Abstract
Background
Environmental exposures to cockroach allergen and endotoxin are recognized epidemiological risk factors for the early development of allergies and asthma in children. Because of this, it is important to examine the role of early life concurrent inhalation exposures to cockroach allergen and endotoxin in the pathogenesis of allergic airways disease.
Objective
We examined the effects of repeated concomitant endotoxin and cockroach allergen inhalation on the pulmonary and systemic immune responses of newborn and juvenile mice.
Methods
C3H/HeBFeJ mice were exposed to inhaled endotoxin and cockroach allergen via intranasal instillation from day 2 to 21 after birth, and systemic and pulmonary responses were examined in serum, bronchoalveolar lavage fluid, and lung tissue.
Results
Cockroach allergen exposures induced pulmonary eosinophilic inflammation, total and allergen specific IgE, IgG1, and IgG2a production, and alveolar remodeling. Co-exposures with endotoxin and cockroach allergen significantly increased serum IgE and IgG1, lung inflammation, and alveolar wall thickness, and decreased airspace volume density. Importantly, compared to exposures with individual substances, the responses to co-exposures were more than additive.
Conclusions
Repeated inhalation exposures of neonatal and juvenile mice to endotoxin and cockroach allergen increased the pulmonary inflammatory and systemic immune responses in a synergistic manner and enhanced alveolar remodeling in the developing lung. These data underscore the importance of evaluating the effect of multiple, concurrent environmental exposures, and of using an experimental model that incorporates clinically relevant timing and route of exposures.
Keywords: endotoxin, cockroach allergen, lung inflammation, allergy, enzyme hypothesis, synergistic effect
INTRODUCTION
House dust is a complex mixture of mostly organic agents, ranging from inert to highly inflammatory [1]. Inhalation exposures to house dust necessarily imply concomitant exposures to its components, such as cockroach allergen and endotoxin; the health effects of exposure to these agents are likely to differ with concurrent rather than individual or sequential exposures [2]. Indoor environmental exposures to house dust containing endotoxin are associated with early-life establishment of asthma [1-4]. Of all common aeroallergens, early-life, environmental exposures to cockroach allergens appear to play a particularly significant role in the pathogenesis and exacerbation of symptoms of allergic asthma [1, 5-7].
While the individual effects of endotoxin and allergens on the respiratory system have been studied in detail, little is known about the health effects of concurrent-inhalation exposures [6]. Human data on concurrent endotoxin and allergen inhalation exposures are limited to adult human subject studies of inhalation with endotoxin-contaminated allergen extracts [8]. Animal studies of concurrent allergen and endotoxin exposures show conflicting results, as some researchers observed suppressed, while others observed amplified systemic and pulmonary responsiveness after co-administration [9-12]. The choice of antigen may explain the differences in the results. Studies that utilized endotoxin along with ovalbumin showed suppressed responses [9, 10], while studies that used common environmental allergens, such as Aspergillus fumigatus or cat allergen, showed augmented responses [11, 12]. The latter aeroallergens are active proteases, as are some components of cockroach and house dust mite allergen (the cysteine proteases from Group 1 in mite extracts and serine proteases in mite – Groups 3, 6, and 9, and cockroach extracts) [13-16]. The proteolytic activity of these allergens may enhance their trans-epithelial delivery by disrupting the airway epithelial cells tight junctions [17]. Proteolytic allergens also exert direct pro-inflammatory effects by promoting secretion of airway inflammatory cytokines [15] and endogenous proteases that in turn augment the pro-inflammatory and immunogenic effects of the allergens [18].
Endotoxin inhalation alone also induces secretion of airway inflammatory cytokines, including TNF-α, IL-6, as well as endogenous proteases [19], all of which can augment the pro-inflammatory and immunogenic properties of aeroallergens. To this end, it is important to examine the role of concurrent-inhalation exposures to cockroach allergen and endotoxin in the pathogenesis of allergic airways disease, specifically during early life when such exposures are most likely to affect the development of asthma.
To elucidate the role of simultaneous exposures with endotoxin (lipopolysaccharide, LPS) and cockroach allergen, and to model human indoor environmental exposures utilizing timing and route of exposures relevant to humans, we developed a model in young mice with exposures to an endotoxin and cockroach allergen mixture. We hypothesized that responses induced by these simultaneous-inhalation exposures would exceed those induced by allergen exposures alone. Our experiments were designed to test for interaction between endotoxin and cockroach allergen in the development of airways inflammation, systemic sensitization in early life, and structural remodeling of the alveolar regions of the lungs in young mice.
Some of the results of these studies have been previously reported in the form of abstracts [20-23].
METHODS
Animals
C3H/HeBFeJ mice (Jackson Laboratories, Bar Harbor, ME), strain extensively utilized in our endotoxin responsiveness studies [24 - 26] were maintained and bred in a pathogen-free, AAALAC-approved animal facility, and were provided food and water ad libitum. All protocols were approved by the Institutional Animal Care and Use Committee; all animal care and housing requirements of the National Institutes of Health Committee on Care and Use of Laboratory Animals were followed.
Neonatal and Juvenile Inhalation Exposures with Endotoxin and Cockroach Allergen
Mice, under short-term isoflurane-induced anesthesia, were exposed to 300 EU/day (30 ng) endotoxin (lipopolysaccharide from Escherichia coli O111:B4, Sigma-Aldrich, St. Louis, MO); an average of 10 ng/day of cockroach allergen extract (CRA, Allergenic Extract 0048, German Cockroach, Blatella germanica, Allermed Laboratories, Inc., San Diego, CA); or both substances concurrently by intranasal instillation (i.n.) [24, 25] daily from day two to twenty one after birth. The dose of 300 EU was selected based on previous studies of endotoxin exposures to adult mice [24 - 26], taking into consideration the anatomical and physiological features of newborn mice, such as body mass, size and shape of the upper and lower airways, and ventilation rate. We aimed to deliver approximately 10-15 EU to the lungs, the level reported for optimal pulmonary responsiveness in mice [26]. Using published methods to estimate lung deposited dose and the range of endotoxin measured in airborne house dust in homes, this level also falls within the range of estimated human indoor endotoxin exposures (0.05 – 163 EU/day—range of estimated endotoxin dose deposited in the lower respiratory tract) [25, 27].
In order to render the model more physiologically valid and representative of human exposures, these studies did not include systemic sensitization or use of an exogenous adjuvant. Age-matched unexposed sentinel animals and animals exposed to a vehicle solution (solution, in which the cockroach allergen was supplied: 0.25% sodium chloride, 0.125% sodium bicarbonate, 0.4% phenol, and 50% glycerol, N=3) served as controls. Mice were euthanized and biological samples collected 2-4 hours after the last exposure on day 21. Each treatment group consisted of 6-8 animals. Experiments were conducted on a second set of 6-8 animals per group and confirmed the findings. Data from one representative experiment are presented.
Endotoxin contamination of commercially available allergen extracts is an acknowledged phenomenon that has been linked to altered pulmonary responses upon inhalation [12]. Therefore we measured the endotoxin concentration in cockroach allergen extract and examined the lungs for evidence of endotoxin-specific inflammatory changes in non-sensitized mice inhaling cockroach allergen alone. Cockroach allergen extract was serially diluted in endotoxin-free LAL water with 0.05% Tween 20, and assayed using the kinetic chromogenic Limulus Amebocyte Lysate (LAL) assay (Kinetic-QCL; Cambrex Bio Science, Inc., Walkersville, MD) modified as previously described [6]. Endotoxin concentration was determined based upon the maximum slope of absorbance versus time from the concentrations falling in the linear range of the standard curve. We determined that the 25 μl of cockroach allergen extract solution that we used for intranasal challenges contained 76 EU (7.6 ng) of endotoxin. Exposure with cockroach allergen extract solution containing this amount of endotoxin only slightly increased the total, but not the neutrophil bronchoalveolar lavage (BAL) cell counts over sentinel mice (BAL neutrophils, mean ± SEM: 3 ±2%, CRA; 1 ± 1%, sentinels), suggesting that the endotoxin content of the cockroach allergen inhalation solution was too low to induce endotoxin-specific lung inflammation. Taking into consideration the amount of endotoxin contained in the cockroach allergen solution, the Figure legends reflect the amounts of endotoxin in each inhalation dose as follows: Endo = 300 EU (30 ng) of endotoxin; CRA = 10 ng of cockroach allergen extract with 76 EU (7.6 ng) endotoxin; Endo + CRA = 10 ng of cockroach allergen extract and 376 EU (37.6 ng) of endotoxin.
Peripheral Blood and BAL Fluid Collection and Processing
Mice were euthanized by an injection of Nembutal. Blood was collected from the retroorbital venous plexus and serum aliquots were stored at -80°C. BAL fluid was collected and processed as described previously [24]. Briefly, lungs were washed with total of 1.8 - 2 ml sterile pyrogen-free saline and recovered BAL fluid was centrifuged (2,400 rpm for 5 min at 4°C); supernatant was aliquoted and stored at -80°C, and cell pellets were processed for total and differential cell counts.
Cytokine and chemokine levels in the BAL fluid were determined by bead-based multiplex assay (Bio-Rad Laboratories, Inc., Hercules, CA) in individual mice according to manufacturer's specifications. Briefly, beads were plated in 96-well filter plates pre-wetted with assay buffer and washed thrice by vacuum filtration. Fifty μl of samples, serially-diluted cytokine/chemokine standards, and blanks were added and incubated overnight at 4°C. The plate was vacuum washed 3x, detection antibody solution was added followed by 3x vacuum wash; streptavidin-PE solution was added followed by 3x vacuum wash; finally the beads were reconstituted with 125 μl of assay buffer and the plate was read on the Bio-Plex System (Bio-Rad Laboratories, Inc., Hercules, CA). Because the volume of the BAL fluid varied slightly between mice, we normalized the cytokine and chemokine levels by calculating the total amount of cytokine and chemokine produced by each mouse (pg/mouse).
Total IgE, IgG1 and IgG2a, and CRA-specific IgE were assayed with commercially available ELISA reagents (IgE: BD OptEIA™, BD Biosciences, Pharmingen, San Diego, CA; IgG1 and IgG2a: Bethyl Laboratories, Inc, Montgomery, TX) according to manufacturer's specifications and as previously described [28] with the following modification: For CRA-specific IgE, the ELISA plate was coated with 100 ul cockroach allergen extract diluted 1:100 with PBS and incubated overnight at 4° C. The plate was washed and blocked with PBS containing 10% FCS for 1 h at room temperature (RT). Serially-diluted sera were added to plate and incubated for 2 h at RT. Plate was washed and incubated sequentially with biotinylated anti-IgE detection antibody, then avidin-HRP reagent for 1 h at RT. The plate was washed; substrate solution was added and incubated for 30 min. The reaction was stopped by adding 0.18M H2SO4, and the absorbance was read at 450 nm with 570 nm wavelength correction.
Histological and Morphometric Evaluation of the Lungs
Lungs were perfused via the trachea with zinc formalin at a pressure of 20 cm H2O, excised from the chest cavity, processed, and embedded in paraffin. Five μm thick sections were stained with hematoxylin and eosin and examined in a blinded manner [24]. Periodic acid-Schiff stain was used to visualize the mucus-containing cells [19]. Lung morphometric analyses were performed as described previously [29]. Briefly, three randomly chosen non-overlapping alveolar tissue fields of H&E stained lung sections of all animals in each group were photographed at 200X. Uniformly enlarged photographic prints were overlaid with transparent measuring grids and the morphometric measurements were performed by an investigator blinded to the treatment assignment. The volume densities of airspaces and tissue were calculated [29], and alveolar wall thickness was determined. Means and SEMs were calculated for each treatment and statistical analysis was performed.
Statistical Analysis
Results were expressed as mean ± standard error of the mean (SEM). Statistically significant differences (P≤ 0.05) between groups were determined by analysis of variance using Fisher's protected least significant difference test and unpaired Student's t test (SAS 9.1, Cary, NC), or Mann-Whitney Rank Sum test for non-parametric data (SigmaStat 3.0.1., SPSS Inc., Chicago, IL).
RESULTS
BAL Pleocytosis Reflects Synergistic Interaction between Cockroach Allergen and Endotoxin
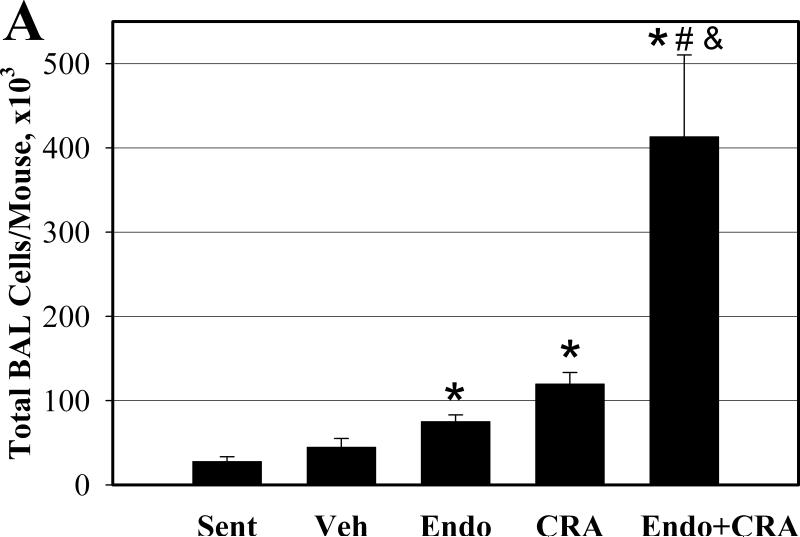
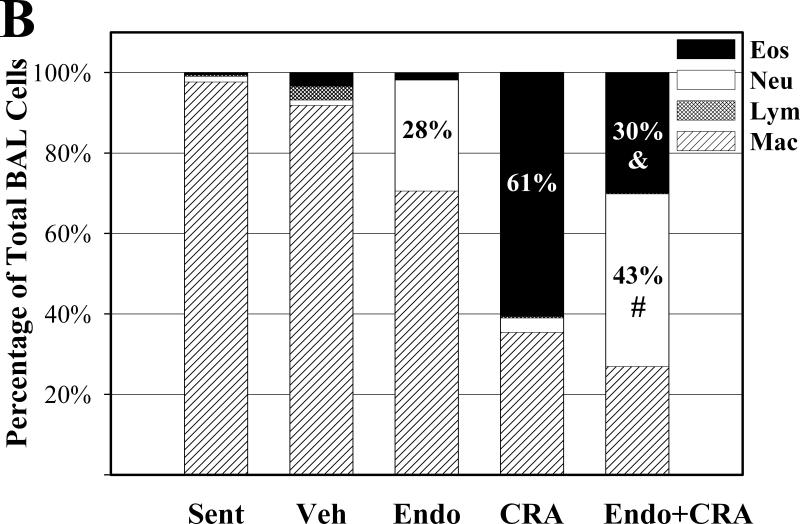
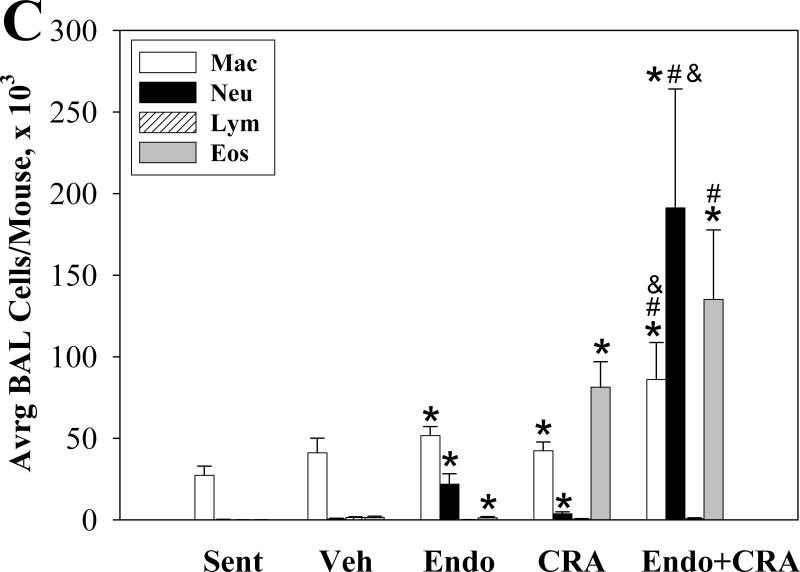
We determined the number and type of the inflammatory cells recovered from the BAL fluid to assess the inflammatory processes in the lungs. Inhalation of endotoxin, cockroach allergen, and the mixture of the two all resulted in a significant influx of total inflammatory cells into the airways (all P<0.001, compared to sentinels) (Figure 1A). The mixture induced 3.5-fold more total BAL cells than cockroach allergen alone, and 5.5-fold more than endotoxin alone (both P<0.02). Eosinophils, the principal effector cells in allergic lung inflammation, constituted 61% of BAL cells in animals exposed to cockroach allergen (Figure 1B). Neutrophils, on the other hand, constituted 28% of BAL cells in endotoxin-exposed animals. Cockroach allergen exposure also induced recruitment of macrophages and neutrophils (both P<0.05). Endotoxin exposure produced an increase in BAL fluid macrophages (P<0.01) and eosinophils (P<0.05) compared to sentinels (Figure 1C).
Figure 1.
BAL fluid total (A) and differential (B and C) cell counts following repeated intranasal exposures of young mice. Data are means ± SEM (A and C) of the absolute number of cells in BAL fluid. * P< 0.05 compared with Sentinel (Sent); # P< 0.05 compared with Endotoxin (Endo); & P<0.05 compared with Cockroach Allergen (CRA) Group. N=6-7 animals per group; Vehicle exposed animals (Veh, N=3).
While inhalation of the mixture resulted in recruitment of all BAL cell types into the airways compared to endotoxin alone or cockroach allergen inhalation alone, this increase was significant only for neutrophils and macrophages (Figure 1C). Specifically, the absolute number of neutrophils increased about 10-fold in mixture-exposed animals compared to mice exposed to endotoxin alone (P<0.01). This change represents about a 2-fold increase in the proportion of neutrophils among all BAL cells (P<0.001). While the number of eosinophils in BAL after inhalation of the mixture also increased, the change was not statistically significant (P=0.1).
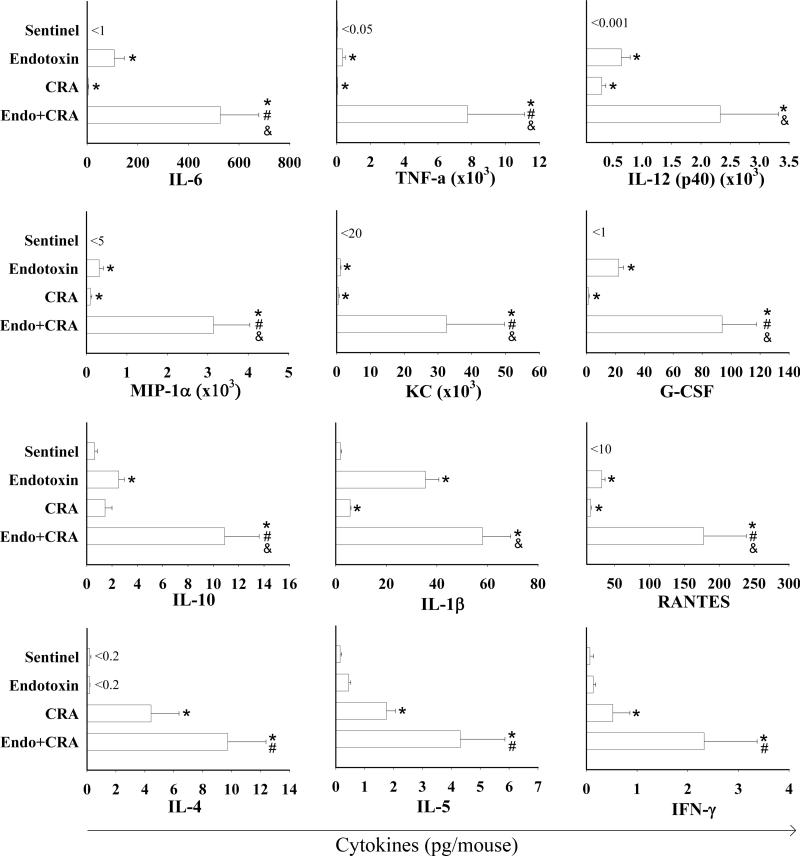
LPS and CRA Synergistically Up-regulate Secretion of Inflammatory Cytokines and Chemokines in the Lungs
Secretion of cytokines by activated inflammatory and epithelial cells in the airways contributes to exacerbation of asthmatic airways disease. Inhalation of endotoxin and cockroach allergen individually induced secretion of numerous inflammatory cytokines and chemokines in the lungs, many of which have been implicated in pathogenesis of asthma. Specifically, BAL of mice exposed to cockroach allergen alone and endotoxin alone showed elevated levels of IL-6, TNF-α, IL-12(p40), IL-1β, G-CSF, RANTES, MIP-1α, and KC (Figure 2). Inhalation of the LPS+CRA mixture enhanced the secretion of all these cytokines and chemokines several-fold. Specifically, levels of TNF-α increased 18-fold after co-exposure compared to endotoxin only, and the levels of the other cytokines and chemokines (except IL-1β) increased by 4-fold to 12-fold. The increase was statistically significant for all (compared to CRA), or most (compared to endotoxin) of these cytokines and chemokines (P<0.05) (Figure 2).
Figure 2.
Cytokines and chemokines (pg/mouse) measured in BAL fluid of young mice exposed to inhaled endotoxin, cockroach allergen (CRA) and the mixture of endotoxin and CRA. Data are expressed as mean + SEM. Symbols are: < below the indicated level of detection; * P<0.05 compared with Sentinels; # P<0.05 compared with Endotoxin; & P<0.05 compared with CRA Group.
Cockroach allergen also induced secretion of IL-4 (P<0.05), a cytokine important for stimulation of activated B cells and differentiation of T cells to Th2 lineage and IL-5 (P<0.005), a Th2 cytokine playing an important role in eosinophil function. Inhalation of the mixture increased the levels of IL-4 and IL-5 by more than 2-fold.
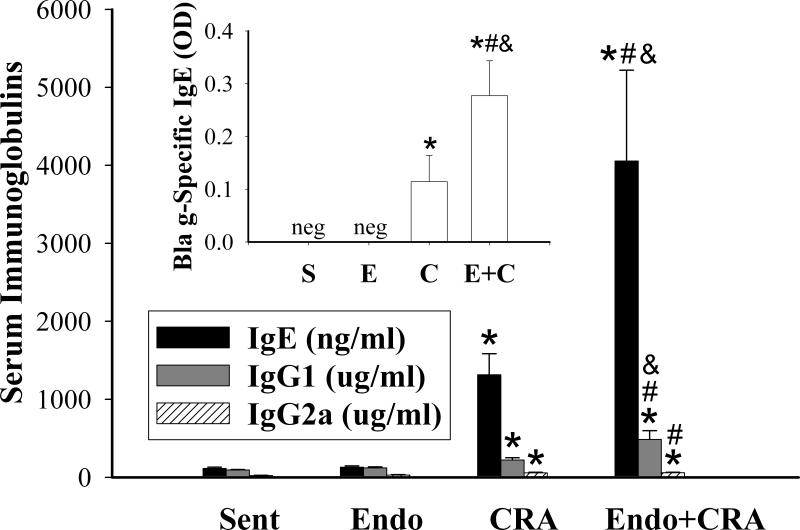
Endotoxin Augments Cockroach Allergen Induced Systemic IgE and IgG1 Production
Nasal mucosal exposures to cockroach allergen resulted in systemic allergic sensitization, as evidenced by increased levels of total IgE (P<0.001), cockroach allergen-specific IgE (P=0.002), and total IgG1 (P<0.003) (Figure 3). In addition, inhalation of cockroach allergen induced significant production of IgG2a (P<0.002). On the other hand, repeated inhalation of endotoxin did not affect the serum immunoglobulin levels compared to sentinels. When endotoxin was added to the cockroach allergen inhalation exposures, secretion of IgE and IgG1, but not IgG2a was significantly enhanced. Specifically, the levels of total IgE were 3-fold higher (P<0.03), the allergen-specific IgE levels were 2.5-fold higher (P<0.04), and the IgG1 levels were 2.2-fold higher (P<0.03) in the group exposed to the mixture compared to allergen alone. In contrast, the levels of IgG2a were not different between the mixture and cockroach exposure group. Thus, as evidenced by systemic levels of immunoglobulins IgE, IgG1 and IgG2a, the addition of endotoxin to inhaled cockroach allergen augmented primarily the Th2 systemic immune response.
Figure 3.
Levels of immunoglobulins (total IgE, IgG1, and IgG2a), in serum of mice exposed by inhalation to endotoxin, cockroach allergen, or mixture of the two. Inset: cockroach allergen-specific IgE in serum. Data are expressed as mean + SEM. Symbols are: neg = negative; * P<0.01, compared with Sentinels (S); # P<0.05 compared with Endotoxin (E); & P<0.05, compared with Cockroach Allergen Group (CRA).
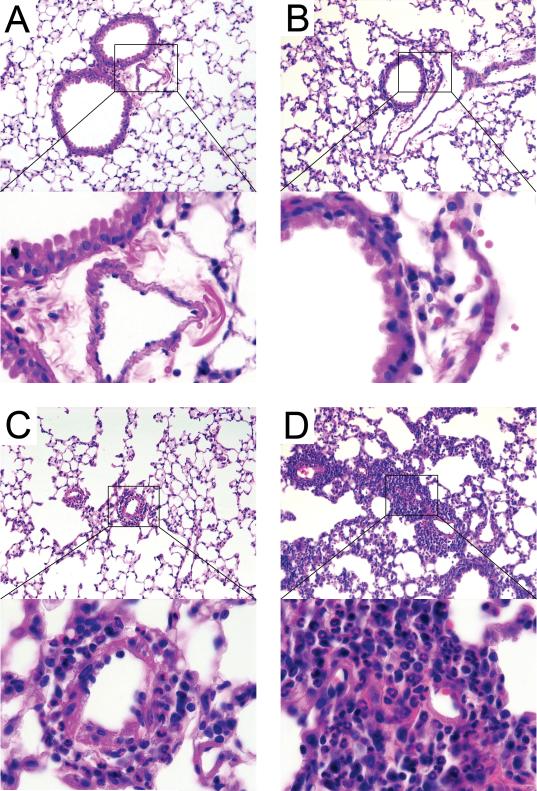
Cockroach Allergen- and Endotoxin-Induced Inflammatory Changes in the Lungs
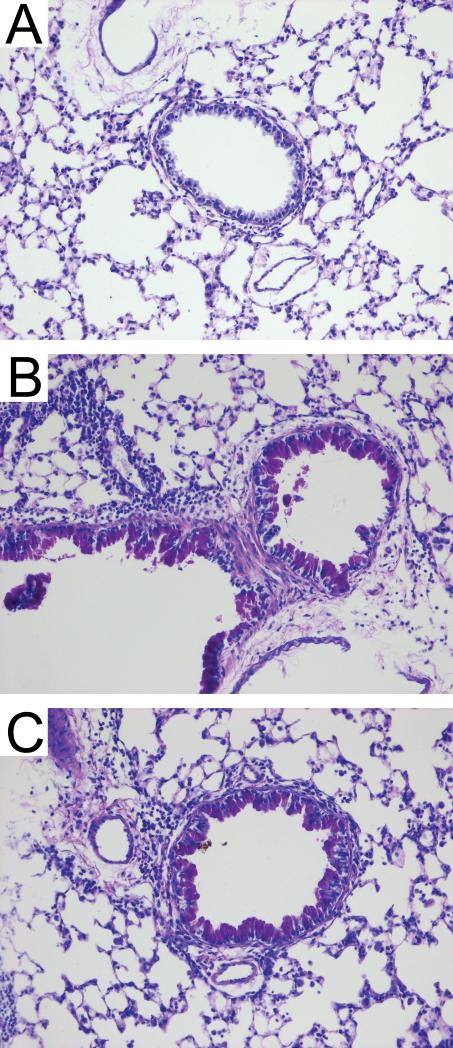
We examined lung histology to determine the effect of exposures on lung tissue in situ. Compared to non-exposed sentinel animals (Figure 4A), mice inhaling endotoxin developed neutrophilic inflammatory infiltrates surrounding primarily the small airways (Figure 4B). Mice inhaling cockroach allergen demonstrated pulmonary accumulation of eosinophils predominantly in perivascular regions of the lungs (Figure 4C). Mice inhaling a mixture of both endotoxin and allergen developed more extensive dense perivascular and peribronchiolar inflammatory infiltrates compared to mice inhaling either substance alone (Figure 4D). Finally, we noted the presence of numerous periodic acid-Schiff-positive goblet cells in lungs from mice given cockroach allergen alone and mice given the mixture of endotoxin and allergen (Figure 5), but not endotoxin alone (not shown).
Figure 4.
Representative photomicrographs of lung sections from an animal of each treatment group. Areas selected by the rectangle are shown at higher magnification (magnifications 20X and 100X, respectively). A – Sentinels; B – Endotoxin induced peribronchiolar (open arrow) neutrophilic infiltrates; C – Cockroach allergen induced predominantly perivascular (closed arrow) eosinophilic infiltrates; D – Mixture of endotoxin and cockroach allergen induced thick focal, both perivascular and peribronchiolar inflammatory infiltrates. H&E staining.
Figure 5.
Representative photomicrographs of lung sections from an animal of each treatment group: Sentinels (A); the epithelial cells metaplasia in juvenile mice exposed to cockroach allergen (B) and mixture of endotoxin and cockroach allergen (C). Periodic acid-Schiff staining; mucin producing goblet cells stain bright purple; magnification 20X.
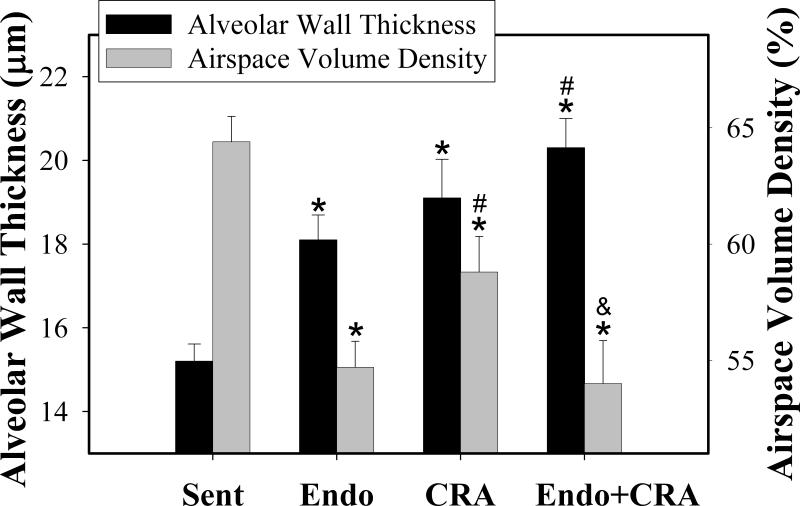
Inhaled Cockroach Allergen and Endotoxin Induce Alveolar Remodeling in Young Mice
Airways remodeling is an important consequence of asthmatic airways inflammation [30]. Repeated inhalation exposures to endotoxin and cockroach allergen during early life induced profound changes in the alveolar structure of the lungs. Morphometric analysis revealed significantly increased alveolar wall thickness in all the exposed groups with the greatest changes occurring in concomitantly exposed animals (expressed as mean ± SEM μm; endotoxin, 18.1 ± 0.6; cockroach allergen, 19.1 ± 0.9; co-exposure, 20.3± 0.7 μm; all P<0.003, compared to sentinel animals, 15.2 ± 0.4) (Figure 6). Furthermore, the airspace volume density was significantly decreased in endotoxin-exposed (mean % volume density ± SEM; 55±1.1 %; P<00.01), cockroach allergen-exposed (59±1.5 %; P<0.01), and, greater yet, in co-exposed animals (54±1.9 %; P<0.001), compared to sentinels (64±1.1 %) (Figure 6).
Figure 6.
Results of morphometric analysis of the alveolar tissues in the juvenile lungs. Alveolar wall thickness and airspace volume density were evaluated following repeated inhalation exposures with endotoxin, cockroach allergen, or the mixture of the two. Data are expressed as mean + SEM. Symbols are: *, P<0.05 compared with Sentinels; # P<0.05 compared with Endotoxin; & P<0.05, compared with CRA.
DISCUSSION
Our findings have three important ramifications. First, cockroach allergen alone, when administered by inhalation and without alum adjuvant, can induce systemic sensitization, allergic lung disease, and airway remodeling. Secondly, concurrent inhalation of endotoxin with cockroach allergen synergistically augments the immune, pulmonary inflammatory and remodeling responses. Thirdly, these data demonstrate the value of an animal model of allergic airways disease that utilizes concurrent inhalation of two common indoor environmental pollutants to mimic relevant early-life environmental exposures.
Exposure to cockroach allergen and endotoxin are independent major risk factors for the early development of allergies and asthma in inner-city children [1-7]. Inhalation exposures to these indoor-air pollutants begin in early infancy and may be especially relevant for children younger than 5 years [1, 3, 5]. As important constituents of house dust, endotoxin and cockroach allergen are most likely to be inhaled concurrently [1, 4]. The pulmonary and systemic effects of inhaled endotoxin have been thoroughly documented [24-26]. However, there is little information on the pulmonary and systemic effects of inhaled cockroach allergen. Nor is there information on the effect of concurrent exposures to endotoxin and cockroach allergen under conditions that mimic children's exposure— inhalation during early life.
To address this gap, we developed a model of early-life exposure to asthma triggers and examined the pulmonary and systemic immune responses to inhaled endotoxin and cockroach allergen, individually and combined. To model the relevant route and timing of children's exposures to house dust endotoxin and allergens we employed repeated intranasal exposures, initiated from early after birth to 3 weeks of age. Importantly, we limited exposure to the intranasal route. We demonstrated that exposure to inhaled cockroach allergen alone—in the absence of prior immunization with alum adjuvant— induced airway remodeling, pulmonary eosinophilia and neutrophilia, Th1-, Th2-associated cytokines, and innate cytokine and chemokine responses, and systemic production of class IgE, IgG1 and IgG2a immunoglobulins. Concurrent inhalation of cockroach allergen and endotoxin synergistically augmented lung innate immune responses and systemic IgE and IgG1 responses.
Our findings of pulmonary eosinophilia, Th2 cytokines in the BAL, and systemic allergen-specific IgE in response to inhaled cockroach allergen alone are comparable to studies of house dust mite allergen-inhalation induced lung disease [30, 32], which similarly do not require prior immunization. Our cockroach allergen model and the house dust mite allergen model stand in sharp contrast to the OVA-induced lung disease mouse model, which requires intraperitoneal or subcutaneous immunization with antigen plus alum adjuvant prior to OVA inhalation in order to elicit ovalbumin-specific IgE and eosinophilic response in the lungs [28]. In the latter model, repeated inhalation with OVA alone fails to elicit ovalbumin-specific responses, inducing mucosal and systemic antigen-specific tolerance instead [31].
It has been hypothesized that the enzymatic activity of an allergen is an important factor in its ability to induce allergen-specific IgE responses [16, 33]. The reports of allergic sensitization to inhaled Der p 1, a house dust mite allergen with protease activity, as well as the reports of immune tolerance to inhaled OVA, which lacks protease activity, support the “enzyme hypothesis” [13, 14, 16, 31, 32]. Moreover, blocking the proteolytic activity of Der p 1 reduces allergen-specific inflammatory effects [13], and adding an exogenous protease to inhaled OVA promotes an allergic inflammatory response [14]. However, many important allergens do not have enzymatic activity [16].
Whether the enzyme hypothesis can extend to cockroach allergen extract remains open to debate. Proteolytic activity has not been reported for the cockroach allergens cloned so far [16]; for example, the cockroach allergen Bla g 2 which is an aspartic protease homolog, showed no activity in a standard aspartic protease assay [33]. However, serine protease activity has been measured in extract of whole body of American cockroach [34, 35]. Furthermore, the ability of commercial preparations of whole body German cockroach extract to augment secretion of IL-6 and IL-8 by in vitro cultured human epithelial airway cells depended on serine protease activity [15]. These data suggest that cockroach extract contains allergens with active serine proteases. We point out that we utilized whole body German cockroach extract as our allergen.
In our model, cockroach allergen extract inhalation induced predominantly eosinophilic pulmonary inflammation with a neutrophilic component, secretion of numerous pro-inflammatory cytokines and chemokines in the airways, and systemic production of IgG1, IgG2a, and total and cockroach allergen-specific IgE immunoglobulins. Types of secreted cytokines and chemokines indicated that the both lung innate and adaptive immune responses were activated. Our finding of increased levels of TNF-α, IL-12(p40), IL-1β, IFN-γ, IL-4, G-CSF, RANTES, and KC in response to inhaled cockroach allergen is novel. We also detected increased production of MIP-1α, IL-5, and IL-6 in BAL in response to inhaled cockroach allergen, confirming previous reports [36, 37].
We detected significantly higher levels of IL-4, IL-5, and IFN-γ cytokine in BAL fluid of cockroach allergen-treated mice compared to sentinel mice, albeit at levels in the picogram/BAL range. Rarely did we detect lymphocytes in BAL of treated mice. Therefore the relative contribution of Th2 and Th1 cellular immunity to the local pulmonary response to cockroach allergen is difficult to ascertain from sampling of the BAL. Future studies of allergen-specific cytokine production by lymphocytes isolated from lung tissue would help clarify the role that local allergen-specific Th2 and Th1 CD4+ T-cells play in our model.
On the other hand, the total and allergen-specific IgE, and IgG1 antibodies levels indicate a role for Th2 cells in the systemic response. Cockroach-allergen treated mice showed increased levels of circulating allergen-specific IgE as well as total IgE and IgG1. Total IgE and IgG1 are valid surrogate markers for allergen-specific IgE and IgG1 [38]. Although we also measured increased levels of total IgG2a in cockroach-allergen treated mice, total IgG2a does not predict allergen-specific IgG2a levels [38]. Moreover, IgG2a production does not always require the Th1 cytokine, INF- γ [39, 40].
Co-exposure to endotoxin and cockroach allergen resulted in a synergistic increase in airway neutrophilia, most innate immune cytokines and chemokines, and the Th2-associated immunoglobulins (total and allergen-specific IgE and total IgG1) compared to endotoxin or cockroach allergen alone. Co-exposure also resulted in higher IL-4, IL-5 and IFN-γ levels in the BAL, though the increase was not synergistic.
Our finding of synergistically augmented innate immune responses in the lungs and Th2 responses in sera to inhaled mixture of endotoxin and cockroach allergen is novel. Previous murine studies of concurrent allergen and endotoxin exposures have reported either suppressed or enhanced systemic and pulmonary inflammatory responses depending on the type of allergen and route of exposure [9 - 12]. Ormstad et al. reported that concurrent administration of endotoxin and cat allergen induced significantly greater levels of allergen specific IgE, IgG1, and IgG2a [11]. However, the authors utilized subcutaneous injection into the footpad for the route of allergen and endotoxin exposure rather than inhalation, and they did not measure pulmonary responses. Studies utilizing co-exposures with endotoxin and ovalbumin have previously found lower immune responses in mice. For example, exposure to endotoxin-containing ovalbumin resulted in reduced allergic airway inflammation, decreased airway hyper-reactivity, and lower IgE production compared to the endotoxin-free ovalbumin [9, 10]. In the present studies we limited our investigation to the C3H/HeBFeJ because this strain has been extensively utilized in our endotoxin responsiveness studies in the past [24 - 26]. Future studies comparing the responses between C3H/HeBFeJ and BALB/c mice would be important to determine whether our findings are generalizable to allergy-prone strains.
Several mechanisms have been proposed by which endotoxin may augment responses to allergens. Specifically, engagement and activation of the LPS receptor TLR4 yields an enhanced dendritic cell maturation and expression of dendritic cell co-stimulatory molecules CD80 and CD86 [41]. This results in improved antigen presentation and more effective T-cell priming [42, 43]. Furthermore, endotoxin up-regulates expression and function of LPS- binding protein (LBP) that plays a crucial role in the process of allergic sensitization, as both TLR4- and LBP-knockout mice were unresponsive to allergen [44]. In addition, pretreatment of airway epithelial cells with TNF-α increased secretion of allergen-specific cytokines upon stimulation with cockroach allergen [15]. Finally, the endogenous proteolytic enzymes secreted by LPS-activated neutrophils can further augment allergen-specific immune and inflammatory responses. Specifically, neutrophil elastase and matrix metalloproteinase-9 secreted by activated neutrophils and other inflammatory cells contribute to destruction of epithelial tissues and facilitate transepithelial allergen delivery, thus perpetuating the inflammatory and immunogenic processes [18]. Altogether, this evidence suggests that endotoxin-induced inflammatory mediators can up-regulate allergen-specific pulmonary inflammation.
Interestingly, we noted nearly a 10-fold increase of neutrophils in BAL from mice exposed to the mixture compared to mice exposed to endotoxin alone. This increase exceeds what one would expect from the additional endotoxin content of the cockroach allergen. Because pulmonary neutrophilia is a common response to inhaled endotoxin [45, 46], our results suggest that cockroach allergen augmented the inflammatory effects of endotoxin. Compared to well-documented evidence for the role of endotoxin in augmenting the allergen responsiveness, there is relative paucity of data on allergen-driven augmentation of endotoxin-specific responses. Several mechanisms underlying this phenomenon have been proposed. Inhalation of proteolytic allergens, such as pollen and house dust mite, were documented to increase expression of CD14 on macrophages and neutrophils, and secretion of soluble CD14 (sCD14) into the airways lumen [47, 48]. Ragweed allergen, in addition, induced airway secretion of lipopolysaccharide binding protein [48]. Being important mediators of LPS-responsiveness, CD14, sCD14, and LBP facilitate LPS-TLR4 engagement, and up-regulate production of inflammatory cytokines, such as TNF-α and IL-6, among others.
Consistent with BAL indicators of lung inflammation, the magnitude of histological changes was significantly more pronounced after inhalation of the mixture. Importantly, we documented that inhalation of endotoxin and cockroach allergen both individually and as a mixture resulted in significant alveolar remodeling in the lungs. Animals exposed to LPS and CRA had significantly thicker alveolar walls and significantly decreased airspace volume density. Similar findings were described by others in mice inhaling endotoxin or house dust mite allergen, but not ovalbumin [30, 49]. Moreover, pulmonary remodeling changes in these studies were chronic and irreversible, persisting long after cessation of inhalation exposures, and resulted in pulmonary hyperresponsiveness [30]. Altogether, these findings are consistent with evidence in humans that suggests that airway remodeling begins early in life in infants with asthmatic airways disease, and is the basis for altered pulmonary responsiveness later in life [30].
In conclusion, to our knowledge this is the first report of using a clinically relevant exposure model to examine the effect of co-administered endotoxin and cockroach allergen on pulmonary and systemic responses. In this model of juvenile experimental lung disease, newborn and juvenile inhalation exposures with mixture of endotoxin and cockroach allergen induced an allergic pulmonary inflammation with significant neutrophilic component, alveolar remodeling, and robust systemic allergic sensitization. The effects of these combined exposures significantly exceeded effects induced by endotoxin and cockroach allergen inhaled individually. Importantly, effects of the combined exposures were greater than the sum of the individual effects, suggesting synergistic interaction between the endotoxin and cockroach allergen in development of airways disease and systemic allergic sensitization. The augmentation process between the endotoxin and cockroach allergen was bi-directional, with endotoxin augmenting allergen-specific effects and cockroach allergen augmenting endotoxin-specific effects, possibly employing diverse complementary mechanisms. Our findings underscore the importance of evaluating the effects of multiple and simultaneously acting environmental risk factors of asthma as they occur in real human environments. Such experimental designs will enhance the relevancy to human environmental exposures leading to environmentally-induced asthma and allergies.
Acknowledgments
Sources of Support: This study was supported by NIH P30 ES05605, NIH R01 HL059324.
REFERENCES
- 1.Litonjua AA, Milton DK, Celedon JC, Ryan L, Weiss ST, Gold DR. A longitudinal analysis of wheezing in young children: the independent effects of early life exposure to house dust endotoxin, allergens, and pets. J Allergy Clin Immunol. 2002;110(5):736–742. doi: 10.1067/mai.2002.128948. [DOI] [PubMed] [Google Scholar]
- 2.Michel O, Kips J, Duchateau J, Vertongen F, Robert L, Collet H, Pauwels R, Sergysels R. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1641–1646. doi: 10.1164/ajrccm.154.6.8970348. [DOI] [PubMed] [Google Scholar]
- 3.Park JH, Gold DR, Spiegelman DL, Burge HA, Milton DK. House dust endotoxin and wheeze in the first year of life. Am J Respir Crit Care Med. 2001;163:322–8. doi: 10.1164/ajrccm.163.2.2002088. [DOI] [PubMed] [Google Scholar]
- 4.Thorne PS, Kulhankova K, Yin M, Cohn R, Arbes SJ, Jr, Zeldin DC. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am J Resp Crit Care Med. 2005;172(11):1371–1377. doi: 10.1164/rccm.200505-758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn PW, Boudreau JO, He H, Wang Y, Chapman MD, Vincent C, Burge HA, Weiss ST, Perkins DL, Gold DR. Children at risk for asthma: home allergen levels, lymphocyte proliferation, and wheeze. J Allergy Clin Immunol. 2000;105(5):933–942. doi: 10.1067/mai.2000.106546. [DOI] [PubMed] [Google Scholar]
- 6.Gruchalla RS, Pongracic J, Plaut M, Evans R, 3rd, Visness CM, Walter M, Crain EF, Kattan M, Morgan WJ, Steinbach S, Stout J, Malindzak G, Smartt E, Mitchell H. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115(3):478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Arruda LK, Vailes LD, Ferriani VPL, Santos ABR, Pomés A, Chapman MD. Cockroach allergens and asthma. J Allergy Clin Immunol. 2001;107:419–428. doi: 10.1067/mai.2001.112854. [DOI] [PubMed] [Google Scholar]
- 8.Hunt LW, Gleich GJ, Ohnishi T, Weiler DA, Mansfield ES, Kita H, Sur S. Endotoxin contamination causes neutrophilia following pulmonary allergen challenge. Am J Resp Crit Care Med. 1994;149(6):1471–1475. doi: 10.1164/ajrccm.149.6.8004300. [DOI] [PubMed] [Google Scholar]
- 9.Delayre-Orthez C, Becker J, de Blay F, Frossard N, Pons F. Exposure to endotoxins during sensitization prevents further endotoxin-induced exacerbation of airway inflammation in a mouse model of allergic asthma. Int Arch Allergy Immunol. 2005;138(4):298–304. doi: 10.1159/000088867. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe J, Miyazaki Y, Zimmerman GA, Albertine KH, McIntyre TM. Endotoxin contamination of ovalbumin suppresses murine immunologic responses and development of airway hyper-reactivity. J Biol Chem. 2003;278(43):42361–42368. doi: 10.1074/jbc.M307752200. [DOI] [PubMed] [Google Scholar]
- 11.Ormstad H, Groeng EC, Duffort O, Lovik M. The effect of endotoxin on the production of IgE, IgG1 and IgG2a antibodies against the cat allergen Fel d 1 in mice. Toxicology. 2003;188(2-3):309–318. doi: 10.1016/s0300-483x(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 12.Pirie RS, Dixon PM, McGorum BC. Endotoxin contamination contributes to the pulmonary inflammatory and functional response to Aspergillus fumigatus extract inhalation in heaves horses. Clin Exp Allergy. 2003;33(9):1289–1296. doi: 10.1046/j.1365-2745.2003.01651.x. [DOI] [PubMed] [Google Scholar]
- 13.Gough L, Campbell E, Bayley D, Van Heeke G, Shakib F. Proteolytic activity of the house dust mite allergen Der p 1 enhances allergenicity in a mouse inhalation model. Clin Exp Allergy. 2003;33(8):1159–1163. doi: 10.1046/j.1365-2222.2003.01716.x. [DOI] [PubMed] [Google Scholar]
- 14.Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169(10):5904–5911. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 15.Bhat RK, Page K, Tan A, Hershenson MB. German cockroach extract increases bronchial epithelial cell interleukin-8 expression. Clin Exp Allergy. 2003;33(1):35–42. doi: 10.1046/j.1365-2222.2002.01481.x. [DOI] [PubMed] [Google Scholar]
- 16.Chapman MD, Pomes A, Breiteneder H, Ferreira F. Nomenclature and structural biology of allergens. J Allergy Clin Immunol. 2007;119(2):414–420. doi: 10.1016/j.jaci.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Wan H, Winton HL, Soeller C, Gruenert DC, Thompson PJ, Cannell MB, Stewart GA, Garrod DR, Robinson C. Quantitative structural and biochemical analyses of tight junction dynamics following exposure of epithelial cells to house dust mite allergen Der p 1. Clin Exp Allergy. 2000;30:685–698. doi: 10.1046/j.1365-2222.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- 18.Page K, Hughes VS, Bennett GW, Wong HR. German cockroach proteases regulate matrix metalloproteinase-9 in human bronchial epithelial cells. Allergy. 2006;61(8):988–995. doi: 10.1111/j.1398-9995.2006.01103.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Lee SY, Bak SM, Suh IB, Lee SY, Shin C, Shim JJ, In KH, Kang KH, Yoo SH. Effects of matrix metalloproteinase inhibitor on LPS-induced goblet cell metaplasia. Am J Physiol Lung Cell Mol Physiol. 2004;287:L127–L133. doi: 10.1152/ajplung.00047.2003. [DOI] [PubMed] [Google Scholar]
- 20.Kulhankova K, George CL, Kline JN, Thorne PS. Pulmonary and systemic responses to inhaled allergens are aggravated by inhaled endotoxin. J Allergy Clin Immunol. 2006;117(2):1226. [Google Scholar]
- 21.Kulhankova K, George CL, Kline JN, Thorne PS. Synergistic effect of cockroach allergens and endotoxin in development of airway disease and systemic sensitization. Proc Am Thoracic Soc. 2005;2:A98. [Google Scholar]
- 22.Kulhankova K, George CL, Kline JN, White M, Thorne PS. Inhalation of allergen results in systemic sensitization and upper and lower airways disease. Proc Am Thoracic Soc. 2005;2:A98. [Google Scholar]
- 23.Kulhankova K, Thorne PS, George CL, White M, O'Neill ME. Neutrophilic lung inflammation induced by aerosol inhalation and nasal aspiration of endotoxin in a newborn murine model. Proc Am Thoracic Soc. 2005;2:A531. [Google Scholar]
- 24.George CL, Jin H, Wohlford-Lenane CL, O'Neill ME, Phipps JC, O'Shaughnessy P, Kline JN, Thorne PS, Schwartz DA. Endotoxin responsiveness and subchronic grain dust-induced airway disease. Am J Physiol Lung Cell Mol Physiol. 2001;280(2):L203–213. doi: 10.1152/ajplung.2001.280.2.L203. [DOI] [PubMed] [Google Scholar]
- 25.Thorne PS. Inhalation toxicology models of endotoxin- and bioaerosol-induced inflammation. Toxicology. 2000;152(1-3):13–23. doi: 10.1016/s0300-483x(00)00287-0. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz DA, Thorne PS, Jagielo PJ, White GE, Bleuer SA, Frees KL. Endotoxin responsiveness and grain dust-induced inflammation in the lower respiratory tract. Am J Physiol Lung Cell Mol Physiol. 1994;267:L609–L617. doi: 10.1152/ajplung.1994.267.5.L609. [DOI] [PubMed] [Google Scholar]
- 27.Park JH, Spiegelman DL, Gold DR, Burge HA, Milton DK. Predictors of Airborne Endotoxin in the Home. Environ Health Perspect. 2000;108(11):1023–1028. doi: 10.1289/ehp.001081023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kline JN, Kitagaki K, Businga TR, Jain VV. Treatment of established asthma in a murine model using CpG oligodeoxynucleotides. Am J Physiol Lung Cell Mol Physiol. 2002;283(1):L170–179. doi: 10.1152/ajplung.00402.2001. [DOI] [PubMed] [Google Scholar]
- 29.Snyder JM, Jenkins-Moore M, Jackson SK, Goss KL, Dai HH, Bangsund PJ, Giguere V, McGowan SE. Alveolarization in retinoic acid receptor-beta-deficient mice. Pediatr Res. 2005;57(3):384–391. doi: 10.1203/01.PDR.0000151315.81106.D3. [DOI] [PubMed] [Google Scholar]
- 30.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004;169(3):378–385. doi: 10.1164/rccm.200308-1094OC. [DOI] [PubMed] [Google Scholar]
- 31.Van Hove CL, Maes T, Joos GF, Tournoy KG. Prolonged inhaled allergen exposure can induce persistent tolerance. Am J Respir Cell Mol Biol. 2007;36:573–584. doi: 10.1165/rcmb.2006-0385OC. [DOI] [PubMed] [Google Scholar]
- 32.Yu CK, Shieh Ch-M, Lei H-Y. Repeated intratracheal inoculation of house dust mite (Dermatophagoides farinae) induces pulmonary eosinophilic inflammation and IgE antibody production in mice. J Allergy Clin Immunol. 1999;104(1):228–236. doi: 10.1016/s0091-6749(99)70140-5. [DOI] [PubMed] [Google Scholar]
- 33.Pomes A, Chapman MD, Vailes LD, Blundell TL, Dhanaraj V. Cockroach allergen Bla g 2. Structure, Function, and Implications for Allergic Sensitization. Am J Respir Crit Care Med. 2002;165:391–397. doi: 10.1164/ajrccm.165.3.2104027. [DOI] [PubMed] [Google Scholar]
- 34.Wongtim S, Lehrer SB, Salvaggio JE, Horner WE. Protease Activity in Cockroach and Basidiomycete Allergen Extracts. Allergy Asthma Proc. 1993;14:(4)263–268(6). doi: 10.2500/108854193778811946. [DOI] [PubMed] [Google Scholar]
- 35.Iraneta SG, Duschak VG, Rodriguez SM, Seoane MA, Albonico JF, Alonso A. Proteinase and gelatinolytic activities of house dust mite and cockroach extracts. J Investig Allergol Clin Immunol. 1999;9(4):235–40. [PubMed] [Google Scholar]
- 36.Papouchado BG, Chapoval SP, Marietta EV, Weiler CR, David CS. Cockroach allergen-induced eosinophilic airway inflammation in HLA-DQ/human CD4(+) transgenic mice. J Immunol. 2001;167(8):4627–4634. doi: 10.4049/jimmunol.167.8.4627. [DOI] [PubMed] [Google Scholar]
- 37.Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol. 1998;161(12):7047–7053. [PubMed] [Google Scholar]
- 38.Lewkowich IP, Rempel JD, HayGlass KT. Antigen-specific versus total immunoglobulin synthesis: Total IgE and IgG1, but not IgG2a levels predict murine antigen-specific responses. Int Arch Allergy Immunol. 2004;133:145–153. doi: 10.1159/000076440. [DOI] [PubMed] [Google Scholar]
- 39.Markine-Goriaynoff D, van der Logt JTM, Truyens C, Nguyen TD, Heessen FWA, Bigaignon G, Carlier Y, Coutelier J-P. IFN-γ-independent IgG2a production in mice infected with viruses and parasites. Int Immunol. 2000;12(2):223–230. doi: 10.1093/intimm/12.2.223. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimoto T, Okada K, Morishima N, Kamiya S, Owaki T, Asakawa M, Iwakura Y, Fukai F, Mizuguchi J. Induction of IgG2a Class Switching in B Cells by IL-27. J Immunol. 2004;173:2479–2485. doi: 10.4049/jimmunol.173.4.2479. [DOI] [PubMed] [Google Scholar]
- 41.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196(12):1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dabbagh K, Dahl ME, Stepick-Biek P, Lewis DB. Toll-like receptor 4 is required for optimal development of Th2 immune responses: role of dendritic cells. J Immunol. 2002;168(9):4524–4530. doi: 10.4049/jimmunol.168.9.4524. [DOI] [PubMed] [Google Scholar]
- 43.Barrios CS, Johnson BD, Henderson JR JD, Fink JN, Kelly KJ, Kurup VP. The costimulatory molecules CD80, CD86 and OX40L are up-regulated in Aspergillus fumigatus sensitized mice. Clin Exp Immunol. 2005;142(2):242–250. doi: 10.1111/j.1365-2249.2005.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strohmeier GR, Walsh JH, Klings ES, Farber HW, Cruikshank WW, Center DM, Fenton MJ. Lipopolysaccharide binding protein potentiates airway reactivity in a murine model of allergic asthma. J Immunol. 2001;166(3):2063–2070. doi: 10.4049/jimmunol.166.3.2063. [DOI] [PubMed] [Google Scholar]
- 45.Koyama S, Rennard SI, Leikauf GD, Shoji S, Von Essen S, Claassen L, Robbins RA. Endotoxin stimulates bronchial epithelial cells to release chemotactic factors for neutrophils. A potential mechanism for neutrophil recruitment, cytotoxicity, and inhibition of proliferation in bronchial inflammation. J Immunol. 1991;147(12):4293–4301. [PubMed] [Google Scholar]
- 46.Snella MC, Rylander R. Endotoxin inhalation induces neutrophil chemotaxis by alveolar macrophages. Inflamm Res. 1985;16(6):521–526. doi: 10.1007/BF01983657. [DOI] [PubMed] [Google Scholar]
- 47.Virchow JC, Jr, Julius P, Matthys H, Kroegel C, Luttmann W. CD14 expression and soluble CD14 after segmental allergen provocation in atopic asthma. Eur Respir J. 1998;11:317–323. doi: 10.1183/09031936.98.11020317. [DOI] [PubMed] [Google Scholar]
- 48.Dubin W, Martin TR, Swoveland P, Leturcq DJ, Moriarty AM, Tobias PS, et al. Asthma and endotoxin: lipopolysaccharide-binding protein and soluble CD14 in bronchoalveolar compartment. Am J Physiol. 1996;270(5):L736–744. doi: 10.1152/ajplung.1996.270.5.L736. [DOI] [PubMed] [Google Scholar]
- 49.Vernooy JHJ, Dentener MA, van Suylen RJ, Buurman WA, Wouters EFM. Long-term intratracheal lipopolysaccharide exposure in mice results in chronic lung inflammation and persistent pathology. Am J Respir Cell Mol Biol. 2002;26:152–159. doi: 10.1165/ajrcmb.26.1.4652. [DOI] [PubMed] [Google Scholar]