Abstract
The cell’s ability to sense and respond to DNA damage is critical to maintain homeostasis and prevent the development of cancer. Paradoxically, Economopoulou et al. recently reported that a DNA damage response protein, H2AX, promotes tumor growth and angiogenesis.
The DNA damage response pathway is crucial to maintaining genomic stability and cellular homeostasis. Toxic DNA lesions such as DNA double-strand breaks (DSBs) are frequently generated by endogenous sources, including the byproductsof metabolism, e.g., reactive oxygen species (ROS), spontaneous depurination of DNA strands, and replication fork collapse. Exogenous agents such as chemicals, ultraviolet radiation, and ionizing radiation also contribute to the formation of DSBs. Importantly, the inability to repair DSBs can result in genomic instability, cell death, and cancer (Fillingham et al., 2006).
Immediately following the generation of a DSB, a highly conserved DNA damage response pathway is activated to halt cell-cycle progression and repair the lesion. The molecular response to DNA damage begins with the recognition of the DSB followed by the activation of phosphatidyl inosital-3-kinase-like kinase family members ATM, ATR, and DNA-PK. Once activated, these kinases phosphorylate a number of effector molecules that regulate cell-cycle progression, DNA damage repair, and apoptosis. Histone H2AX is an important effector of the DNA damage response pathway that recruits DSB break recognition and repair proteins to the break (Bonner et al., 2008).
Consistent with a role for H2AX in DNA damage responses, recent studies have suggested that H2AX may function as a tumor suppressor. The chromosomal region (11q23) harboring H2AX is mutated or deleted in a variety of human cancers, including leukemia, breast, and head and neck cancers (Bonner et al., 2008). In addition, genetic inactivation of H2AX results in increased tumor burden in p53-deficient mice (Bassing et al., 2003).
Unexpectedly, a recent study in Nature Medicine has revealed a positive role for H2AX in tumorigenesis. Economopoulou et al. demonstrate that genetic inactivation of H2AX is sufficient to suppress tumor angiogenesis and growth in xenograft models (Economopoulou et al., 2009). Moreover, the authors demonstrate that specific inactivation of H2AX in endothelial cells similarly suppressed tumor angiogenesis and growth, indicating that H2AX and the DNA damage response in endothelial cells (ECs) play significant roles in tumor angiogenesis. Because hypoxia plays a critical role in the induction of tumor angiogenesis and has been previously shown to activate H2AX (Bencokova et al., 2009; Hammond et al., 2003), the authors examined the contribution of hypoxia to H2AX activation in ECs. In vitro studies revealed that hypoxia is sufficient to induce H2AX phosphorylation (γ-H2AX) and H2AX-dependent EC proliferation. Furthermore, the authors provide strong genetic data demonstrating a role for H2AX in additional models of hypoxia-induced neovascularization, including pathologic proliferative retinopathy and hind limb ischemia (Economopoulou et al., 2009). Together, these findings demonstrate that H2AX is an important component of hypoxia-induced angiogenesis and raise important questions regarding the mechanisms of H2AX-induced angiogenesis.
γ-H2AX may be induced in hypoxic ECs through replicative stress. Recent studies haveidentified hypoxia as aunique cellular stress that has the capacity to activate the DNA damage response pathway through damage-independent mechanisms. Indeed, we found that severe hypoxia (0.02% oxygen) is sufficient to activate ATR and subsequent H2AX phosphorylation in the absence of DNA breaks (Bencokova et al., 2009; Hammond et al., 2003). In this setting, activation of ATR is thought to result from the accumulation of single-stranded DNA at stalled replication forks (Bencokova et al., 2009). Interestingly, Economopoulou et al. found that endothelial cells exposed to moderate hypoxia (1% oxygen) also induced γ-H2AX in an ATR-dependent manner. Additionally, they found that γ-H2AX foci colocalized with the single-strand DNA-binding protein RPA, indicating that the accumulation of single-stranded DNA may be responsible for H2AX phosphorylation in hypoxic endothelial cells. Whether these findings can be extrapolated in vivo requires further investigation. It is important to note that the in vivo models used in this study include subcutaneous-implanted tumors, pathologic proliferative retinopathy, and hind limb ischemia, which induce hypoxia and ischemia/ reperfusion. In tumors, cellular ischemia/reperfusion occurs as a result of heterogeneous blood flow due to the irregularity of vessels (Brown and Giaccia, 1998). It is clear that reoxygenation, through the production of ROS, can induce significant levels of DNA damage and DNA damage repair (Hammond et al., 2003). Interestingly, the production of ROS following ischemia/reperfusion has also been linked to angiogenesis (Maulik, 2002). Whether reoxygenation and ROS contribute to H2AX-mediated angiogenesis remains to be determined. Understanding the mechanism(s) of H2AX phosphorylation during hypoxia-induced angiogenesis will aid in the identification of new therapeutic targets for the inhibition of angiogenesis.
Additionally, understanding the molecular mechanisms by which H2AX regulates hypoxia-induced angiogenesis will aid in elucidating the role of the DNA damage response pathway in angiogenesis. The authors hypothesized that H2AX, through its DNA repair function, would play an important role in maintaining EC proliferation during hypoxia-induced neovascularization. In support of this hypothesis, H2AX inactivation significantly reduced EC proliferation under hypoxic conditions. Moreover, H2AX deficiency was associated with decreased endothelial cell proliferation in all in vivo models that they tested. However, it remains unclear whether the DNA repair function of H2AX is involved in the regulation of EC proliferation and angiogenesis under hypoxia. During the DNA damage response, H2AX recruits a number of DNA repair factors, including NuA4, INO80, SWRC, MCPH1, BRCA1, CHK1, NBS1, and cohesin (Bonner et al., 2008). If H2AX repair function is involved in hypoxia-induced angiogenesis, one would hypothesize that downstream signaling proteins may also contribute to angiogenesis.
Regardless of the mechanistic details, Economopoulou et al. have identified a novel role for histone H2AX in hypoxia-triggered angiogenesis. These findings suggest that, in addition to the transcriptional induction of proangiogenic gene expression, hypoxia can also influence angiogenesis through alternate routes such as the DNA repair pathway (Figure 1). Given the central role for hypoxia-inducible transcription factors (HIF-1 and HIF-2) in the cellular response to hypoxia (Maxwell and Ratcliffe, 2002), it will be important to determine whether the hypoxic regulation of H2AX activity is also HIF dependent. The findings outlined in this study have important clinical implications for human disease, as aberrant activation of angiogenesis contributes to many pathologic conditions, including cancer, proliferative retinopathies, and inflammatory disorders (Carmeliet, 2005). An intriguing finding from this study is that pathological, but not developmental, hypoxia-driven angiogenesis is regulated by H2AX. This implies that either the activation of H2AX or the role of H2AX in angiogenesis differs between these states. Elucidating the molecular mechanisms of H2AX activation and H2AX-mediated angiogenesis will aid in the development of novel angiogenic inhibitors.
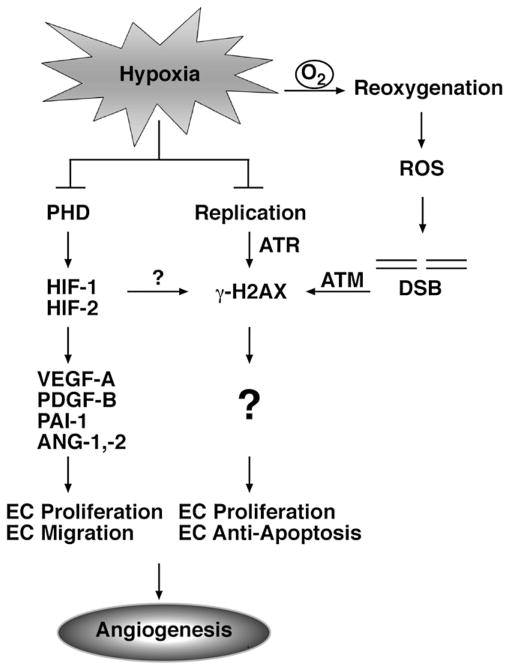
Figure 1. Hypoxia-Inducible Transcription Factors and γ-H2AX Are Mediators of Hypoxia-Induced Angiogenesis.
Hypoxia-inducible transcription factors (HIF-1 and HIF-2) are central mediators of hypoxia-induced angiogenesis. HIF activity is induced during hypoxia through the inhibition of oxygen-dependent proteasomal degradation. Prolyl-4-hydroxylase (PHD) enzymes utilize molecular oxygen as a substrate to hydroxylate and target HIF for proteasomal degradation. Under hypoxia, HIF-1 and HIF-2 are stabilized and activate the expression of proangiogenic genes, including Angiopoietin 1 (ANG-1); Angiopoietin 2 (ANG-2); Plasminogen activator inhibitor-1 (PAI-1); platelet-derived growth factor-B (PDGF-B); and vascular endothelial growth factor (VEGF).
Economopoulou et al. have revealed an important role for H2AX in hypoxia-induced angiogenesis. H2AX activity can be induced under hypoxia through ATR- and ATM-dependent mechanisms. Under hypoxia, ATR phosphorylates H2AX in response to the accumulation of single-stranded DNA at stalled replication forks. Additionally, ATM phosphorylates H2AX in response to DSBs generated by ROS during reoxygenation. Economopoulou et al. demonstrate that H2AX is important for maintaining endothelial cell proliferation under hypoxic conditions.
References
- Bassing CH, Suh H, Ferguson DO, Chua KF, Manis J, Eckersdorff M, Gleason M, Bronson R, Lee C, Alt FW. Cell. 2003;114:359–370. doi: 10.1016/s0092-8674(03)00566-x. [DOI] [PubMed] [Google Scholar]
- Bencokova Z, Kaufmann MR, Pires IM, Lecane PS, Giaccia AJ, Hammond EM. Mol Cell Biol. 2009;29:526–537. doi: 10.1128/MCB.01301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. Nat Rev Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Giaccia AJ. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- Carmeliet P. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Economopoulou M, Langer HF, Celeste A, Orlova VV, Choi EY, Ma M, Vassilopoulos A, Callen E, Deng C, Bassing CH, et al. Nat Med. 2009;15:553–558. doi: 10.1038/nm.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingham J, Keogh MC, Krogan NJ. Biochem Cell Biol. 2006;84:568–577. doi: 10.1139/o06-072. [DOI] [PubMed] [Google Scholar]
- Hammond EM, Dorie MJ, Giaccia AJ. J Biol Chem. 2003;278:12207–12213. doi: 10.1074/jbc.M212360200. [DOI] [PubMed] [Google Scholar]
- Maulik N. Antioxid Redox Signal. 2002;4:805–815. doi: 10.1089/152308602760598963. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Ratcliffe PJ. Semin Cell Dev Biol. 2002;13:29–37. doi: 10.1006/scdb.2001.0287. [DOI] [PubMed] [Google Scholar]