Abstract
The glycan β-galactosamine-(1–4)-3-O-methyl-D-chiro-inositol, called INS-2, was previously isolated from liver as a putative second messenger-modulator for insulin. Synthetic INS-2 injected intravenously in rats is both insulin-mimetic and insulin-sensitizing. This bioactivity is attributed to allosteric activation of pyruvate dehydrogenase phosphatase (PDHP) and protein phosphatase 2Cα (PP2Cα). Towards identification of potentially metabolically stable analogues of INS-2 and illumination of the mechanism of enzymatic activation, C-INS-2, the exact C-glycoside of INS-2, and C-INS-2-OH the deaminated analog of C-INS-2, were synthesized and their activity against these two enzymes evaluated. C-INS-2 activates PDHP comparable to INS-2, but failed to activate PP2Cα. C-INS-2-OH was inactive against both phosphatases. These results and modeling of INS-2, C-INS-2 and C-INS-2-OH into the 3D structure of PDHP and PP2Cα, suggest that INS-2 binds to distinctive sites on the two different phosphatases to activate insulin signaling. Thus the carbon analog could selectively favor glucose disposal via oxidative pathways.
Keywords: C-glycoside, insulin mediator-modulator, pyruvate dehydrogenase, protein phosphatase
1. Introduction
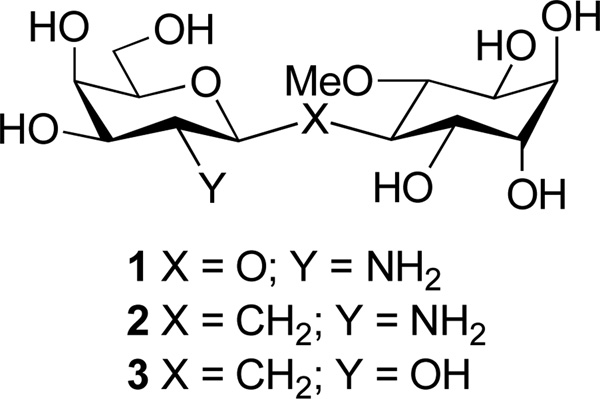
Soluble inositol-glycans have been proposed to operate as insulin messengers-modulators in concert with the insulin receptor tyrosine kinase signaling system to control intracellular oxidative and non-oxidative glucose disposal.1 By analogy with the PIP3 signaling pathway, the insulin receptor is thought to regulate, via heterotrimeric G proteins, specifically Gq,2 the GPI-phospholipase cleavage of a glycolipid to generate diacylglycerol and inositol glycans as messengers-modulators. In earlier studies, we isolated INS-2, 1 from beef liver as a novel inositol glycan pseudo-disaccharide (Figure 1). The INS-2 structure was determined by 2D NMR and confirmed by chemical synthesis.3 Synthetic INS-2 was insulin-mimetic and insulin-sensitizing when injected in vivo, and when added to living cells.3 In biochemical assays, INS-2 activated two related Mg2+ dependent protein phosphatases, mitochondrial pyruvate dehydrogenase phosphatase (PDHP) and a Ser/Thr protein phosphatase, PP2Cα.3,4 These protein phosphatases respectively dephosphorylate the rate limiting enzymes of intracellular oxidative glucose disposal (i.e. PDH), and non-oxidative glucose disposal (glycogen synthase, GS).5 Both PDH and GS are classical targets of activation by insulin via dephosphorylation.6,7 When docked into the X-ray crystal structure of PP2Cα, INS-2 bound to an allosteric pocket adjoining the catalytic pocket that was occupied by a phosphopeptide substrate.4 INS-2 enhanced reaction with the phosphopeptide substrate, but not with the small molecule substrate p-nitrophenyl phosphate. A point mutation D163A in PP2Cα completely eliminated allosteric activation by INS-2, with no loss of catalytic activity.4 Thus a mechanism of allosteric activation of PP2Cα was proposed.4
Figure 1.
1. INS-2, 2. C-INS-2 and 3. C-INS-2-OH
To further define the structure-activity relationship of INS-2, we synthesized C-INS-2, 2, the C-glycoside of 1 in which the glycosidic oxygen is replaced with a methylene and C-INS-2-OH, 3, the corresponding C-glycoside in which the 2-amino substituent is replaced with a hydroxyl group. These analogues were compared to INS-2 for activation of the two protein phosphatases, PP2Cα and PDHP. C-glycoside probes like 2 and 38 may provide insight on the chemical and conformational requirements for activity,9 and are of special interest as possible therapeutic agents that are stable to hydrolysis.10,11,12
2. Synthesis
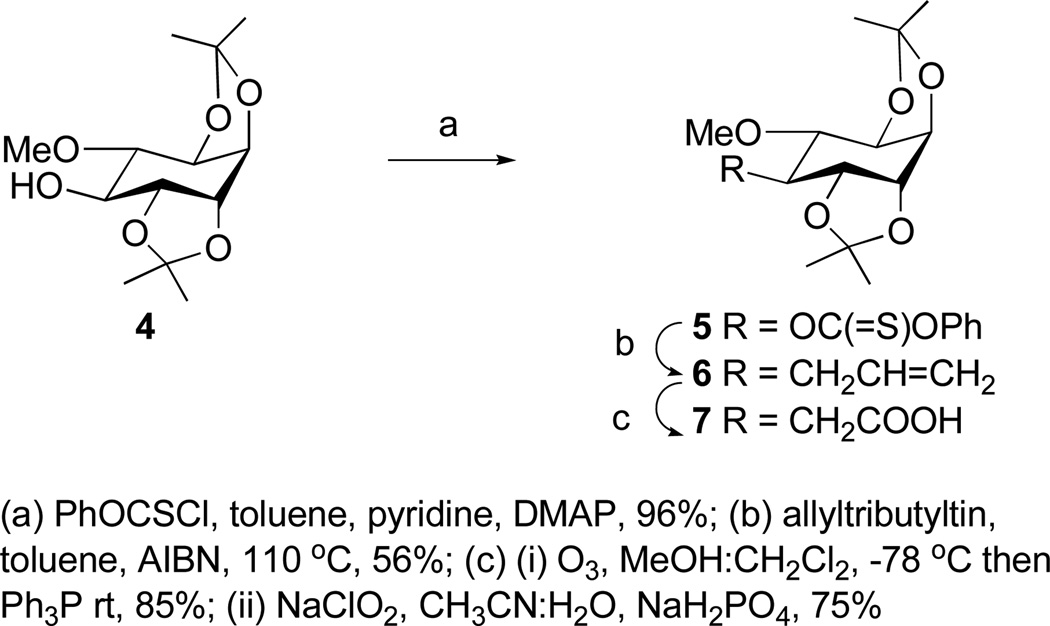
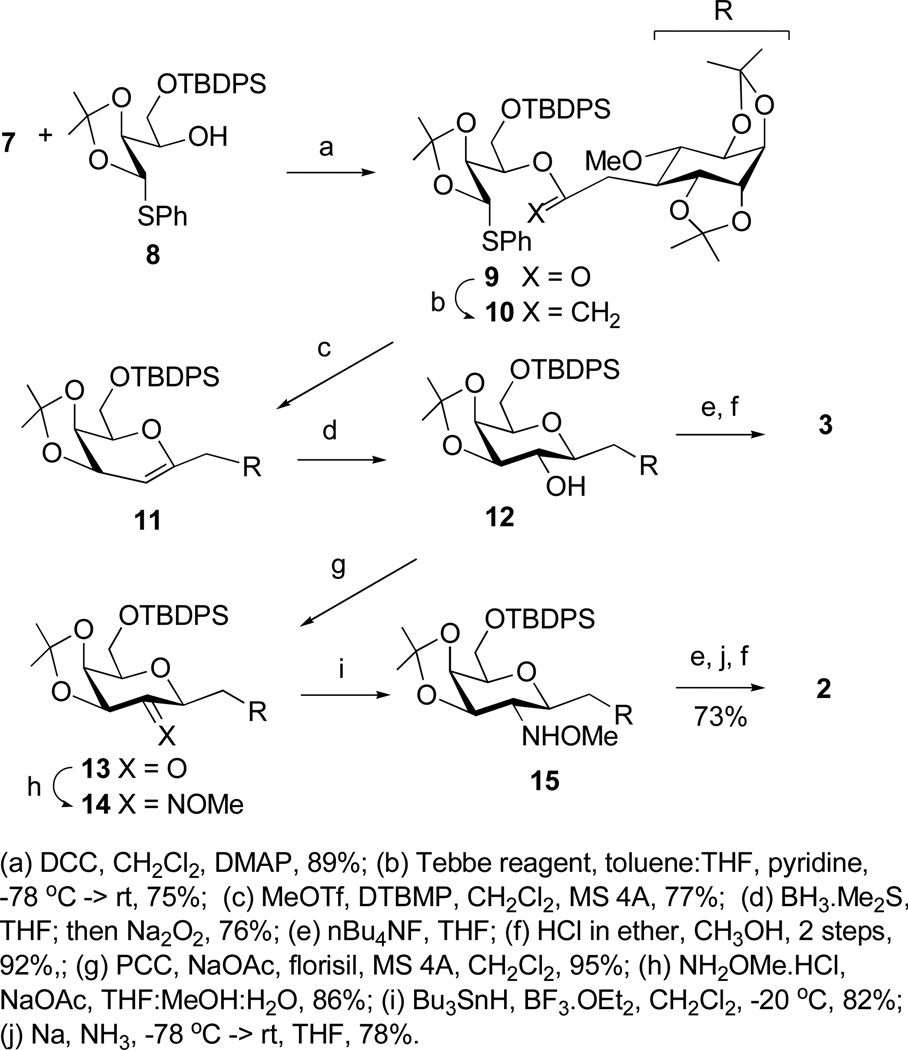
C-glycoside 3 was prepared using our protocol for β-C-galactosides and the aminated analog 2 was obtained from an intermediate en route to 3.13 Accordingly, di-O-isopropylidene pinitol 4 was transformed to the thiocarbonate 5, which when subjected to the Keck radical allylation procedure gave the equatorial C-propenyl derivative 6 in greater than 90% stereoselectivity as determined by 1H NMR.14 (Scheme 1). Oxidative processing of 6 led to the C-branched-ethanoic acid 7. Acid 7 and known 1-thio-1,2-O-isopropylidene acetal 8 were next subjected to the C-glycosidation sequence (Scheme 2). Thus, DCC mediated esterification on 7 and 8, followed by Tebbe olefination of the derived ester 9 afforded enol ether 10. In the key step, treatment of 10 with methyl triflate in the presence of 2,6-di-tert-butyl-4-methylpyridine (DTBMP), provided glycal 11 in 77% yield. Hydroboration of 11 afforded C-pseudodisaccharide 12 as a single diastereomer. Removal of the isopropylidene protecting groups provided C-INS-2-OH 3. For the synthesis of 2, alcohol 12 was first oxidized to ketone 13 using PCC. Treatment of 13 with O-methylhydroxylamine afforded a mixture of oximes 14. Attempted reduction of 14 with standard hydride reducing agents produced complex mixtures with low yield of the desired hydroxylamine ether 15.15,16 The optimal conditions were found to be Bu3SnH in the presence of BF3.OEt2, which afforded 15 in 82% isolated yield, and a minor compound (less than 5%) for which the physical data supported the axial isomer.17 Cleavage of the silylether in 15 followed by methoxylamine reduction and cleavage of acetal protecting groups provided C-INS-2 in 73% overall yield from 15. The structures of 2 and 3 were confirmed by 2D NMR and HRMS analysis (Supporting Information).
Scheme 1.
Synthesis of the C-inositol segment
Scheme 2.
Assembly of C-INS-2 and C-INS-2-OH
3. Enzyme assays
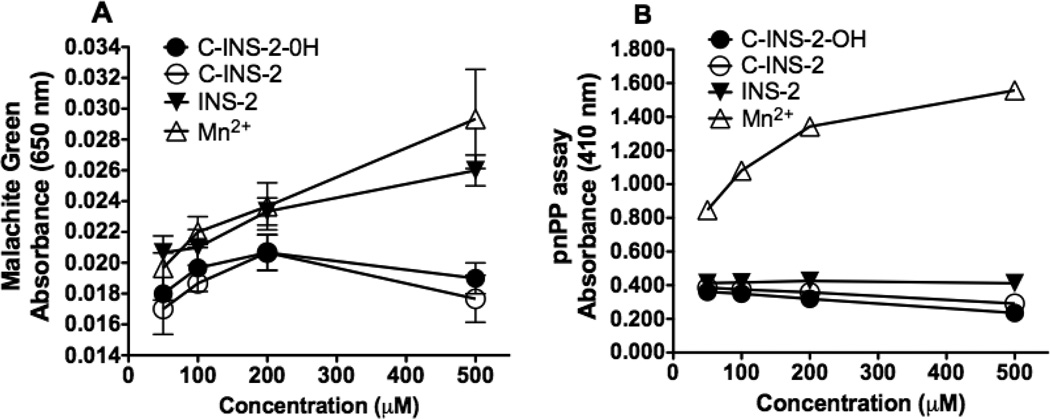
PP2Cα was assayed using two separate substrates with and without added INS-2 analogues. p-Nitrophenyl phosphate (pNPP) as substrate assayed the activity of the enzyme catalytic center and this activity is unaffected by the allosteric site for INS-2. PP2Cα activity with an octapeptide-phosphate substrate (RRRRPp-TPA) was sensitive to the allosteric site regulating the active site.4 As seen in Fig 2A, with the octapeptide substrate and Malachite green, as previously shown, Mn2+ alone and INS-2 showed phosphate release assayed by dose-dependent increase in PP2Cα activity, while the C-glycosides 2 and 3 were inactive. As shown in Fig 2B, with the pNPP substrate, as previously shown,4 only Mn2+ increased the catalytic activity of PP2Cα, not INS-2 or its analogues.
Figure 2.
Protein phosphatase 2Cα activity in response to added INS-2, C-INS-2-OH and C-INS-2. The recombinant GST-PP2C fusion protein was assayed as described in the experimental section, using either phosphopeptide (A) or p-nitrophenyl phosphate (B) as the substrate. Samples were assayed by triplicate and the average values plotted ± SD as a function of the concentration of added compounds. A: The PP2C activity using a phosphopeptide as substrate adding increasing concentrations of MnCl2, INS-2, C-INS-2-OH or C-INS-2. B: The PP2C activity using the p-nitrophenyl phosphate as a substrate adding various concentrations of MnCl2, INS-2, C-INS-2-OH or C-INS-2.
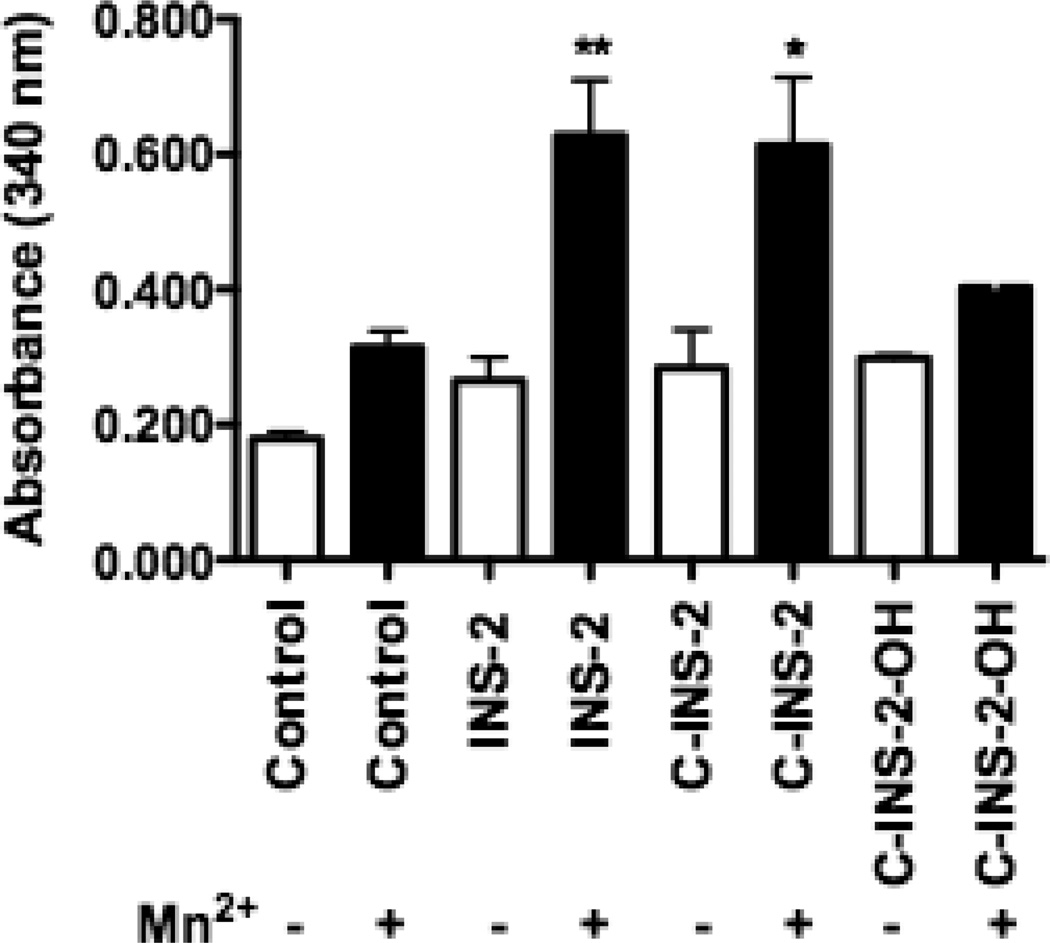
As shown in Figure 3, INS-2, C-INS-2, C-INS-2-OH were assayed ± Mn2+ for their activation of PDHP. Only INS-2 and C-INS-2 activated PDHP significantly, and activation was seen only in the presence of Mn2+, relative to control. The C-INS-2-OH analog was inactive in the presence or absence of Mn2+. The results were combined from two separate sets of experiments with two separate chemically synthesized samples. These data show equal activation of PDHP by INS-2 and its carbon analog, C-INS-2. Thus while C-INS-2-OH was inactive with both phosphatases, there was a marked dichotomy with C-INS-2 which activated PDHP, but not PP2Cα.
Figure 3.
PDHP assay of INS-2, C-INS-2 and C-INS-2-OH ± 5 mM Mn2+. Activity is expressed as change in absorbance at 340 nm. Mean + s.e.m. (n=6) * p < 0.016; ** p < 0.004 vs. Control.
4. Computer Modeling
Docking of INS-2 and its analogues was accomplished using the FlexX flexible docking suite in the SYBYL shell.4,18 The crystal structure for PP2Cα (1ASQ) and PDHP1 (2PNQ) were acquired from the Protein Data Bank. The allosteric sites for the two proteins were detailed through a residue selection followed by an 8 Å radius selection. Compound structures were then prepared in the SYBYL shell and minimized using the conjugate gradient method with an endpoint of 0.01 kcal. The top 30 conformations of each molecule were then analyzed using G-score, D-score, PMF-score as previously described.4 Multiple scoring functions were then combined for analysis by the C-score function to rate the conformations.19 In each case the top scoring two to three conformations of the molecule overlap well, but then the scores of the conformations drop off quickly and the position of the molecule is less reproducible (Supporting Information). Putative hydrogen bonds were analyzed through the SYBYL shell by the addition of hydrogen atoms to the protein structure with the Biopolymer function.
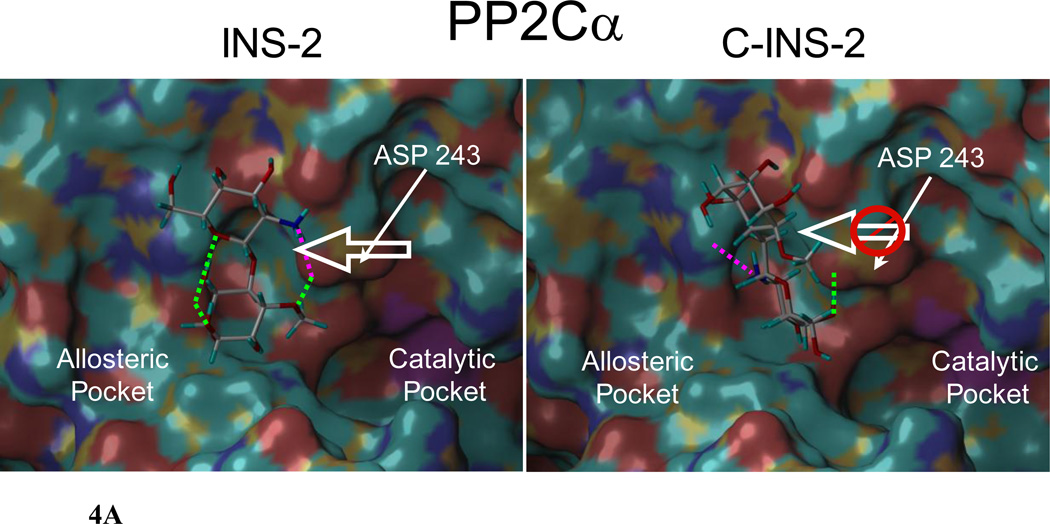
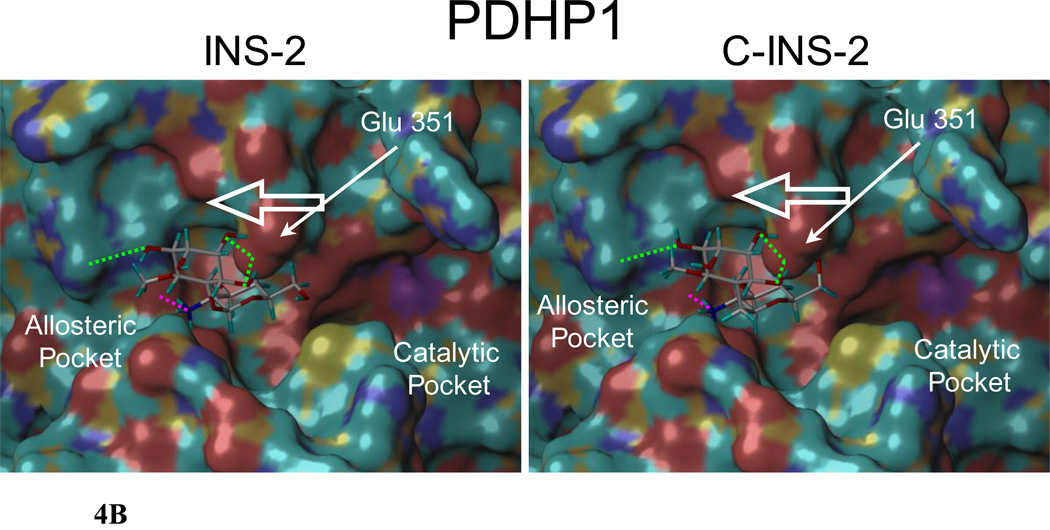
Docking of INS-2 and C-INS-2 into the X-ray crystal structure of PP2Cα revealed very different orientations and conformations (with respect to the intersaccharide torsions) for the two glycans (Figure 4A and 4B).11 The intersaccharide torsions Φ,Ψ for INS-2 and C-INS-2 are (270, 64) and (300, 210) respectively.20 In particular, Asp 243, which is proximal to the catalytic site (vide infra), interacts with different residues on the two ligands, the sugar 2-amino and 3-hydroxyl on INS-2, and the sugar 4-hydroxyl and the inositol 2- and 3- hydroxyls on C-INS-2 (Figures 4A, 4B and Supporting Information). The orientation of INS-2 suggests that Asp 243 is moved toward the allosteric pocket and away from the catalytic site. In contrast, bound C-INS-2 does not appear to have a significant effect on the position of Asp 243. However, both INS-2 and C-INS-2 dock very similarly into the crystal structure for PDHP1. The respective intersaccharide torsions are (244, 60) and (240, 60) and both ligands appear to pull Glu 351 (the comparable acidic residue to Asp 243 in PPC2α) from the active site (Figure 4B and Supporting Information).
Figure 4.
A. INS-2 and C-INS-2 docked in silico into the allosteric site of PP2Cα. H-bonds to the amino group indicated in pink. For the enzyme to be fully active Asp 243 moves to open up the catalytic site. Note the significant difference in position of Asp 243 with the two glycans. INS-2 pulls the acidic residue Asp 243 toward the allosteric pocket and away from the catalytic site favoring hydrolysis. C-INS-2 still binds to this region, but does not pull Asp 243 towards the allosteric pocket, and is inactive.
B. INS-2 and C-INS-2 docked in silico into the allosteric site of PDHP1. H-bonds to the amino group indicated in pink. Both are similarly positioned to pull the acidic residue Glu 351 towards the allosteric pocket and away from the catalytic site favoring hydrolysis.
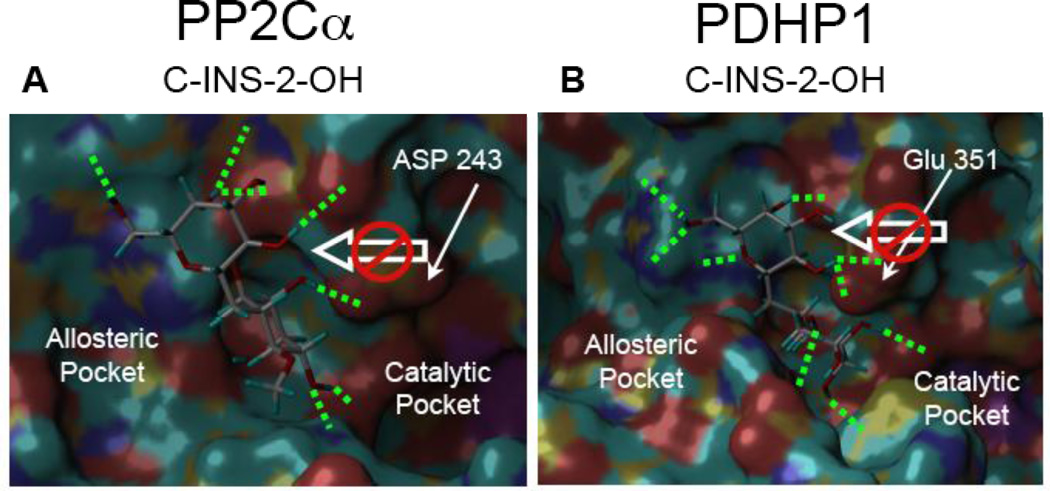
When C-INS-2-OH was docked into the X-ray crystal structures of PP2Cα (Fig 5A) and PDHP1 (Fig 5B), it bound to both enzymes but not in a fashion that would be expected to pull the acidic residue (either Asp 243 or Glu 351) away from the catalytic pocket.
Figure 5.
A: C-INS-2-OH docked into the allosteric site of PP2Cα. Note that C-INS-2-OH still binds but is blocked from pulling Asp 243 toward the allosteric pocket. B: C-INS-2-OH docked in silico into the allosteric site of PDHP1. Note again that C-INS-2-OH still binds but is blocked from pulling Glu 351 toward the allosteric pockets.
5. Discussion
Carbon in place of oxygen bridges in carbohydrates are commonly synthesized to produce analogues that are resistant to intestinal hydrolytic breakdown.8,10 A similar strategy was imagined for INS-2, a natural inositol glycan pseudo-disaccharide, initially isolated from liver and then chemically synthesized and shown to be an insulin-mimetic and sensitizing agent.3
INS-2, in vitro activates two phosphoprotein phosphatases, mitochondrial PDHP and cytosolic PP2Cα,3,4 both members of the same PPM family and with ~ 20% amino acid sequence identity.21 Both have been crystallized and X-ray structures determined.21,22 By computer modeling, we have previously reported that INS-2 docked into an allosteric site on PP2Cα adjacent to the catalytic site. With a point mutant D163A in the allosteric site, we showed a specific loss of allosteric activation of the enzyme with INS-2, but with full retention of catalytic activity measured with a non-peptide substrate.4 Thus we were able to propose a mechanism of allosteric action. By occupying the allosteric site, INS-2 would hydrogen bond to Asp 243 and thus prevent Asp 243 from interfering with positioning of the phosphopeptide substrate at the catalytic site.4
In the present study, C-INS-2, the C-glycoside analog of INS-2 was found to be inactive on PP2Cα (Fig 3). To get insight into the molecular basis for the difference in activity of INS-2 and C-INS-2 on PP2Cα, the binding of the two ligands to the X-ray crystal structure of PP2Cα was modeled (Fig 4A). Significant differences in their binding suggest that INS-2 but not C-INS-2 pulls Asp 243 into the allosteric site and away from the catalytic pocket, thereby providing a possible explanation for the inactivity of C-INS-2. In contrast to their activity on PP2Cα, INS-2 and C-INS-2 showed similar activity on PDHP1. This result was somewhat surprising because PDHP1 and PP2Cα are related enzymes. We therefore modeled the binding of INS-2 and C-INS-2 to the recently published 3D structure of PDHP1 (Fig 4B) and compared the results with those for PP2Cα (Fig 4A). INS-2 and C-INS-2 bound similarly to PDHP1 and in a way that suggests they both pull Glu-351 (the comparable residue to Asp 243 on PP2Cα), away from the catalytic pocket. Interestingly, the bound conformations of both ligands on PDHP1 are particularly high energy rotamers with respect to the intersaccharide torsions, whereas PP2Cα selects relatively low energy conformations. The selection of high energy conformers by PDHP1 may be a reflection of an intrinsically higher binding affinity with this enzyme. Such conformational variations in bound glycans are influenced by both glycan structure and receptor architecture and have previously been noted.11,23,24 C-INS-2-OH was inactive with both phosphatases and its inactivity is consistent with the absence of an appropriately placed amino group that contributes to pulling Asp 243 or Glu 351 toward the allosteric site. (Fig 5A and B). However, it is also possible that the 2’-amino group may function to chelate Mg2+ or Mn2+ in conjunction with an OH on the pinitol. This provides an alternative possible mechanism for the inactivity of C-INS-2-OH.
It is of interest that INS-2 alone activates PP2Cα, but requires addition of Mn2+ for activating PDHP1. PP2Cα is purified and crystallized in the presence of Mn2+.22 Two binding sites in a bimetallic center are well established. In contrast, PDHP1catalytic subunit is isolated in the presence of Mg2+.21 A Mg2+ bimetallic center has been established. However, the PDHP1 catalytic subunit is known to interact with its regulatory subunit, which also has a low affinity Mg2+ binding site that is inhibitory. We propose that in the case of PP2Cα, sufficient Mn2+ is present bound to the bimetallic center of the enzyme to chelate to added INS-2 to activate the enzyme. In contrast, INS-2 and C-INS-2 need addition of Mn2+ to activate PDHP1 and the effect is possibly to displace loosely bound Mg2+ in the regulatory subunit.21
6. Conclusion
The relative activity of the inositol glycan INS-2 and its C-glycoside analog C-INS-2 on PP2Cα and PDHP, two enzymes that are implicated in insulin modulation was evaluated. Both glycans activated PDHP similarly, but PP2Cα was only activated by INS-2. This result suggests that the inositol-glycan binding sites on the two enzymes are distinct. Preliminary modeling experiments appear to support this conclusion. What possible benefit may be derived from these results? Since PDHP is a selective activator of mitochondrial pyruvate oxidation, C-INS-2, in contrast to INS-2, would selectively activate one of two pathways of glucose disposal, glucose oxidation, bypassing the other pathway, glycogen synthesis. Such a selective tool would be useful in investigating insulin and insulin-mimetic agents for partitioning of intracellular carbon disposal between oxidation and glycogen synthesis. Presently this may be done with inhibitors of oxidation or of glycogen synthesis, which tend to be nonspecific. In terms of a potential therapeutic agent, selective glucose disposal via oxidation without glycogen storage may be beneficial against insulin resistance and diabetes for disposal of carbon overload. For future studies, in the context of the advancement of analogues of INS-2 as potential clinical agents, it will be of interest to determine whether C-INS-2 does escape intestinal breakdown and manifests insulin-mimetic effects in vivo.
7. Experimental
7.1 General – Synthesis
Unless otherwise stated, all reactions were carried out under a nitrogen atmosphere in oven-dried glassware using standard syringe and septa technique. 1H and 13C NMR spectra were obtained on a Varian Unity Plus 500 (500 MHz) spectrometer. Chemical shifts are relative to the deuterated solvent peak or the tetramethylsilane (TMS) peak at (δ 0.00) and are in parts per million (ppm). Assignments for selected nuclei were determined from 1H COSY experiments. Unless otherwise stated, thin layer chromatography (TLC) was done on 0.25 mm thick precoated silica gel HF254 aluminum sheets. Chromatograms were observed under UV (short and long wavelength) light, and were visualized by heating plates that were dipped in a solution of ammonium (VI) molybdate tetrahydrate (12.5 g) and cerium (IV) sulfate tetrahydrate (5.0 g) in 10% aqueous sulphuric acid (500 mL). Flash column chromatography (FCC) was performed using silica gel 60 (230–400 mesh) and employed a stepwise solvent polarity gradient, correlated with TLC mobility.
7.2. 1,2,5,6-di-O-isopropylidene-3-O-methyl-4-O-phenylcarbonothioyl-D-chiro-inositol 5
Phenyl chlorothionoformate (8.75 mL, 64.7 mmol) was added dropwise to a suspension of alcohol 4 (8.05 g, 29.4 mmol) and DMAP (0.72 g, 5.87 mmol), in dry toluene (120 mL) at rt. Pyridine (12.0 mL, 147 mmol) was then added and the resulting suspension was stirred for 2h at rt. The reaction mixture was then washed with HCl (0.1M) and saturated NaHCO3, and the organic extracts were dried (Na2SO4) and concentrated in vacuo. FCC of the residue gave 5 (11.5 g, 95%). Rf = 0.43 (10% ethyl acetate: petroleum ether). 1H NMR (CDCl3) ™ 2.40 (s, 6H, 2 × CH3-C), 2.60 (two s, 6H, 2 × CH3-C), 3.40 (dd, 1H, J = 7.7, 10.9 Hz, H-3), 3.58 (s, 3H, CH3O), 4.39 (t, 1H, J = 7.7 Hz, H-2), 4.48–4.51 (m, 3H. H-1, H-5, H-6), 5.60 (dd, 1H, J = 7.1, 10.9 Hz, H-4), 7.16 (d, 2H, J = 8.4 Hz, Ar-H), 7.31 (t, 1H, J = 7.4 Hz, Ar-H), 7.44 (t, 2H, J = 7.9 Hz, Ar-H); 13C NMR (CDCl3) ™ 25.4 (CH3-C), 25.5 (CH3-C), 27.5 (CH3-C), 27.8 (CH3-C), 60.2 (CH3O), 75.4, 75.5, 76.0, 78.4, 80.4, 82.5, 109.5 (OCO), 110.0(OCO), 122.0 (Ar), 126.6 (Ar), 129.5 (Ar), 153.6 (Ar), 195.0 (C=S). HRMS (ESI) m/z calcd for C20H27O7S (M + H)+ 411.1472, found 411.1471.
7.3 4-Deoxy-4-C-(3’-propenyl)-1,2,5,6-di-O-isopropylidene-3-O-methyl-D-chiro-inositol 6
A solution of 5 (4.17 g, 10.2 mmol), allyltributyltin (9.35 mL, 30.6 mmol), AIBN (0.33 g, 2.04 mmol) and toluene (18 mL) was heated at reflux for 4 h at which point TLC showed complete consumption of the starting material. The solution was concentrated in vacuo. FCC of the residue afforded 6 (1.70 g, 56%). Rf = 0.64 (10% ethyl acetate: petroleum ether). 1H NMR (5% CDCl3/C6D6) ™ 1.40 (two s, 6H, 2 × CH3-C), 1.51 (two s, 6H, 2 × CH3-C), 1.79–1.87 (m, 1H, H-4), 2.54–6.67 (m, 2H, CH2-3’), 3.02 (dd, 1H, J = 6.8, J = 12.4 Hz, H-3), 3.54 (s, 3H, CH3O), 4.06 (dd, 1H, J = 6.9, 9.2 Hz, H-5), 4.15 (t, 1H, J = 6.3 Hz, H-6), 4.21 (t, 1H, J = 7.1 Hz, H-2), 4.30 (t, 1H, J = 6.6 Hz, H-1), 5.20 (d, 1H, J = 10.1 Hz, ½ × =CH2), 5.27 (d, 1H, J = 17.1 Hz, ½ × =CH2); 6.10 (m, 1H, -CH=) 13C NMR (5% CDCl3/C6D6) ™ 25.1 (CH3-C), 25.2 (CH3-C), 27.8 (CH3-C), 27.8 (CH3-C), 32.7 (C-3’), 41.5 (C-4), 58.4 (CH3O), 75.9, 77.2, 77.8, 79.2, 81.1, 108.7 (OCO), 108.9 (OCO), 117.3 (=CH2), 135.2 (-CH=).
7.4 4-Deoxy-4-C-(2’-ethanoic acid)-1,2,5,6-di-O-isopropylidene-3-O-methyl-D-chiro-inositol 7
Alkene 6 (1.35 g, 4.55 mmol) was dissolved in a 5/1 mixture of CH2Cl2/MeOH (24 mL). The solution was cooled to −78 °C and treated with a stream of O3 in O2 until TLC indicated complete disappearance of the starting material. The reaction was then purged with nitrogen, and Ph3P (2.64 g, 9.09 mmol) was added. The mixture was warmed to rt, stirred for 1 h at this temperature, and concentrated under reduced pressure. FCC of the residue gave the derived aldehyde (1.15 g, 85%). Rf = 0.16 (10 % ethyl acetate: petroleum ether). 1H NMR (CDCl3) ™ 1.30 (two s, 6H, 2 × CH3-C), 1.51 (two s, 6H, 2 × CH3-C), 2.09–2.18 (m, 1H, H-4), 2.48 (m, 1H, H-2’), 2.23 (dd, 1H, J = 4.7, 16.2 Hz, H-2’), 3.15 (dd, 1H, J = 6.7, 12.1 Hz, H-3), 3.45 (s, 3H, CH3O), 4.04 (dd, 1H, J = 6.5, 9.7 Hz, H-5), 4.21 (m, 2H, H- 2, 6), 4.36 (dd, 1H, J = 4.7, 6.7 Hz, H-1), 9.65 (d, 1H, J = 2.1 Hz, CH=O); 13C NMR (CDCl3) ™ 25.3 (CH3-C), 25.4 (CH3-C), 27.8 (2 × CH3-C), 39.1 (CH2), 43.9 (C-4), 59.1 (CH3O), 76.4, 76.8, 76.9, 80.2, 80.5, 109.3 (2 × OCO), 200.9 (C=O). HRMS (ESI) m/z calcd for C15H24NaO6 (M + Na)+ 323.1468, found 323.1468.
To a solution of aldehyde from the previous step (1.4 g, 4.67 mmol) in a 5:1 mixture of CH3CN:H2O (85:17mL) at 0 °C, was added NaH2PO4 3.H2O (6.46 g, 46.8 mmol), and a solution of NaClO2 (0.51 g, 5.62 mmol) in H2O (85 mL) and H2O2 (0.55 mL). The mixture was stirred at 0 °C until TLC indicated the disappearance of the starting material. Solid Na2SO3 was then added to the reaction. The reaction mixture was diluted with water and the organic phase was extracted with ether. The combined extract was dried (Na2SO4), the solvent removed under reduced pressure, and the residue purified by FCC to give 7 (1.10 g, 75%). Rf = 0.16 (25% ethyl acetate: petroleum ether). 1H NMR (CDCl3) ™ 1.35 (s, 3H, CH3-C), 1.38, (s, 3H, CH3-C), 1.45 (s, 3H, CH3-C), 1.53 (s, 3H, CH3-C), 2.05 (m, 1H, H-4), 2.52 (dd, 1H, J = 7.0, 15.7 Hz, H-2’), 2.71 (dd, 1H, J = 4.3, 15.7 Hz, H-2’), 3.16 (dd, 1H, J = 7.3, 12.2 Hz, H-3), 3.54 (s, 3H, CH3O), 4.14 (dd, 1H, J = 6.4, 9.9 Hz, H-5), 4.25 (m, 2H, H- 2, 6), 4.38 (dd, 1H, J = 4.7, 6.7 Hz, H-1); 13C NMR (CDCl3) ™ 25.4 (2 × CH3-C), 27.7 (CH3-C), 27.8 (CH3-C), 33.5 (CH2CO), 39.7 (C-4), 59.5 (CH3O), 76.4, 76.5, 76.7, 80.3, 80.4, 109.3 (2 × OCO), 176.9 (C=O).
7.5 Monothioacetal Ester 9
DCC (0.50 g, 2.42 mmol) was added at 0 °C to a mixture of acid 7 (0.64 g, 2.02 mmol), alcohol 8 (1.23 g, 2.42 mmol), and DMAP (74.0 mg, 0.61 mmol) in dry dichloromethane (10 mL). The reaction mixture was warmed to rt and stirred for 6 h. The mixture was then diluted with ether and filtered. The filtrate was successively washed with 0.1 N aqueous HCl and brine, dried (Na2SO4), filtered, and evaporated in vacuo. FCC of the residue gave 9 (1.45 g, 89%) as a colorless oil. Rf = 0.38 (10% ethyl acetate: petroleum ether). 1H NMR (CDCl3) ™ 1.05 (s, 9H, (CH3)3C), 1.26 (s, 3H, CH3-C), 1.27 (s, 3H, CH3-C), 1.28 (s, 3H, CH3-C), 1.56 (two s, 6H, 2 × CH3-C), 2.13 (m, 1H, H-4), 2.34 (dd, 1H, J = 7.9, 16.5 Hz, ½ × CH2C=O), 2.69 (dd, 1H, J = 3.8, 15.4 Hz, ½ × CH2C=O), 3.06 (dd, 1H, J = 7.1, 12.2 Hz, H-3), 3.20 (s, 3H, CH3O), 3.80 (dd, 1H, J = 7.3, 10.0 Hz, ½ × CH2O), 3.85 (dd, 1H, J = 6.0, 9.9 Hz, ½ × CH2O), 4.17 (dd, 1H, J = 6.6, 9.8 Hz, H-5), 4.19 (m, 2H, H-2, 6), 4.29 (t, J = 7.0 Hz, H-1), 4.42 (dd, 1H, J = 2.4, 7.7 Hz, H-2’), 5.25 (m, H, H-3’), 5.50 (d, 1H, J = 6.8 Hz, H-1’), 7.24–7.34 (m, 3H, ArH), 7.37–7.49 (m, 6H, ArH), 7.56 (m, 2H, ArH), 7.67–7.74 (m, 4H, ArH); 13C NMR (CDCl3) ™ 20.0 (Me3C-Si), 25.3 (CH3-C), 25.5 (CH3-C), 26.2 (CH3-C), 26.7 ((CH3)3C), 27.3 (CH3-C), 27.7 (CH3-C), 27.9 (CH3-C), 34.5 (CH2CO), 39.9 (C-4), 59.0 (CH3O), 62.0 (CH2O), 70.8, 76.5, 76.6, 78.9, 80.4, 80.5, 84.4 (OCS), 109.3 (2 × OCO), 111.5 (OCO), 127.5, 127.8, 127.9, 128.9, 129.7 (two signals), 132.3, 133.1 (two signals), 133.7, 135.6, 135.7, 171.4 (C=O). HRMS (ESI) m/z calcd for C44H58O10SSiNa (M + Na)+ 829.3420, found 829.3423.
7.6 Monothioacetal enol ether 10
To a mixture of ester 9 (0.592 g, 0.735 mmol), and pyridine (0.3 mL) in anhydrous 3:1 toluene:THF (10:5 mL), was added under an argon atmosphere at −78 °C, the Tebbe reagent (3.7 mL, 0.5M in THF). The reaction mixture was warmed to rt and stirred at this temperature for 1 h. The mixture was then slowly poured into 1N aqueous NaOH at 0 °C, and the resulting suspension extracted with ether. The combined organic phase was washed with brine, dried (Na2SO4), filtered and concentrated in vacuo. FCC of the residue on basic alumina provided 10 (0.44g, 75%) as light yellow oil. Rf = 0.49 (basic alumina, 10% ethyl acetate: petroleum ether). 1H NMR (CDCl3) ™ 1.07 (s, 9H, (CH3)3C), 1.29 (s, 3H, CH3-C), 1.35 (s, 6H, 2 × CH3-C), 1.48 (s, 3H, 2 × CH3-C), 1.50 (s, 3H, CH3-C), 1.51 (s, 3H, CH3-C), 1.86 (m, 1H, H-4), 2.31 (dd, 1H, J = 5.4, 14.4 Hz, ½ × CH2C=), 2.45 (dd, 1H, J = 5.4, 14.4 Hz, ½ × CH2C=), 3.14 (dd, 1H, J = 6.9, 12.5 Hz, H-3), 3.34 (s, 3H, CH3O), 3.81 (d, 1H, J = 2.2 Hz, ½ × =CH2), 3.85–3.93 (m, 3H, CH2O, ½ × CH2C=), 3.97 (t, 1H, J = 6.3 Hz, H-6), 4.10 (dd, 1H, J = 7.9, 8.9 Hz, H-5), 4.16 (m, 2H, H-1, 2), 4.25 (m, 1H, H-3’), 4.49 (dd, 1H, J = 3.2, 8.0 Hz, H-2’), 5.49 (d, 1H, J = 7.0 Hz, H-1’), 7.25–7.34 (m, 4H, ArH), 7.37–7.48 (m, 4H, ArH), 7.51–7.58 (m, 2H, ArH), 7.67–7.73 (m, 5H, ArH); 13C NMR (CDCl3) ™ 19.1 (Me3C-Si), 25.2 (CH3-C), 25.3 (CH3-C), 26.1 (CH3-C), 26.7 ((CH3)3C), 27.4 (CH3-C), 27.8 (2 × CH3-C), 34.3 (CH2C=), 39.3 (C-4), 58.6 (CH3O), 61.1 (CH2O), 74.2, 76.0, 76.9, 79.6, 79.7, 81.0, 83.7, 84.3, 109.3 (OCO), 111.3 (OCO), 112.0 (OCO), 127.3, 127.7, 127.8, 129.0, 129.7, 129.8, 131.8 (two signals), 133.0, 133.3, 134.1, 135.6, 159.4 (OC=CH2). HRMS (ESI) m/z calcd for C45H60O9NaSSi (M + Na)+ 827.3625, found 827.3625.
7.7. 4-Deoxy-4-C-(2,6-anhydro-7-O-tertbutyldiphenylsilyl-4,5-O-isopropylidene-1,3-dideoxy-D-galacto-hept-2-enit-1-C-yl)-1,2,5,6-di-O-isopropylidene-3-O-methyl-D-chiro-inositol 11
A mixture of enol ether 10 (5.22 g, 6.49 mmol), 2,6-di-tert-butyl-4-methylpyridine (20.0 g, 97.3 mmol), and freshly activated, powdered 4 Å molecular sieves (15.3 g) in anhydrous CH2Cl2 (200 mL), was stirred for 15 min at rt, under an atmosphere of argon, then cooled to 0 °C. Methyl triflate (9.50 mL, 84.4 mmol) was then introduced, and the mixture warmed to rt, and stirred for an additional 18 h, at which time, triethylamine (15 mL) was added. The mixture was diluted with ether, washed with saturated aqueous NaHCO3 and brine, dried (Na2SO4), filtered and evaporated in vacuo. FCC of the residue provided recovered 10 (1.22 g) and 11 (2.66 g, 77% based on recovered 10) as a light yellow oil. Rf = 0.58 (basic alumina, 10 % ethyl acetate: petroleum ether). 1H NMR (C6D6) ™ 1.29 (s, 9H, (CH3)3C), 1.30 (s, 3H, CH3-C), 1.34 (s, 3H, CH3-C), 1.50 (s, 3H, CH3-C), 1.56 (s, 3H, CH3-C), 1.66 (s, 3H, CH3-C), 2.16 (m, 2H, H-4, ½ × CH2C=), 2.89 (bd, 1H, J = 10.2 Hz, ½ × CH2C=), 3.07 (dd, 1H, J = 6.9, 12.0 Hz, H-3), 3.56 (s, 3H, CH3O), 4.10 (t, 1H, J = 6.9 Hz, H-5), 4.25, 4.33, 4.36 (m, m, t, J = 6.4 Hz, 3H, 2H, 1H resp. H-1, H-2, H-6, H-6’, CH2-7’), 4.47 (d, 1H, J = 6.4 Hz, H-5’), 4.73 (dd, 1H, J = 2.5, 6.4 Hz, H-4’), 4.94 (d, 1H, J = 2.5 Hz, H-3’), 7.31–7.41 (m, 6H), 7.87 (m, 4H); 13C NMR (C6D6) ™ 19.3 (Me3C-Si), 25.2 (CH3-C), 25.4 (CH3-C), 26.8 ((CH3)3C), 27.0 (CH3-C), 27.8 (CH3-C), 28.0 (CH3-C), 28.4 (CH3-C), 34.1 (CH2C=), 39.8 (C-4), 58.5 (CH3O), 63.8 (CH2O), 70.1, 72.0, 75.9, 80.0, 81.1, 100.5 (C-3’), 108.5 (OCO), 108.8 (OCO), 110.0 (OCO), 128.5, 129.8, 133.6 (two signals), 135.8 (two signals), 157.1. HRMS (ESI) m/z calcd for C39H54O9NaSi (M + Na)+ 717.3429, found 717.3422.
7.8 4-Deoxy-4-C-(2,6-anhydro-7-O-tertbutyldiphenylsilyl-4,5-O-isopropylidene-1-deoxy-D-galacto-D-glycero-heptit-1-C-yl)-1,2,5,6-di-O-isopropylidene-3-O-methyl-D-chiro-inositol 12
BH3.Me2S (1.1 mL, 1M solution, mmol) was added at 0 °C to a solution of glycal 11 (0.191 g, 0.275 mmol) in anhydrous THF (8 mL) under an atmosphere of argon. The mixture was warmed to rt and stirred for an additional 1 h. At that time the solution was recooled to 0 °C and treated with a mixture of 3N NaOH (2 mL) and 30% aqueous H2O2 (2 mL) for 30 min. The solution was then diluted with ether and washed with saturated aqueous NaHCO3 and brine, dried (Na2SO4), filtered and evaporated under reduced pressure. FCC of the residue provided 12 (0.149 g, 76 %). Rf = 0.51 (25 % ethyl acetate: petroleum ether). 1H NMR (CDCl3) ™ 1.06 (s, 9H, (CH3)3C)), 1.32 (s, 3H, CH3-C), 1.34 (s, 3H, CH3-C), 1.40 (s, 3H, CH3-C), 1.44 (s, 3H, CH3-C), 1.51 (s, 3H, CH3-C), 1.54 (s, 3H, CH3-C), 1.75 (m, 1H, ½ × CH2), 2.01 (m, 2H, H-4, ½ × CH2), 2.42 (d, 1H, J = 3.4 Hz, OH), 3.01 (dd, 1H, J = 6.3, 12.2 Hz, H-3), 3.45 (m, 2H), 3.49 (s, 3H, CH3O), 3.86 (m, 3H), 4.03 (t, 1H, J = 5.4 Hz, H-3’), 4.07 (m, 2H), 4.20 (m, 2H), 4.39 (dd, 1H, J = 1.8, 5.4 Hz, H-4’), 7.35–7.46 (m, 6H), 7.67–7.75 (m, 4H); 13C NMR (CDCl3) ™ 19.2 (Me3C-Si), 25.3 (CH3-C), 25.4 (CH3-C), 26.4 (CH3-C), 26.7 ((CH3)3C),, 27.7 (CH3-C), 27.8 (CH3-C), 28.4 (CH3-C), 32.7 (CH2-1’), 37.5 (C-4), 58.7 (CH3O), 62.7 (CH2O), 73.8, 74.8, 75.5, 76.1, 77.2, 79.1, 80.1, 80.3 (two signals), 109.2 (OCO), 109.4 (OCO), 109.6 (OCO), 127.6, 127.7, 129.6, 133.2, 133.4, 135.6 (two signals). HRMS (ESI) m/z calcd for C39H56O10NaSi (M + Na)+ 735.3534, found 735.3537.
7.9 C-INS-2-OH 3
To a solution of TBDPS protected alcohol 12 (0.065 g, 0.09 mmol) in dry THF (1 mL), was added TBAF (0.18 mL, 0.18 mmol) at rt. The reaction was stirred for 5 h at rt. The mixture was then diluted with saturated aqueous NaHCO3 and extracted with ether. The combined organic phase was dried (Na2SO4) and concentrated in vacuo. FCC of the residue afforded the derived primary alcohol (0.041 g, 95%) as a colorless oil. The product (0.040 g, 0.09 mmol) was dissolved in dry methanol (3 mL). The pH of the solution was adjusted to 2–3 by addition of a 2M solution of HCl in anhydrous ether, and the mixture stirred for 18 h. The solution was then diluted with methanol and evaporated under reduced pressure. The dilution-evaporation procedure was repeated three times and the residue dried in vacuo to afford 3 (0.029 g, 97%) as a clear gum. Rf = 0.45 (50% methanol:ethyl acetate). 1H NMR (D2O) ™ 1.68–1.78 (m, 1H, H-1’a), 1.98 (m, 1H, H-4), 2.05 (m, 1H, H-1’b), 3.23 (t, 1H, J = 9.6 Hz, H-3), 3.33 (t, 1H, J = 9.5 Hz, H-3’), 3.43 (s, 3H, CH3O), 3.45 (m, 1H, H-2’), 3.49–3.67 (m, 4H, H-6’, 4’, CH2-7’), 3.75 (dd, 1H, J = 3.1, 9.6 Hz, H-2), 3.89–3.79 (m, 3H, H-1, 5, 5’), 3.89 (t, 1H, J = 3.3 Hz, H-6); 13C NMR (CDCl3) δ 28.8 (C-1’), 37.9 (C-4), 58.5 (CH3O), 61.4 (C-7’), 69.1 (C-1/5/5’), 69.9 (C-1/5/5’), 71.2 (C-2), 71.3 (C-3’), 71.8 (C-6), 72.0 (C-1/5/5’), 73.9 (C-4’), 77.8 (C-2’), 78.2 (C-6’), 79.9 (C-3). HRMS (ESI) m/z calcd for C14H26NaO10 (M + Na)+ 377.1425, found 377.1425.
7.10 C-pseudodisaccharide ketone 13
To a mixture of PCC (0.401 g, 1.87 mmol), florisil (0.996 g), sodium acetate (0.153 g, 1.87 mmol), freshly activated, powdered 4Å molecular sieves (0.996 g) and Celite (0.996 g) in dry dichloromethane (15 mL), was added dropwise a solution of alcohol 12 (0.322 g, 0.466 mmol) in dry dichloromethane (5 mL). The reaction mixture was stirred at rt until TLC indicated total consumption of the starting material. The mixture was then diluted with ether and filtered through a column of florisil. The filtrate was evaporated in vacuo and the residue purified by FCC to afford ketone 13 (0.315 g, 95%) as colorless oil. Rf = 0.51 (25% ethyl acetate: petroleum ether). 1H NMR (CDCl3) ™ 1.07 (s, 9H, (CH3)3C)), 1.30 (s, 3H, CH3-C), 1.35 (s, 3H, CH3-C), 1.42 (s, 3H, CH3-C), 1.45 (2s, 6H, 2 × CH3-C), 1.51 (s, 3H, CH3-C), 1.80 (ddd, 1H, J = 3.8, 10.5, 14.4 Hz, H-1a’), 1.90 (m, 1H, H-4), 2.21(ddd, 1H, J = 2.5, 9.2, 14.4 Hz, H-1’b), 3.01 (dd, 1H, J = 6.3, 12.1 Hz, H-3), 3.49 (s, 3H, CH3O), 3.93 (m, 2H, CH2-7’), 4.12 (m, 3H, H- 1, 5, 6’), 4.20 (m, 2H, H-2, 6), 4.26 (dd, 1H, J = 3.8, 9.2 Hz, H-2’), 4.47 (d, 1H, J = 5.8 Hz, H-4’), 4.77 (dd, 1H, J = 1.7, 5.8 Hz, H-5’), 7.32–7.56 (m, 6H, ArH), 7.67–7.99 (m, 4H, ArH); 13C NMR (CDCl3) ™ 19.2 (Me3C-Si), 25.3 (CH3-C), 25.4 (CH3-C), 26.1 (CH3-C),, 26.8 ((CH3)3C), 27.2 (CH3-C), 27.7 (CH3-C), 27.9 (CH3-C), 29.8 (C-1’), 38.3 (C-4), 58.9 (CH3O), 62.5 (C-7’), 76.7, 77.1, 77.2, 77.5, 77.8, 79.0, 79.1, 80.0, 80.3, 109.2 (OCO), 109.4 (OCO), 110.8 (OCO), 127.7 (two signals), 128.3, 129.7, 133.2, 133.3, 135.5, 135.6, 204.6 (C=O). HRMS (ESI) m/z calcd for C39H54O10SiNa (M + Na)+ 733.3393, found 733.3393.
7.11 Pseudodisaccharide oxime 14
To a solution of ketone 13 (0.312 g, 0.439 mmol) in a 1:1 mixture of THF/MeOH (5.5 mL), was added a mixture of O-methyl hydroxylamine hydrochloride (0.367 g, 4.39 mmol) and NaOAc (0.397 g, 4.82 mmol) in water (2.75 mL), which was adjusted to pH = 4.5 with a few drops of glacial acetic acid. The reaction mixture was stirred at rt for 4 h then diluted with EtOAc and washed with saturated aqueous NaHCO3 and water, dried (Na2SO4), and filtered. The solvent was removed in vacuo to give 14 (0.280 g, 86%, 1.5:1 mixture) as a colorless oil. Rf = 0.60 (20 % ethyl acetate: petroleum ether). For major isomer: 1H NMR (CDCl3) ™ 1.07 (s, 9H, (CH3)3C), 1.32 (s, 3H, CH3-C), 1.34 (s, 3H, CH3-C), 1.36 (s, 3H, CH3-C), 1.42 (s, 3H, CH3-C), 1.49 (s, 3H, CH3-C), 1.50 (s, 3H, CH3-C), 1.83 (dd, 1H, J = 9.5, 14.0 Hz, H-1’a), 2.05 (m, 1H, H-4), 2.40 (ddd, J = 1.9, 10.6, 14.0 Hz, H-1’b), 3.12 (dd, 1H, J = 7.0, 12.5 Hz, H-3), 3.45 (dt, 1H, J = 1.7, 6.4 Hz, H-6’), 3.48 (s, 3H, CH3O), 3.82 (m, 2H, CH2-7’), 3.93 (s, 3H, CH3O), 4.03 (t, 1H, J = 7.0 Hz, H-6), 4.15 (t, 1H, J = 7.0 Hz, H-1), 4.23 (t, 1H, J = 7.0 Hz, H-2), 4.35 (t, 1H, J =7.0 Hz, H-5), 4.53 (dd, 1H, J = 1.7, J = 7.8 Hz, H-5’), 4.78 (d, 1H, 7.8 Hz, H-4’), 5.09 (d, 1H, J = 9.5 Hz, H-2’), 7.36–7.48 (m, 6H), 7.68–7.75 (m, 4H); 13C NMR (CDCl3) ™ 19.2 (Me3C-Si), 25.3 (2 × CH3-C), 25.4 (CH3-C), 26.0 (CH3-C), 26.8 ((CH3)3C), 27.8 (CH3-C), 27.9 (CH3-C), 34.0 (C-1’), 38.6 (C-4), 58.4 (CH3O), 62.3 (CH3ON), 62.5 (CH2-7’), 72.5, 74.2, 75.7, 76.7, 77.1, 77.5, 78.9, 79.5, 109.2 (OCO), 109.5 (OCO), 111.2 (OCO), 127.6, 127.7, 133.4, 133.7, 135.7, 135.8, 156.1 (C=N). HRMS (ESI) m/z calcd for C40H57NO10NaSi (M + Na) + 762.3643, found 762.3643.
7.12 C-Pseudodisaccharide methoxylamine 15
To a mixture of oxime 14 (0.092 g, 0.124 mmol), and Bu3SnH (0.065 mL, 0.248 mmol) in CH2Cl2 (3 mL), was added at −20 °C, BF3.OEt2 (0.015 mL, 0.124 mmol). The mixture was maintained at this temperature for 2 h, then diluted with saturated aqueous NaHCO3 and extracted with CH2Cl2. The combined organic phase was dried (Na2SO4) and concentrated in vacuo. FCC of the residue afforded 15 (0.075 g, 82%) as a colorless oil. Rf = 0.81 (25 % ethyl acetate: petroleum ether). 1H NMR (CDCl3) ™ 1.06 (s, 9H, (CH3)3C), 1.33 (s, 3H, CH3-C), 1.35 (s, 3H, CH3-C), 1.38 (s, 3H, CH3-C), 1.46 (s, 3H, CH3-C), 1.49 (s, 3H, CH3-C), 1.51 (s, 3H, CH3-C), 1.82 (ddd, 1H, J = 1.7, 10.0, 11.8 Hz, H-1’a), 2.05 (m, 2H, H-1’b, H-4), 2.59 (dd, 1H, J = 8.1, 9.7 Hz, H-3’), 3.05 (dd, 1H, J = 6.1, 12.3 Hz, H-3), 3.49 (s, 3H, CH3O), 3.56 (s, 3H, CH3O), 3.73 (dt, 1H, J = 1.7, 9.9 Hz, H-2’), 3.84 (m, 2H, CH2-7’), 3.91 (dd, 1H, J = 7.5, 9.0 Hz, H-6’), 4.06 (t, 1H, J = 6.5 Hz, H-6), 4.13 (dd, 1H, J = 7.2, 8.6 Hz, H-5), 4.19 (m, 2H, H-1, 2), 4.40 (m, 2H, H-4’, 5’), 6.01 (bs, 1H, NH), 7.31–7.51 (m, 6H, ArH), 7.71–7.82 (m, 4H, ArH); 13C NMR (CDCl3) ™ 19.2 (Me3C-Si), 25.3 (2 × CH3-C), 26.4 (CH3-C), 26.8 ((CH3)3C),, 27.8 (CH3-C), 27.9 (CH3-C), 28.5 (CH3-C),, 33.2 (C-1’), 37.8 (C-4), 58.5 (CH3O), 62.3 (CH3O), 63.0 (CH2-O), 65.7 (CH-N), 72.9, 73.2, 73.7, 75.9, 77.4, 79.1, 79.9, 80.4, 109.2 (OCO), 109.3 (OCO), 109.4 (OCO), 127.6, 127.7, 129.6, 133.5, 133.6, 135.5, 135.6. HRMS (ESI) m/z calcd for C40H59NO10SiNa (M + Na)+ 764.3688, found 764.3688.
7.13 C-INS-2-hydrochloride
To a solution of silylether 15 (0.033 g, 0.046 mmol) in dry THF (1 mL), was added TBAF (0.09 mL, 0.09 mmol) at rt. The reaction mixture was stirred for 5 h at rt then diluted with saturated aqueous NaHCO3 and extracted with ether. The combined organic phase was dried (Na2SO4) and concentrated in vacuo. FCC of the residue afforded the primary alcohol derivative (0.022 g, 95%) as a colorless oil. Rf = 0.20 (50 % ethyl acetate: petroleum ether). 1H NMR (CDCl3) ™ 1.35 (s, 3H, CH3-C), 1.36 (s, 3H, CH3-C), 1.37 (s, 3H, CH3-C), 1.50 (s, 3H, CH3-C), 1.51 (s, 3H, CH3-C), 1.53 (s, 3H, CH3-C), 1.80 (dd, 1H, J = 3.9, 10.8, 13.9 Hz, H-1’a), 2.10 (m, 2H, H-1’b, H-4), 2.44 (dd, 1H, J = 1.9, 9.4 Hz, OH), 2.66 (t, 1H, J = 7.7 Hz, H-3’), 3.10 (dd, 1H, J = 6.5, 12.3 Hz, H-3), 4.06 (t, 1H, J = 7.1 Hz, H-6), 4.12 (t, 1H, J = 6.9 Hz, H-5), 4.17 (dd, 1H, J = 1.8, 5.5 Hz, H-1), 4.21 (t, 1H, J = 7.4 Hz, H-5’), 4.25 (t, 1H, J = 4.2 Hz, H-2), 4.39 (dd, 1H, J = 5.5, 8.2 Hz, H-4’), 5.97 (d, 1H, J = 1.3 Hz, NH); 13C NMR (CDCl3) ™ 25.3 (CH3-C), 25.5 (CH3-C), 26.4 (CH3-C), 27.8 (CH3-C), 28.0 (CH3-C),, 28.3 (CH3-C), 33.4 (C-1’), 37.8 (C-4), 58.6 (CH3O), 62.4 (CH3O), 63.0 (CH2-7’), 65.7 (CH-N), 69.8, 73.3 (two signals), 76.5, 77.4, 77.5, 78.3, 80.2, 80.5, 109.5 (OCO), 109.7 (OCO), 109.9 (OCO).
Liquid NH3 was condensed into a solution of the product from the previous step (0.122 g, 0.242 mmol) in THF (4 mL) at −78 °C, under an atmosphere of argon. Sodium was then carefully added to the solution until a blue color persisted for several minutes. After slowly warming to rt, the reaction was quenched with solid NH4Cl, and filtered. The filtrate was concentrated in vacuo. FCC of the residue afforded the amine derivative (0.090 g, 78%) as a colorless oil. Rf = 0.48 (25 % methanol:ethyl acetate). 1H NMR (CDCl3) ™ 1,30 (s, 3H), 1.35 (3, 6H), 1.47 (s, 3H), 1.50 (s, 6H), 2.05 (m, 2H), 2.70 (t, 1H, J = 8.8 Hz), 3.05 (dd, 1H, J = 6.4, 12.3 Hz), 3.23 (t, 1H, J = 9.2 Hz), 3.46 (s, 3H), 3.66 (m, 2H), 3.83 (m, 2H), 4.04 (t, 1H, J = 7.0 Hz), 4.05 (m, 2H), 4.15 (t, 1H, J = 7.4 Hz), 4.23 (t, 1H, J = 7.0 Hz); 13C NMR (CDCl3) δ 25.2, 25.5, 26.5, 27.8, 28.0, 28.3, 32.9, 37.6, 56.7, 58.5, 62.9, 73.5, 76.1, 76.7, 76.8, 77.4, 77.5, 78.2, 80.0, 80.6, 80.7, 109.5, 109.7, 110.0.
To a solution of product from the previous step (52 mg, 0.11 mmol) in dry methanol (3 mL) was added a 2M solution of HCl in anhydrous ether to a pH of 2–3. The reaction mixture was stirred for 18 h. The mixture was then diluted with methanol and evaporated under reduced pressure. The dilution-evaporation procedure was repeated three times and the residue dried in vacuo to give 2 as the hydrochloride salt (38 mg, 98%). Rf = 0.67 (4:4:1:2 of 2-propanol: pyridine: acetic acid: water). 1H NMR (500 MHz, D2O) ™ 1.77 (ddd, 1H, J = 3.0, 7.5, 14.8 Hz, H-1’a), 1.85 (ddd, 1H, J = 1.8, 9.9, 14.8 Hz, H-1’b), 2.05 (m, 1H, H-4), 3.05 (t, 1H, J = 10.3 Hz, H-3’), 3.52 (t, 1H, J = 3.2 Hz, H-3), 3.40 (s, 3H, CH3O), 3.57 (dd, 1H, J = 3.9, 8.1 Hz, H-6’), 3.61 (dd, 1H, J = 3.9, J =11.8 Hz, H-7’), 3.69 (dd, 1H, J = 8.1, 11.8 Hz, H-7’), 3.75 (dd, 1H, J = 3.0, 10.6 Hz, H-2’), 3.77 – 3.81 (m, 4H, H-1, 2, 5, 6), 3.87 (d, 1H, J = 3.5 Hz, H-5’), 3.90 (t, 1H, J = 3.5 Hz, H-4’); 13C NMR (CDCl3) ™ 30.9 (C-1’), 36.6 (C-4), 54.0 (CH-N), 57.5 (CH3O), 61.3 (CH2-7’), 68.0 (C-5’), 70.3 (C-1/2/5/6), 70.4 (C-2’), 71.2 (C-1/2/5/6), 71.9 (C-4’), 72.1 (C-1/2/5/6), 75.2 (C-1/2/5/6), 78.5 (C-6’), 79.8 (C-3). HRMS (ESI) m/z calcd for C14H28NO9 (RNH3)+ 354.1759, found 354.1762.
7.14. PP2Cα Assays
The mouse wild type GST-PP2Cα fusion protein was expressed and purified as previously described.4 Phosphatase activity was assayed at rt in 0.1 M Tris-HCl (pH 8.0), 2 mM dithiothreitol, and various concentrations of added MnCl2, INS-2, C-INS-2-OH or C-INS-2, in a 96-well plate in 50 µL reaction mixtures with 1 µg of GST-PP2Cα fusion protein and 5 mM p-nitrophenyl phosphate (PNPP). After 30 min, the reaction was stopped by addition of 200 µL of 0.5% SDS and the absorbance at 410 nm recorded with an Eldex microplate reader. Alternatively, activity of 2 µg of GST-PP2Cα was assayed in the same volume of the same buffer with a synthetic phosphopeptide (R-R-R-R-P-pT-P-A) at a final concentration of 5 µM. After 15 min, the reaction was stopped by addition of 100 µL of a malachite green solution (Millipore). After color development for 15 min, the absorbance at 650 nm was determined with a microplate reader. Values were corrected by subtracting the absorbance of blanks that were reactions without added enzyme.
7.15. PDHP Assay
PDHP was assayed in triplicate samples on a 96 well plate.3 An ATP inhibition curve was first established to determine the 50% inhibition concentration. 10 µL of assay buffer (1mM imidazole pH 7, 2 mM DTT, 10 mM MgCl2, 0.1mM CaCl2, 105 mg/mL BSA) was added to each well. 10 µL of 0.5 to 5 mM ATP is added and the volumes all adjusted with aliquots of distilled water (DW). 10 µL PDHP is added and the plate gently shaken. 10 µL PDH was added, the plate gently shaken and incubated at 37 °C for 30 minutes. 10 µL of 110 mM NaF was added and the plate gently shaken. 10 µL pyruvate-ADP (30 mM sodium pyruvate, 30 mM ADP) was added with gentle shaking. 45 µL NAD mix (83 mM imidazole pH 7, 83 mM ®-NAD, 1.65 mM cocarboxylase, 3.3 mM DTT, 3.3 mM CoA) was added with gentle shaking and incubated at rt for 2 min. The plate was read for change in O.D. at 340 nM. Activity was expressed as % of basal, e.g. ATP concentration for 50% inhibition.
Supplementary Material
Acknowledgment
This investigation was supported by grants R01 GM57865 from the National Institute of General Medical Sciences of the National Institutes of Health (NIH). "Research Centers in Minority Institutions" award RR-03037 from the National Center for Research Resources of the NIH, which supports the infrastructure and instrumentation of the Chemistry Department at Hunter College, is also acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Larner J, Galasko G, Cheng K, DePaoli-Roach A, Huang L, Daggy P, Kellogg J. Science. 1979;206:1408–1410. doi: 10.1126/science.228395. [DOI] [PubMed] [Google Scholar]
- 2.Sleight S, Wilson B, Heimark D, Larner J. Biochem Biophys Res Commun. 2002;295:561–569. doi: 10.1016/s0006-291x(02)00701-5. [DOI] [PubMed] [Google Scholar]
- 3.Larner J, Price JD, Heimark D, Smith L, Rule G, Piccariello T, Fonteles MC, Pontes C, Vale D, Huang L. J Med Chem. 2003;46:3283–3291. doi: 10.1021/jm030071j. [DOI] [PubMed] [Google Scholar]
- 4.Brautigan D, Brown M, Grindrod S, Chinigo G, Kruszewski A, Lukasik S, Bushweller J, Horal M, Keller S, Tamura S, Heimark D, Price J, Larner A, Larner J. Biochemistry. 2005;44:11067–11073. doi: 10.1021/bi0508845. [DOI] [PubMed] [Google Scholar]
- 5.Hiraga A, Kikuchi K, Tamura S, Tsuiki S. Eur J Biochem. 1981;119:503–510. doi: 10.1111/j.1432-1033.1981.tb05636.x. [DOI] [PubMed] [Google Scholar]
- 6.Villar-Palasi C, Larner J. Biochim Biophys Acta. 1960;39:171–173. doi: 10.1016/0006-3002(60)90142-6. [DOI] [PubMed] [Google Scholar]
- 7.Linn T, Pettit F, Reed L. Proc Natl Acad Sci U S A. 1969;62:234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) For C-glycoside reviews: Postema MHD. C-Glycoside Synthesis. Boca Raton, Fl: CRC Press; 1995. Levy DE, Tang C. The Synthesis of C-Glycosides. Oxford: Pergamon; 1995. Beau J-M, Gallagher T. Top. Curr. Chem. 1997;187:1–54. Togo H, He W, Waki Y, Yokoyama M. Synlett. 1998:700–717. Du Y, Linhardt RJ, Vlahov JR. Tetrahedron. 1998;54:9913–9959. Yuan X, Linhardt RJ. Curr. Top. Med. Chem. 2005;5:1393–1430. doi: 10.2174/156802605774643033. Levy DE. In: The Organic Chemistry of Sugars. Levy DE, Fügedi P, editors. Boca Raton: CRC Press / Taylor & Francis; 2005. pp. 269–348.
- 9.Jiménez-Barbero J, Espinosa JF, Asensio JL, Cañada FJ, Poveda A. Adv. Carbohydr. Chem. Biochem. 2000;56:235–284. doi: 10.1016/s0065-2318(01)56006-0. [DOI] [PubMed] [Google Scholar]
- 10.For examples of relative activity of O-glycosides and their exact C-glycoside analogues: Acton EM, Ryan KJ, Tracy M, Arora SK. Tetrahedron Letters. 1986;27:4245–4248. Wei A, Boy KM, Kishi Y. J. Am. Chem. Soc. 1995;117:9432–9436. Uchiyama T, Vassilev VP, Kajimoto T, Wong W, Huang H, Lin C-C, Wong C-H. J. Am. Chem. Soc. 1995;117:5395–5396. Xin Y-C, Zhang Y-M, Mallet J-M, Glaudemans CPJ, Sinaÿ P. Eur. J. Org. Chem. 1999:471–476. Link JT, Sorensen BK. Tetrahedron Lett. 2000;41:9213–9217. Yang G, Schmieg J, Tsuji M, Franck RW. Angew. Chem. Int. Ed. 2004;43:3818–3822. doi: 10.1002/anie.200454215. Postema MH, Piper JL, Betts RL, Valeriote FA, Pietraszkewicz H. J Org Chem. 2005;70:829–836. doi: 10.1021/jo040254t.
- 11.For an example where O- and C- glycosides bind in different conformations with respect to the intersaccharide torsions: Espinosa JF, Montero E, Vian A, Garcia JL, Dietrich H, Schmidt RR, Martín-Lomas M, Imberty A, Cañada FJ, Jimenéz-Barbero J. J. Am. Chem. Soc. 1998;120:1309–1318.
- 12.(a) Gabius HJ. Pharm Res. 1998;15:23–30. doi: 10.1023/a:1011936300845. [DOI] [PubMed] [Google Scholar]; (b) Dam TK, Brewer CF. Chem. Rev. 2002;102:387–429. doi: 10.1021/cr000401x. [DOI] [PubMed] [Google Scholar]
- 13.Khan N, Cheng X, Mootoo DR. J. Am. Chem. Soc. 1999;121:4918–4919. [Google Scholar]
- 14.Keck GE, Enholm EJ, Yates JB, Wiley MR. Tetrahedron. 1985;41:4079–4094. [Google Scholar]
- 15.Zunszain PA, Varela O. Tetrahedron: Asym. 1998;9:1269–1276. [Google Scholar]
- 16.Afarinkia K, Bahar A. Tetrahedron Asym. 2005;16:1239–1287. [Google Scholar]
- 17.Ueda M, Miyabe H, Namba M, Nakabayashi T, Naito T. Tetrahedron Lett. 2002;43:4369–4371. [Google Scholar]
- 18.Rarey M, Kramer B, Lengauer T, Klebe GA. J. Mol. Biol. 1996;261:470–489. doi: 10.1006/jmbi.1996.0477. [DOI] [PubMed] [Google Scholar]
- 19.For definition of scoring functions see ref. 4.
- 20.For Φ: O5-C1-X-C4’, Ψ:C1-X-C4’-H4’ where X the glycosidic oxygen or “CH2”
- 21.Vassylyev D, Symersky J. J Mol Biol. 2007;370:417–426. doi: 10.1016/j.jmb.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das A, Helps N, Cohen P, Barford D. EMBO J. 1996;15:6798–6809. [PMC free article] [PubMed] [Google Scholar]
- 23.Mikkelsen LM, Hernáiz MJ, Martín-Pastor M, Skrydstrup T, Jiménez-Barbero J. J. Am. Chem. Soc. 2002;124:14940–14951. doi: 10.1021/ja020468x. [DOI] [PubMed] [Google Scholar]
- 24.García-Herrero A, Montero E, Muñoz JL, Espinosa JF, Vián A, García JL, Asensio JL, Cañada FJ, Jiménez-Barbero J. J. Am. Chem. Soc. 2002;124:4804–4810. doi: 10.1021/ja0122445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.