Summary
Attention is crucial for visual perception because it allows the visual system to effectively use its limited resources by selecting behaviourally and cognitively relevant stimuli from the large amount of information impinging on the eyes. Reflexive, stimulus-driven attention is essential for successful interactions with the environment because it can, for example, speed up responses to life threatening events. It is commonly believed that exogenous attention operates in the retinotopic coordinates of the early visual system. Here, using a novel experimental paradigm [1], we show that a non-retinotopic cue improves both accuracy and reaction times in a visual search task. Furthermore, the influence of the cue is limited both in space and time, a characteristic typical of exogenous cueing. These and other recent findings show that many more aspects of vision are processed non-retinotopically than previously thought.
Results and discussion
Attention is a key feature of vision. We usually perceive or remember objects only when we pay attention to them [2]. Two types of attention can be distinguished [3, 4]. Endogenous attention is directed by voluntary effort, for example, when intentionally looking at a star in a clear night. Exogenous attention, instead, is drawn automatically to the location of salient events, such as a shooting star. This attentional capture is usually assumed to be based on retinotopic coordinates, i.e., allocated to the location where the event is projected on the retina. Here, we show that attentional capture can occur in a non-retinotopic frame of reference. This grouping-based non-retinotopic capture shows the same characteristics as exogenous attention hitherto characterized by retinotopic paradigms.
Experimentally, exogenous attention is investigated with cueing paradigms where a cue precedes a target [4–9]. Speeded responses to the target are faster when the cue and the target appear at the same retinotopic location compared to when they occur at different locations. Here, we combined a cueing paradigm with a variant of the Ternus-Pikler display [10, 11] that we proposed as a sensitive test for non-retinotopic processing [1]. Three gray squares were followed by an inter-stimulus interval (ISI) and then shifted by one position randomly to the left or to the right (in Figure 1A, a left-to-right motion is shown). A “group motion” percept is elicited where the three squares appear to move laterally in tandem as a group [12]. Because of the group motion, only one central square is perceived [1]. To investigate the effect of non-retinotopic cueing, we presented a cue in the central square of the first frame and a visual search display in the central square of the second frame. Observers had to indicate the orientation (clockwise versus counter-clockwise) of a tilted red bar (target) among upright red and tilted green bars (distractors).
Figure 1.
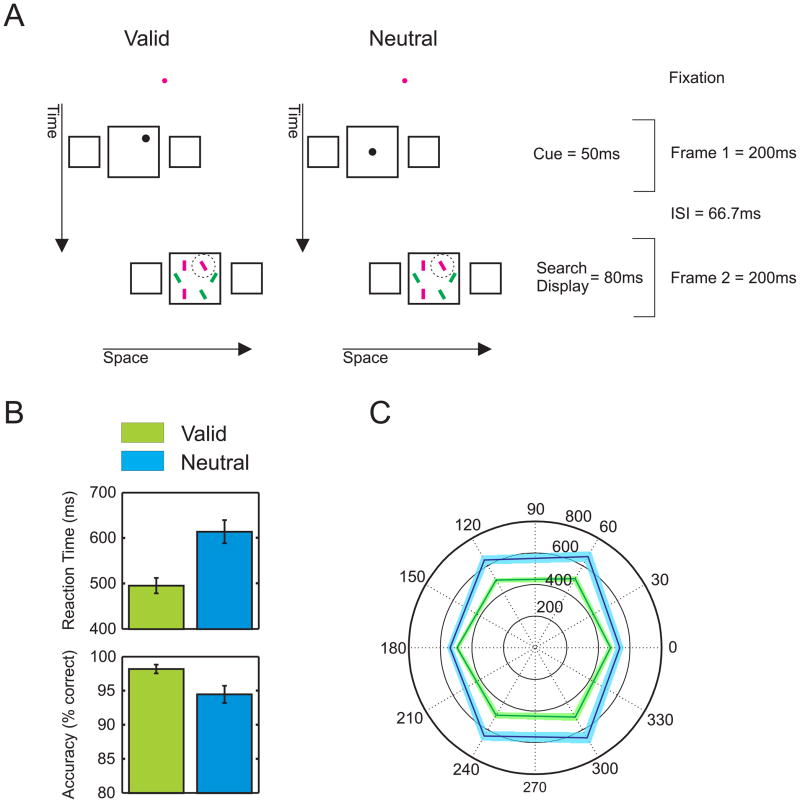
Non-retinotopic cueing. A) Observers fixated on a dot throughout the experiment. In a Ternus-Pikler display, a cue appeared in the central square of the first frame while a visual search display appeared in the central square of the second frame. Observers were asked to indicate the orientation of the tilt of a red bar (target, highlighted by a circle not shown in the actual display) among upright red and tilted green bars (distractors). In “valid” trials, the cue and the target appeared at the same relative position with respect to the surrounding central square. In “neutral” trials, the cue appeared always at the center of the central square. Neither in the valid nor in the neutral trials, the cue and the target overlapped retinotopically. B) Collapsed over the individual target positions, responses are faster and more accurate for valid than for neutral cues. Error bars show the standard error of the mean of six observers. C) Reaction times for the individual target positions. The black outer circle indicates the target position (in degrees). The inner circles provide a scale for the reaction times. The corners of the hexagons represent the average reaction times for each target position. The closer the corners are to the center, the faster the reaction times are. For all target positions, reaction times are faster in valid (green) than in neutral (blue) trials. Colored bands indicate standard errors.
Experiment 1
The target was presented randomly at one of six possible locations. The cue was presented either at the center of the square (neutral cue) or at the same position as the target with respect to non-retinotopic, “square coordinates” (valid cue). When, for example, the cue was at the upper right corner of the central square in the first frame, the target was in the same corner in the second frame. Because of the group motion and the corresponding non-retinotopic frame of reference, the cue and the search display were perceived in the same central square. Still, cues never overlapped with the target retinotopically.
Even though the cue and the target never overlapped retinotopically, observers were much faster and accurate when the cue was valid compared to when the cue was neutral (Figures 1B, C; paired t-test, reaction times, t(5) = 7.8, p < 0.001; accuracy, t(5) = 3.19, p = 0.024). Hence, the cue effectively captured attention non-retinotopically. In the neutral condition, reaction times along the horizontal meridian were faster than along the vertical one (116.12ms difference; paired t-test, t(5) = 6.52, p < 0.005). This difference largely disappeared in the “valid cue” condition, well in line with findings in retinotopic paradigms [7].
Experiment 2
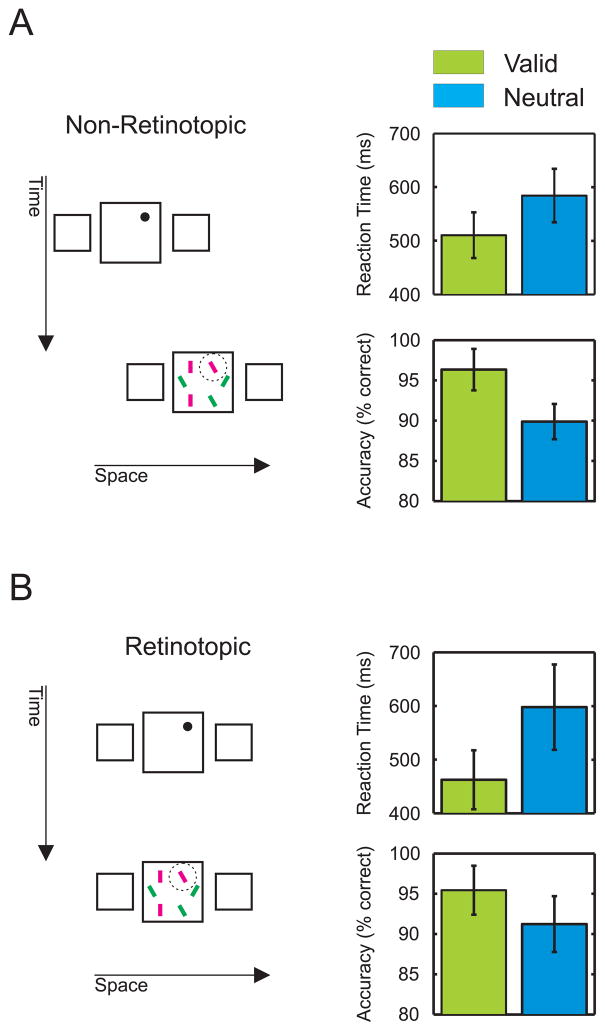
The advantage of the valid over the neutral cue in Experiment 1 is not due to the training with the non-retinotopic Ternus-Pikler paradigm. Three new observers performed 1000 trials using the same paradigm as in Experiment 1. Then, we presented 200 trials using a retinotopic paradigm where the display appeared twice at the same location (Figure 2A). The pattern of results is similar as in the non-retinotopic condition. The cueing effect with the retinotopic display was even larger than the one in the last 200 trials of the non-retinotopic condition (Figures 2A, B; paired t-test: t(2) = 4.15, p = 0.053).
Figure 2.
Effects of training. A) In Experiment 2, three new observers performed a total of 1000 trials using the same paradigm as in Experiment 1 (left). Average reaction times and accuracy are shown for the last 200 of the 1000 trials (right). B) After performing the 1000 non-retinotopic trials in the non-retinotopic condition, observers performed 200 trials in the retinotopic condition, where the squares appeared twice at the same location. Reaction times and accuracy show the same pattern as in the non-retinotopic condition. Error bars represent SEM.
Experiment 3
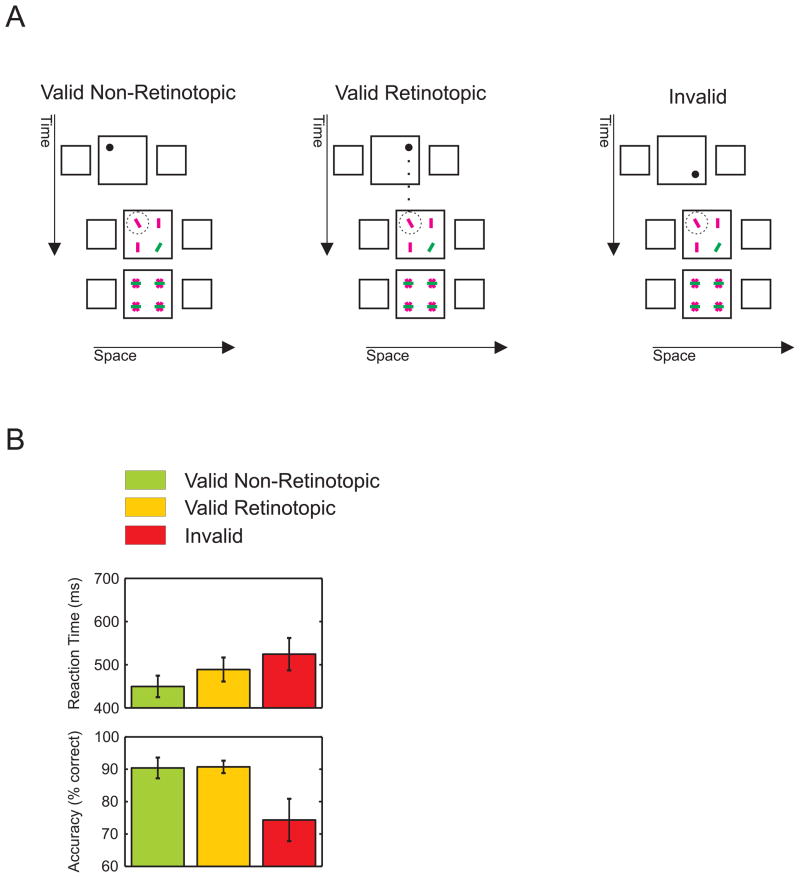
To further contrast the effect of retinotopic and non-retinotopic cueing, we decreased the inter-frame displacement to make the central squares of the first and second frames partially overlap. We presented a search display composed of four elements preceded by a cue flashed at one of the four corners of the central square of the first frame (Figure 3A). The cue was predictive (80% valid) of the row (top or bottom) where the target would appear in the second frame, but not for the horizontal target position, which was selected randomly at each trial. Thus, the cue was equally predictable non-retinotopically and retinotopically. This setup resulted in valid trials (either retinotopic or non-retinotopic) and invalid trials. Observers were faster when the target was cued non-retinotopically than when it was cued retinotopically (Figure 3B; retinotopic vs. non-retinotopic, reaction times, paired t-test, t(4) = 3.98, p = 0.016; accuracy, t(4) = 0.13, p = 0.9). For invalid trials (20% of all trials), reaction times were longer than for valid ones and accuracy was much lower.
Figure 3.
Retinotopic vs. non-retinotopic cueing. A) In Experiment 3, the central square of the first and second frames partially overlapped. A cue, which could appear at each one of the corners of the central square in the first frame, was in 80% of the occurrences predictive for the row where the target (tilted red line) were to appear in the second frame. For example, when the cue appeared in the left corner of the upper row, the target appeared with 40% probability at this position or with 40% probability at the right corner (in the second frame). In the remaining 20% of trials, the target was presented at either position in the lower row. With this set-up, non-retinotopic and retinotopic cue validity was the same for targets presented at the overlap region. The search display was followed by a mask to increase task difficulty. B) Only trials with targets presented at the overlap region are shown. Among the valid trials, responses were faster for non-retinotopic than for retinotopic valid trials. Error bars represent SEM.
Experiment 4
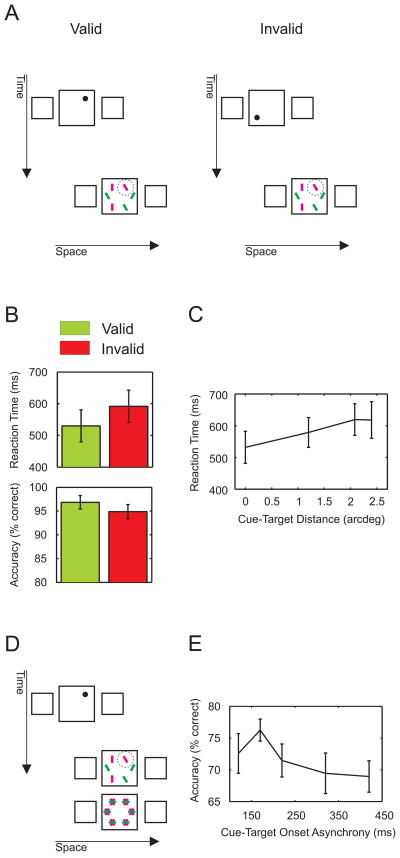
Next, we show that a non retinotopic cue captures attention even when non-informative. The cue appeared randomly at one of the six possible positions in the central square of the first frame independently of the target position. This arrangement accidentally produces “valid” trials when the cue is presented at the same position as the target and “invalid” ones otherwise (Figure 4A). Notwithstanding the fact that the cue was non-predictive and that observers were informed about this, responses to “valid” trials were faster than responses to “invalid” ones (Figure 4B; paired t-test, t(2) = 6.06, p = 0.026; accuracy, paired t-test, t(2) = 2.31, p = 0.14). A regression analysis showed that reaction times increased with the distance between the cue and the target by 38ms/arcdeg (Figure 4C; one sample t-test on the slope of the regression line, t(2) = 7.40, p = 0.017). Hence, even though the cue did not carry any information about the position of the target, the cue still attracted exogenous attention similarly to findings in retinotopic paradigms [13]. The effect of the uninformative cue is smaller than the one in Experiment 1 (61.71ms and 118.79ms, respectively).
Figure 4.
Uninformative cue and time course. A) In Experiment 4, the cue and target positions were selected independently from each other. Thus, only in one sixth of the trials, the cue was valid. Observers were informed that the cue was uninformative. B) Responses in the “valid” trials are faster and more accurate than responses in the “invalid” trials. However, the cueing effect is smaller than in Experiment 1. C) Reaction times increase with the distance between the cue and the target (in non-retinotopic coordinates). D) Example of a trial in Experiment 5. E) Attentional capture is transient. Accuracy is plotted as a function of cue-target onset asynchrony (CTOA). Performance peaks at 170ms and decreases at longer CTOAs. Error bars represent SEM.
Experiment 5
To investigate the time course of non-retinotopic cueing, we presented the cue in the first frame with one out of five Cue Target Onset Asynchronies (CTOAs). In this experiment, the target location was cued non-retinotopically (100% valid). In addition, the search display was masked (Figure 4D). Motion direction was kept constant within each block of trials. An ANOVA showed a significant performance difference among the levels of CTOA (repeated measures ANOVA, n=9, F(4) = 2.7, p = 0.048). A set of multiple comparisons (Tukey-Kramer test, p = 0.048) showed that performance was better at shorter (170ms) than at longer CTOAs (420ms). These results are in agreement with an early, transient exogenous attention deployment similar to findings in retinotopic paradigms [4, 8, 9]. For purely endogenous attention, accuracy should monotonically increase with CTOA. Our results do not exclude the involvement of an endogenous component, but the transient peak around CTOA=170ms strongly supports the engagement of exogenous attention.
The early visual system is organized retinotopically and most visual processing is thought to take place within retinotopic representations. Retinotopic accounts of attention predict cueing benefits when the cue and the target occupy the same retinotopic location. In contrast to this prediction, we have shown significant cueing effects in non-retinotopic coordinates.
Because, in our experiments, observers’ head and body positions were fixed, our results rule out not only retinotopic but also all egocentric (e.g., body- and head-centered reference frames) and static allocentric (e.g., spatiotopic) reference frames. Instead, our results show that exogenous cueing occurs in a coordinate system that moves according to perceptual grouping relations, as in an object centered reference frame. Previous studies showed non-retinotopic cueing effects to be non-specific and related to the entire object [14, 15, but see 16]. Our results show that non-retinotopic cueing is specific to the location within the object (the central square) where the cue is presented.
Recently, eye movement paradigms were used to study the frame of reference of endogenous attention across saccades with conflicting results [17, 18], some of them favouring non-retinotopic accounts. Here, we have shown that exogenous attention also can be processed in non-retinotopic, object-based coordinates.
Our results show that models of attention need to take non-retinotopic processing into account. Non-retinotopic reference frames might be conceptualized in different ways and attention can come into play at various time points, namely before, during or after the non-retinotopic frame of reference is established. For example, according to a hierarchical retinotopic account, objects may be computed first in retinotopic coordinates and the effect of the exogenous cue may shift automatically in conjunction with the retinotopic shift of the moving object. Alternatively, a non-retinotopic frame of reference may provide the fundamental representation where attention operates. In this case, cueing occurs in a non-retinotopic reference frame along with the computation of the object. In a natural environment, most objects undergo continuous motion, necessitating the computation of objects over continuously changing retinotopic locations. Although pursuit eye movements can retinotopically stabilize a target, all stimuli having a different velocity than the pursuit target will require computations over continuously changing retinotopic locations [19]. In agreement with these observations, it has been shown that the computation of fundamental object attributes such as form [20], color [21], brightness [22], and size [23] takes place in non-retinotopic representations. Thus, unlike the aforementioned retinotopically-based hierarchical processing, we suggest that the computation of objects and the operation of attention take place simultaneously within the same non-retinotopic space-time representation. These space-time representations are computationally beneficial for the visual system, because the computations become invariant with respect to continuous movements of the eyes, head, body, and objects. Furthermore, non-retinotopic, grouping based space-time reference frames provide the natural bases for event representations and analyses.
Taken together, our results, along with recent discoveries of non-retinotopic visual processing [18–39], may pave the way toward a novel conceptualization of visual computations wherein non-retinotopic frames of reference provide the computational framework for many processes hitherto thought to operate in retinotopic coordinates.
Experimental procedures
Apparatus. Subjects observed the stimuli on a PHILIPS 201B4 CRT monitor 1280 by 1024 pixels at 75 Hz refresh rate (Experiment 5: 1024 by 768 at 100Hz). We used the iViewX-HiSpeed eye tracker (SMI), set up for binocular mode at 500 Hz sampling frequency. Signals of both eyes were averaged in order to reduce noise.
A total of seventeen subjects (fourteen naïve) were tested. Observers had normal or corrected-to-normal vision at least for one eye, as assessed with the Freiburg Visual Acuity Test.
All experiments were approved by the local ethics commission.
Experiment 1
Six subjects (five naïve) were tested. We trained the observers until they reached 90% accuracy in two consecutive blocks. This needed a maximum of 400 trials.
In the first frame, three gray squares were presented for 200ms followed by a 66.7ms ISI and a second frame, displayed again for 200ms. The display shifted during the ISI by 3.7arcdeg, either left-to-right or right-to-left, selected randomly in each trial.
The squares had 3arcdeg side and a luminance of 36.4cd/m2 on a 4.55cd/m2 black background. The central square was surrounded by a brighter (54.6cd/m2 luminance) 0.29arcdeg frame. The squares were separated by a 3.7arcdeg center-to-center distance.
In the central square of the second frame, six bars (24cd/m2 luminance) were presented for 80ms from the onset of the second frame. The bars were 0.4arcdeg long and 300arcsec wide and were presented at 0, 60, 120, 180 or 240 degrees along an imaginary circle centered on the square (1.2arcdeg radius, Figure 1A). Distractors were either red and upright or green and tilted by 30deg. The target was red and tilted. Subjects were instructed to indicate the direction of the target tilt by pressing one of two buttons as quickly as possible.
In the central square of the first frame, a cue was presented consisting of a black dot (4.55cd/m2 luminance) of 8.79arcmin diameter, flashed for 53.3ms at three different cue target onset asynchronies (120ms, 160ms or 213.4ms). The results from the different CTOAs were pooled together. The cue was presented either centrally (neutral cue) or peripherally (valid cue) in the central square. When peripheral, the cue appeared at the same position as the target in the second frame with respect to the center of the central square.
A 2.9arcmin central fixation dot was presented at the center of the screen before each trial and disappeared before stimulus onset. Observers had to fixate within a 1arcdeg window around the dot for at least 300 ms to allow the trial to start. Trials were discarded when eye movements exceeded the 1arcdeg window during the presentation of the trial. This procedure led to the elimination of 10.25% of the trials in Experiment 1.
Experiment 2 (Training Control)
Three subjects (two naïve) were tested. The stimuli and procedure were the same as in Experiment 1. Subjects started with 400 trials of training. After the training stage, subjects were tested with 600 trials using the same stimuli and procedure. Only the last 200 trials were used for the analysis. Finally, subjects were tested for another 200 trials in a retinotopic condition. Stimuli and procedure in this condition were identical to the non-retinotopic condition except that the three squares flickered twice at the same position. In this experiment, 22.39% and 0.34% of the trials were discarded because they exceeded the fixation limit in the non-retinotopic and retinotopic condition, respectively.
Experiment 3 (Retinotopic vs. non-retinotopic cueing)
Five subjects (three naïve) were tested. Four of them also participated in other experiments of this contribution. Stimuli were similar to those of Experiment 1. The length of each side of the squares was 4arcdeg, and the lateral shift of the squares was 2.26arcdeg. The central squares of the two frames partially overlapped. Four search items were presented in the second frame at an angle of 45, 135, 225, and 315 degrees, at a distance of 1.6arcdeg from the square’s center, preceded by a cue (20arcmin diameter) presented in the first frame. The central square of the first frame was centered at fixation so that all the cue locations in the overlapping region had the same eccentricity. The cue and target could overlap at two of the four locations. To increase the difficulty of the task, we followed the search display (presented for 70ms) by a mask of 300ms composed of a 20deg tilted red “x” overlaid with a 20deg green “+” (or the other way around randomly). An ISI of 50ms was presented between the search display and the mask. For one subject, no ISI was presented, because the task was too easy with an ISI between the search display and the mask.
The cue predicted whether the target appeared in the upper or lower half of the square with 80% validity. Yet, the cue was uninformative as to the horizontal position of the target. With this arrangement, the target at the overlap region was cued retinotopically and non-retinotopcially in an equal percentage of trials. Subjects were informed the predictability of the target was limited to its vertical position. In this experiment 1.69% of the trials were excluded because they exceeded the fixation criterion.
Experiment 4 (Uninformative Cue)
Three subjects (two naïve) were tested in this experiment. All these subjects had participated in Experiment 1. Stimuli and procedure were as in Experiment 1 except that the cue was not predictive of the location of the upcoming target. The cue was randomly presented at one of the target locations, producing 17% (1/6) valid trials and 83% (5/6) invalid trials. Subjects were informed that the cue was uninformative. We discarded 8.53% of the trials because the fixation criterion was exceeded.
Experiment 5 (Time Course)
Nine subjects (seven naïve) were tested. One of these subjects had participated in Experiment 2, and four subjects also participated in Experiment 3. Frame duration and ISI were set to 400ms and 70ms, respectively. Motion direction was fixed within a block, always to the right or to the left. A valid cue (20arcmin diameter) was presented 50, 150, 250, 300 or 350ms after the onset of the first frame and stayed on throughout the duration of the first frame. This produced five different cue-target onset asynchrony levels: 120, 170, 220, 320 and 420ms. A search display was briefly presented in the second frame and was immediately followed by a mask, similar to the mask used in Experiment 2. Target display duration and mask duration were chosen individually for each subject to produce a performance level close to 70% (target duration ranging from 30 to 100ms and mask duration ranging from 300 to 370 ms) as previously done by Muller and Rabbit [9]. Subjects were required to be as accurate as possible without any emphasis on response speed. Eye movement analysis led to the elimination of 10.56% of the trials.
Highlights.
Spatial cueing can occur in a non-retinotopic frame of reference
Attention is captured by non-retinotopic, non-informative cues
Non-retinotopic capture of attention is transient
Acknowledgments
This work was supported by the Pro*Doc project “Mechanisms of Human Perception” of the Swiss National Science Foundation (SNF) and in part by award R01 EY018165 from the National Institutes of Health (NIH). We thank the anonymous reviewers for their insightful and helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boi M, et al. A (fascinating) litmus test for human retino- vs. non-retinotopic processing. J Vis. 2009;9(13):51–11. doi: 10.1167/9.13.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mack A, Rock I. Inattentional blindness. Cambridge, Mass: MIT Press; 1998. p. xiv.p. 273. [Google Scholar]
- 3.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 4.Nakayama K, Mackeben M. Sustained and transient components of focal visual attention. Vision Res. 1989;29(11):1631–47. doi: 10.1016/0042-6989(89)90144-2. [DOI] [PubMed] [Google Scholar]
- 5.Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;109(2):160–74. [PubMed] [Google Scholar]
- 6.Carrasco M, Ling S, Read S. Attention alters appearance. Nature Neuroscience. 2004;7(3):308–13. doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrasco M, Giordano AM, McElree B. Temporal performance fields: visual and attentional factors. Vision Res. 2004;44(12):1351–65. doi: 10.1016/j.visres.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Cheal M, Lyon DR. Central and peripheral precuing of forced-choice discrimination. The Quarterly journal of experimental psychology A, Human experimental psychology. 1991;43(4):859–80. doi: 10.1080/14640749108400960. [DOI] [PubMed] [Google Scholar]
- 9.Muller HJ, Rabbitt PM. Reflexive and voluntary orienting of visual attention: time course of activation and resistance to interruption. Journal of experimental psychology. Human perception and performance. 1989;15(2):315–30. doi: 10.1037//0096-1523.15.2.315. [DOI] [PubMed] [Google Scholar]
- 10.Pikler J. Sinnesphysiologische Untersuchungen. Leipzig, Germany: Barth; 1917. [Google Scholar]
- 11.Ternus J. Experimentelle untersuchungen über phänomenale Identität. Psychological Research. 1926;7:81–136. [Google Scholar]
- 12.Pantle AJ, Petersik JT. Effects of spatial parameters on the perceptual organization of a bistable motion display. Percept Psychophys. 1980;27(4):307–12. doi: 10.3758/bf03206119. [DOI] [PubMed] [Google Scholar]
- 13.Jonides J. Attention and Performance IX1981. Hillsdale, N.J: Lawrence Erlbaum Associates; Voluntary vs. Automatic control over the mind’s eye’s movement. [Google Scholar]
- 14.Pertzov Y, Zohary E, Avidan G. Rapid formation of spatiotopic representations as revealed by inhibition of return. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(26):8882–7. doi: 10.1523/JNEUROSCI.3986-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tipper SP, Driver J, Weaver B. Object-centred inhibition of return of visual attention. The Quarterly journal of experimental psychology A. Human experimental psychology. 1991;43(2):289–98. doi: 10.1080/14640749108400971. [DOI] [PubMed] [Google Scholar]
- 16.Souto D, Kerzel D. Involuntary cueing effects during smooth pursuit: facilitation and inhibition of return in oculocentric coordinates. Exp Brain Res. 2009;192(1):25–31. doi: 10.1007/s00221-008-1555-x. [DOI] [PubMed] [Google Scholar]
- 17.Golomb JD, Chun MM, Mazer JA. The Native Coordinate System of Spatial Attention Is Retinotopic. Journal of Neuroscience. 2008;28(42):10654–10662. doi: 10.1523/JNEUROSCI.2525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathot S, Theeuwes J. Evidence for the predictive remapping of visual attention. Experimental Brain Research. 2010;200(1):117–122. doi: 10.1007/s00221-009-2055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogmen H, Herzog MH. The Geometry of Visual Perception: Retinotopic and Nonretinotopic Representations in the Human Visual System. Proceedings of the Ieee. 2010;98(3):479–492. doi: 10.1109/JPROC.2009.2039028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogmen H, Otto TU, Herzog MH. Perceptual grouping induces non-retinotopic feature attribution in human vision. Vision Res. 2006;46(19):3234–42. doi: 10.1016/j.visres.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Nishida S, et al. Human visual system integrates color signals along a motion trajectory. Current Biology. 2007;17(4):366–372. doi: 10.1016/j.cub.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 22.Shimozaki SS, Eckstein M, Thomas JP. The maintenance of apparent luminance of an object. J Exp Psychol Hum Percept Perform. 1999;25(5):1433–53. doi: 10.1037//0096-1523.25.5.1433. [DOI] [PubMed] [Google Scholar]
- 23.Kawabe T. Spatiotemporal feature attribution for the perception of visual size. J Vis. 2008;8(8):71–9. doi: 10.1167/8.8.7. [DOI] [PubMed] [Google Scholar]
- 24.Cavanagh P, et al. Visual stability based on remapping of attention pointers. Trends Cogn Sci. 2010;14(4):147–53. doi: 10.1016/j.tics.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255(5040):90–2. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- 26.d’Avossa G, et al. Spatiotopic selectivity of BOLD responses to visual motion in human area MT. Nat Neurosci. 2007;10(2):249–55. doi: 10.1038/nn1824. [DOI] [PubMed] [Google Scholar]
- 27.Fischer J, Spotswood N, Whitney D. The emergence of perceived position in the visual system. J Cogn Neurosci. 2011;23(1):119–36. doi: 10.1162/jocn.2010.21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melcher D. Spatiotopic transfer of visual-form adaptation across saccadic eye movements. Curr Biol. 2005;15(19):1745–8. doi: 10.1016/j.cub.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 29.Melcher D. Predictive remapping of visual features precedes saccadic eye movements. Nat Neurosci. 2007;10(7):903–7. doi: 10.1038/nn1917. [DOI] [PubMed] [Google Scholar]
- 30.Melcher D, Morrone MC. Spatiotopic temporal integration of visual motion across saccadic eye movements. Nature Neuroscience. 2003;6(8):877–881. doi: 10.1038/nn1098. [DOI] [PubMed] [Google Scholar]
- 31.Burr D, Tozzi A, Morrone MC. Neural mechanisms for timing visual events are spatially selective in real-world coordinates. Nature Neuroscience. 2007;10(4):423–425. doi: 10.1038/nn1874. [DOI] [PubMed] [Google Scholar]
- 32.Herzog MH, Koch C. Seeing properties of an invisible object: feature inheritance and shine-through. Proc Natl Acad Sci U S A. 2001;98(7):4271–5. doi: 10.1073/pnas.071047498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merriam EP, Genovese CR, Colby CL. Spatial updating in human parietal cortex. Neuron. 2003;39(2):361–73. doi: 10.1016/s0896-6273(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 34.Merriam EP, Genovese CR, Colby CL. Remapping in human visual cortex. J Neurophysiol. 2007;97(2):1738–55. doi: 10.1152/jn.00189.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura K, Colby CL. Updating of the visual representation in monkey striate and extrastriate cortex during saccades. Proc Natl Acad Sci U S A. 2002;99(6):4026–31. doi: 10.1073/pnas.052379899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishida S. Motion-based analysis of spatial patterns by the human visual system. Current Biology. 2004;14(10):830–839. doi: 10.1016/j.cub.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 37.Ong WS, et al. Psychophysical evidence for spatiotopic processing in area MT in a short-term memory for motion task. J Neurophysiol. 2009;102(4):2435–40. doi: 10.1152/jn.00684.2009. [DOI] [PubMed] [Google Scholar]
- 38.Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. I. Predictive visual responses. J Neurophysiol. 1997;78(3):1373–83. doi: 10.1152/jn.1997.78.3.1373. [DOI] [PubMed] [Google Scholar]
- 39.Whitney D, et al. Flexible retinotopy: motion-dependent position coding in the visual cortex. Science. 2003;302(5646):878–81. doi: 10.1126/science.1087839. [DOI] [PMC free article] [PubMed] [Google Scholar]