTTTCACCTTGTAAACAAATG). To generate pMA3411, this double mutant cDNA was inserted into pMA1662. Retrovirus- and lentivirus-containing supernatants were produced by CaPO4-mediated transfection of the Phoenix ampho and HEK293FT cell lines, respectively, using established protocols (35). Gag, Pol, and Env functions for lentiviral constructs were provided in trans by cotransfection of the vector plasmid with two helper plasmids, psPAX2 and pMD2.G. In all instances when PCR was employed to amplify DNA fragments, the fidelity of amplification was confirmed by sequencing.
Bacterial Strains
P. aeruginosa strains have been described in detail elsewhere (24, 27). Two strains of P. aeruginosa were used, one with an active ExoY toxin (PA103 exoUexoT::Tc pUCPexoY or P. aeruginosa ExoY) and one with an inactive ExoY exotoxin (PA103ΔexoUexoT::Tc pUCPexoYK81M or P. aeruginosa ExoYK81M). Bacteria were taken from frozen explants, grown overnight on solid agar/carbenicillin (400 μg/ml), and resuspended in PBS to an optical density (OD540) of 0.25. This was previously determined to equal 2 × 108 bacteria/ml (27). Bacteria were subsequently diluted in PBS to achieve the desired multiplicity of infection (m.o.i.).
For bacterial infection, endothelial cells were trypsinized and counted using a Coulter counter (Beckman Coulter) as reported previously (40). Endothelial cells were grown to 12–24 h post-confluence and then infected with P. aeruginosa ExoY or P. aeruginosa ExoYK81M at an m.o.i. of 20:1 and incubated for up to 6 h at 37 °C in 21% oxygen and 5% carbon dioxide as described previously (27).
Measurement of 3′-5′-cAMP and 3′-5′-cGMP
Cyclic nucleotides levels were assessed by standard radioimmunoassay (catalogue No. BT-300 for cAMP and BT-340 for cGMP, Biomedical Technologies, Inc., Stoughton, MA) following the manufacturer's protocol. After bacterial infection, endothelial cells were lysed with 1 n HCl in the presence of 500 μm 3-isobutyl-1-methylxanthine. The reaction was neutralized with 1 n NaOH. Lysates were stored at −70 °C for further analyses (27).
Pulmonary microvascular endothelial cells were grown to 12–24 h post-confluence. Cells were incubated for up to 6 h at 37 °C and 5% CO2. Cell integrity was determined by the presence or absence of inter-endothelial cells gaps using phase contrast microscopy (Nikon IX70) at the end of this time period. When required, time lapse microscopy (SPOT Advanced software) (27) was also used. For this purpose, cells were maintained under environmentally controlled conditions (37 °C, 5% CO2), and micrographs were taken using a Nikon 80i upright microscope. NIS-Elements was used to process image sequences (41).
Antibodies, Immunoblot, Immunoprecipitation, and Densitometry
Cell lysates were generated as described previously (33). Briefly, cells were rinsed with cold (4 °C) 1× PBS followed by lysis with radioimmune precipitation assay buffer (catalogue No. BP-115, Boston Bioproducts, Worcester, MA) with a 1:100 protease inhibitor mixture (catalogue No. P8340, Sigma-Aldrich) and 1:100 phosphatase inhibitors (phosphatase inhibitor mixture I (catalogue No. BP-479) and phosphatase inhibitor mixture II (catalogue No. BP-480), Boston Bioproducts). Cell lysates were normalized for protein concentration using the Lowry protein assay kit (procedure P5656, Sigma-Aldrich), resolved in 4–12% bis-Tris polyacrylamide gels (catalogue No. NP0321, Invitrogen), and then transferred to 0.2-mm nitrocellulose membranes (catalogue No. 162–0213, Bio-Rad). Membranes were incubated with the appropriate antibodies (phospho-Tau Ser-214 at 1:500, catalogue No. 44-742G, Invitrogen; pan-Tau (TAU-5) at 1:1000, catalogue No. AT-5004, MBL International Corp., Woburn, MA; Myc-Tag (9B11) at 1:1000, catalogue No. 2276, Cell Signaling, Danvers, MA). All antibodies were diluted in 5% bovine serum albumin and incubated overnight. Membranes were probed with species-appropriate HRP-conjugated secondary antibody (1:6000 for 1 h at room temperature) and developed using SuperSignal West Femto chemiluminescent substrate (catalogue No. 34096, Thermo Scientific).
For immunoprecipitation (catalogue No 26149, Thermo Scientific) the manufacturer's instructions were followed. P. aeruginosa ExoY+-infected cells were washed with ice-cold PBS and lysed using Pierce immunoprecipitation lysis buffer with protease and phosphatase inhibitors (1:100). Protein concentration was measured, and 80 μl of cell lysate (∼20 μg of protein) was incubated with 40 μl of phospho-Tau Ser-214 antibody overnight. Samples were eluted and analyzed by Western blot (see above) using a TAU-5 antibody.
Western blot densitometries were measured using ImageJ software (National Institutes of Health, Bethesda, MD) by the method published previously (33). Densitometries are expressed as area in arbitrary units.
PKA and PKG Inhibition
PKI-(6–22)-amide, a short synthetic PKI-derived peptide (catalogue no. sc-201160, Santa Cruz Biotechnology, Santa Cruz, CA) (42), and PKGI, a short synthetic PKG inhibitory peptide (catalogue No. sc-201161, Santa Cruz Biotechnology) (43), were delivered using the protein delivery system ChariotTM (catalogue No. 30025, Active Motif North America, Carlsbad, CA) following the manufacturer's instructions at 1 h prior to Pseudomonas infection.
Sodium Nitroprusside (SNP), Doxycycline, and Shield1
SNP (catalogue No. 228710, Sigma-Aldrich) was diluted following the manufacturer's instructions to aliquots of 10 mm. On the day of treatment, pulmonary microvascular endothelial cells were washed with 1× PBS and treated with SNP diluted to 100 μm in 1× Tyrode's buffer. Doxycycline and Shield1 were purchased from Clontech (Mountain View, CA) and used according to the manufacturer's instructions.
Macromolecular Permeability Assays
Endothelial permeability was assayed by measuring the transmonolayer flux to a FITC-labeled dextran tracer (catalogue No. FD40S, Sigma-Aldrich) using a modified version of the protocol described by Lampugnani and Dejana (44). Pulmonary microvascular endothelial cells engineered to conditionally overexpress either sGCα1-β1 or sGCαQ-β-KD were grown on clear polyester Transwell insert membranes (catalogue No. 3470, Corning Inc.) in phenol red-free DMEM (catalogue No. 21063, Invitrogen) with 10% heat-inactivated fetal bovine serum and 1% penicillin/streptomycin at 37 °C in 21% oxygen and 5% carbon dioxide. Twenty-four hours before the experiment day, cells were treated with doxycycline at 1 μg/ml. On experiment day (12–24 h post-confluency), cells were incubated with the tracer and treated with 100 μm SNP for up to 2 h. Fluorescence was read using the Expectra Max M5 plate reader (Molecular Devices, Sunnyvale, CA) at emission 485 nm and excitation 539 nm. Data were acquired as fluorescence units with Softmax Pro V5 (Molecular Devices) and arbitrarily reported as normalized fluorescence (x/baseline fluorescence).
Sarkosyl Extraction
Purification of Sarkosyl-insoluble Tau was based on a previously published protocol (45). Briefly, following Pseudomonas infection, endothelial cells were homogenized in a 6× volume of the following (in mm): 50 Tris base, pH 8.0, 274 NaCl, 5 KCl, 2 EGTA, and 2 EDTA with protease inhibitor mixture (catalogue No. P8340, Sigma-Aldrich) and phosphatase inhibitors (phosphatase inhibitor mixture I, catalogue No. BP-479, and phosphatase inhibitor mixture II, catalogue No. BP-480, Boston Bioproducts). The extract (homogenate) was spun for 15 min at 13,000 × g, and supernatant was used as a total fraction. The amount of starting material was adjusted for protein concentration. The supernatant was then centrifuged further at 150,000 × g for 15 min to separate proteins into soluble and insoluble (pellet) fractions. A pellet was re-extracted and centrifuged at 150,000 × g for 15 min. The pellet was discarded, and the supernatant was incubated with 1% Sarkosyl at 37 °C for 1 h and centrifuged at 150,000 × g for 30 min, washed briefly with the same buffer, and centrifuged again. The pellet (solubilized in Tris-EDTA buffer) contained Sarkosyl-insoluble Tau.
Statistical Analyses
Data are presented as mean ± S.E. Data were analyzed by one-way ANOVA, two-way ANOVA, and Bonferroni's multiple comparisons test as appropriate. A value of p < 0.05 was considered statistically significant. GraphPad Prism 4.0 software (GraphPad Software, Inc., La Jolla, CA) was used for statistical analysis.
RESULTS
Pseudomonas aeruginosa ExoY Increased Intracellular Levels of Both cAMP and cGMP
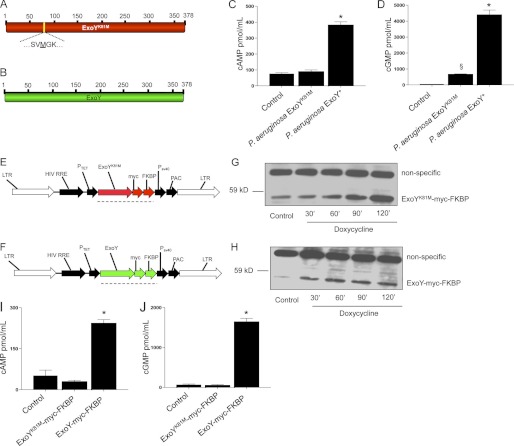
A recent report resolved that other bacterial adenylyl cyclase exotoxins similar to ExoY are capable of simultaneously synthesizing more than one cyclic nucleotide (29). Therefore, we tested whether ExoY would increase the levels of cGMP in addition to those of cAMP. We found that both cAMP and cGMP increased pulmonary microvascular endothelial cells infected with the P. aeruginosa ExoY+ but not P. aeruginosa ExoYK81M strain (Fig. 1, C and D). Of note, ExoY+ generated roughly 10-fold more cGMP than cAMP.
FIGURE 1.
P. aeruginosa ExoY is sufficient to increase the intracellular accumulation of cAMP and cGMP. A, schematic representation of ExoYk81M. B, schematic representation of ExoY. C, mean ± S.E. of global cAMP levels in pulmonary microvascular endothelial cells 6 h post-P. aeruginosa ExoY+ infection. D, mean ± S.E. of global cGMP levels in pulmonary microvascular endothelial cells 6 h post-P. aeruginosa ExoY+ infection. E and F, general maps of vectors used to deliver codon-optimized ExoY and ExoYK81M. LTR, retroviral/lentiviral long-terminal repeat; HIV RRE, human immunodeficiency virus response element; Ptet, doxycycline-regulated promoter; FKBP, protein destruction domain (FKBP); PSV40, SV40 promoter; PAC, puromycin resistance gene. G, conditional expression of codon-optimized ExoYK81M. H, conditional expression of codon-optimized ExoY. I, intracellular cAMP levels of pulmonary microvascular endothelial cells conditioned to express codon-optimized ExoY-myc-FKBP or ExoYK81M-myc-FKBP 2 h after induction with 2 μm Shield1 and 2 μg/ml doxycycline. J, intracellular cGMP levels of pulmonary microvascular endothelial cells conditioned to express codon-optimized ExoY-myc-FKBP or ExoYK81M-myc-FKBP 2 h after induction with 2 μm Shield1 and 2 μg/ml doxycycline. *, p < 0,001 versus P. aeruginosa ExoYK81M; §, p < 0.05 versus control. Data represent five independent experiments. Statistical significance was determined by one-way ANOVA followed by Bonferroni's multiple comparisons test.
To test whether ExoY was sufficient to increase the cytoplasmic levels of both cAMP and cGMP, we engineered pulmonary microvascular endothelial cells to conditionally express ExoY. First, we tried to express the full-length exoY gene using an adenoviral delivery system in pulmonary microvascular endothelial cells, but we could not detect the expression of ExoY protein. Even though degeneracy is a fundamental attribute of the genetic code, bacterial genes use codons that are rarely employed by mammalian cells (37). Suspecting codon mismatching, we next engineered pulmonary microvascular endothelial cells to conditionally express a codon-optimized P. aeruginosa ExoY with a hemagglutinin (HA) tag at the C terminus (see supplemental material for ExoY codon-optimized sequence). Although the expression of ExoY-HA was successful (supplemental Fig. S1), we were not able to detect enzymatic activity. We then tested whether HA or Myc (ExoY-myc) tags in the C terminus of bacteria-delivered ExoY reduced ExoY enzymatic activity when compared with wild-type ExoY and found that ExoY-HA had the least activity, with ExoY-myc having the most (although intermediate when compared with the wild type (data not shown)). Thereafter, we conditionally expressed a codon-optimized P. aeruginosa ExoY with a Myc tag but detected enough background expression of the transgene to intoxicate the cells, suggesting that enzymatic activity was present. This result revealed the need to systematically regulate gene and protein expression.
To control gene and protein expression, we developed a two-tier control protein expression system to conditionally express codon-optimized ExoY by combining doxycyline-inducible gene expression (35) with protein stability regulation by small molecules (36–38). Two constructs were developed: ExoY-myc-FKBP (Fig. 1E) and ExoYK81M-myc-FKBP (Fig. 1F). Following induction with both doxycycline and Shield1, we detected both ExoY-myc-FKBP and ExoYK81M-myc-FKBP at the predicted molecular mass of ∼55 kDa (Fig. 1, G and H). ExoY was catalytically active, as pulmonary microvascular endothelial cells expressing codon-optimized ExoY-myc-FKBP, but not cells expressing ExoYK81M-myc-FKBP, accumulated both cAMP and cGMP (Fig. 1, I and J); once again, ExoY generated more cGMP than cAMP. Hence, ExoY is enzymatically active in the absence of bacteria and in the absence of intoxication through the type III secretion system; expression within the host cell is sufficient for ExoY to bind its necessary cofactor and synthesize cAMP and cGMP.
Pseudomonas aeruginosa ExoY Was Sufficient to Induce Inter-endothelial Cell Gaps
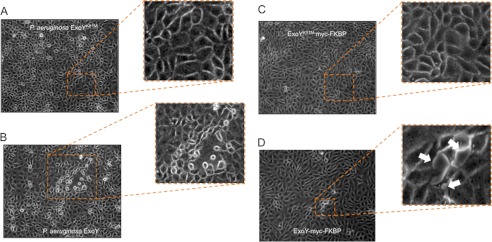
Consistent with a previous report from our group (27), pulmonary microvascular endothelial cells monolayers developed gaps after infection with a P. aeruginosa strain that expressed an active ExoY toxin (P. aeruginosa ExoY+), but not when they were infected with a P. aeruginosa strain that expressed an inactive toxin (P. aeruginosa ExoYK81M) (Fig. 2, A and B). To determine whether ExoY, in the absence of bacteria, was sufficient to induce inter-endothelial gap formation in pulmonary microvascular endothelial cells, we tested the response in cells engineered to express codon-optimized ExoY-myc-FKBP and codon-optimized ExoYK81M-myc-FKB. Induction of ExoY, but not ExoYK81M, resulted in inter-endothelial gap formation in parallel with an intracellular accumulation of cAMP and cGMP (Fig. 2, C and D, and supplemental Movie 1).
FIGURE 2.
P. aeruginosa ExoY is sufficient to induce inter-endothelial gap formation. A, pulmonary microvascular endothelial cells 6 h after P. aeruginosa ExoYK81M infection at m.o.i. 20:1 in serum-free medium. Magnification, ×20. No endothelial gap formation was observed. B, pulmonary microvascular endothelial cells 6 h after P. aeruginosa ExoY+ infection at m.o.i. 20:1 in serum-free medium. Magnification, ×20. Note the disruption of the monolayers. Photograph are representative of 10 or more experiments. C, pulmonary microvascular endothelial cells that conditionally expressed codon-optimized ExoYK81M-myc-FKBP 2 h after induction with 2 μm Shield1 and 2 μg/ml doxycycline. No inter-endothelial cell gaps were observed. These photographs are representative of five experiments. D, pulmonary microvascular endothelial cells that conditionally expressed codon-optimized ExoY-myc-FKBP 2 h after induction with 2 μm Shield1 and 2 μg/ml doxycycline. White arrows point to inter-endothelial cell gaps. These photographs are representative of four experiments.
Two Isoforms of Tau Are Detected in Pulmonary Microvascular Endothelial Cells When Compared with Brain Homogenates
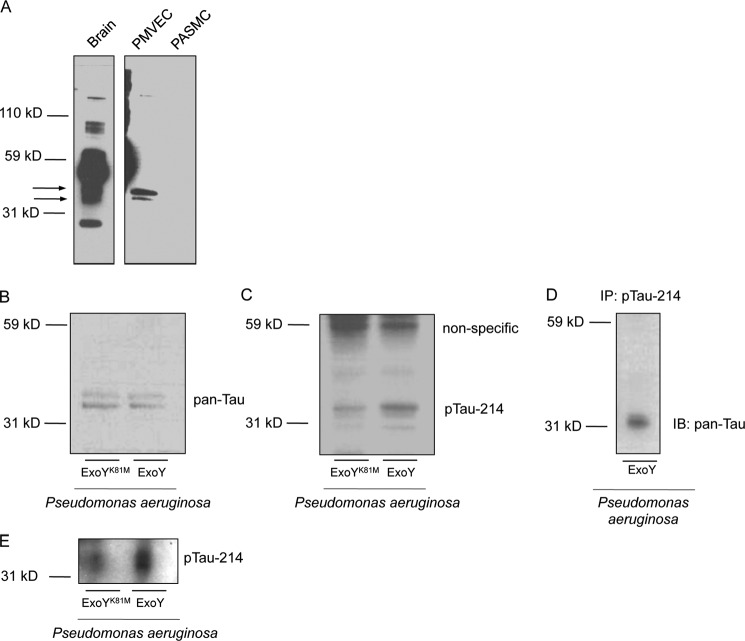
Previous studies from our group (25, 46, 47) and others (48) have resolved that Tau or a Tau-like protein is expressed in pulmonary microvascular endothelial cells. It is not clear, however, whether endothelial Tau is similar to those Tau isoforms found in the central nervous system. We compared Tau expression in endothelial cells against brain homogenates as well as pulmonary artery smooth muscle cells using a pan-Tau antibody. We found that the pan-Tau antibody revealed multiple bands in brain homogenates, as expected, with a major band at ∼40 kDa. Additionally, we found that pulmonary microvascular endothelial cells expressed two isoforms found in brain extracts at ∼33 and ∼35 kDa (Fig. 3A). We did not detect Tau expression in pulmonary artery smooth muscle cells.
FIGURE 3.
P. aeruginosa ExoY+ infection results in the hyperphosphorylation of endothelial Tau Ser-214. A, immunoblot to detect Tau using a pan-Tau antibody. Pulmonary microvascular endothelial cells (PMVEC) express two isoforms of Tau also found in brain homogenates. Pulmonary artery smooth muscle cells (PASMC) do not express Tau. B and C, P. aeruginosa ExoY+ infection results in Tau Ser-214 hyperphosphorylation when compared with P. aeruginosa ExoYK81M. Hyperphosphorylation occurs on the band resolved at ∼33 kDa. D, immunoprecipitation revealed that pan-Tau and Ser-214-specific antibodies recognize the same product resolved at ∼33 kDa. Pulmonary microvascular endothelial cells were infected with P. aeruginosa ExoY+, and an immunoprecipitation of cellular extracts was performed with Tau Ser-214-specific antibody and an immunoblot against pan-Tau was performed. E, P. aeruginosa ExoY+ infection results in increased Sarkosyl-insoluble Tau as detected by Ser-214 phospho-antibody.
These studies and those that follow (see below), as well as the published literature (25, 46–48), support the notion that endothelial cells express Tau. However, this immunotypification presently lacks confirmation by comparative molecular biology and protein sequencing. In future studies it will be imperative to clone and sequence the endothelial Tau.
P. aeruginosa ExoY+, but Not P. aeruginosa ExoYK81M, Infection Resulted in hyperphosphorylation of Endothelial Tau Ser-214 in Pulmonary Microvascular Endothelial Cells
To follow the signaling events downstream to the simultaneous increase in cyclic nucleotide levels, we monitored the phosphorylation state of endothelial Tau Ser-214. Our laboratory had previously identified Tau ser-214 as a readout for cytosolic cAMP accumulation (25, 46, 47). We found that the increased levels of cAMP and cGMP that paralleled gap formation in P. aeruginosa ExoY+-infected pulmonary microvascular endothelial cells resulted in increased Tau Ser-214 phosphorylation, whereas cells infected with P. aeruginosa ExoYK81M did not display increased Tau Ser-214 phosphorylation (Fig. 3, B and C). Of note, the band demonstrating Tau hyperphosphorylation was that resolved at ∼33 kDa. To confirm that Tau was in fact being hyperphosphorylated, an immunoprecipitation experiment was performed. The Ser-214 antibody was used to pull down the endothelial Tau, and immunoblots were performed using the pan-Tau antibody. As shown in Fig. 3D, immunoprecipitation experiments demonstrate that the endothelial Tau resolved at ∼33 kDa is phosphorylated following ExoY activation.
P. aeruginosa ExoY+ Infection Resulted in Increased Levels of Insoluble Endothelial Tau Ser-214 when Compared with P. aeruginosa ExoYK81M
Hyperphosphorylation of Tau is a key event in the development of Alzheimer disease, a neurodegenerative tauopathy (30). In neurons, hyperphosphorylated Tau becomes insoluble, and insoluble Tau is unable to interact with tubulin (49). Consistent with a tauopathy, P. aeruginosa ExoY+-infected cells accumulated more hyperphosphorylated Tau Ser-214, which was insoluble after Sarkosyl extraction when compared with P. aeruginosa ExoYK81M (Fig. 3E). As shown, the insoluble endothelial cell Tau was resolved at ∼33 kDa, the same molecular mass resolved in Western analysis. Hence, whereas ExoY induced Tau hyperphosphorylation and insolubility, it did not produce Tau aggregates, at least over the time course tested. To further examine whether hyperphosphorylated and aggregated Tau could be resolved, we tested AT8 and AT100 antibodies. The AT8 antibody recognized paired helical filamentous Tau doubly phosphorylated at Ser-202/Thr-205, Ser-199/Ser-202, or Ser-205/Ser-208. The AT100 antibody recognized the Thr-212/Ser-214 doubly phosphorylated Tau. Despite multiple attempts, neither the AT8 nor the AT100 antibody revealed bands in ExoY-inoculated endothelium (data not shown). These findings suggest ExoY either does not cause phosphorylation of the residues that are recognize by AT8 and AT100 (PKA and PKG are not the principal kinases targeting these dual phosphorylation sites; see below), or it does not induce Tau aggregation.
Protein Kinase A and Protein Kinase G Inhibition Indicated That Both cAMP and cGMP Are Implicated in Tau Hyperphosphorylation Caused by P. aeruginosa ExoY
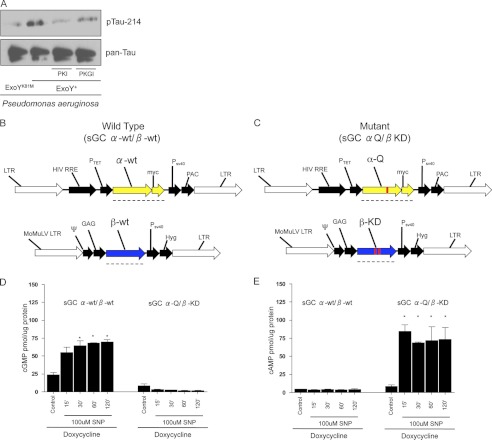
To evaluate the contribution of cAMP and cGMP to Tau Ser-214 phosphorylation in ExoY-intoxicated cells, pulmonary microvascular endothelial cells were infected with P. aeruginosa ExoY+ in the presence of the PKA inhibitor PKI-(6–22) (42, 50, 51) or the PKG inhibitor PKGI (52, 53). Treatment of P. aeruginosa ExoY+-infected cells with PKI-(6–22) inhibited Tau Ser-214 phosphorylation (all versus ExoY alone) (Fig. 4). PKGI treatment modestly reduced Tau Ser-214 phosphorylation (all versus ExoY alone) (Fig. 4). These observations were confirmed by pharmacological inhibition using H89 and KT-5823 (supplemental Fig. S2). Importantly, PKI-(6–22) and PKGI did not affect the base-line phosphorylation of Tau Ser-214 (supplemental Fig. S3).
FIGURE 4.
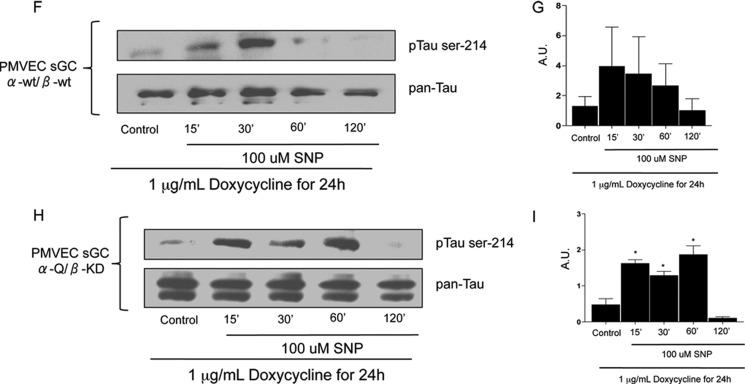
cAMP and cGMP independently mediate Tau Ser-214 hyperphosphorylation. A, the specific PKA inhibitor PKI-(6–22) (10 μm) prevented the increase in Tau Ser-214 phosphorylation promoted by P. aeruginosa ExoY+. PKG inhibition with 10 μm PKGI modestly reduced Tau Ser-214 phosphorylation. B and C, general maps of vectors used to deliver the α- and β-subunits of rat sGC. These vectors were delivered to endothelial cells already expressing the advanced Tet-on system as described in Ref. 35. Therefore, these were triple mutant pulmonary microvascular endothelial cells. LTR, retroviral/lentiviral long terminal repeat; HIV RRE, human immunodeficiency virus response element; Ptet, doxycycline-regulated promoter; α-wt, wild-type α-1 subunit of rat sGC; PSV40, SV40 promoter; PAC, puromycin resistance gene; MoMuLV, Moloney murine leukemia virus; ψ, packaging psi; CAG β-wt, wild-type β-1 subunit of rat sGC; Hyg, hygromycin resistance cassette; α-Q, α-1 R592Q subunit of sGC; β-KD, E473K/C541D subunit of sGC. Black dashed lines show the sequence translated by ribosome. D, cGMP levels in both sGC α-wt/β-wt and sGC α-Q/β-KD after 24 h of doxycycline induction and 2 h of treatment with 100 μm SNP. E, cAMP levels in both sGC α-wt/β-wt and sGC α-Q/β-KD after 24 h of doxycycline induction and 2 h of treatment with 100 μm SNP. *, p < 0.001 versus control. Data represent four independent experiments. Statistical significance was determined by one-way ANOVA followed by Bonferroni's multiple comparisons test. F, immunoblot blot analysis of pulmonary microvascular endothelial cells conditionally overexpressing rat sGC α-Q/β-KD, induced with doxycycline, and treated with 100 μm SNP. G, densitometries revealed average response from three independent experiments. H, immunoblot blot analysis of pulmonary microvascular endothelial cells (PMVEC) conditionally overexpressing rat sGC α-wt/β-wt, induced with doxycycline, and treated with 100 μm SNP. I, densitometry confirms a sustained increase in Tau Ser-214 phosphorylation. Images are representative of three independent experiments. A.U., arbitrary units.
Because high levels of cAMP can cross-activate PKG and high levels of cGMP can cross-activate PKA (54–56), the specific signaling modules that lead to Tau hyperphosphorylation in endothelial cells infected with P. aeruginosa ExoY+ cannot be resolved using this bacteria-soluble cyclase. To approach this problem, we developed pulmonary microvascular endothelial cell lines to generate exclusively either cytosolic cAMP or cGMP in response to SNP (see above and Fig. 4, B and C) (57).
Accumulation of Cytoplasmic cAMP and cGMP Mediated Tau Ser-214 Hyperphosphorylation
To disentangle the contributions of cAMP and cGMP in the signaling events that lead to Tau Ser-214 phosphorylation, we developed a pulmonary microvascular cell line in which sGC was conditionally overexpressed to synthesize either cGMP or cAMP in response to 100 μm SNP (Fig. 4, B and C) (57). Three mutations were made to the wild-type rat sGC (R592Q in the α-subunit and E473K as well as C541D in the β-subunit) that made sGC generate cAMP exclusively (Fig. 4, D and E). In cells expressing the wild-type sGC, SNP induced a time-dependent increase in cGMP over a 2-h time course. Tau Ser-214 phosphorylation was induced by 15 min, but this effect was not sustained past the 30-min time point (Fig. 4F). In the mutated sGC, SNP induced a time-dependent increase in cAMP over a 2-h time course. However, in this instance, Tau Ser-214 phosphorylation was prominently increased by 15 min, and this effect was observed until the 60-min time point (Fig. 4F). This approach revealed that both cytosolic cAMP and cGMP can mediate Tau Ser-214 hyperphosphorylation. However, the cAMP-dependent Tau phosphorylation signal was sustained over time.
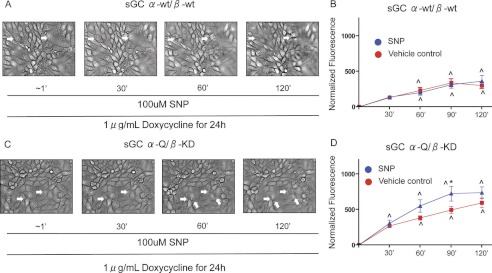
SNP Treatment of sGCα-Q/β-KD Mutant Cells, but Not sGCα-wt/β-wt, Resulted in Large Inter-endothelial Gap Formation
After doxycycline induction, sGCα-Q/β-KD mutant cells developed inter-endothelial gaps over a 2-h period in response to 100 μm SNP (Fig. 5C and supplemental Movie 2). sGCα-wt/β-wt cells also developed inter-endothelial gaps; these gaps, however, were smaller, and some of them resealed within the 2-h time period (Fig. 5A, and supplemental Movie 3).
FIGURE 5.
Intracellular accumulation of cAMP, but not cGMP, results in large inter-endothelial gap formation and increased endothelial permeability. A, sGC α-wt/β-wt mutant cells induced with doxycycline and treated with 100 μm developed smaller inter-endothelial gaps (see also supplemental Movie 3). B, doxycycline induction and treatment with 100 μm sGC α-wt/β-wt mutant cells did not increase endothelial cell permeability as measured by macromolecular flux to a 40-kDa FITC-labeled dextran tracer. n = 7 independent experiments. C, sGC α-Q/β-KD mutant cells induced with doxycycline and treated with 100 μm SNP developed large inter-endothelial gaps over a 2-h time period (see also supplemental Movie 2). D, doxycycline induction and treatment with 100 μm SNP in sGC α-Q/β-KD mutant cells increased endothelial cell permeability as measured by macromolecular flux to a 40-kDa FITC-labeled dextran tracer. n = 8 independent experiments. One-way ANOVA was used to assess significance for each condition over the 120-min time course. Two-way ANOVA was used to make comparisons between treatments. *, p < 0.05 versus vehicle control; [caret], p < 0.05 versus base line.
SNP Treatment of sGCα-Q/β-KD Mutant Cells, but Not of sGCα-wt/β-wt, Increased Endothelial Permeability
To evaluate the independent contribution of these cyclic nucleotides to pulmonary microvascular endothelial permeability, we investigated the transmonolayer flux to a 40-kDa FITC-labeled dextran tracer in response to 100 μm SNP. We found that SNP treatment increased endothelial permeability in sGCα-Q/β-KD cells but not in sGCα-wt/β-wt cells, as revealed by the increase in transcellular dextran flux (Fig. 5, B and D).
DISCUSSION
ExoY is an edema factor that causes endothelial hyperpermeability (28, 58). To date, however, the study of ExoY inside eukaryotic cells has relied solely on bacterial delivery of the toxin through the type III secretion system. This has limited the thorough examination of the function and dynamics of the toxin, as confounding variables such as the contribution of the type III secretion system to cellular toxicity (59) cannot be eliminated using a bacterial delivery system. By generating a two-tier protein expression system in pulmonary microvascular endothelial cells to conditionally express a codon-optimized ExoY toxin, we resolved that ExoY, by itself, is sufficient to induce inter-endothelial gap formation and that an active ExoY is enough to elevate intracellular levels of both cAMP and cGMP. Although astonishing, this last finding is in line with a recent report by Göttle et al. (29) showing that edema factor from B. anthracis and CyaA from Bordetella pertussis exhibit adenylyl, cytidylyl, and uridylyl cyclase activity within the same catalytic core. Thus, the requirement for ExoY to generate multiple cyclic nucleotides warrants further study.
The synthesis of cGMP by prokaryotes has been documented only in Cyanobacteria (60). Therefore, ours is the first report on the ability of Proteobacteria to synthesize cGMP, albeit via a type III secretion system exotoxin. In the hallmark description of ExoY as an adenylyl cyclase, Yahr et al. (24) did not detect cGMP in a purified ExoY cyclic nucleotide-generating reaction when GTP was substituted for ATP, although cell extract was not present in this purified system. These earlier results are in contrast to our present findings in which ExoY was exposed to the cytosolic compartment of the cell, where the cofactor exists. Indeed, ExoY becomes active only when it binds to an unknown mammalian cofactor (24); it remains to be seen whether this cofactor can modify the substrate specificity of ExoY. This idea is consistent with the recent findings of Göttle et al. (29), who report that cells display different substrate specificities for edema factor-induced cyclic nucleotides. Another possibility is that the ExoY catalytic core is nonspecific for nucleotides and binds to those that are readily available. In the case of endothelial cells, ATP and GTP are both key physiological substrates.
Both transmembrane and soluble mammalian cyclases display substrate specificity, yet two to three residue substitutions in their catalytic cores reverse this specificity (61). The ability of ExoY, edema factor, and CyaA to synthesize multiple cyclic nucleotides suggests evolutionary specification in mammalian enzymes. Several lines of evidence suggest vertical soluble cyclase gene transfer from bacteria to mammals (62), and reports indicate that both sAC and sGC could have evolved separately (63). However, Roelofs et al. (64) identified an ortholog of sAC in Dictyostelium that is an sGC, suggesting, at least in this case, that sGC may have evolved from sAC. In consequence, one could argue that the nucleotide specificity of soluble cyclases is a trait that could have evolved from bacteria (via eukaryotic microorganisms) to mammals. Although the evolutionary pressures that determined the specificity of mammalian soluble cyclases are unclear, our studies indicate that prokaryotic exotoxin-soluble cyclases are not discriminatory enzymes, in stark contrast to their mammalian counterparts.
In the past, cAMP and cGMP were considered to have opposing influences in the regulation of biological processes; that is, the physiological events that were facilitated by cAMP were suppressed by cGMP (and vice versa). This is known as the “Yin Yang hypothesis of biological regulation,” advanced by Goldberg et al. (65, 66) in the 1970s based upon empirical evidence. Data from the microvascular permeability field clearly support this idea (67). However, it is now apparent that this theory is incomplete. New evidence has resolved that cAMP can either decrease or promote microvascular permeability, depending on whether it is synthesized at the plasma membrane or cytosolic compartment, respectively. This phenomenon was first described using ExoY (27) and subsequently confirmed using a chimeric mammalian sAC (68, 69). Although this subplasmalemmal versus cytoplasmic hypothesis was recently offered to explain the contradictory data on how cGMP regulates microvascular permeability (19), we were not able to demonstrate increased permeability with high levels of cGMP, despite detecting small and transient inter-endothelial gaps. These observations do not rule out the possibility that cytoplasmic accumulation of cGMP results in transendothelial hyperpermeability to compounds of lesser molecular mass (we used a 40-kDa FITC-labeled dextran) or that cytoplasmic accumulation of cGMP results in endothelial hyperpermeability under conditions of hydrostatic pressure, especially in response to ExoY, which generates more cGMP than cAMP. This unresolved issue warrants further study. What is clear, however, is that in our model of equivalent increases in cAMP and cGMP, it was the cytoplasmic cAMP signal, and not the cytoplasmic cGMP signal, that resulted in large inter-endothelial gaps that paralleled an increase in permeability.
The ExoY-generated cyclic nucleotides induce Tau hyperphosphorylation, which causes a conformational change in Tau making it insoluble and unable to interact with microtubules (70). Tau hyperphosphorylation and insolubility are hallmarks of neurodegenerative tauopathies such as Alzheimer disease (71), argyrophilic grain disease, Pick disease, and hereditary frontotemporal dementias (72, 73). In these conditions, excessive kinase activity, reduced phosphatase activity, or both cause hyperphosphorylated Tau (30). Although PKA and other kinases phosphorylate Tau Ser-214 (74), at present what signal or signals activate these kinases remain unknown, although infectious causes have been proposed (75–77). We now report that toxins such as ExoY are sufficient to cause Tau hyperphosphorylation and insolubility, at least in endothelial cells, an effect that leads to endothelial cell retraction.
Insoluble Tau is prone to aggregation. The further processing of aggregated Tau by proteases such as caspase generates paired helical filaments in neurodegenerative diseases. The term “tauopathy” has been used to describe any abnormal Tau behavior, whether referring to hyperphosphorylation and insolubility or aggregation and paired helical formation (31, 78). Indeed, the mere process of hyperphosphorylation causes a constellation of changes in the physical property of Tau, which cause dysfunction even before Tau forms aggregates. To this end, we have described the endothelial cell consequences of ExoY activity as a tauopathy. ExoY-induced Tau hyperphosphorylation was detected specifically on the Ser-214 position, a phosphorylation site that not only causes microtubule disassembly but also impairs the formation of paired helical filaments (79). Consistent with this idea, we could not detect Tau aggregates in our studies, perhaps because the Ser-214 site prevented aggregation (79) or perhaps because our studies were conducted over acute (i.e. hours) and not chronic (i.e. days to weeks) time periods (80).
Just recently, de Calignon et al. (81) have suggested that fibrillar Tau is not a cause of apoptosis in neurons. Rather, they suggest that fibrillar Tau is indicative of surviving neurons. Hyperphosphorylated Tau may transition from soluble to insoluble to aggregate forms. However, some evidence suggests that the mechanisms responsible for Tau phosphorylation and paired helical filament formation may be altogether different (82, 83). We propose that ExoY induces Tau hyperphosphorylation, generating an insoluble form of the protein that impairs microtubule growth without the formation of larger aggregates and paired helical filaments and, as a consequence, causing endothelial retraction and increased permeability.
Interestingly, several reports have detected a perivascular accumulation of hyperphosphorylated Tau in brain specimens from patients with Alzheimer disease (84). In these cases, the origin of Tau is presumed to be neural, although it is not clear whether other cells such as endothelium could also be a source. In neurons, abnormal Tau impairs cognition (30); in endothelium, it increases permeability (47) and may increase the susceptibility to apoptosis.
In summary, we have documented a new two-tier protein expression system specially designed to express cytotoxic proteins. We also report that P. aeruginosa ExoY is a promiscuous cyclase that simultaneously synthesizes cAMP and cGMP; that ExoY is sufficient to cause inter-endothelial cell gaps; and that both cAMP and cGMP mediate Tau Ser-214 phosphorylation. Furthermore, we show in our model that cAMP, and not cGMP, mediates increased macromolecular permeability. Finally, we present the first evidence for an infectious endothelial tauopathy.
Supplementary Material
Acknowledgments
We thank Linn Ayers and Anna Buford for their contribution to the development of this work.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-60024 and HL-66299 (to T. S.), HL-76125 and HL-107122 (to C. D. O.), and 1RO1RR031286 (to M. A.).

This article contains supplemental “Experimental Procedures,” Codon-optimized Sequences, Figs. S1–S3, and Movies 1–3.
R. Balczon, N. Prasain, C. D. Ochoa, J. Prater, B. Zhu, M. Alexeyev, S. Sayner, D. W. Frank, and T. Stevens, submitted for publication.
I. N. Shokolenko, R. Fayzulin, V. Pastukh, J. Hill, and M. Alexeyev, unpublished data.
- ExoY
- exotoxin Y
- m.o.i.
- multiplicity of infection
- bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- SNP
- sodium nitroprusside
- ANOVA
- analysis of variance
- sGC
- soluble guanylyl cyclase
- sAC
- soluble adenylyl cyclase.
REFERENCES
- 1. Stevens T. (2007) Microheterogeneity of lung endothelium. In Endothelial Biomedicine (Airdm W. C., ed) pp. 1160–1170, Cambridge University Press, Cambridge, UK [Google Scholar]
- 2. Prasain N., Stevens T. (2009) The actin cytoskeleton in endothelial cell phenotypes. Microvasc. Res. 77, 53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ofori-Acquah S. F., King J., Voelkel N., Schaphorst K. L., Stevens T. (2008) Heterogeneity of barrier function in the lung reflects diversity in endothelial cell junctions. Microvasc. Res. 75, 391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stevens T., Phan S., Frid M. G., Alvarez D., Herzog E., Stenmark K. R. (2008) Lung vascular cell heterogeneity: endothelium, smooth muscle, and fibroblasts. Proc. Am. Thorac. Soc. 5, 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parker J. C., Stevens T., Randall J., Weber D. S., King J. A. (2006) Hydraulic conductance of pulmonary microvascular and macrovascular endothelial cell monolayers. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L30–L37 [DOI] [PubMed] [Google Scholar]
- 6. Jacobson J. R., Garcia J. G. N. (2010) Chapter 6 appendix: endothelial cell function. In Murray and Nadel's Textbook of Respiratory Medicine (Mason R. J., Broaddus B. C., Martin T. R., King T. E., Jr., Schraufnagel E., Murray J. F., Nadel J. A., eds) 5th Ed., p. 1–6, W. B. Saunders Co., Philadelphia, PA [Google Scholar]
- 7. Mehta D., Malik A. B. (2006) Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 86, 279–367 [DOI] [PubMed] [Google Scholar]
- 8. Adamson R. H., Ly J. C., Sarai R. K., Lenz J. F., Altangerel A., Drenckhahn D., Curry F. E. (2008) Epac/Rap1 pathway regulates microvascular hyperpermeability induced by PAF in rat mesentery. Am. J. Physiol. Heart Circ. Physiol. 294, H1188–H1196 [DOI] [PubMed] [Google Scholar]
- 9. Fukuhara S., Sakurai A., Sano H., Yamagishi A., Somekawa S., Takakura N., Saito Y., Kangawa K., Mochizuki N. (2005) Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol. Cell. Biol. 25, 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stelzner T. J., Weil J. V., O'Brien R. F. (1989) Role of cyclic adenosine monophosphate in the induction of endothelial barrier properties. J. Cell. Physiol. 139, 157–166 [DOI] [PubMed] [Google Scholar]
- 11. Adamson R. H., Liu B., Fry G. N., Rubin L. L., Curry F. E. (1998) Microvascular permeability and number of tight junctions are modulated by cAMP. Am. J. Physiol. 274, H1885–H1894 [DOI] [PubMed] [Google Scholar]
- 12. Rentsendorj O., Mirzapoiazova T., Adyshev D., Servinsky L. E., Renné T., Verin A. D., Pearse D. B. (2008) Role of vasodilator-stimulated phosphoprotein in cGMP-mediated protection of human pulmonary artery endothelial barrier function. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L686–L697 [DOI] [PubMed] [Google Scholar]
- 13. Moldobaeva A., Welsh-Servinsky L. E., Shimoda L. A., Stephens R. S., Verin A. D., Tuder R. M., Pearse D. B. (2006) Role of protein kinase G in barrier-protective effects of cGMP in human pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L919–L930 [DOI] [PubMed] [Google Scholar]
- 14. Berthiaume Y., Staub N. C., Matthay M. A. (1987) β-Adrenergic agonists increase lung liquid clearance in anesthetized sheep. J. Clin. Invest. 79, 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahluwalia A., MacAllister R. J., Hobbs A. J. (2004) Vascular actions of natriuretic peptides. Cyclic GMP-dependent and -independent mechanisms. Basic Res. Cardiol. 99, 83–89 [DOI] [PubMed] [Google Scholar]
- 16. Kemp S. F., Lockey R. F., Simons F. E., and World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis (2008) Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy 63, 1061–1070 [DOI] [PubMed] [Google Scholar]
- 17. Sayner S. L. (2011) Emerging themes of cAMP regulation of the pulmonary endothelial barrier. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L667–L678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen Q., Rigor R. R., Pivetti C. D., Wu M. H., Yuan S. Y. (2010) Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc. Res. 87, 272–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuebler W. M. (2011) The Janus-faced regulation of endothelial permeability by cyclic GMP. Am. J. Physiol. Lung Cell. Mol. Physiol. 301, L157–L160 [DOI] [PubMed] [Google Scholar]
- 20. Lory S. (2004) The multi-talented bacterial adenylate cyclases. Int. J. Med. Microbiol. 293, 479–482 [DOI] [PubMed] [Google Scholar]
- 21. Ahuja N., Kumar P., Bhatnagar R. (2004) The adenylate cyclase toxins. Crit. Rev. Microbiol. 30, 187–196 [DOI] [PubMed] [Google Scholar]
- 22. Weber D. J., Rutala W. A., Sickbert-Bennett E. E., Samsa G. P., Brown V., Niederman M. S. (2007) Microbiology of ventilator-associated pneumonia compared with that of hospital-acquired pneumonia. Infect. Control Hosp. Epidemiol. 28, 825–831 [DOI] [PubMed] [Google Scholar]
- 23. Feltman H., Schulert G., Khan S., Jain M., Peterson L., Hauser A. R. (2001) Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147, 2659–2669 [DOI] [PubMed] [Google Scholar]
- 24. Yahr T. L., Vallis A. J., Hancock M. K., Barbieri J. T., Frank D. W. (1998) ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. U.S.A. 95, 13899–13904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sayner S. L., Balczon R., Frank D. W., Cooper D. M., Stevens T. (2011) Filamin A is a phosphorylation target of membrane but not cytosolic adenylyl cyclase activity. Am. J. Physiol. Lung Cell. Mol. Physiol. 301, L117–L124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cowell B. A., Evans D. J., Fleiszig S. M. (2005) Actin cytoskeleton disruption by ExoY and its effects on Pseudomonas aeruginosa invasion. FEMS Microbiol. Lett. 250, 71–76 [DOI] [PubMed] [Google Scholar]
- 27. Sayner S. L., Frank D. W., King J., Chen H., VandeWaa J., Stevens T. (2004) Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ. Res. 95, 196–203 [DOI] [PubMed] [Google Scholar]
- 28. Lowe K., Alvarez D. F., King J. A., Stevens T. (2010) Perivascular fluid cuffs decrease lung compliance by increasing tissue resistance. Crit. Care Med. 38, 1458–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Göttle M., Dove S., Kees F., Schlossmann J., Geduhn J., König B., Shen Y., Tang W. J., Kaever V., Seifert R. (2010) Cytidylyl and uridylyl cyclase activity of Bacillus anthracis edema factor and Bordetella pertussis CyaA. Biochemistry 49, 5494–5503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Querfurth H. W., LaFerla F. M. (2010) Alzheimer's disease. N. Engl. J. Med. 362, 329–344 [DOI] [PubMed] [Google Scholar]
- 31. Bouchard M., Suchowersky O. (2011) Tauopathies: one disease or many? Can. J. Neurol. Sci. 38, 547–556 [DOI] [PubMed] [Google Scholar]
- 32. King J., Hamil T., Creighton J., Wu S., Bhat P., McDonald F., Stevens T. (2004) Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc. Res. 67, 139–151 [DOI] [PubMed] [Google Scholar]
- 33. Ochoa C. D., Stevens T., Balczon R. (2011) Cold exposure reveals two populations of microtubules in pulmonary endothelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L132–L138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gairhe S., Bauer N. N., Gebb S. A., McMurtry I. F. (2011) Myoendothelial gap junctional signaling induces differentiation of pulmonary arterial smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 301, L527–L535 [DOI] [PubMed] [Google Scholar]
- 35. Alexeyev M. F., Fayzulin R., Shokolenko I. N., Pastukh V. (2010) A retro-lentiviral system for doxycycline-inducible gene expression and gene knockdown in cells with limited proliferative capacity. Mol. Biol. Rep. 37, 1987–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Banaszynski L. A., Chen L. C., Maynard-Smith L. A., Ooi A. G., Wandless T. J. (2006) A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell 126, 995–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lorimer D., Raymond A., Walchli J., Mixon M., Barrow A., Wallace E., Grice R., Burgin A., Stewart L. (2009) Gene composer: database software for protein construct design, codon engineering, and gene synthesis. BMC Biotechnol. 9, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grimley J. S., Chen D. A., Banaszynski L. A., Wandless T. J. (2008) Synthesis and analysis of stabilizing ligands for FKBP-derived destabilizing domains. Bioorg. Med. Chem. Lett. 18, 759–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. (1989) Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77, 61–68 [DOI] [PubMed] [Google Scholar]
- 40. Parra-Bonilla G., Alvarez D. F., Al-Mehdi A. B., Alexeyev M., Stevens T. (2010) Critical role for lactate dehydrogenase A in aerobic glycolysis that sustains pulmonary microvascular endothelial cell proliferation. Am. J. Physiol. Lung Cell. Mol. Physiol. 299, L513–L522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Creighton J., Jian M., Sayner S., Alexeyev M., Insel P. A. (2011) Adenosine monophosphate-activated kinase alpha1 promotes endothelial barrier repair. FASEB J. 25, 3356–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murray A. J. (2008) Pharmacological PKA inhibition: all may not be what it seems. Sci. Signal. 1, re4. [DOI] [PubMed] [Google Scholar]
- 43. Wei J. Y., Jin X., Cohen E. D., Daw N. W., Barnstable C. J. (2002) cGMP-induced presynaptic depression and postsynaptic facilitation at glutamatergic synapses in visual cortex. Brain Res. 927, 42–54 [DOI] [PubMed] [Google Scholar]
- 44. Lampugnani M. G., Dejana E. (2004) Endothelial cell permeability assays is culture. In Methods in Endothelial Cell Biology (Augustin H. G., ed) pp. 103–112, Springer, New York [Google Scholar]
- 45. Berger Z., Roder H., Hanna A., Carlson A., Rangachari V., Yue M., Wszolek Z., Ashe K., Knight J., Dickson D., Andorfer C., Rosenberry T. L., Lewis J., Hutton M., Janus C. (2007) Accumulation of pathological Tau species and memory loss in a conditional model of tauopathy. J. Neurosci. 27, 3650–3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Creighton J., Zhu B., Alexeyev M., Stevens T. (2008) Spectrin-anchored phosphodiesterase 4D4 restricts cAMP from disrupting microtubules and inducing endothelial cell gap formation. J. Cell Sci. 121, 110–119 [DOI] [PubMed] [Google Scholar]
- 47. Zhu B., Zhang L., Creighton J., Alexeyev M., Strada S. J., Stevens T. (2010) Protein kinase A phosphorylation of tau-serine 214 reorganizes microtubules and disrupts the endothelial cell barrier. Am. J. Physiol. Lung Cell. Mol. Physiol. 299, L493–L501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Birukova A. (2004) Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J. 18, 1879–1890 [DOI] [PubMed] [Google Scholar]
- 49. Hirata-Fukae C., Li H. F., Ma L., Hoe H. S., Rebeck G. W., Aisen P. S., Matsuoka Y. (2009) Levels of soluble and insoluble Tau reflect overall status of Tau phosphorylation in vivo. Neurosci. Lett. 450, 51–55 [DOI] [PubMed] [Google Scholar]
- 50. Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. (1984) Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry 23, 5036–5041 [DOI] [PubMed] [Google Scholar]
- 51. Cheli Y., Luciani F., Khaled M., Beuret L., Bille K., Gounon P., Ortonne J. P., Bertolotto C., Ballotti R. (2009) αMSH and cyclic AMP-elevating agents control melanosome pH through a protein kinase A-independent mechanism. J. Biol. Chem. 284, 18699–18706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kase H., Iwahashi K., Nakanishi S., Matsuda Y., Yamada K., Takahashi M., Murakata C., Sato A., Kaneko M. (1987) K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem. Biophys. Res. Commun. 142, 436–440 [DOI] [PubMed] [Google Scholar]
- 53. Komalavilas P., Shah P. K., Jo H., Lincoln T. M. (1999) Activation of mitogen-activated protein kinase pathways by cyclic GMP and cyclic GMP-dependent protein kinase in contractile vascular smooth muscle cells. J. Biol. Chem. 274, 34301–34309 [DOI] [PubMed] [Google Scholar]
- 54. White R. E., Kryman J. P., El-Mowafy A. M., Han G., Carrier G. O. (2000) cAMP-dependent vasodilators cross-activate the cGMP-dependent protein kinase to stimulate BK(Ca) channel activity in coronary artery smooth muscle cells. Circ. Res. 86, 897–905 [DOI] [PubMed] [Google Scholar]
- 55. Khaled M., Larribere L., Bille K., Aberdam E., Ortonne J. P., Ballotti R., Bertolotto C. (2002) Glycogen synthase kinase 3β is activated by cAMP and plays an active role in the regulation of melanogenesis. J. Biol. Chem. 277, 33690–33697 [DOI] [PubMed] [Google Scholar]
- 56. Hucho T. B., Dina O. A., Levine J. D. (2005) Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J. Neurosci. 25, 6119–6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sunahara R. K., Beuve A., Tesmer J. J., Sprang S. R., Garbers D. L., Gilman A. G. (1998) Exchange of substrate and inhibitor specificities between adenylyl and guanylyl cyclases. J. Biol. Chem. 273, 16332–16338 [DOI] [PubMed] [Google Scholar]
- 58. Prasain N. (2009) Soluble adenylyl cyclases disassemble microtubules and disrupt the pulmonary endothelial barrier. Ph.D. dissertation, University of South Alabama, Mobile, AL [Google Scholar]
- 59. Sawa T., Wiener-Kronish J. (2004) A therapeutic strategy against the shared virulence mechanism utilized by both Yersinia pestis and Pseudomonas aeruginosa. Anesthesiol. Clin. North Am. 22, 591–606 [DOI] [PubMed] [Google Scholar]
- 60. Rauch A., Leipelt M., Russwurm M., Steegborn C. (2008) Crystal structure of the guanylyl cyclase Cya2. Proc. Natl. Acad. Sci. U.S.A. 105, 15720–15725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tucker C. L., Hurley J. H., Miller T. R., Hurley J. B. (1998) Two amino acid substitutions convert a guanylyl cyclase, RetGC-1, into an adenylyl cyclase. Proc. Natl. Acad. Sci. U.S.A. 95, 5993–5997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Roelofs J., Van Haastert P. J. (2002) Deducing the origin of soluble adenylyl cyclase, a gene lost in multiple lineages. Mol. Biol. Evol. 19, 2239–2246 [DOI] [PubMed] [Google Scholar]
- 63. Baker D. A., Kelly J. M. (2004) Structure, function and evolution of microbial adenylyl and guanylyl cyclases. Mol. Microbiol. 52, 1229–1242 [DOI] [PubMed] [Google Scholar]
- 64. Roelofs J., Meima M., Schaap P., Van Haastert P. J. (2001) The Dictyostelium homologue of mammalian soluble adenylyl cyclase encodes a guanylyl cyclase. EMBO J. 20, 4341–4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. (1970) Elevation of guanosine 3′,5′-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc. Natl. Acad. Sci. U.S.A. 66, 398–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Goldberg N. D., Haddox M. K., Nicol S. E., Glass D. B., Sanford C. H., Kuehl F. A., Jr., Estensen R. (1975) Biologic regulation through opposing influences of cyclic GMP and cyclic AMP: the Yin Yang hypothesis. Adv. Cyclic Nucleotide Res. 5, 307–330 [PubMed] [Google Scholar]
- 67. Michel C. C., Curry F. E. (1999) Microvascular permeability. Physiol. Rev. 79, 703–761 [DOI] [PubMed] [Google Scholar]
- 68. Sayner S. L., Alexeyev M., Dessauer C. W., Stevens T. (2006) Soluble adenylyl cyclase reveals the significance of cAMP compartmentation on pulmonary microvascular endothelial cell barrier. Circ. Res. 98, 675–681 [DOI] [PubMed] [Google Scholar]
- 69. Prasain N., Alexeyev M., Balczon R., Stevens T. (2009) Soluble adenylyl cyclase-dependent microtubule disassembly reveals a novel mechanism of endothelial cell retraction. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L73–L83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Matenia D., Mandelkow E. M. (2009) The Tau of MARK: a polarized view of the cytoskeleton. Trends Biochem. Sci. 34, 332–342 [DOI] [PubMed] [Google Scholar]
- 71. Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. (1986) Abnormal phosphorylation of the microtubule-associated protein Tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. U.S.A. 83, 4913–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Frank S., Clavaguera F., Tolnay M. (2008) Tauopathy models and human neuropathology: similarities and differences. Acta Neuropathol. 115, 39–53 [DOI] [PubMed] [Google Scholar]
- 73. Spillantini M. G., Goedert M., Crowther R. A., Murrell J. R., Farlow M. R., Ghetti B. (1997) Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial Tau filaments. Proc. Natl. Acad. Sci. U.S.A. 94, 4113–4118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hanger D. P., Anderton B. H., Noble W. (2009) Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 15, 112–119 [DOI] [PubMed] [Google Scholar]
- 75. Balin B. J., Little C. S., Hammond C. J., Appelt D. M., Whittum-Hudson J. A., Gérard H. C., Hudson A. P. (2008) Chlamydophila pneumoniae and the etiology of late-onset Alzheimer's disease. J. Alzheimers Dis. 13, 371–380 [DOI] [PubMed] [Google Scholar]
- 76. Kinoshita J. (2004) Pathogens as a cause of Alzheimer's disease. Neurobiol. Aging 25, 639–640 [DOI] [PubMed] [Google Scholar]
- 77. Itzhaki R. F., Wozniak M. A., Appelt D. M., Balin B. J. (2004) Infiltration of the brain by pathogens causes Alzheimer's disease. Neurobiol. Aging 25, 619–627 [DOI] [PubMed] [Google Scholar]
- 78. Williams D. R. (2006) Tauopathies: classification and clinical update on neurodegenerative diseases associated with microtubule-associated protein tau. Intern. Med. J. 36, 652–660 [DOI] [PubMed] [Google Scholar]
- 79. Schneider A., Biernat J., von Bergen M., Mandelkow E., Mandelkow E. M. (1999) Phosphorylation that detaches Tau protein from microtubules (Ser-262, Ser-214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry 38, 3549–3558 [DOI] [PubMed] [Google Scholar]
- 80. King M. E., Gamblin T. C., Kuret J., Binder L. I. (2000) Differential assembly of human Tau isoforms in the presence of arachidonic acid. J. Neurochem. 74, 1749–1757 [DOI] [PubMed] [Google Scholar]
- 81. de Calignon A., Fox L. M., Pitstick R., Carlson G. A., Bacskai B. J., Spires-Jones T. L., Hyman B. T. (2010) Caspase activation precedes and leads to tangles. Nature 464, 1201–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sato S., Tatebayashi Y., Akagi T., Chui D. H, Murayama M., Miyasaka T., Planel E., Tanemura K., Sun X., Hashikawa T., Yoshioka K., Ishiguro K., Takashima A. (2002) Aberrant Tau phosphorylation by glycogen synthase kinase-3β and JNK3 induces oligomeric Tau fibrils in COS-7 cells. J. Biol. Chem. 277, 42060–42065 [DOI] [PubMed] [Google Scholar]
- 83. Bandyopadhyay B., Li G., Yin H., Kuret J. (2007) Tau aggregation and toxicity in a cell culture model of tauopathy. J. Biol. Chem. 282, 16454–16464 [DOI] [PubMed] [Google Scholar]
- 84. Williams S., Chalmers K., Wilcock G. K., Love S. (2005) Relationship of neurofibrillary pathology to cerebral amyloid angiopathy in Alzheimer's disease. Neuropathol. Appl. Neurobiol. 31, 414–421 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.