Abstract
The classical cadherins are known to have both adhesive and signaling functions. It has also been proposed that localized regulation of cadherin activity may be important in cell assortment during development. In the context of eye development, it has been suggested that cadherins are important for separation of the invaginated lens vesicle from the surface ectoderm. To test this hypothesis, we conditionally deleted N-cadherin or E-cadherin from the presumptive lens ectoderm of the mouse. Conditional deletion of either cadherin alone did not produce a lens vesicle separation defect. However, these conditional mutants did exhibit common structural deficits, including microphthalmia, severe iris hyperplasia, persistent vacuolization within the fibre cell region, and eventual lens epithelial cell deterioration. To assess the co-operative roles of E-cadherin and N-cadherin within the developing lens, double conditional knockout embryos were generated. These mice displayed distinct defects in lens vesicle separation and persistent expression of another classical cadherin, P-cadherin, within the cells of the persistent lens stalk. Double mutant lenses also exhibited severe defects in lens epithelial cell adhesion and survival. Finally, the severity of the lens phenotype was shown to be sensitive to the number of wild-type E- and N-cadherin alleles. These data suggest that the co-operative expression of both E- and N-cadherin during lens development is essential for normal cell sorting and subsequent lens vesicle separation.
INTRODUCTION
The formation of tissues during embryogenesis depends largely upon close interactions between neighboring cells. The ocular lens is no exception, as this tissue relies on the maintenance of epithelial cell-cell contacts throughout the morphogenetic process (Zelenka, 2004). The vertebrate lens arises from a series of interactions and inductive signals between the surface ectoderm, and the underlying mesenchyme and neuroectoderm (Lang, 2004). The surface ectoderm, a thin epithelial sheet, thickens to give rise to the lens placode. The placodal epithelial cells invaginate and separate from the overlying ectoderm to give rise to the lens vesicle. As the lens vesicle matures, the posteriorly positioned epithelial cells elongate and differentiate giving rise to primary fibre cells. Thus, all of the cells comprising the mature lens are derived from a common ancestor that is epithelial in origin and it is the adhesive properties of these epithelial cells that maintain the general lens architecture during and following lens morphogenesis. Furthermore, cell adhesion molecules that maintain this adhesiveness are not only required for maintaining the structural/architectural properties of the cell, but are also critical to the regulation of cell differentiation and development (Fagotto and Gumbiner, 1996).
One of the most influential adhesive interactions necessary for the development and maintenance of tissues is that mediated by the cadherins. Cadherins comprise a family of calcium-dependent cell adhesion molecules which undergo homophilic interactions in order to maintain cell-cell contacts at adherens junctions (Perez-Moreno et al., 2003). The classical cadherins, including E-, N-, P- and R-cadherin, are the most well studied members of the cadherin family and share a great degree of structural similarity (Goodwin and Yap, 2004). They are single-pass transmembrane glycoproteins that mediate cell-cell adhesion through their extracellular amino terminus, consisting of tandem repeats of domains carrying negatively charged amino acids. The cytoplasmic tail, in turn, associates with various intracellular proteins, including the catenins (β-catenin, α-catenin, p120-catenin). This complex of proteins, comprise the “core” cadherin-catenin complex and serves as a scaffold to which other proteins bind and link the cadherin molecule to the actin cytoskeleton (Abe and Takeichi, 2008; Kemler, 1993). Thus, the cadherin-catenin adhesion complex is essential for the polarization and function of epithelial cells and for the integrity of various cell strata (Tepass et al., 2000).
Cadherins are differentially expressed during development, and their expression is often restricted to specific cell types within a tissue (Edelman, 1985; Edelman and Crossin, 1991; Takeichi, 1988; Takeichi, 1991; Takeichi et al., 1990). In the lens these classical cadherin molecules (E, N, P) possess distinct expression patterns throughout development as evidenced by in situ hybridization and immunofluorescent analyses (Pontoriero et al., 2008; Takeichi, 1988; Xu et al., 2002). At early stages, both E- and P-cadherin are highly expressed in the surface ectoderm and developing lens placode. As the lens vesicle separates from the overlying surface ectoderm, P-cadherin expression is gradually lost while E-cadherin expression is maintained in the lens vesicle. At the same time, this event is accompanied by increased expression of N-cadherin within the developing lens vesicle. As the lens matures, E-cadherin expression is preserved within the lens epithelium while N-cadherin is conserved in both lens epithelial and fibre cell compartments. The implications of this differential expression are not known, but one proposed function is to mediate cell sorting, whereby the switching in expression of different cadherins results in the aggregation of cells into separate populations that express the same cadherin (Friedlander et al., 1989; Nose et al., 1988; Steinberg and Takeichi, 1994; Takeichi, 1988; Takeichi et al., 1981). It has been recently proposed that cadherin switching at this stage contributes to the events leading to lens vesicle separation (Pontoriero et al., 2008; Wheelock et al., 2008). However, this hypothesis has yet to be directly tested, as loss of function germline mutation of E-cadherin and N-cadherin results in embryonic lethality that precedes lens vesicle separation (Larue et al., 1994; Radice et al., 1997).
Although E-cadherin and N-cadherin are highly expressed in the lens during development, little is known about the intrinsic role(s) for E-cadherin- and N-cadherin-mediated cellular adhesion and signaling during lens morphogenesis. Thus, the aim of this study was to eliminate E-cadherin and N-cadherin expression specifically from the developing lens utilizing Cre-recombinase-mediated conditional deletion. Lens-Cre-mediated deletion (Ashery-Padan et al., 2000) of either E- or N-cadherin resulted in severe microphthalmia and iris hyperplasia along with lens epithelial and fibre cell defects. However, no defects in lens separation were observed. Cadherin molecules have been previously shown to functionally compensate for one another. Ectopic expression of E-cadherin within the myocardium of N-cadherin-null mice restored myocyte adhesion and cardiac looping (Luo et al., 2001). Considering E- and N-cadherin possess an overlapping expression pattern within the developing lens, the possibility of cadherin compensation could thus not be ruled out. As such, simultaneous conditional deletion of E- and N-cadherin was performed and resulted in complete lens deterioration during postnatal development. Interestingly, the lens phenotype observed within these mice varied depending on the number of genetic copies of either E- or N-cadherin. Analysis of embryonic stages of double mutant mice revealed residual adhesion of the lens vesicle with the surface ectoderm, suggesting that E- and N-cadherin play an important role during lens vesicle separation.
MATERIALS AND METHODS
Animal Maintenance and Use
Animals were housed in a pathogen-free vivarium in accordance with institutional policies. All animal studies were carried out in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and the Canadian Council on Animal Care guidelines. Gestational age was determined through detection of a vaginal plug. Day 0.5 of embryogenesis was defined as noon of the day of the appearance of the vaginal plug (E0.5). At specific gestational ages, fetuses were removed by hysterectomy after the dams had been anesthetized with isoflurane.
Generation of Mouse Lines
In order to generate a strains of mice possessing either single or double conditional knockouts of cadherin-1 (Cdh1), the gene encoding E-cadherin, and/or cadherin-2 (Cdh2), the gene encoding N-cadherin, specifically within the developing lens placode the following transgenic mouse lines were generated previously and employed: heterozygous Lens-Cre mice (Ashery-Padan et al., 2000), heterozygous E-cadherin knockout mice (Larue et al., 1994), homozygous E-cadherin floxed mice (Boussadia et al., 2002), heterozygous N-cadherin mutant mice (Radice et al. 1997), and homozygous N-cadherin floxed mice (Kostetskii et al., 2005). Resultant mutant progeny were compared with control littermates lacking expression of the Cre transgene.
Genotyping
DNA from embryonic yolk sac or adult mouse ear tissue was extracted using the DNeasy tissue kit (Qiagen). Mice genotypes were determined with PCR using protocols that have been previously described. For the detection of the Cre transgene, forward primer, Cre1 (5′-GCT GGT TAG CAC CGC AGG TGT AGA G-3′), and reverse primer, Cre3 (5′-CGC CAT CTT CCA GCA GGC GCA CC-3′), were used. PCRs ran for 35 cycles (45 seconds at 95°C, 45 seconds at 67°C, and 1.5 minutes at 72°C) generating a 420 bp fragment. For the detection of the E-cadherin floxed allele, forward primer, pE10.2 (5′-CTT ATA CCG CTC GAG AGC CGG A-3′), and reverse primer, pE11as.2 (5′-GTG TCC CTC CAA ATC CGA TA-3′), were used. PCRs ran for 35 cycles (45 seconds at 94°C, 45 seconds at 65°C, and 1.5 minutes at 72°C) producing products of 900 and 980 bp for the wild-type and floxed alleles, respectively. To detect the Cdh1 knockout allele, forward primer, NeoF (5′-AGG TGA GAT GAC AGG AGA TC-3′), and reverse primer, NeoR (5′-CTT GGG TGG AGA GGC TAT TC-3′), detecting the neor cassette were used. PCRs ran for 35 cycles (30 seconds at 94°C, 45 seconds at 62°C, and 1 minute at 72°C) generating a 280 bp fragment. To detect the Cdh2 mutant allele, the primers, 5′-CGT GTT CCG GCT GTC AGC GCA GG-3′ and 5′-CAA CGC TAT GTC CTG ATA GCG GTC C-3′, detecting the neor cassette were used. PCRs ran for 30 cycles (1 minute at 94°C, 2 minutes at 65°C, and 3 minutes at 72°C). To detect the Cdh2 floxed allele, forward primer, L07 (5′-TGC TGG TAG CAT TCC TAT GG-3′), and reverse primer L08 (5′-TAC AAG TTT GGG TGA CAA GC-3′), were utilized. PCRs ran for 35 cycles (30 seconds at 94°C, 45 seconds at 65°C, and 1 minute at 72°C) generating 640 bp and 600 bp fragments for the floxed and wild-type alleles, respectively.
Histology
Specimens were collected from mutant and control mice at various embryonic and postnatal timepoints. Tissue was either fixed in 10% neutral buffered formalin overnight at 4°C, processed and embedded in paraffin or fixed in 4% paraformaldehyde overnight at 4°C, cryo-preserved in a 30% sucrose/PBS solution and embedded in Tissue-Tek® OCT compound. Serial sections were cut at 5 μm in thickness and stained with hematoxylin and eosin (H&E), or used for subsequent experiments.
Immunohistochemistry
Indirect immunofluorescence was used to detect protein expression in wild-type and mutant embryonic mouse eyes at various stages. 5 μm thick frozen tissue sections were washed in PBS. Sections were blocked with normal serum and incubated with either mouse monoclonal anti-E-cadherin (1:200, BD Transduction Laboratories, Franklin Lakes, NJ), rabbit polyclonal anti-N-cadherin (1:500, Abcam Inc., Cambridge, MA), mouse monoclonal anti-ZO-1 (1:500, Invitrogen), mouse monoclonal anti-β-catenin (1:200, BD Transduction Laboratories), rabbit polyclonal anti-MIP26 (1:200, kindly provided by Dr. Joseph Horwitz (Jules Stein Eye Institute, Los Angeles, CA)), mouse anti-βB1-crystallin (1:200, kindly provided by Dr. Joseph Horwitz), rabbit polyclonal anti-Pax6 (Covance, Princeton, NJ, 1:250), goat polyclonal anti-calretinin (Santa Cruz Biotechnology, Santa Cruz, CA, 1:800), mouse anti-syntaxin-1 (Sigma-Aldrich, Oakville, ON, 1:2000), mouse monoclonal anti-AP-2α (3B5; University of Iowa, used neat), or rabbit anti-Foxe3 (1:2000, kindly provided by Dr. Peter Carlsson (University of Gothenburg, Gothenburg, Sweden)) primary antibodies overnight at 4°C. The locations of these antigens were then revealed using Alexa Fluor-labelled fluorescent secondary antibodies (Invitrogen – Molecular Probes, Burlington, ON). After repeated washing, sections were either counterstained with Alexa-Fluor-labelled phalloidin (Invitrogen – Molecular Probes) and Hoechst 33342 (Sigma-Aldrich) or mounted with Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA).
Direct immunofluorescence was used to detect α-smooth muscle actin protein expression. A monoclonal anti-α-smooth muscle actin antibody conjugated to fluorescein isothiocyanate (FITC) (Sigma-Aldrich, 1:100) was incubated with tissue sections for 1 hour at room temperature. Slides were then mounted with Vectashield containing DAPI (Vector Laboratories).
Terminal Uridine Deoxynucleotidyl Transferase dUTP Nick End Labeling
The ApopTag® In Situ Apoptosis Fluorescein Detection Kit (Chemicon International, Temecula, CA) was used to detect apoptosis in wild-type and E-cadherin conditional knockout mutant embryos. Firstly, frozen tissue sections were air dried and rinsed with PBS. The tissue was then pretreated with proteinase K (20 μg/ml) for 15 minutes at room temperature. The sections were then incubated in equilibration buffer for 10 seconds at room temperature and incubated with TdT enzyme for 1 hour at 37°C in a humidity chamber. Following washing, slides were incubated in anti-digoxigenin-fluorescein conjugate for 30 minutes at room temperature, washed and mounted with Vectashield mounting medium containing DAPI (Vector Laboratories).
RESULTS
Lens-Cre-mediated deletion of Cdh1 or Cdh2 results in conditional loss of E-cadherin and N-cadherin protein expression during lens development
Genomic wide elimination of either E-cadherin or N-cadherin results in early embryonic lethality (Larue et al., 1994; Radice et al., 1997). In order to bypass the requirement for E-cadherin and N-cadherin in the early embryo and address the roles that E-cadherin and N-cadherin possess in vertebrate lens development, conditional knockout (cKO) mouse strains of both E-and N-cadherin in the lens placode were created using the Cre/loxP recombination approach (Gu et al., 1994). The Lens-Cre mouse line, which directs expression of Cre-recombinase within the early lens placode (embryonic day 9 (E9)) was used to induce lens-specific deletion of E- and N-cadherin (Ashery-Padan et al., 2000). Lens-cre mice were bred with those possessing either the Cdh1 or Cdh2 genes flanked by two loxP sites that have been used previously for deletion of E- and N-cadherin in the embryonic mammary gland and myocardium (Boussadia et al., 2002; Kostetskii et al., 2005).
Compared to wild-type littermates at E9.5 (Fig. 1A), in which normal expression of E-cadherin was observed in the overlying surface ectoderm, reduced E-cadherin protein expression was present within the lens placode of E-cadherin cKO embryos (Fig. 1B). Similarly, at E10.5, little E-cadherin expression was observed within the lens pit or head ectoderm of E-cadherin cKOs (Fig. 1D) compared with control embryos (Fig. 1C). While normal expression of E-cadherin was observed within the anterior lens epithelium and surface ectoderm of wild-type embryos at E12.5 (Fig. 1E) and E14.5 (Fig. 1G), no observable E-cadherin expression was evident in these regions in E-cadherin cKO embryos at both E12.5 (Fig. 1F) and E14.5 (Fig. 1H), indicating successful lens-specific deletion of the protein.
Figure 1.
Loss of E-cadherin and N-cadherin in lens ectoderm derivatives. Normal expression of Ecadherin (red) in wild-type (WT) mice is evident within the lens placode at E9.5 (A) and invaginating lens pit at E10.5 (C). E-cadherin is maintained within the lens epithelium and overlying surface ectoderm at both E12.5 (E) and E14.5 (G). Reduced E-cadherin expression is observed within the lens placode and lens pit of E-cadherin cKO mice (Lens-Cre+,Cdh1flox/KO) at E9.5 (B) and E10.5 (D), respectively. E-cadherin expression is lost within the lens epithelium and overlying surface ectoderm of E-cadherin cKO mice at both E12.5 (F) and E14.5 (H). Normal expression of N-cadherin (red) is evident within the optic vesicle of wild-type mice at E9.5 (I) and the invaginating lens pit and optic cup at E10.5 (K). N-cadherin expression is maintained within the lens epithelial and primary fibre cells of control mice at both E12.5 (M) and E14.5 (O). In N-cadherin cKO mice (Lens-Cre+,Cdh1flox/neo), N-cadherin expression is reduced within the lens pit at E10.5 (L) and lost throughout the lens epithelium and fibre cell region as development progressed (N,P). Nuclei are counterstained with either DAPI (blue) or Hoechst (blue) staining.
Examination of N-cadherin protein expression in both wild-type (Fig. 1I) and N-cadherin cKO (Fig. 1J) embryos at E9.5 revealed normal expression of N-cadherin within the underlying optic vesicle. At E10.5, N-cadherin was observed within the invaginating lens placode and optic cup of control embryos (Fig. 1K). In N-cadherin cKO embryos, N-cadherin protein expression was eliminated from lens pit but was maintained within the invaginating optic cup (Fig. 1L). Similarly, successful deletion of N-cadherin protein expression was observed within the developing lenses of N-cadherin mutant embryos at E12.5 (Fig. 1N) and E14.5 (Fig. 1P), while its expression was maintained in the underlying optic cup.
Microphthalmia and iris hyperplasia in either single E-cadherin or N-cadherin cKO mice
Macroscopic examination of three-week old E-cadherin cKO mice revealed significant defects in eye development as compared with wild-type counterparts. E-cadherin cKO mice displayed severe microphthalmia, a phenotype that was consistently observed bilaterally and in all mutants examined at this stage of postnatal development (Fig. 2A). Additionally, these mutant mice also exhibited severe iris hyperplasia and lacked an observable pupil (Fig. 2A,E). Histological examination of E-cadherin cKO mice at P21 indicated that these mutants possessed significantly smaller lenses than their wild-type counterparts (Fig. 2E). Furthermore, these E-cadherin mutant lenses appeared to be severely vacuolated and lacked a distinct lens epithelial cell layer (Fig. 2F). N-cadherin cKO embryos at E14.5 also exhibited microphthalmia but no other overt ocular phenotypes at this stage (Fig. 2B). Histological examination of N-cadherin cKO mice at P21 also revealed smaller lenses as compared with control mice, along with severe iris hyperplasia and no pupil (Fig. 2G). Additionally, N-cadherin cKO mice exhibited severe defects in both lens epithelial and fibre cell integrity (Fig. 2H).
Figure 2.
Conditional knockout of either Cdh1 (E-cadherin) or Cdh2 (N-cadherin) within the developing lens results in distinct lens defects. (A) At P21, E-cadherin cKO mice (Lens-Cre+,Cdh1flox/KO) exhibit severe microphthalmia along with distinct iris hyperplasia. (E,F) As compared to wild-type (WT) littermates (C,D), histological examination of E-cadherin cKO lenses at P21 exhibited distinct fibre cell vacuolization and deterioration of the lens epithelial cell layer. Macroscopic examination of N-cadherin cKO (Lens-Cre+,Cdh2flox/neo) embryos at E14.5 revealed small eye phenotype (B), while histological examination of N-cadherin cKO mice at P21 indicated a severe lens deterioration accompanied by hyperplastic iris development (G,H).
E-cadherin is required for maintenance of the lens epithelial cell phenotype
In order to better assess the lens defects associated with E-cadherin cKO mice, mutants were examined at various embryonic and postnatal stages and compared with wild-type littermates. Histological examination of E-cadherin cKO mice at E14.5 (Fig 3B) revealed a marked decrease in the overall size of the mutant lens as compared to control littermates (Fig. 3A). Aside from this phenotype, no other significant defects in the general architecture of the lens epithelium or fibre cell region were observed at this developmental stage (Fig. 3C). Similarly at E16.5, lens specific deletion of E-cadherin resulted in a small lens with no other structural abnormalities (data not shown). In contrast, analysis of mutant lenses at P0 indicated significant defects in secondary lens fibre cell differentiation and development, as compared to wild-type pups at this stage (Fig. 3D). A large number of vacuoles were observed within the transitional zone of differentiating fibre cells, extending towards the posterior aspect of the lens (Fig. 3E,F). Two weeks later at P14, while control animals displayed normal lens morphology (Fig. 3G), the lenses of E-cadherin cKO mutants were significantly smaller and exhibited more evident vacuolization (Fig. 3H,I). Similar to mice at P21, the irises of E-cadherin cKO mice at P14 appeared to be severely hyperplastic and formed a continuous layer over the lens with no evident pupil. Additionally, a distinct lens epithelial layer appeared to be absent from the lenses of E-cadherin mutant mice, while an abnormal accumulation of nuclei within the posterior fibre cell compartment was observed.
Figure 3.
Inactivation of E-cadherin within the developing lens leads to defects in lens integrity. Although the lenses of E-cadherin cKO (Lens-Cre+,Cdh1flox/KO) mice (B,C) appeared smaller, no apparent alterations in lens structure were observed at E14.5 as compared with wild-type (WT) littermates (A). (E,F) P0 E-cadherin cKO lenses exhibited fibre cell defects as evidenced by the presence of numerous vacuoles within the transitional zone. (H,I) At P14, E-cadherin cKO mouse lenses possessed discontinuous lens epithelia, with a severely vacuolized lens fibre cell region. Furthermore mutant mice developed hyperplastic irises that extended across the entire anterior face of the lens. Scale bars are 100 μm.
In order to determine whether E-cadherin deficient lens epithelial cells in the cKO mice display defects in cell polarity, the expression pattern of the tight junction molecule, zona occludens-1 (ZO-1), was examined. ZO-1 has previously been shown to localize to the apical membrane of epithelial cells within the developing lens (Nguyen et al., 2005; Nielsen et al., 2003). Strong expression of ZO-1 was observed at the apical aspect of lens epithelial cells in wild-type mice at E16.5 (Fig. 4A), P0 (Fig. 4C) and P14 (Fig. 4E). Similarly, positive ZO-1 immunoreactivity was observed on the apical cell surface of lens epithelial cells at E16.5 in E-cadherin cKO mice (Fig. 4B), albeit less intense than wild-type littermates. Conversely, ZO-1 expression was substantially disrupted within the lenses of postnatal E-cadherin mutant mice. Although ZO-1 expression was somewhat maintained on the apical aspect of the mutant lens epithelium at P0, it was also found to be abnormally distributed among various cells within the transitional zone (Fig. 4D). Additionally, this expression pattern deteriorated over time, as ZO-1 immunoreactivity was completely absent from the lens epithelial cell region at P14 and appeared to accumulate within the fibre cell compartment of mutant lenses (Fig. 4F).
Figure 4.
E-cadherin is essential for maintenance of the lens epithelial cell phenotype. ZO-1 is localized on the apical aspect of the lens epithelial cell layer (red) in wild-type (WT) animals at E16.5 (A), P0 (C) and P14 (E). Expression of ZO-1 is somewhat maintained at the apical lens epithelial membrane in E-cadherin cKO embryos (Lens-Cre+,Cdh1flox/KO) at E16.5 (B). In contrast, E-cadherin cKO lenses at P0 (D) and P14 (F) exhibit disrupted ZO-1 lens expression at apical lens epithelial cell junctions. Expression of β-catenin (green) was observed within the developing lenses of control mice at E14.5 (G), P0 (I), and P14 (K). β-catenin expression was maintained within the lens epithelium and fibre cell region of E-cadherin cKO mice at E16.5 (H). As the lens deteriorated at P0 (J) and continuing to P14 (L), β-catenin expression was maintained at the cell surface of lens epithelial cells only in places in which the epithelium had not yet deteriorated. Positive α-SMA immunoreactivity (green) was observed within the irises of wild-type embryos at E16.5 (M), P0 (O), P14 (Q). An accumulation of α-SMA expression within the lens epithelium of E-cadherin cKO mice was observed at E16.5 (N). α-SMA immunoreactivity was further up-regulated within the anterior lens epithelium of P0 (P) and P14 (R).Dotted lines outline the lens capsule. Nuclei are counterstained with DAPI (blue). Abbreviations: LE, lens epithelium; IR, iris.
The expression pattern of β–catenin was also examined within the lenses of E-cadherin cKO mice, as this molecule is closely associated with E-cadherin at the cell surface. At E16.5, normal expression of β–catenin was observed at the cell membrane within the lenses of both wild-type (Fig. 4G) and E-cadherin cKO mice (Fig. 4H). A similar finding was observed in the lens epithelium of cKO mice at P0 (Fig. 4J). At P14, strong β–catenin immunostaining was evident at the periphery of lens epithelial cells in wild-type animals (Fig. 4K), and was also observed at the surface of cells localized to the discontinuous anterior lens epithelium of mutant mice (Fig. 3L). In portions of the epithelium where lens epithelial cells appeared to be absent, no β–catenin expression was evident.
A loss in E-cadherin expression has been intimately linked with the phenomenon known as epithelial to mesenchymal transition (EMT), in which epithelial cells transform and differentiate to express a mesenchymal cell phenotype (Huber et al., 2005). Cells undergoing this process generally lose their cell polarity and one hallmark feature of this transformation is the presence of the contractile element, alpha-smooth muscle actin (α-SMA) (Zavadil and Bottinger, 2005). As such, α-SMA protein expression was examined within the lenses of E-cadherin cKO mice and wild-type littermates. While wild-type animals express α-SMA normally within the overlying iris tissue (Fig. 4M), an accumulation of α-SMA was observed within the lens epithelium of E-cadherin cKO animals beginning at E16.5 (Fig. 4N) and continuing to P0 (Fig. 4P). At P14, a substantial up-regulation of α-SMA expression was observed within the lenses of E-cadherin cKO mutants (Fig. 4R) as compared to control mice (Fig. 4Q). Positive staining for α-SMA protein expression within the anterior region of the mutant lens extended across the entire lens epithelial surface. Surprisingly, positive α-SMA immunoreactivity was also present along the posterior aspect of the lens suggesting abnormal migration of cells to the posterior lens fibre cell region (data not shown).
N-cadherin is required for lens fibre morphogenesis and elongation, but is not required for differentiation
N-cadherin is maintained within the lens fibre cell compartment throughout morphogenesis. Histological examination of N-cadherin cKO mice during embryogenesis revealed no overt defects in lens morphogenesis as compared with wild-type littermates. At E14.5, N-cadherin cKO lenses appeared to be smaller in size but still developed distinct epithelial and fibre cell compartments (Fig. 5B). Similarly, at E17.5, N-cadherin mutant lenses (Fig. 5D) were substantially smaller than the lenses of wild-type littermates (Fig. 5C) yet sill appeared to develop normally. Conversely, postnatal examination of N-cadherin cKO lenses revealed gross defects in fibre cell and iris development, phenotypes which varied in severity from one mutant to another. At P14, N-cadherin cKO mice exhibited distinct iris hyperplasia in the absence of a pupil (Fig. 5F). Additionally, the lenses of N-cadherin mutant mice exhibited extensive vacuolization throughout the entire fibre cell compartment (Fig. 5F,G). Furthermore, N-cadherin mutant lenses also possessed an abnormal accumulation of nuclei within the transitional zone, also suggesting defects in fibre cell development (Fig. 5G).
Figure 5.
Conditional deletion of N-cadherin within the developing lens is associated with defects in lens development. The lenses of N-cadherin cKO (Lens-Cre+,Cdh2flox/neo) embryos at E15.5 (B) and E17.5 (D) were substantially smaller than their wild-type (WT) counterparts (A,C) yet did not exhibit any other overt defects in lens development as they developed distinct lens epithelial and fibre cell compartments. At P14, N-cadherin cKO mice (F,G) possess vacuolated lenses that had accumulated nuclei within the fibre cell region. Additionally, mutant mice also exhibited defects in iris development, as mutant irises displayed significant hyperplasia.
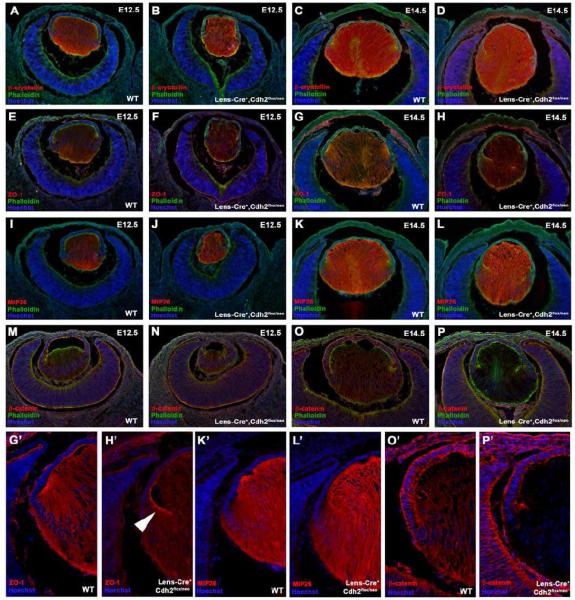
In order to further examine the changes in lens fibre cell differentiation and architecture within N-cadherin cKO mice, a number of markers for fibre cell development were assessed. Lens epithelial cells begin to differentiate as early as embryonic stage E10.5, where β-crystallin, one of the first detectable proteins involved in fibre cell differentiation, is expressed. At E12.5, β-crystallin expression is retained in both wild-type (Fig. 6A) and N-cadherin cKO lenses (Fig. 6B), revealing that lens fiber differentiation had occurred. A similar finding was observed at E14.5, in which β-crystallin immunoreactivity was maintained in the lenses of both N-cadherin mutant (Fig. 6C) and control littermates (Fig. 6D). Additionally, two major fibre cell membrane components, lens major intrinsic protein (MIP26) and ZO-1, were also examined and shown to be equally expressed in both wild-type (Fig. 6E,I) and N-cadherin cKO embryos (Fig. 6F,J) at E12.5. Similarly at E14.5, the intensity of MIP26 and ZO-1 expression was equivalent between the lenses of N-cadherin mutant (Fig. 6H’,L’) and control embryos (Fig. 6G’,K’). However, the distribution and arrangement of lens fibre cells in N-cadherin cKO lenses appeared to be disrupted when compared to wild-type counterparts at this stage. Wild-type lens fibre cells were well organized and appeared to form “Y” sutures as they joined and elongated through the lens capsule (Fig. 6G’,K’). In contrast, the fibre cells of N-cadherin cKO lenses failed to properly elongate as evidenced by the disrupted expression patterns of both ZO-1 (arrowhead in Fig. 6H’) and MIP26 (Fig. 6L) and altered phalloidin distribution.
Figure 6.
N-cadherin is required for normal fibre cell development. Comparable β-crystallin protein expression was observed in both wild-type (WT) (A) and N-cadherin cKO (Lens-Cre+,Cdh2flox/neo) (B) lenses at E12.5. Similarly, positive β-crystallin expression was observed within the fibre cell region of N-cadherin cKO embryos at E14.5 (D), suggesting that lens fiber differentiation had occurred. At E12.5 (E) and E14.5 (G,G’), positive ZO-1 immunoreactivity was observed on the apical aspect of lens epithelial cells of wild-type embryos. A similar intensity of ZO-1 expression was observed within the lenses of N-cadherin cKO mice at both E12.5 (F) and E14.5 (H,H’). The fibre cell marker, MIP26, also appeared to exhibit equivalent immunoreactivity between N-cadherin cKO and wild-type embryos at both E12.5 (compare (I) and (J)) and E14.5 (compare (K’) and (L’)). However, immunolocalization of ZO-1 and MIP26, along with phalloidin staining, suggested a disruption in the elongation and arrangement of fibre cells in N-cadherin cKO mice at E14.5 (H,H’,L,L’). (M,O,O’) β-catenin expression was observed at the cell membranes of lens epithelial and fibre cells of wild-type embryos at both E12.5 and E14.5. Expression of β-catenin was maintained within the lenses of N-cadherin cKO mice at E12.5 (N) but was lost within the fibre cell compartment at E14.5 (P’). Panels G’,H’,K’,L’,O’ and P’ are increased magnifications of panels G,H,K,L,O and P, respectively.
β-catenin was also examined as this molecule is required for cell adhesion during lens development and has shown to play an important part in the coordination of morphogenesis (Smith et al., 2005). Junctional β-catenin expression was observed within the lens epithelia and fibre cell compartments of wild-type embryos at both E12.5 (Fig. 6M) and E14.5 (Fig. 6O’). While the expression of β-catenin was maintained within the lenses of N-cadherin cKO mice at E12.5 (Fig. 6N), distinct β-catenin expression was absent, specifically from the fibre cell region, of N-cadherin mutant embryos at E14.5 (Fig. 6P’). Interestingly, β-catenin expression was maintained within the lens epithelial layer of N-cadherin cKO mice at all stages examined. Thus, in the absence of N-cadherin, β-catenin expression is eventually lost at fibre cell membrane junctions, which in turn may account for the observed disruption and disorganization of the fibre cell region at this stage.
E- and N-cadherin dosage has a significant impact on lens development
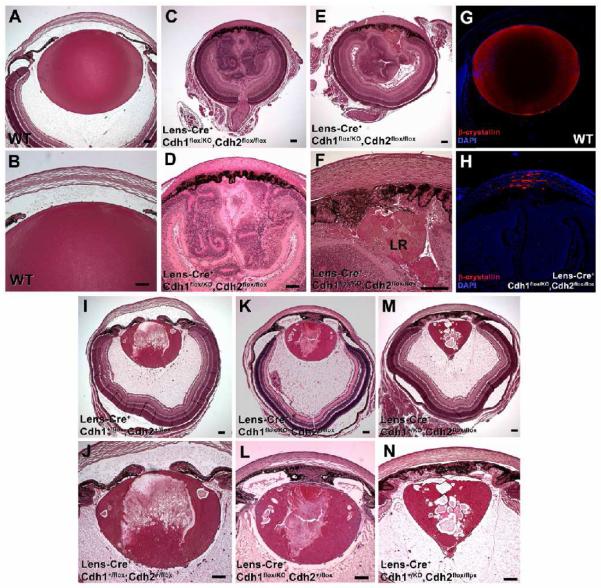
N-cadherin expression was maintained within the lenses of E-cadherin cKO mice and E-cadherin was maintained within N-cadherin cKO lenses (data not shown). Thus, in order to examine the possibility of cadherin compensation in either the E-cadherin conditional or N-cadherin cKO lens, both E- and N-cadherin were simultaneously eliminated from the presumptive lens ectoderm using the Lens-Cre transgenic mouse line. Analysis of mutant progeny and control littermates revealed that at P7, double conditional E- and N-cadherin cKO mice exhibited ocular defects more severe than either single cKO mouse model (Fig. 7C,E). In addition to microphthalmia, double cKO mice lacked a distinct lens (Fig. 7D). However, some double mutants possessed remnants of lens material positioned either directly beneath the iris (LR in Fig. 7F) or distributed throughout the anterior compartment as evidenced by distinct βB1-crystallin immunoreactivity (Fig. 7H). The retinas of double cKO mice appeared to be overgrown, occupying the majority of the ocular space (Fig. 7D). Furthermore, these retinas also appeared to be completely laminated as evidenced by positive immunoreactivity for the retinal cell markers, Pax6, calretinin, and syntaxin (data not shown). Double cKO animals also lacked an observable pupil, possessing iris tissue that had abnormally migrated and infiltrated across the entire anterior chamber, in a manner analogous to either the E- or N-cadherin single cKO mouse models. Additionally, the iris appeared to fuse with the overlying cornea which in turn lacked an endothelium and stratified epithelium.
Figure 7.
Lens growth and integrity is sensitive to the number of E- and N-cadherin alleles. (A,B) Histological examination of wild-type (WT) animals at P7 revealed normal lens, cornea and retinal morphology. (C,D,E,F) E- and N-cadherin double cKO mice (Lens-Cre+,Cdh1flox/KO,Cdh2flox/flox) at P7 were severely microphthalmic as compared with all littermates examined. In most cases, these mice lacked normal lens formation (aphakia) but did exhibit remnants of lens material within the eye (LR in panel F). Instead E- and N-cadherin double cKO mice possessed retinas which exhibited severe overgrowth and folding within the orbit of the eye (D). (G) Normal expression of βB1-crystallin (red) in wild-type mice was observed within the lens fibre cell region at P7. (H) Abnormal accumulation of βB1-crystallin-positive lens material was observed within the anterior ocular compartment of E- and N-cadherin double cKO mice. Nuclei are counterstained with DAPI (blue). (I,J) Conditional knockout of one copy of both E- and N-cadherin (Lens-Cre+,Cdh1+/flox,Cdh2+/flox) in P7 mice resulted in microphthalmia, a smaller lens, extensive vacuolization within the lens fibre cell region. (K,L) Conditional elimination of both copies of E-cadherin and one N-cadherin allele (Lens-Cre+,Cdh1flox/flox,Cdh2+/flox) also induced microphthalmia, a decrease in lens size, substantial lens vacuolization and iris hyperplasia. (M,N) Mice lacking one copy of E-cadherin and both N-cadherin alleles (Lens-Cre+,Cdh1+/flox,Cdh2flox/neo) also exhibited microphthalmia, with smaller lenses, extensive lenticular vacuolization and iris hyperplasia. Scale bars are 100 μm.
The nature of the breeding scheme employed to generate the double cKO mice allowed us to determine the allelic dosage effect through the elimination of various combinations of E- and N-cadherin during lens development. Mice deficient for only one copy of each of these cadherin molecules (Fig. 7I,J) did not lack a lens or pupil, but lenses were reduced in size. These lenses also exhibited distinct vacuolization, a phenotype that was previously observed in either of the single E- or N-cadherin cKO mouse models. Mice possessing only one functional copy of N-cadherin but lacking both E-cadherin alleles (Fig. 7K,L) or mice possessing one functional E-cadherin allele but lacking both copies of N-cadherin within the lens (Fig. 7M,N) were also microphthalmic and possessed lenses that were much smaller as compared with mice either lacking only one copy of each cadherin or wild-type littermates. These data clearly demonstrate that the combined loss of E-cadherin and N-cadherin within the lens results in gradual aphakia and that the allelic dosage of E-and N-cadherin has a significant impact on the regulation of lens growth.
E-cadherin and N-cadherin are required for lens vesicle separation and survival of the lens epithelium
Histological examination of E- and N-cadherin double cKO mice was next performed at various embryonic and postnatal stages to assess the co-operative roles for these cadherins during lens morphogenesis. At E10.5, the lens placode of wild-type littermates invaginated along with the optic vesicle to give rise to the lens pit and optic cup, respectively (Fig. 8A). Similarly, in E- and N-cadherin double cKO, the lens placode appeared to develop and invaginate towards the optic vesicle (Fig. 8B). At E11.5, normal development of the lens vesicle was observed in wild-type embryos (Fig. 8C). Conversely, significant defects in lens vesicle development were consistently observed in double cKO mice (Fig. 8D). A homogeneous population of cells infiltrated and occupied the normally vacant lumen of the lens vesicle. Additionally, the epithelium comprising the anterior region of the lens vesicle was substantially thinner than its respective littermates and appeared to possess a lens stalk remnant, suggesting defects in lens vesicle separation. At E13.5, distinct defects in lens vesicle separation became more evident. While wild-type littermates (Fig. 8E) possessed normal lenses which separated from the surface ectoderm, the lens vesicle of double cKO embryos at E13.5 (Fig. 8F) remained continuous with the overlying surface ectoderm, exhibiting the formation of a persistent lens stalk. H&E staining of E15.5 embryos revealed that unlike control littermates, which possessed a defined lens epithelium and underlying fibre cell region (Fig. 8G), the lenses of double cKO animals lacked a distinct lens epithelial layer (Fig. 8H). Instead, double cKO mice possessed a nucleated fibre cell mass. This vacuolated lentoid structure also appeared to migrate anteriorly into the periocular space, suggesting defects in lens capsule formation. Additionally, cells from the periocular mesenchyme also abnormally migrated behind the lens, into the vitreal space. Similarly at P0, as compared to wild-type lenses (Fig. 8I), double cKO mice were severely microphthalmic and possessed nucleated fibre cell-like material which had infiltrated the anterior chamber (Fig. 8J). Not surprising, double cKO lenses at P0 also lacked an observable lens epithelium. Furthermore, double cKO eyes at this stage exhibited overt retinal overgrowth in the absence of a distinct lens.
Figure 8.
Conditional inactivation of E- and N-cadherin within the developing lens leads to a failure in lens vesicle separation. At E10.5, both wild-type (WT) (A) and E- and N-cadherin double cKO (Lens-Cre+,Cdh1flox/KO,Cdh2flox/flox) mice (B) exhibited normal lens placode invagination and lens pit formation. While wild-type mice (C) developed normally double cKO embryos (D) at E11.5 exhibited a distinct lens stalk remnant that appeared continuous with the overlying surface ectoderm (arrowhead), along with an accumulation of cells within the lumen of the lens vesicle. (F) Similarly at E12.5, E- and N-cadherin double cKO embryos exhibited persistent lens stalk formation suggesting a failure in lens vesicle separation (arrowhead). While wild-type embryos at E15.5 (G) developed a distinct organized lens epithelial layer and fibre cell region, the lenses of E- and N-cadherin double cKO mice (H) lacked an epithelial layer. Moreover, the lens material abnormally infiltrated the anterior chamber of the eye, which was correlated with the abnormal migration of the perioptic mesenchyme (arrowhead in (H)). (J) At P0, double cKO mice lacked a cuboidal epithelial layer and possessed lens material mispositioned within the anterior chamber.
Histological examination of double cKO embryos revealed severe defects in lens vesicle formation. A large population of cells had infiltrated the lumen of the developing lens vesicle. As such, in order to determine the origin of these abnormally displaced cells, markers of lens epithelial cell development were immunolocalized in the double cKO embryo at E11.5. As shown in Fig. 9A, localization of Pax6 protein within wild-type embryos at E11.5 revealed the characteristic broad expression pattern of Pax6 throughout surface ectoderm, lens vesicle and optic cup. Assessment of Pax6 protein expression in double cKO embryos at E11.5 revealed that in addition to the characteristic Pax6 expression profile within the cells comprising the lens vesicle, distinct Pax6 immunostaining was also evident in the cells displaced within the developing lens vesicle (Fig. 9B). The expression pattern of the transcription factor, AP-2α, was also localized within the lenses of double cKO and control embryos as this protein has been shown to localize to lens epithelial cells positioned more anteriorly than Pax6 (Makhani et al., 2007). In wild-type embryos at E11.5, expression of AP-2α was observed within the anterior portion of the lens vesicle and the overlying surface ectoderm (Fig. 9C). In double cKO embryos, expression of AP-2α was also evident with the abnormally displaced lens epithelial cells within the lumen of the lens vesicle (Fig. 9D). Finally, the expression of Foxe3, a lens epithelial-specific cell marker, was characterized within the lenses of both wild-type (Fig. 9E) and double cKO embryos (Fig. 9F). Distinct Foxe3 immunoreactivity was also observed within the cells situated within the lumen of the double cKO lens vesicle (Fig. 9F). These findings demonstrate that the cells in the lumen of double mutant lenses are of lens epithelial origin and that in the absence of E- and N-cadherin, the anteriorly positioned cells are likely those that are lost. Corroborating this hypothesis, the anterior region of the lens vesicle in double cKO mice appeared to be significantly thinner than control embryos at this stage.
Figure 9.
Expression of lens epithelial markers in E- and N-cadherin double cKO embryos. Wild-type (WT) embryos (A) at E11.5 exhibited normal expression of Pax6 (red) in all the cells comprising the lens vesicle, surface ectoderm and neural retina. Immunolocalization of Pax6 within E- and N-cadherin cKO (Lens-Cre+,Cdh1flox/KO,Cdh2flox/flox) embryos (B) was also observed within the abnormally displaced cells positioned in the lumen of the lens vesicle. AP-2α (green) protein expression was evident within the anterior lens epithelium of the lens vesicle in wild-type embryos (C), while it was also immunolocalized within the cells of the lumen of double cKO lens vesicles (D). (E) Lens-specific expression of Foxe3 (green) was observed within wild-type embryos at E11.5. (F) Distinct Foxe3 immunoreactivity was evident within the cells of the abnormal lens vesicle of E- and N-cadherin double cKO mutant embryos. In wild-type embryos (G) at E11.5, very few TUNEL (green) positive lens epithelial cells were observed within the lens vesicle. Conversely in double cKO embryos (H), TUNEL positive cells were present within the abnormally displaced cells of the lens vesicle. Normal expression of β-catenin (green) was observed at cell-cell junctions within the developing lens and neural retina of wild-type (WT) mice at E10.5 (I), E11.5 (K) and E13.5 (M). Expression of β-catenin was reduced within the lens pit of double cKO (Lens-Cre+,Cdh1flox/KO,Cdh2flox/flox) mice at E10.5 (J) and was eventually lost within the lenses of double cKO embryos by E11.5 (L) and E13.5 (N). (O) Wild-type (WT) embryos at E13.5 exhibit positive expression of P-cadherin (red) within the developing surface ectoderm and retinal pigmented epithelium. (P) E- and N-cadherin double cKO (Lens-Cre+,Cdh1flox/KO,Cdh2flox/flox) embryos at E13.5 exhibit a failure in lens vesicle separation that correlated with positive immunoreactivity for P-cadherin (red) within the cells of the persistent lens stalk. Abbreviations: RPE, retinal pigmented epithelium; LS, lens stalk. Nuclei are counterstained with DAPI (blue).
When epithelial cells are prohibited from maintaining cellular adhesion with the extracellular matrix or surrounding cells for extended periods of time, they can undergo apoptosis by a phenomenon known as anoikis (Fouquet et al., 2004; Shanmugathasan and Jothy, 2000). In order to address the hypothesis that loss of epithelial cells into the lumen of the lens vesicle leads to subsequent apoptosis, a TUNEL analysis was performed at E11.5. As shown in Fig. 9G, very few epithelial cells located within the lens vesicle of wild-type embryos appeared to undergo programmed cell death, as evidenced by the lack of TUNEL-positive cells. In contrast, a substantial increase in TUNEL-labeling was observed with the lens vesicles of embryos harboring various genetic copies of either E- or N-cadherin (data not shown). Similarly, a large number of TUNEL-positive cells were observed within the lens vesicle of double cKO embryos, with the greatest proportion located within the lumen (Fig. 9H). These data suggest that E- and N-cadherin are required for lens epithelial cell survival and for the prevention of detachment-mediated apoptosis within the lens vesicle and that the combined loss of various cadherin molecules within the lens results in a greater degree of apoptosis.
The expression profile of β-catenin was also examined within the developing lens of E- and N-cadherin double cKO mice. At E10.5, wild-type embryos exhibited strong immunoreactivity for β-catenin at cell-cell junctions within the developing lens pit (Fig. 9I). In double cKO lenses, while normal expression of β-catenin was maintained within the developing optic cup, β-catenin expression was substantially down-regulated within the invaginating lens pit as compared with control littermates (Fig. 9J). Normal expression of β-catenin was observed within the developing lens at both E11.5 (Fig. 9K) and E13.5 (Fig. 9M). In contrast to littermate controls, expression of β-catenin was completely lost within the lens vesicle of double mutant embryos at E11.5 (Fig. 9L) and E13.5 (Fig. 9N). Interestingly, no nuclear localization of β-catenin was observed at either stage examined, suggesting that liberated β-catenin was likely targeted for proteasomal degradation in double mutant mice.
The alteration in the distribution of cadherin molecules has been hypothesized to play an important role during the separation of various tissues (Wheelock et al., 2008). P-cadherin, another cadherin family member is also expressed within the presumptive lens ectoderm during embryonic development and is usually lost within the lens vesicle as the lens separates. Analysis of P-cadherin expression was performed in the E-cadherin and N-cadherin double cKO mice and revealed that normal expression of P-cadherin was observed within the developing surface ectoderm and retinal pigmented epithelium of both normal control (Fig. 9O) and double cKO embryos (Fig. 9P) at E13.5. However, distinct P-cadherin immunoreactivity was also observed at the junctions of cells comprising the residual lens stalk of double cKO mice (Fig. 9P). Taken together, these findings suggest that both E- and N-cadherin are required for normal lens vesicle separation and that the maintenance of P-cadherin expression within the residual stalk cells may also play an important role in preventing normal lens separation.
DISCUSSION
The classical cadherins are important mediators of cell adhesion that maintain cell-cell contacts throughout the life of an organism. The importance of these molecules during embryogenesis is demonstrated from studies of germline knockout mice. For example, homozygous N-cadherin-null embryos die by E10 of gestation (Radice et al., 1997). The most dramatic cell adhesion defect in these mice is observed in the primitive heart, where myocardial tissue initially develops but rapidly deteriorates as myocytes lose adhesion and dissociate. Homozygous negative E-cadherin embryos die around the time of implantation and fail to form a trophoectodermal epithelium or a blastocyst cavity (Larue et al., 1994). These results clearly demonstrate the pivotal roles of these adhesion molecules in the development of multicellular vertebrate organisms. However, the early embryonic lethality associated with these mouse models prevents their use for examining the roles cadherins play in lens development.
Previous studies have shown a correlation between loss in the expression of cadherin molecules during lens development and failure in lens vesicle separation (Pontoriero et al., 2008). To directly address the hypothesis that loss of cadherin expression within the lens results in defects in lens separation, the Lens-Cre transgene was employed to induce a lens specific knockout of either E-cadherin or N-cadherin within the developing lens. Lens-Cre-mediated recombination of either Cdh1 or Cdh2 floxed alleles resulted in successful elimination of E-cadherin and N-cadherin expression within the developing lens by E10.5. In both mutant models, defects in lens vesicle separation were not observed. Both the E-cadherin and N-cadherin cKO mice did however exhibit severe microphthalmia with smaller vacuolated lenses, along with a hyperplastic iris. The vacuolization observed in E-cadherin cKO lenses originated within the transitional zone and extended throughout the cortical fibre cell region during postnatal development. Additionally, the epithelial layer of E-cadherin cKO mice appeared to lose polarity over time. A disruption in ZO-1 expression became apparent along with the accumulation of α-SMA within the anterior and posterior lens regions. Thus, these findings show that E-cadherin is essential for maintaining the integrity of the lens epithelial cell layer and maintenance of the lens epithelial cell phenotype. Examination of N-cadherin cKO lenses also revealed extensive vacuolization within the fibre cell region. Defects in normal fibre cell elongation were also observed and correlated with a loss of β-catenin expression within the fibre cell compartment of N-cadherin cKO embryos. Thus, the E-cadherin and N-cadherin single cKO mice exhibited both overlapping and distinct lens phenotypes. This may be partly due to the fact that these proteins have both similar and unique expression patterns in the developing lens, with E- and N-cadherin expressed in the lens epithelium and only N-cadherin expressed in the fibre cell compartment. However, it may also be that E- and N-cadherin have both similar and discrete roles during lens development. Supporting this hypothesis, Kan and colleagues expressed N-cadherin from the E-cadherin locus (Kan et al., 2007) and demonstrated that while N-cadherin cDNA was able to rescue the loss of E-cadherin for morula compaction, it was not able to functionally compensate for E-cadherin during the ensuing formation of the trophoectoderm.
In order to address the possibility of cadherin compensation and to determine the co-operative roles for these cadherins in lens development, a double E and N-cadherin lens-specific knockout mouse was generated. Notably, double cKO embryos exhibited a defect in lens vesicle formation, as compared to their wild-type littermates. This defect appeared to involve a failure in separation of the lens from that overlying ectoderm, which was correlated with sustained expression of P-cadherin within the remaining lens stalk region. Interestingly, cKO mice lacking the transcription factor, AP-2α, from the developing lens, exhibit a persistent lens stalk phenotype that is also correlated with a loss of E- and N-cadherin and sustained expression of P-cadherin within the cells comprising the lens stalk region (Pontoriero et al., 2008). From the morphological data alone, however, it cannot be determined whether the defect in the double cadherin cKO mice was due to failure of the lens vesicle, once closed, to separate from the overlying ectoderm or was caused by failure in proper closure of the vesicle, perhaps due to inadequate adhesion of cells lining either side of the lens pit. Furthermore, whether the sustained expression of P-cadherin within the corneal-lenticular adhesion of these mutant mice is simply a consequence/remnant or a contributing factor to lens stalk formation remains to be elucidated. One could, however, envision that the persistent expression of P-cadherin within the lens stalk cells prevented proper cell sorting as these cells are now more phenotypically similar to the epithelial cells of the surface ectoderm. Future studies aimed at either conditionally inactivating or overexpressing P-cadherin specifically within the presumptive lens ectoderm will help to further determine its contribution to the formation of this developmental defect. A number of other mouse mutants exhibit failure in lens vesicle separation with a persistent lens stalk (Blixt et al., 2000; Collinson et al., 2001; Favor et al., 1997; Hill et al., 1991; Rieger et al., 2001; Wurm et al., 2008; Yoshimoto et al., 2005). It may therefore be useful to examine the expression profiles of E-, N-, and P-cadherin within the lenses of these mutant mice in order to further determine the contribution of cadherins to lens vesicle separation.
Histological analysis of double cKO embryos at E11.5 revealed an accumulation of cells with the lumen of the developing lens vesicle. These abnormally displaced cells expressed characteristics of the anterior lens epithelium, including the expression of the lens epithelial cell markers, Pax6 and AP-2α, and were also labeled positively in TUNEL analyses. Importantly, the displaced cells were also found to express FoxE3, an additional lens epithelial cell marker, which unlike AP-2α and Pax6, is not expressed in the overlying ectoderm following lens separation. This further suggests that these cells were derived from the lens epithelium rather than being derived from the overlying surface ectoderm during vesicle closure. Thus, these data suggest that there is a co-requirement for E-cadherin and N-cadherin in maintenance of the developing anterior lens epithelium. In the absence of both E- and N-cadherin, these cells appear to lose their ability to maintain cell-cell and/or cell-matrix contacts and are lost to the lumen of the developing lens vesicle where they undergo apoptosis. Interestingly, lenses lacking other combinations of either E-cadherin or N-cadherin displayed increased TUNEL reactivity, as compared to wild-type embryos, but did not appear to lose epithelial cells to the lens vesicle lumen, suggesting that at least one copy of either cadherin, E or N, can prevent the loss of anterior lens epithelial cells during early lens development. The biological process, in which cells lose their contact with the underlying extracellular matrix and undergo programmed cell death, has been termed detachment-induced apoptosis or anoikis (Frisch and Francis, 1994; Grossmann, 2002). The importance of cadherins in the maintenance of cell-cell adhesion and prevention of detachment-induced apoptosis has been demonstrated elsewhere. For example, treatment of intestinal epithelial cells with a blocking anti-E-cadherin antibody increased the rate of anoikis, whereas the activation of E-cadherin using a dimeric E-cadherin-IgG Fc chimera protein reduced anoikis (Fouquet et al., 2004). More recently, activation of Src family kinases in a chick lens model of cortical cataracts resulted in the disassembly of N-cadherin junctions, followed by subsequent apoptosis (Zhou et al., 2007). Cadherins have also been shown to directly interact with molecules, such as the integrins, which are important for linking cells to the basement membrane. For example, the fifth cadherin ectodomain has been shown to be essential for heterophilic contact with integrins (Shiraishi et al., 2005), and cadherins and integrins are also thought to crosstalk and mediate differentiation via epidermal growth factor signaling and downstream Akt kinase activity (Muller et al., 2008). Thus, the deletion of cadherins within the developing lens in the cKO mice may have perturbed common downstream signaling targets of integrins that are important for mediating cell anchorage to the basement membrane and the prevention of apoptosis.
Cadherins, at adherens junctions, link the extracellular environment to the actin cytoskeleton via their interactions with the armadillo repeat protein, β-catenin (Perez-Moreno and Fuchs, 2006; Perez-Moreno et al., 2003). As such, β-catenin protein expression was examined within the developing lenses of cKO mice. Normal expression of β-catenin was observed at the cell membranes of lens epithelial and fibre cells in E-cadherin cKO mice during early development. This finding is not surprising given that normal expression of N-cadherin was also observed in E-cadherin cKO lenses at various developmental timepoints (data not shown). Interestingly, β-catenin expression was lost within the developing lens vesicle of double cKO mice as early as E10.5, while in N-cadherin cKO mice, β-catenin expression was only notably down-regulated within the fibre cell compartment. In the presence of an extracellular Wnt signal, β-catenin has the potential to translocate to the nucleus where it acts as a co-activator associating with TCF/LEF family of DNA-binding proteins (Behrens et al., 1996; Brunner et al., 1997; Perez-Moreno and Fuchs, 2006). In the absence of these exogenous signals, excess β-catenin is normally phosphorylated by the multiprotein complex, glycogen synthase kinase-3β, Axin, and APC, which targets it for ubiquitination and subsequent proteasomal degradation (Akiyama, 2000; Nakamura et al., 1998). In this way, excess β-catenin is prevented from accumulating within the cytoplasm. As no nuclear localization of β-catenin was observed within the cells of the invaginating lens placode or lens vesicle in double cKO mice nor in the fibre cell compartment of the N-cadherin cKO lens, it is thought that excess accumulation of β-catenin within these cells was targeted for proteasomal degradation. Recently, the role of β-catenin function in lens development has been directly addressed through the use of Cre/loxP technology (Kreslova et al., 2007; Smith et al., 2005). In agreement with our findings, lens-specific deletion of β-catenin did not influence lens induction or the appearance of various lens fate markers (Pax6 and AP-2α) but did have a significant impact on lens epithelial cell adhesion and morphogenesis. Taken together, these data suggest that deletion of E- and N-cadherin within the developing lens leads to degradation of the cytosolic β-catenin pool, subsequent cytoskeletal disorganization and perturbation of lens epithelial cell-cell contacts.
Examination of the morphology of postnatal E- and N-cadherin double cKO mouse eyes revealed that the number of functional E- and N-cadherin alleles plays an important role during vertebrate lens development. By P7, double mutant eyes were nearly aphakic, with vast retinal overgrowth in the posterior chamber and only remnants of lens material displaced within the anterior chamber, as evidenced by the positive expression of the lens fibre cell marker, βB1-crystallin. This phenotype has been previously observed in mice lacking the Pitx3 transcription factor (Semina et al., 2000). These Pitx3-null mice, during embryonic development, possess defects in lens vesicle separation, a phenotype that eventually leads to lens deterioration and retinal overgrowth later in development. At P7, mice lacking one copy of each of the cadherins possessed lenses that were smaller and vacuolated as compared to wild-type littermates, while mice losing one more copy of either Cdh1 or Cdh2 still developed a somewhat structured lens, albeit smaller with greater deterioration. Interestingly, these phenotypes resembled the lenses of single (E or N) cKO mice. Both E-cadherin heterozygous mice and N-cadherin heterozygous mice develop lenses that are comparable to wild-type lenses in terms of lens size and integrity (data not shown), suggesting that loss of either one E- or N-cadherin allele is still sufficient to maintain an adequate level of E and N-cadherin protein and preserve the normal cellular interactions between lens epithelial cells and among fibre cells. Thus, these data indicate that the allelic dosage of cadherin molecules within the developing lens likely influences the severity of lens deterioration during development and that E and N-cadherin can compensate for one another during lens morphogenesis.
Finally, single E- and N-cadherin cKO mice, along with E- and N-cadherin double cKO exhibited distinct iris hyperplasia. Considering neither E-cadherin, N-cadherin or the Lens-Cre transgene are expressed within the developing iris, the observed defect in iris formation likely reflects an indirect non-autonomous effect relating to the defects associated with the loss of these cadherin molecules within the lens. Interestingly, the lens has been previously shown to induce and influence the growth of anterior ocular structures in the chick eye (Genis-Galvez, 1966; Thut et al., 2001). More recently in mice, lens ablation experiments have shown that the lens “organizes” development of tissues within the anterior chamber (Zhang et al., 2007). This study showed lens-specific expression of diphtheria toxin resulted in mice with severe microphakia that was also correlated with an undifferentiated iris. Other studies have shown that soluble factors released from the developing lens can signal the surrounding mesenchyme and possibly influence iris development (Reneker et al., 1995; Srinivasan et al., 1998). Thus, the data implies that loss of E-cadherin and N-cadherin within the developing lens leads to the abnormal modulation of lens-secreted signaling molecules which likely play pivotal roles in regulation normal iris migration. A number of additional knockout mouse models have been created which result in defects in iris development (hypoplasia or hyperplasia), yet their relationship to E-cadherin and N-cadherin or other factors that regulate iris development is not yet known (Ramaesh et al., 2003; Swamynathan et al., 2007; Zaki et al., 2006).
In summary, the data suggest that E- and N-cadherin regulate vertebrate lens vesicle separation from the overlying surface ectoderm. Dual elimination of these cadherin molecules within the presumptive lens ectoderm results in loss of cell-cell contacts within the anterior region of the lens vesicle and subsequent detachment-induced apoptosis (anoikis). Thus, E- and N-cadherin function as survival factors within developing lens epithelial cells and their absence results in complete deterioration of the anterior lens epithelium during mid-embryogenesis. Furthermore, loss of various combinations of cadherin molecules within the lens resulted in less severe lens defects, suggesting that not only can E- and N-cadherin can compensate for one another during lens morphogenesis but that the dosage of cadherin molecules within the developing lens likely dictates the severity of the lenticular defects.
ACKNOWLEDGMENTS
The following individuals are acknowledged for their outstanding technical assistance: Ms. Lauren Kirkby, Mr. Paul Speeg, and Mrs. Paula Deschamps. We would also like to thank Dr. Ruth Ashery-Padan (Tel Aviv University, Ramat Aviv, Israel) for providing the Lens-Cre transgenic mouse line, Dr. Joseph Horwitz (Jules Stein Eye Institute, Los Angeles, CA) for providing the rabbit polyclonal anti-MIP26 and mouse anti-βB1-crystallin antibodies and Dr. Peter Carlsson (University of Gothenburg, Gothenburg, Sweden) for providing the rabbit polyclonal anti-Foxe3 antibody. This work was supported by the following: NIH R01 EY11910 (J.W.M.), NIH R01’s EY15766 (R.A.L.), EY16241 (R.A.L.), EY17848 (R.A.L.) and CA131270 (R.A.L.), by funds from the Abrahamson Pediatric Eye Institute Endowment at Children’s Hospital Medical Center of Cincinnati (R.A.L.) and a Natural Sciences and Engineering Research Council of Canada – Postgraduate Scholarship (G.F.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A. 2008;105:13–9. doi: 10.1073/pnas.0710504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T. Wnt/beta-catenin signaling. Cytokine Growth Factor Rev. 2000;11:273–82. doi: 10.1016/s1359-6101(00)00011-3. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–11. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–42. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Blixt A, Mahlapuu M, Aitola M, Pelto-Huikko M, Enerback S, Carlsson P. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 2000;14:245–54. [PMC free article] [PubMed] [Google Scholar]
- Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev. 2002;115:53–62. doi: 10.1016/s0925-4773(02)00090-4. [DOI] [PubMed] [Google Scholar]
- Brunner E, Peter O, Schweizer L, Basler K. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature. 1997;385:829–33. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Quinn JC, Buchanan MA, Kaufman MH, Wedden SE, West JD, Hill RE. Primary defects in the lens underlie complex anterior segment abnormalities of the Pax6 heterozygous eye. Proc Natl Acad Sci U S A. 2001;98:9688–93. doi: 10.1073/pnas.161144098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman GM. Expression of cell adhesion molecules during embryogenesis and regeneration. Exp Cell Res. 1985;161:1–16. doi: 10.1016/0014-4827(85)90485-9. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Crossin KL. Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem. 1991;60:155–90. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- Fagotto F, Gumbiner BM. Cell contact-dependent signaling. Dev Biol. 1996;180:445–54. doi: 10.1006/dbio.1996.0318. [DOI] [PubMed] [Google Scholar]
- Favor J, Grimes P, Neuhauser-Klaus A, Pretsch W, Stambolian D. The mouse Cat4 locus maps to chromosome 8 and mutants express lens-corneal adhesion. Mamm Genome. 1997;8:403–6. doi: 10.1007/s003359900456. [DOI] [PubMed] [Google Scholar]
- Fouquet S, Lugo-Martinez VH, Faussat AM, Renaud F, Cardot P, Chambaz J, Pincon-Raymond M, Thenet S. Early loss of E-cadherin from cell-cell contacts is involved in the onset of Anoikis in enterocytes. J Biol Chem. 2004;279:43061–9. doi: 10.1074/jbc.M405095200. [DOI] [PubMed] [Google Scholar]
- Friedlander DR, Mege RM, Cunningham BA, Edelman GM. Cell sorting-out is modulated by both the specificity and amount of different cell adhesion molecules (CAMs) expressed on cell surfaces. Proc Natl Acad Sci U S A. 1989;86:7043–7. doi: 10.1073/pnas.86.18.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genis-Galvez JM. Role of the lens in the morphogenesis of the iris and cornea. Nature. 1966;210:209–10. doi: 10.1038/210209a0. [DOI] [PubMed] [Google Scholar]
- Goodwin M, Yap AS. Classical cadherin adhesion molecules: coordinating cell adhesion, signaling and the cytoskeleton. J Mol Histol. 2004;35:839–44. doi: 10.1007/s10735-004-1833-2. [DOI] [PubMed] [Google Scholar]
- Grossmann J. Molecular mechanisms of “detachment-induced apoptosis--Anoikis”. Apoptosis. 2002;7:247–60. doi: 10.1023/a:1015312119693. [DOI] [PubMed] [Google Scholar]
- Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994;265:103–6. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–5. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–58. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Kan NG, Stemmler MP, Junghans D, Kanzler B, de Vries WN, Dominis M, Kemler R. Gene replacement reveals a specific role for E-cadherin in the formation of a functional trophectoderm. Development. 2007;134:31–41. doi: 10.1242/dev.02722. [DOI] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–21. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Kostetskii I, Li J, Xiong Y, Zhou R, Ferrari VA, Patel VV, Molkentin JD, Radice GL. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ Res. 2005;96:346–54. doi: 10.1161/01.RES.0000156274.72390.2c. [DOI] [PubMed] [Google Scholar]
- Kreslova J, Machon O, Ruzickova J, Lachova J, Wawrousek EF, Kemler R, Krauss S, Piatigorsky J, Kozmik Z. Abnormal lens morphogenesis and ectopic lens formation in the absence of beta-catenin function. Genesis. 2007;45:157–68. doi: 10.1002/dvg.20277. [DOI] [PubMed] [Google Scholar]
- Lang RA. Pathways regulating lens induction in the mouse. Int J Dev Biol. 2004;48:783–91. doi: 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A. 1994;91:8263–7. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Ferreira-Cornwell M, Baldwin H, Kostetskii I, Lenox J, Lieberman M, Radice G. Rescuing the N-cadherin knockout by cardiac-specific expression of N- or E-cadherin. Development. 2001;128:459–69. doi: 10.1242/dev.128.4.459. [DOI] [PubMed] [Google Scholar]
- Makhani LF, Williams T, West-Mays JA. Genetic analysis indicates that transcription factors AP-2alpha and Pax6 cooperate in the normal patterning and morphogenesis of the lens. Mol Vis. 2007;13:1215–25. [PubMed] [Google Scholar]
- Muller EJ, Williamson L, Kolly C, Suter MM. Outside-in signaling through integrins and cadherins: a central mechanism to control epidermal growth and differentiation? J Invest Dermatol. 2008;128:501–16. doi: 10.1038/sj.jid.5701248. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Hamada F, Ishidate T, Anai K, Kawahara K, Toyoshima K, Akiyama T. Axin, an inhibitor of the Wnt signalling pathway, interacts with beta-catenin, GSK-3beta and APC and reduces the beta-catenin level. Genes Cells. 1998;3:395–403. doi: 10.1046/j.1365-2443.1998.00198.x. [DOI] [PubMed] [Google Scholar]
- Nguyen MM, Rivera C, Griep AE. Localization of PDZ domain containing proteins Discs Large-1 and Scribble in the mouse eye. Mol Vis. 2005;11:1183–99. [PubMed] [Google Scholar]
- Nielsen PA, Baruch A, Shestopalov VI, Giepmans BN, Dunia I, Benedetti EL, Kumar NM. Lens connexins alpha3Cx46 and alpha8Cx50 interact with zonula occludens protein-1 (ZO-1) Mol Biol Cell. 2003;14:2470–81. doi: 10.1091/mbc.E02-10-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A, Nagafuchi A, Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11:601–12. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–48. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- Pontoriero GF, Deschamps P, Ashery-Padan R, Wong R, Yang Y, Zavadil J, Cvekl A, Sullivan S, Williams T, West-Mays JA. Cell autonomous roles for AP-2alpha in lens vesicle separation and maintenance of the lens epithelial cell phenotype. Dev Dyn. 2008;237:602–17. doi: 10.1002/dvdy.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- Ramaesh T, Collinson JM, Ramaesh K, Kaufman MH, West JD, Dhillon B. Corneal abnormalities in Pax6+/− small eye mice mimic human aniridia-related keratopathy. Invest Ophthalmol Vis Sci. 2003;44:1871–8. doi: 10.1167/iovs.02-0576. [DOI] [PubMed] [Google Scholar]
- Reneker LW, Silversides DW, Patel K, Overbeek PA. TGF alpha can act as a chemoattractant to perioptic mesenchymal cells in developing mouse eyes. Development. 1995;121:1669–80. doi: 10.1242/dev.121.6.1669. [DOI] [PubMed] [Google Scholar]
- Rieger DK, Reichenberger E, McLean W, Sidow A, Olsen BR. A double-deletion mutation in the Pitx3 gene causes arrested lens development in aphakia mice. Genomics. 2001;72:61–72. doi: 10.1006/geno.2000.6464. [DOI] [PubMed] [Google Scholar]
- Semina EV, Murray JC, Reiter R, Hrstka RF, Graw J. Deletion in the promoter region and altered expression of Pitx3 homeobox gene in aphakia mice. Hum Mol Genet. 2000;9:1575–85. doi: 10.1093/hmg/9.11.1575. [DOI] [PubMed] [Google Scholar]
- Shanmugathasan M, Jothy S. Apoptosis, anoikis and their relevance to the pathobiology of colon cancer. Pathol Int. 2000;50:273–9. doi: 10.1046/j.1440-1827.2000.01047.x. [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Tsuzaka K, Yoshimoto K, Kumazawa C, Nozaki K, Abe T, Tsubota K, Takeuchi T. Critical role of the fifth domain of E-cadherin for heterophilic adhesion with alpha E beta 7, but not for homophilic adhesion. J Immunol. 2005;175:1014–21. doi: 10.4049/jimmunol.175.2.1014. [DOI] [PubMed] [Google Scholar]
- Smith AN, Miller LA, Song N, Taketo MM, Lang RA. The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol. 2005;285:477–89. doi: 10.1016/j.ydbio.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Srinivasan Y, Lovicu FJ, Overbeek PA. Lens-specific expression of transforming growth factor beta1 in transgenic mice causes anterior subcapsular cataracts. J Clin Invest. 1998;101:625–34. doi: 10.1172/JCI1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MS, Takeichi M. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc Natl Acad Sci U S A. 1994;91:206–9. doi: 10.1073/pnas.91.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamynathan SK, Katz JP, Kaestner KH, Ashery-Padan R, Crawford MA, Piatigorsky J. Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells. Mol Cell Biol. 2007;27:182–94. doi: 10.1128/MCB.00846-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988;102:639–55. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–5. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M, Atsumi T, Yoshida C, Uno K, Okada TS. Selective adhesion of embryonal carcinoma cells and differentiated cells by Ca2+-dependent sites. Dev Biol. 1981;87:340–50. doi: 10.1016/0012-1606(81)90157-3. [DOI] [PubMed] [Google Scholar]
- Takeichi M, Inuzuka H, Shimamura K, Fujimori T, Nagafuchi A. Cadherin subclasses: differential expression and their roles in neural morphogenesis. Cold Spring Harb Symp Quant Biol. 1990;55:319–25. doi: 10.1101/sqb.1990.055.01.033. [DOI] [PubMed] [Google Scholar]
- Tepass U, Truong K, Godt D, Ikura M, Peifer M. Cadherins in embryonic and neural morphogenesis. Nat Rev Mol Cell Biol. 2000;1:91–100. doi: 10.1038/35040042. [DOI] [PubMed] [Google Scholar]
- Thut CJ, Rountree RB, Hwa M, Kingsley DM. A large-scale in situ screen provides molecular evidence for the induction of eye anterior segment structures by the developing lens. Dev Biol. 2001;231:63–76. doi: 10.1006/dbio.2000.0140. [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121:727–35. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- Wurm A, Sock E, Fuchshofer R, Wegner M, Tamm ER. Anterior segment dysgenesis in the eyes of mice deficient for the high-mobility-group transcription factor Sox11. Exp Eye Res. 2008;86:895–907. doi: 10.1016/j.exer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Xu L, Overbeek PA, Reneker LW. Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Exp Eye Res. 2002;74:753–60. doi: 10.1006/exer.2002.1175. [DOI] [PubMed] [Google Scholar]
- Yoshimoto A, Saigou Y, Higashi Y, Kondoh H. Regulation of ocular lens development by Smad-interacting protein 1 involving Foxe3 activation. Development. 2005;132:4437–48. doi: 10.1242/dev.02022. [DOI] [PubMed] [Google Scholar]
- Zaki PA, Collinson JM, Toraiwa J, Simpson TI, Price DJ, Quinn JC. Penetrance of eye defects in mice heterozygous for mutation of Gli3 is enhanced by heterozygous mutation of Pax6. BMC Dev Biol. 2006;6:46. doi: 10.1186/1471-213X-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–74. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- Zelenka PS. Regulation of cell adhesion and migration in lens development. Int J Dev Biol. 2004;48:857–65. doi: 10.1387/ijdb.041871pz. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Overbeek PA, Govindarajan V. Perinatal ablation of the mouse lens causes multiple anterior chamber defects. Mol Vis. 2007;13:2289–300. [PubMed] [Google Scholar]
- Zhou J, Leonard M, Van Bockstaele E, Menko AS. Mechanism of Src kinase induction of cortical cataract following exposure to stress: destabilization of cell-cell junctions. Mol Vis. 2007;13:1298–310. [PubMed] [Google Scholar]