Abstract
Data on traumatic brain injury (TBI) economic outcomes are limited. We used Rochester Epidemiology Project (REP) resources to estimate long-term medical costs for clinically-confirmed incident TBI across the full range of severity after controlling for pre-existing conditions and co-occurring injuries. All Olmsted County, Minnesota, residents with diagnoses indicative of potential TBI from 1985–2000 (n=46,114) were identified, and a random sample (n=7175) was selected for medical record review to confirm case status, and to characterize as definite (moderate/severe), probable (mild), or possible (symptomatic) TBI. For each case, we identified one age- and sex-matched non-TBI control registered in REP in the same year (±1 year) as case's TBI. Cases with co-occurring non-head injuries were assessed for non-head-injury severity and assigned similar non-head-injury-severity controls. The 1145 case/control pairs for 1988–2000 were followed until earliest death/emigration of either member for medical costs 12 months before and up to 6 years after baseline (i.e., injury date for cases and comparable dates for controls). Differences between case and control costs were stratified by TBI severity, as defined by evidence of brain injury; comparisons used Wilcoxon signed-rank plus multivariate modeling (adjusted for pre-baseline characteristics). From baseline until 6 years, each TBI category exhibited significant incremental costs. For definite and probable TBI, most incremental costs occurred within the first 6 months; significant long-term incremental medical costs were not apparent among 1-year survivors. By contrast, cost differences between possible TBI cases and controls were not as great within the first 6 months, but were substantial among 1-year survivors. Although mean incremental costs were highest for definite cases, probable and possible cases accounted for>90% of all TBI events and 66% of total incremental costs. Preventing probable and possible events might facilitate substantial reductions in TBI-associated medical care costs.
Key words: costs, head injury, outcome measures, traumatic brain injury
Introduction
It is well recognized that traumatic brain injury (TBI) causes substantial morbidity, disability, and mortality. There is also evidence that TBI is associated with high health care costs, both for individuals and society (Berg et al., 2005; Finkelstein et al., 2006; Kayani et al., 2009; Max et al., 1991; McGarry et al., 2002; Schootman et al., 2003; Schneier et al., 2006; Shi et al., 2009; Thurman, 2001). While there is no way to fully describe the human costs of TBI, complete and valid estimates of TBI-associated medical care utilization and costs are essential for informing allocation of scarce resources, targeting efforts toward prevention, identifying best practices, addressing future care needs, and implementing cost-effective treatments.
Existing estimates of TBI-associated costs are problematic for various reasons; several reflect limitations on TBI ascertainment. Although it is increasingly recognized that a majority of TBI events are mild, and that a substantial proportion are diagnosed and managed in office-based settings (Cassidy et al., 2004; Centers for Disease Control and Prevention, 2003; Leibson et al., 2011; Ruff, 2005), the majority of objective population-based cost estimates are limited to injuries admitted to the hospital or emergency department (ED) and/or resulting in death. Case ascertainment often relies on diagnosis codes obtained from billing data or death certificates, and is limited to relatively few highly specific codes recommended for use by the Centers for Disease Control and Prevention (CDC) (i.e., skull fracture, hemorrhage, concussion, cerebral laceration/contusion, or intracranial injury) (Faul et al., 2010). This approach excludes TBI events managed in outpatient settings. Also excluded are events with evidence of brain injury in the medical record, but that were not assigned any CDC-recommended codes. They were instead assigned codes such as post-concussive symptoms, other head injuries, polytrauma, or late effects of injury (Leibson et al., 2011; Centers for Disease Control and Prevention, 2009).
Many published estimates of TBI-associated medical costs are also limited to costs accrued during the initial encounter. Although long-term adverse consequences of TBI on physical and mental health are recognized (Centers for Disease Control and Prevention, 2003, 2010; Hall et al., 1994; Selassie et al., 2008; DeKosky et al., 2010), the long-term economic consequences of TBI are largely unknown. This reflects inherent difficulties of most billing data for identifying and following unique individuals across providers over extended periods of time. Of the few cohort studies that followed unique individuals for costs after the initial encounter, most were limited to persons admitted to the hospital and/or who survived to rehabilitation. Many were limited to specific age groups or mechanisms of injury, and some relied on self-report of injury and/or utilization (Brener et al., 2004; Brooks et al., 1995; Cameron et al., 2008; King et al., 2010; Slomine et al., 2006; Vangel et al., 2005; Whitlock and Hamilton, 1995). Existing estimates of TBI-associated medical care costs have thus been characterized as incomplete and subject to ascertainment, participation, recall, and survivor bias (Berg et al., 2005; Borg et al., 2004; Corrigan et al., 2003; Thurman, 2001).
Previous studies of TBI-associated medical costs are also limited by a shortage of appropriate controls. Patient characteristics, including sex, age, and certain medical conditions (e.g., alcohol abuse and neuropsychiatric conditions) are recognized risk factors for TBI (Bombardier et al., 2002; Vassallo et al., 2007), many of which are independently associated with increased medical costs (Harwood and The Lewin Group, 2002; Welch et al., 2009). Moreover, TBI events are often accompanied by other body system injuries. However, with rare exception (Cameron et al., 2008; McGarry et al., 2002), investigations of TBI-attributable medical costs have insufficiently controlled for costs not attributable to the TBI itself, but which result from pre-existing conditions or co-occurring injuries.
The present study attempts to address several limitations of previous medical care cost estimates by using the unique population-based, medical-records-linkage resources of the Rochester Epidemiology Project (REP) (Melton, 1996). REP resources afford the opportunity to estimate medical care costs associated with TBI: (1) across the full spectrum from symptomatic through fatal events; (2) from before the event until death or emigration; and (3) after controlling for age, sex, calendar year, pre-injury medical conditions, and co-occurring non-head injuries.
Methods
Study setting
Olmsted County (2000 census population=124,277) provides a unique opportunity for investigating the natural history of TBI (Annegers et al., 1980a,1980b,1998; Brown et al., 2004; Flaada et al., 2007; Leibson et al., 2011; Nemetz et al., 1999). As described elsewhere (Melton, 1996), Rochester, the county seat, is approximately 80 miles from the nearest major metropolitan area, and is home to Mayo Clinic, one of the world's largest medical centers. Mayo Clinic and its two hospitals, together with Olmsted Medical Center (OMC), a second group practice and its hospital, provide nearly all of the medical care delivered to local residents. Since 1907, every Mayo patient has been assigned a unique identifier. All information from every contact (office, nursing home, ED, hospital inpatient, and hospital outpatient visits) is contained within a unit record for each patient. Detailed information includes medical history, all clinical assessments, consultation reports, surgical procedures, dismissal summaries, laboratory and radiology results, correspondence, death certificates, and autopsy reports. Diagnoses assigned at each visit are coded and entered into continuously updated files. The coding system was developed for clinical, not billing, purposes, and uses an 8-digit modification of the Hospital Adaptation of the International Classification of Diseases (H-ICDA, 1973), which affords high sensitivity and specificity. Under the auspices of the REP, the unique identifiers, diagnostic index, and records-linkage were expanded to include other providers of medical care to local residents, including OMC and the few private practitioners in the area, thereby linking medical records for community residents. The REP provides the capability for population-based studies of disease risk factors, incidence, and outcomes, that is unique in the United States (Melton, 1996).
Data on medical utilization and costs
As described previously (Leibson et al., 2006), due to the unique circumstances noted above, >95% of all medical care encounters by Olmsted County residents occur at either the Mayo Clinic, OMC, or their affiliated hospitals. Through an electronic data-sharing agreement signed by administration at Mayo Clinic and OMC, patient-level administrative data on health care utilization and associated billed charges incurred at these institutions are shared and archived within the Olmsted County Healthcare Expenditure and Utilization Database (OCHEUD) for use in approved research studies. Individuals can be identified across institutions and over time. Data are electronically linked, affording complete information on all hospital and ambulatory care delivered by these providers to area residents from 1/1/1987 until the present.
The files serve as a major source of health economic information on all patients (i.e., all ages and payer types, including the uninsured), and contain line-item detail on date, type, frequency, and billed charge for every good or service provided. Recognizing discrepancies between billed charges and true resource use, the OCHEUD employs widely accepted valuation techniques to generate a standardized inflation-adjusted estimate of the costs of each service or procedure in constant dollars. Cost estimates in this study were adjusted to 2007 dollars. A detailed description of the costing methodology is provided elsewhere (Leibson et al., 2006).
Case identification
This study was approved by the Mayo Clinic and OMC Institutional Review Boards. The method of case identification has been described previously (Leibson et al., 2011). As part of the Mayo Clinic Traumatic Brain Injury Model System Center research activities funded by the National Institute on Disability and Rehabilitation Research (NIDRR), all Olmsted County residents with any diagnosis suggestive of head injury or TBI in the REP diagnostic index from 1/1/1985 through 12/31/1999 were identified. The extensive list of the International Classification of Diseases, 9th Revision-Clinical Modification (ICD-9-CM) and Mayo adaptation of H-ICDA codes used to identify cases is provided in the supplementary materials (Supplementary Table 1; see online supplementary material at http://www.liebertonline.com). There were 46,114 unique individuals with one or more such codes; the 323 who refused authorization at all REP providers where they were seen were excluded from further review (Melton, 1997). From the remaining 45,791 cases, a 16% sample was randomly selected for manual review of authorized medical records from date first seen until date last seen at any REP provider, to identify, confirm, and characterize each event. Information from medical records was collected by trained nurse abstractors under the direction of a board-certified physiatrist (A.W.B.) and neuropsychologist (J.F.M.). The record review involved all available clinical data including, but not limited to, general history notes, ED notes, hospital records, radiological imaging findings, surgical records, and autopsy reports.
TBI was defined as a traumatically-induced injury that contributed to physiological disruption of brain function. Evidence of physiological disruption included documentation of any of the following: concussion with loss of consciousness (LOC); post-traumatic amnesia (PTA); neurological signs of brain injury and/or evidence of intracerebral, subdural, or epidural hematoma; cerebral or hemorrhagic contusion; brainstem injury; penetrating brain injury; skull fracture; or post-concussive symptoms (dizziness, confusion, blurred vision, double vision, headache, nausea, or vomiting that was not attributable to pre-existing or comorbid conditions). Individuals who only had transient post-concussive symptoms (i.e., lasting<30 min) were excluded.
Incident events were defined as the first event 1/1/1985 through 12/31/1999 for which the individual was a resident of Olmsted County, and for which there was no mention in the medical record of an earlier TBI. All incident TBI events were further characterized by mechanism of injury (Thurman et al., 1995). Incident events were also categorized as either definite, probable, or possible (symptomatic) using the Mayo TBI severity classification system (Malec et al., 2007). The system capitalizes on the strength of evidence of brain injury available within the medical records (Table 1).
Table 1.
Mayo Traumatic Brain Injurya (TBI) Severity Classification System
| A. Classify as definite TBI if one or more of the following criteria apply: |
| 1. Death due to this TBI |
| 2. Loss of consciousness of 30 min or more |
| 3. Post-traumatic anterograde amnesia of 24 h or more |
| 4. Worst Glasgow Coma Scale full score in the first 24 h<13 (unless invalidated upon review; e.g., attributable to intoxication, sedation, or systemic shock) |
| 5. One or more of the following present: |
| • Intracerebral hematoma |
| • Subdural hematoma |
| • Epidural hematoma |
| • Cerebral contusion |
| • Hemorrhagic contusion |
| • Penetrating TBI (dura penetrated) |
| • Subarachnoid hemorrhage |
| B. If none of criteria A apply, classify as probable TBI if one or more of the following criteria apply: |
| 1. Loss of consciousness of momentary to<30 min |
| 2. Post-traumatic anterograde amnesia of momentary to<24 h |
| 3. Depressed, basilar, or linear skull fracture (dura intact) |
| C. If none of criteria A or B apply, classify as possible (symptomatic) TBI if one or more of the following symptoms are documented: |
| 1. Blurred vision |
| 2. Confusion (mental state changes) |
| 3. Dazed |
| 4. Dizziness |
| 5. Focal neurologic symptoms |
| 6. Headache |
| 7. Nausea |
Adapted from Malec et al., 2007.
The detailed medical record review of the 16% sample of Olmsted County residents with any diagnosis suggestive of head injury or TBI from 1/1/1985 through 12/31/1999 revealed 1429 (approximately 1 in 5) individuals who qualified as an incident definite, probable, or possible TBI during that 15-year period. Because administrative claims for Olmsted County residents are only available electronically since 1987, and we wished to examine costs for persons with TBI in the year before injury, the present study is limited to incident cases identified from 1/1/1988 through 12/31/1999 (n=1145).
Selection of controls
Two approaches were used for selecting controls. For the first approach, each TBI case was matched to an individual of same sex and similar birth year (±1 year) who was registered at a REP provider as an Olmsted County resident in the year (±1 year) of the case's TBI. The REP diagnostic index was then used to obtain all diagnosis codes assigned each potential control by any REP provider. Individuals assigned any code associated with head injury from date first seen through date of the case's TBI were excluded as a potential control for that case and another was selected. This first set of controls is subsequently referred to as “regular” controls. The second approach to control selection was intended to reduce potential confounding due to co-occurring non-head injuries. Control selection for this purpose was limited to a subset of “special” TBI cases (i.e., individuals who had presented to the ED or hospital around the time of their TBI, and were assigned a diagnosis code for that encounter that was indicative of a co-occurring non-head injury). For each co-occurring non-head injury, we first assigned a diagnosis code-based empiric measure of severity, and then applied the Trauma Mortality Prediction Model (TMPM-ICD9) to assign an overall measure of non-head injury severity to each individual (Glance et al., 2009). For each “special” case, we randomly selected two controls from the list of all Olmsted County residents of the same sex and birth year as the case, and who had no diagnosis code associated with head injury within or before the year of the case's event, but who were admitted to the ED or hospital in the year of the case's event, and for whom his or her injuries contributed to an overall measure of severity similar to that for the case's co-occurring non-head injuries. These controls are subsequently referred to as “special” controls. For this study of TBI-associated costs, the analysis was limited to one control per case (i.e., a “regular” control for “regular” cases, and one randomly-selected “special” control from the two “special” controls that were assigned each “special case”).
Each subject was assigned a baseline date that served as a referent for obtaining cost data. For cases, baseline was their incident TBI date. For “regular” controls, baseline was the REP registration date closest to the matched case's TBI date. For “special” controls, baseline was the date of their relevant ED or hospital admission. Each case and control was followed forward for costs from 1 year before baseline through the earliest of death, emigration from Olmsted County, or 12/31/2006 (the study's end date). TBI is associated with reduced survival in the acute and subacute phases following injury (Brown et al., 2004; Flaada et al., 2007; Ventura et al., 2010). To ensure similar periods of observation for cases and controls, we censored each member of a matched pair as of the shortest length of follow-up for either member. The control selection process also allowed that a person selected as a control could be assigned a diagnosis code for TBI subsequent to their baseline date, at which point follow-up for both members of the matched pair was also censored.
Statistical analysis
Analyses were conducted with SAS version 8.02 (SAS Institute, Cary, NC). Statistical testing used the two-tailed alpha level of 0.05. The principal outcome was direct medical costs associated with TBI, stratified by level of evidence of brain injury. Costs were assessed from baseline forward for up to 6 years and within each period: 1 day to 6 months, 6 months to 1 year, and 1 year to 6 years. By matching each case to one non-TBI control drawn from the same population who was of same sex and similar age, calendar year, presence and severity of co-occurring non-head injuries, and length of follow-up, we minimized concerns of potential confounding due to these variables. In the primary analytic approach, the costs for each control were subtracted from the costs for his or her case in each time period; statistical significance was assessed using the Wilcoxon signed-rank test to account for the highly skewed nature of cost data and paired observations (Wilcoxon, 1945). Our experience employing this approach is demonstrated with previous REP studies of population-based cost-of-illness estimates for a variety of medical conditions (Leibson et al., 1996,2001,2006; Long et al., 2010; Thompson et al., 2011).
The analysis described above did not control for pre-existing medical conditions. To examine the potential for confounding by these factors in estimating TBI-associated costs, we also compared cases and controls with respect to pre-existing medical conditions and costs in the year before baseline. Pre-existing medical conditions were investigated using Johns Hopkins Adjusted Clinical Groups (ACG) System® software (Johns Hopkins Bloomberg School of Public Health, 2010), which requires ICD-9-CM diagnosis codes. We obtained all ICD-9-CM diagnoses codes assigned each individual in OCHEUD billing data in the year before baseline. We used ACG software to categorize each individual's diagnosis codes into groupings based on persistence, severity, and etiology of the condition, as well as diagnostic certainty, and need for specialty care (Johns Hopkins Bloomberg School of Public Health, 2010). ACG software was also used to assign a Resource Utilization Band (RUB) value to each individual. RUB categories are aggregations of ACGs that have similar expected resource use, with values ranging from 0 (no relevant diagnosis codes) to 5 (diagnosis codes associated with very high use) (Johns Hopkins Bloomberg School of Public Health, 2002). For those TBI severity classification categories with statistically significant differences in the distribution of pre-baseline RUB values between cases and controls, we then used multivariate modeling to examine the extent to which pre-baseline medical conditions accounted for differences in post-baseline costs between cases and controls. This approach employed two-part models to account for zero costs (Buntin and Zaslavsky, 2004; Manning et al., 1981), incorporated a generalized linear model with log link and a gamma distribution for the error term to account for the skewed distribution while enabling coefficients to be directly back-transformed into the original dollar scale (Birnbaum et al., 2009; Mullahy, 1998), and analyzed differences in costs between cases and controls using the method of recycled predictions, setting all individuals as cases with TBI or as controls without TBI, while keeping constant all other individual characteristics (Basu et al., 2006; Esposito et al., 2009). The models of predicted costs as a function of case status were adjusted for baseline age and pre-baseline RUB value. The adjusted cost for each individual as a control was subtracted from his or her adjusted cost as a case; mean values and bootstrapped 95% confidence intervals of the mean difference were calculated.
Results
Baseline case characteristics
For the 1145 randomly sampled individuals who met REP criteria for incident TBI during the 12-year period from 1/1/1988 through 12/31/1999 (i.e., cases), the characteristics at the time of TBI are provided in Table 2. The majority of incident TBI events occurred among males. Individuals age 16 through 64 years accounted for over half of all cases; elderly adults accounted for<10%. Less than 10% of all cases met criteria for definite TBI, as defined in Table 1, while over half of all cases met criteria for possible (symptomatic) TBI. Age and sex distributions differed by TBI severity classification category. Among definite cases, 14% of injuries occurred among youth, and 28% occurred among elderly adults. This pattern was reversed for possible cases: 40% of events occurred in youth, and only 7% occurred among elderly adults. Two hundred five cases (18%) were admitted to the ED or hospital at the time of TBI with co-occurring non-head injuries (i.e., met criteria for “special” cases). The proportions again differed as a function of TBI severity classification category, with the smallest proportion expectedly occurring among possible (symptomatic) cases. Falls and motor vehicle accidents together accounted for over half of all incident events, both overall and in each severity classification category.
Table 2.
Characteristics of Olmsted County Residents Who Met Rochester Epidemiology Project (REP) Criteria for Incident Traumatic Brain Injury (TBI) from 1/1/1988 to 12/31/1999
| Subject characteristics | Total | Definite | Probable | Possible (symptomatic) | p Value |
|---|---|---|---|---|---|
| Number (%) | 1145a | 93 (8%) | 435 (38%) | 617 (54%) | |
| Male, n (%) | 637 (56%) | 54 (58%) | 274 (63%) | 309 (50%) | <0.001 |
| Age distribution, years, n (%) | <0.001 | ||||
| < 16 years | 398 (35%) | 13 (14%) | 137 (31%) | 248 (40%) | |
| 16–64 years | 640 (56%) | 54 (58%) | 259 (60%) | 327 (53%) | |
| > 64 years | 107 (9%) | 26 (28%) | 39 (9%) | 42 (7%) | |
| Special case, n (%)b | 205 (18%) | 25 (27%) | 108 (25%) | 72 (12%) | <0.001 |
| Mechanism of injury, n (%) | <0.001 | ||||
| Fall | 312 (27%) | 33 (35%) | 94 (22%) | 185 (30%) | |
| Motor vehicle accident | 316 (28%) | 32 (34%) | 150 (34%) | 134 (22%) | |
| Hit by object | 64 (6%) | 5 (5%) | 18 (4%) | 41 (7%) | |
| Assault/gunshot | 79 (7%) | 9 (10%) | 38 (9%) | 32 (5%) | |
| Sports/recreation | 283 (25%) | 9 (10 %) | 123 (28%) | 151 (24%) | |
| Other | 91 (8%) | 5 (5%) | 12 (3%) | 74 (12%) |
The number of individuals who were included in the 16% random sample from the list of all 46,114 potential cases 1985 through 1999, and who following detailed medical record review were confirmed as having met REP criteria for first lifetime clinically-recognized definite, probable, or possible TBI (Table 1) from 1/1/1988 through 12/31/1999.
Cases who presented to the emergency department or hospital and received a diagnosis code indicative of co-occurring non-head injury.
Case/control comparisons
At baseline
By virtue of the matched design, cases and controls were similar with respect to baseline age [mean (standard deviation; SD)=27 (22) years for cases, and 28 (22) for controls; p=0.66], percent males (56%), and length of observation [mean (SD), median, and range=7.53 (5.55), 7.26 years, and 0 - 20.97 years]. For the 205 “special” cases, the distribution of injury severity for their non-head injuries was also similar by design to that for the 205 matched “special” controls [mean (SD), median, and range values were 1.46% (4.63%), 0.32%, 0.01–57.58% for cases, and 0.76% (1.20%), 0.30%, <0.01–11.4% for controls; p=0.10].
Before baseline
Table 3A provides results of comparisons between cases and controls for medical costs in the year before baseline. On average, the difference between cases and their matched controls was $49 higher for definite cases and $540 lower for probable cases. For both definite and probable cases, the median difference was<$40; there were no statistically significant cost differences between cases and controls for either of these two groups. By contrast, possible (symptomatic) TBI cases cost an average of $441 more than their matched controls in the year before baseline; the signed rank test was highly significant. As shown in Table 3B, this pre-baseline pattern of costs was consistent with the pattern of between-group differences in pre-baseline medical conditions, as measured using RUB values. There were no significant differences in RUB values in the year before baseline when comparing cases with controls for either definite or probable TBI cases; however, the pre-baseline RUB value for possible (symptomatic) TBI cases was significantly higher than that for their non-TBI controls.
Table 3.
Comparisons between the Random Sample of Olmsted County Residents Who Met Rochester Epidemiology Project (REP) Criteria for Traumatic Brain Injury (TBI; i.e., Cases) and Their Matcheda Non-TBI Controls for Direct Medical Care Costs and Medical Conditions in the Year before Baselineb
|
3A. Medical care costscin the year before baseline | |||||||
|---|---|---|---|---|---|---|---|
| |
|
Mean cost |
Cost difference (case's cost minus control's cost) |
||||
| No. of pairs | Cases | Controls | Mean | Median | 25th-75th percentile | p Valued | |
| Definite (moderate/severe) | 93 | $4071 | $4022 | +49 | +$35 | −$980 to +$1227 | 0.55 |
| Probable (mild) | 435 | $2186 | $2726 | −$540 | $0 | −$451 to +$801 | 0.15 |
| Possible (symptomatic) | 617 | $2163 | $1722 | +$441 | +$132 | −$297 to +$913 | <0.001 |
|
3B. Distribution of Resource Utilization Band (RUB)evalues for cases and controls in the year before baseline, n (%) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | p Valuef | |
| Definite (moderate/severe) | 0.18 | ||||||
| Cases, n=93 | 16 (17%) | 11 (12%) | 20 (22%) | 30 (32%) | 5 (5%) | 11 (12%) | |
| Controls, n=93 | 26 (28%) | 11 (12%) | 13 (14%) | 31 (33%) | 6 (6%) | 6 (6%) | |
| Probable (mild) | 0.14 | ||||||
| Cases, n=435 | 98 (23%) | 83 (19%) | 84 (19%) | 132 (30%) | 27 (6%) | 11 (3%) | |
| Controls, n=435 | 114 (26%) | 75 (17%) | 96 (22%) | 124 (29%) | 20 (5%) | 6 (1%) | |
| Possible (symptomatic) | <0.001 | ||||||
| Cases, n=617 | 108 (17%) | 116 (19%) | 145 (24%) | 211 (34%) | 24 (4%) | 13 (2%) | |
| Controls, n=617 | 178 (29%) | 116 (19%) | 150 (24%) | 134 (22%) | 27 (4%) | 12 (2%) | |
Controls were matched to cases for age, sex, calendar year, severity of non-head injuries at baseline, and length of follow-up.
Date of incident TBI for cases, closest REP registration date for regular controls, and relevant hospital or emergency department encounter for special controls.
Dollar values were adjusted for inflation and geographical wage differences to express costs for each year in 2007 nationally representative constant dollars.
Wilcoxon signed rank test.
Resource Utilization Bands (Johns Hopkins Bloomberg School of Public Health, 2002) are aggregations of diagnosis codes into categories with similar expected costs, with 0=no relevant diagnoses, 1=healthy user, 2=low user, 3=moderate user, 4=high user, 5=very high user.
Asymptotic Wilcoxon p value; exact inference for ordered row and column contingency tables.
The latter finding was explored in greater detail by comparing possible cases with their matched controls for the prevalence of pre-existing medical conditions within each of 34 Johns Hopkins Aggregated Diagnostic Group (ADG) categories (Johns Hopkins Bloomberg School of Public Health, 2010). Even before the incident TBI, possible cases were more likely than controls to have one or more diagnoses for “minor injures/adverse events” (p<0.001). In the category “time limited minor conditions,” possible TBI cases were more likely than their non-TBI controls to have pre-existing diagnoses in both of the sub-categories “primary infections” (p<0.001) and “other than primary infections” (p<0.05). In the category “likely to recur discrete,” possible TBI cases were also more likely than controls to have pre-existing diagnoses in the sub-category “infections” (p<0.05). In the category “psychosocial conditions,” symptomatic TBI cases were more likely than controls to have pre-existing diagnoses in the sub-categories “time limited minor” (p<0.01) and “persistent recurrent, stable” (p<0.01). And in the category “signs and symptoms,” symptomatic TBI cases were more likely than controls to have pre-existing diagnoses within each of the three sub-categories “minor” (p<0.001), “major” (p<0.01), and “uncertain” (p<0.001) (data not shown, available upon request).
After baseline
Table 4 provides the distribution of costs by time period post-baseline for cases who experienced definite (Table 4A), probable (4B), and possible (4C) TBI; cost distributions are similarly provided for their respective controls. The highly skewed nature of cost data is apparent for both cases and controls within each severity classification category, with some individuals experiencing zero costs in almost every time period, and very few individuals contributing a large proportion of costs in every time period. Importantly, 10 (11%) definite cases had zero costs in OCHEUD because they died at the scene or en-route to the hospital or ED. For all severity categories, analyses of costs for the first 6 months after baseline were therefore limited to cases and controls for whom both members of the matched pair survived 1 day after baseline.
Table 4.
Distribution of Direct Medical Care Costsa for the Random Sample of Olmsted County Residents Who Met Rochester Epidemiology Project (REP) Criteria for Traumatic Brain Injury (TBI) (i.e., Cases) and Their Matchedb Non-TBI Controls, for Specified Time Periods after Baselinec
|
4A. Definite TBI cases and matched controls | |||||||
|---|---|---|---|---|---|---|---|
| Time period, no. of pairs (% entering) | Mean | No. with zero costs | 25thpercentile | 50thpercentile | 75thpercentile | Maximum | |
| Full period: Baseline to 6 years, 93 (100%) | Case | 35,244 | 11/93 (12%) | 4169 | 15,781 | 48,146 | 226,699 |
| Control | 14,639 | 3/93 (3%) | 391 | 2755 | 14,102 | 415,802 | |
| 1 day to 6 months, 75 (81%) | Case | 24,838 | 2/75 (3%) | 2153 | 10,741 | 33,146 | 168,340 |
| Control | 1999 | 12/75 (16%) | 87 | 321 | 1631 | 29,340 | |
| 6–12 months, 57 (61%) | Case | 2349 | 9/57 (16%) | 50 | 259 | 935 | 53,053 |
| Control | 966 | 18/57 (32%) | 0 | 215 | 431 | 18,329 | |
| 1–6 years, 53 (57%) | Case | 16,875 | 2/53 (4%) | 855 | 4101 | 16,545 | 155,321 |
| Control | 20,394 | 1/53 (2%) | 1122 | 4772 | 19,027 | 402,190 | |
|
4B. Probable TBI cases and matched controls | |||||||
|---|---|---|---|---|---|---|---|
| Time period, no. of pairs (% entering) | Mean | Percent with zero costs | 25thpercentile | 50thpercentile | 75thpercentile | Maximum | |
| Full period: Baseline to 6 years, 435 (100%) | Case | 16,722 | 0/435 (0%) | 2526 | 6345 | 16,033 | 319,902 |
| Control | 13,221 | 1/435 (0.2%) | 1151 | 3255 | 9712 | 422,483 | |
| 1 day to 6 months, 434 (99.8%) | Case | 3720 | 39/434 (9%) | 195 | 711 | 2781 | 94,100 |
| Control | 2737 | 83/434 (19%) | 43 | 273 | 787 | 301,708 | |
| 6–12 months, 402 (92%) | Case | 1616 | 130/402 (32%) | 0 | 137 | 635 | 72,109 |
| Control | 1196 | 103/402 (26%) | 0 | 144 | 496 | 29,519 | |
| 1–6 years, 373 (86%) | Case | 11,521 | 18/373 (5%) | 643 | 2742 | 8330 | 307,165 |
| Control | 10,505 | 14/373 (4%) | 781 | 2348 | 7339 | 327,086 | |
|
4C. Possible TBI cases and matched controls | |||||||
|---|---|---|---|---|---|---|---|
| Time period, no. of pairs (% entering) | Mean | % with zero costs | 25thpercentile | 50thpercentile | 75thpercentile | Maximum | |
| Full period: Baseline to 6 years, 617 (100%) | Case | 13,319 | 0/617 (0%) | 1750 | 4718 | 13,493 | 335,013 |
| Control | 9789 | 6/617 (1%) | 1042 | 3184 | 9656 | 169,275 | |
| 1 day to 6 months, 616 (99.8%) | Case | 1686 | 70/616 (11%) | 122 | 477 | 1229 | 64,099 |
| Control | 1875 | 125/616 (20%) | 43 | 221 | 726 | 87,873 | |
| 6–12 months, 576 (93%) | Case | 1,154 | 140/576 (24%) | 13 | 205 | 760 | 40,237 |
| Control | 1,198 | 170/576 (30%) | 0 | 119 | 538 | 141,004 | |
| 1–6 years, 537 (85%) | Case | 11,725 | 22/537 (4%) | 1131 | 3785 | 11,464 | 232,117 |
| Control | 7309 | 19/537 (4%) | 814 | 2392 | 6862 | 130,198 | |
Dollar values were adjusted for inflation and geographical wage differences to express costs for each year in 2007 nationally representative constant dollars.
Controls were matched to cases for age, sex, calendar year, severity of non-head injuries at baseline, and length of follow-up; follow-up for each member of the case/control pair was censored as of the earliest death or emigration of either member.
Date of incident TBI for cases, closest REP registration date for regular controls, and relevant hospital or emergency department visit for special controls.
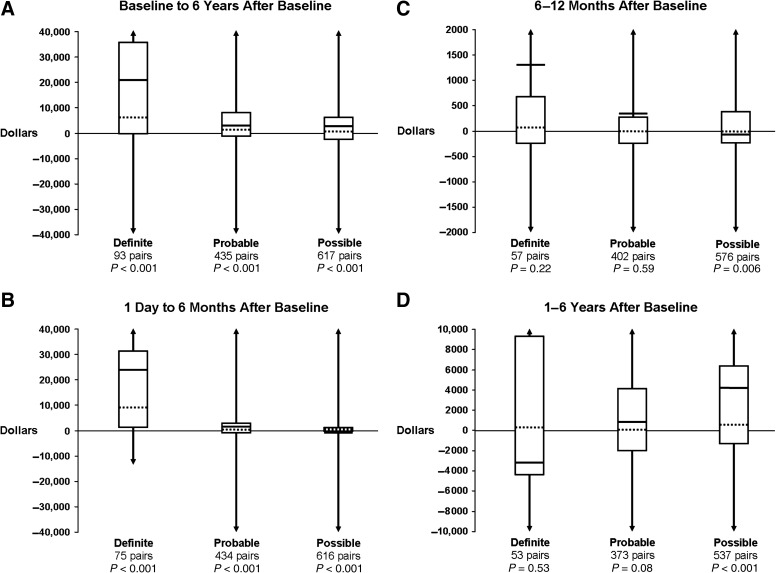
Box and whiskers plots of observed incremental costs (i.e., each case's costs minus costs for his or her matched control) are provided by TBI severity classification category for the four time periods in Figure 1A–D. Over the full period baseline to 6 years, direct medical costs for all persons with TBI were an average of $4906 higher than costs for persons without TBI (data not shown); costs for cases were significantly higher than those for controls within each severity classification category (Fig. 1A). For definite and probable TBI, the majority of excess costs occurred within the first 6 months after baseline; for possible TBI, the costs for cases in the first 6 months were only slightly (although still significantly) higher than for controls (Fig. 1B). In the 6–12 months after baseline, the difference in costs between each case and his or her control appeared slightly higher for cases in each TBI severity classification category, but reached statistical significance only for possible cases (Fig. 1C). Among definite and probable pairs who survived 1 year after baseline, the cost difference between each case and his or her control from 1 to up to 6 years after baseline was again not statistically significant (Fig. 1D). By contrast, possible (symptomatic) cases exhibited greater costs than their matched controls in the period 1 to 6 years, and the difference was highly significant.
FIG. 1.
Summary measures of direct medical care costs for each traumatic brain injury (TBI) case minus costs for his or her matched control are provided for specified time periods after baseline by TBI category (Figs. 1A–1D). Data are presented as box and whiskers plots. Dashed horizontal lines depict the median; boxes depict the interquartile range (25th and 75th percentiles); solid horizontal lines depict the mean; vertical lines (i.e., whiskers) depict the range. In each figure, the y axis was truncated to facilitate visualization (i.e., as depicted by the arrows). Some differences were well above the highest value and well below the lowest value shown on the y axis. (A) The differences in direct medical costs for the full time period from baseline to a maximum of 6 years after baseline. (B) Data for case/control pairs that survived 1 day, and differences in direct medical costs from 1 day to a maximum of 6 months after baseline. (C) Data for case/control pairs that survived 6 months, and differences in direct medical costs from 6 months to a maximum of 12 months after baseline. (D) Data for case/control pairs that survived 1 year, and differences in direct medical costs from 1 year to a maximum of 6 years after baseline.
To investigate whether post-baseline cost differences between possible cases and their controls were confounded by differences in pre-baseline medical conditions observed in Table 3B, we conducted multi-variate analyses, adjusted for pre-baseline RUB values. Mean adjusted costs and mean difference in adjusted costs, together with 95% confidence intervals, are provided for each time period in Table 5. Over the full period baseline to 6 years, the adjusted mean incremental costs associated with possible TBI were $2300. Increased costs were primarily confined to the period 1–6 years after baseline. For both baseline to 6 months and 6–12 months after baseline, adjusted costs for possible TBI cases were similar or slightly less than those for controls. These results are generally consistent with those from univariate analyses for possible cases, with the general exception that estimates of mean incremental costs after adjustment for pre-baseline medical conditions were slightly lower than those observed with univariate signed rank analyses. Because neither definite nor probable TBI cases differed from their matched controls with respect to pre-baseline costs or RUB values, no adjusted analyses were deemed warranted.
Table 5.
Comparisons between the Random Sample of 617 Olmsted County Residents Who Met Rochester Epidemiology Project (REP) Criteria for Possible (Symptomatic) Traumatic Brain Injury (TBI) (i.e., Cases) and Their Matcheda Non-TBI Controls for Costs in the 6 Years after Baselineb Adjusted for Differences between Cases and Controls with Respect to Medical Conditions before Baselinec
| |
|
Adjusted mean costs |
|
|
|---|---|---|---|---|
| Time period | No. of pairs (% entering period) | Cases | Controls | Adjusted mean difference in medical care costsd,e(95% CIf) |
| Full period: Baseline to 6 years | 617 (100%) | $13,018 | $10,718 | +$2300 (+$2190, +$2428) |
| 1 day to 6 months | 616 (99.8%) | $1300 | $1771 | −$471 (−$497, −$440) |
| 6–12 months | 576 (93%) | $993 | $978 | +$15f (+14, +17) |
| 1–6 years | 537 (87%) | $11,645 | $8227 | +$3418 (+$3236, +$3634) |
Controls were matched to cases for age, sex, calendar year, non-head injury severity at baseline, and length of follow-up.
Date of incident TBI for cases and closest REP registration date for controls.
Assessed using Resource Utilization Bands (Johns Hopkins Bloomberg School of Public Health, 2002).
Analyzed using recycled predictions (Basu et al., 2006).
Dollar values were adjusted for inflation and geographical wage differences to express costs for each year in 2007 nationally representative constant dollars.
Bootstrapped values for 95% confidence intervals (CI).
Two-part modeling was used to account for high percentage with zero costs.
Discussion
This population-based historical cohort study revealed significantly higher costs for TBI cases compared to matched controls. Over the full period, from baseline up to 6 years after baseline, medical care costs for persons with TBI were an average of $4906 higher than costs for matched controls without TBI. Findings differed as a function of time since the event and TBI severity classification category. As revealed by Figure 1, from 1 day to a maximum of 6 months post-baseline, a definite TBI case cost an average of $22,838 more than his or her matched control; a probable TBI case cost an average of $983 more than his or her matched control; while a possible TBI case cost an average of $189 less than his or her matched control. Importantly, from 1 year to a maximum of 6 years after baseline, this pattern differed markedly. For definite TBI, the cost for each case was on average less than that for his or her matched control; the difference was not significant. By contrast, persons with probable and possible TBI had higher costs compared to their matched controls (the mean difference was $1016 and $4416, respectively); the difference was highly significant for possible cases. The difference remained highly significant, although the point estimate ($3418) was slightly reduced, after adjusting for the higher prevalence of medical conditions among possible cases compared to their matched controls in the year before baseline (Table 5).
This study addressed several concerns with previous studies of TBI-associated medical costs. A majority of published estimates have relied on administrative claims as the source of case ascertainment, with case status based on assignment of one of the restricted sets of ICD-9-CM discharge diagnosis codes recommended by the CDC (Centers for Disease Control and Prevention, 2005; Thurman, 2001). We and others have demonstrated that milder cases are seriously underestimated using this approach (Leibson et al., 2011; Ryu et al., 2009). The list of potential cases in the present study was generated using a coding system that was designed for clinical rather than reimbursement purposes, and that employs 8-digit codes rather than the 3- to 5-digit ICD-9-CM codes obtained from administrative data. The range of diagnostic codes used was extremely broad. Confirmation and characterization of TBI case status was afforded with access to the complete clinical details contained within the provider-linked medical records of all potential cases and application of standardized criteria. The study included both sexes, all ages, all mechanisms of injury, and the full spectrum of clinically-recognized disease. Cases included both fatal and non-fatal TBI events, as identified from death certificates and from all health care delivery settings (i.e., hospital inpatient, hospital outpatient, ED, and office visits). Access to unique identifiers for all Olmsted County residents over the entire time period and across all REP providers afforded identification of first lifetime clinically-recognized events.
Of the relatively few studies of TBI-associated medical costs, a majority are limited to costs for the event hospitalization and/or rehabilitation. The present study followed cases and controls for all hospital inpatient, hospital outpatient, rehabilitation, ED, and all office visit costs from 1 year before to up to 6 years after baseline. Cost estimates were obtained using line-item billing data that are available for more than 95% of medical care received by Olmsted County residents. Analyses controlled for non-TBI costs by comparing costs for each case with costs for an individual without previous head injury who was drawn from the same population and matched the case for sex, age, calendar year, severity of co-occurring non-head injuries, and length of follow-up, and by statistically adjusting for pre-baseline differences in medical conditions between cases and controls. Our analyses attempted to account for the highly skewed nature of economic data and instances of zero costs.
Our study has a number of limitations. The estimates are for a single geographic population, which in 2000 was 90% white. The age, sex, and racial distribution of Olmsted County is similar to that for Minnesota, the upper midwest, and the U.S. white population; however, residents of Olmsted County have a higher median income and education level compared to these other geographic regions (Melton, 1996; St. Sauver, 2012). While no single geographic area is representative of all others, the under-representation of minorities and the fact that essentially all medical care is delivered by a few providers compromises the generalizability of our study findings to different racial and ethnic groups and to health care environments different from those represented here. The identification of TBI is based on retrospective analysis of medical records; thus individuals who did not seek medical attention are not included. Data from cross-sectional surveys suggest that from 25–42% of non-fatal cases of self-reported head injury do not seek any medical care (Setnik and Bazarian, 2007; Sosin et al., 1996). Our study does not include nursing home costs. Although the proportion of persons admitted to a nursing home following TBI is relatively small, the stays can be long (Mellick et al., 2003). The risk of admission is likely higher for definite cases. Our study also does not include indirect costs. Studies that have reported both direct and indirect costs reveal that TBI-associated indirect costs were substantially higher than direct costs (Borg et al., 2004; Finkelstein et al., 2006; Kalsbeek et al., 1980; Max et al., 1991; Miller et al., 1994).
The present study analyzed differences in medical costs between each TBI case and his or her matched control to estimate excess costs associated with TBI within severity classification categories. Classification categories were based on evidence of brain injury available in the medical record (Table 1). The marked heterogeneity with respect to the range of TBI severity and how it is defined in the literature is well recognized (Barker-Collo and Feigin, 2009; Cassidy et al., 2004). While several definitions, including those employed in earlier REP studies of TBI incidence (Annegers et al., 1980a), required evidence of at least minimal LOC or PTA, others, including the present study, did not. In this study, individuals who presented to medical care following head injury, and whose medical record documentation was limited to self-report of post-concussive symptoms (e.g., dizziness and/or headache), were included within the category of possible TBI. Individuals for whom it was determined on record review that their symptoms were associated with a prior condition were excluded; however, the extent to which our estimates of symptomatic cases may have been inflated by patients' attribution of pre-injury symptoms to recent head trauma (Mittenberg et al., 1992) cannot be determined. In this regard, our finding that, compared to matched controls, persons with possible TBI had a higher prevalence of medical conditions before injury and higher medical costs both before injury and in the period 1–6 years after the injury is of interest. Although our analyses attempted to adjust for pre-existing medical conditions, it remains unclear whether the symptomatic event resulted in mild neurologic injury that contributed new medical issues and/or exacerbated existing medical issues, or whether a trend of gradually increasing medical issues would have occurred even absent the event of TBI. Further investigation is needed.
Implications
Other studies have frequently concluded that medical costs for persons with TBI increase with increasing TBI severity and are highest for cases who do not survive (Brener et al., 2004; Max et al., 1991; McGarry et al., 2002; Schneier et al., 2006; Shi et al., 2009). These reports are consistent with visual comparisons of box and whisker plots in Figure 1B, which suggest that excess costs in the first 6 months after baseline were higher for definite compared to probable or possible cases. And because of the dramatic difference in costs between definite cases and their matched controls in the first 6 months, the incremental costs for definite cases over the full period baseline to 6 years (Fig. 1A) appear to remain much higher compared to incremental costs for probable and possible cases. However, our longer-term investigations suggest that incremental medical costs for possible TBI may not become apparent until 1 year post-injury, after which time they become significantly higher than medical costs for their matched controls. By contrast, cost differences between definite TBI cases and matched controls are no longer significant 1–6 years after baseline. Previous reports were largely limited to comparison of mean or total costs, which do not account for the highly skewed distribution apparent in Table 4. Subjects were often limited to hospitalized cases and therefore excluded cases who died before admission (zero costs), or were managed solely in the ambulatory setting. Follow-up was often limited to the event hospitalization; longer-term estimates typically did not distinguish early and late time periods, and comparisons often failed to control for costs unrelated to the TBI (e.g., patient characteristics or co-occurring injuries). Although these limitations were addressed in our estimates of TBI-associated excess costs within each category, visual comparisons across categories and over time must be interpreted with caution. We did not perform statistical tests across injury severity classification categories. Such analyses would require adjustment for marked differences across categories in pre-baseline age, sex, and co-morbidity (Table 2), consideration of marked differences in length of follow-up, and tests for three-way interactions between case status, injury severity category, and observation period.
Importantly, a full appreciation of where and when TBI-associated medical care costs are occurring requires consideration of not only the average incremental cost, but also the numbers of persons for whom that difference applies, and the length of time over which the cost differences accrue. We multiplied the mean difference in cost over the full period from baseline to a maximum of 6 years for each TBI severity classification category by the number of individuals in each category within our population-based 16% random sample. The total incremental cost for possible cases was $2,178,627, 43% higher than the $1,522,935 for probable cases, and 14% higher than the $1, 916,172 for definite cases. These findings suggest that conclusions from previous studies that TBI-associated medical costs are directly related to severity and inversely related to survival merit some reconsideration, and that substantial reductions in TBI-associated medical care costs at the level of the population and over the long term might be achieved by targeting possible (symptomatic) cases for prevention.
Supplementary Material
Acknowledgments
This research was supported by TBI Model System grants to the Mayo Clinic from the National Institute on Disability and Rehabilitation Research (H133A020507 and H133A070013), and a National Research Service Award from the National Institutes of Health (training grant no. HD-07447). The study was made possible by the Rochester Epidemiology Project (grant no. RO1 AG034676 from the National Institute on Aging). The authors thank Patricia K. Perkins and Nancy N. Diehl for invaluable and expert assistance in medical record abstraction and data collection and analysis.
Author Disclosure Statement
No competing financial interests exist.
References
- Annegers J.F. Grabow J.D. Groover R.V. Laws E.R., Jr. Elveback L.R. Kurland L.T. Seizures after head trauma: a population study. Neurology. 1980a;30:683–689. doi: 10.1212/wnl.30.7.683. [DOI] [PubMed] [Google Scholar]
- Annegers J.F. Grabow J.D. Kurland L.T. Laws E.R., Jr. The incidence, causes, and secular trends of head trauma in Olmsted County, Minnesota, 1935–1974. Neurology. 1980b;30:912–919. doi: 10.1212/wnl.30.9.912. [DOI] [PubMed] [Google Scholar]
- Annegers J.F. Hauser W.A. Coan S.P. Rocca W.A. A population-based study of seizures after traumatic brain injuries. N. Engl. J. Med. 1998;338:20–24. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- Barker-Collo S.L. Feigin V.L. Capturing the spectrum: Suggested standards for conducting population-based traumatic brain injury incidence studies. Neuroepidemiology. 2009;32:1–3. doi: 10.1159/000170084. [DOI] [PubMed] [Google Scholar]
- Basu A. Arondekar B.V. Rathouz P.J. Scale of interest versus scale of estimation: comparing alternative estimators for the incremental costs of a comorbidity. Health Econ. 2006;15:1091–1107. doi: 10.1002/hec.1099. [DOI] [PubMed] [Google Scholar]
- Berg J. Tagliaferri F. Servadei F. Cost of trauma in Europe. Eur. J. Neurol. 2005;12:85–90. doi: 10.1111/j.1468-1331.2005.01200.x. [DOI] [PubMed] [Google Scholar]
- Birnbaum H.G. Ben-Hamadi R. Greenberg P.E. Hsieh M. Tang J. Reygrobellet C. Determinants of direct cost differences among US employees with major depressive disorders using antidepressants. Pharmacoeconomics. 2009;27:507–517. doi: 10.2165/00019053-200927060-00006. [DOI] [PubMed] [Google Scholar]
- Bombardier C. Rimmel C. Zintel H. The magnitude and correlates of alcohol and drug use before traumatic brain injury. Arch. Phys. Med. Rehabil. 2002;83:1765–1773. doi: 10.1053/apmr.2002.36085. [DOI] [PubMed] [Google Scholar]
- Borg J. Holm L. Peloso P.M. Cassidy J.D. Carroll L.J. von Holst H. Paniak C. Yates D. Non-surgical intervention and cost for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004;36(Suppl. 43):76–83. doi: 10.1080/16501960410023840. [DOI] [PubMed] [Google Scholar]
- Brener J. Harman J.S. Kelleher K.J. Yeates K.O. Medical costs of mild to moderate traumatic brain injury in children. J. Head Trauma Rehabil. 2004;19:405–412. doi: 10.1097/00001199-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Brooks C.A. Lindstrom J. McCray J. Whiteneck G.G. Cost of medical care for a population-based sample of persons surviving traumatic brain injury. J. Head Trauma Rehabil. 1995;10:1–13. [Google Scholar]
- Brown A.W. Leibson C.L. Malec J.F. Perkins P.K. Diehl N.N. Long-term survival after traumatic brain injury: a population-based analysis. Neurorehabilitation. 2004;19:37–43. [PubMed] [Google Scholar]
- Buntin M.B. Zaslavsky A.M. Too much ado about two-part models and transformation? Comparing methods of modeling Medicare expenditures. J. Health Econ. 2004;23:525–542. doi: 10.1016/j.jhealeco.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Cameron C.M. Purdie D.M. Kliewer E.V. McClure R.J. Ten-year outcomes following traumatic brain injury: a population-based cohort. Brain Inj. 2008;22:437–449. doi: 10.1080/02699050802060621. [DOI] [PubMed] [Google Scholar]
- Cassidy J.D. Carroll L.J. Peloso P.M. Borg J. von Holst H. Holm L. Krause J. Coronado V.G. Incidence, risk factors, and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004;36:28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National Center for Health Statistics. ICD-9-CM Official Guidelines for Coding and Reporting. 2009. http://www.cdc.gov/nchs/data/icd9/icdguide09.pdf http://www.cdc.gov/nchs/data/icd9/icdguide09.pdf
- Centers for Disease Control and Prevention. National Center for Health Statistics. The Barell Injury Diagnosis Matrix, Classification by Body Region and Nature of the Injury. 2005. http://www.cdc.gov/nchs/data/ice/final_matrix_post_ice.pdf http://www.cdc.gov/nchs/data/ice/final_matrix_post_ice.pdf
- Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta: Centers for Disease Control and Prevention; 2003. [Google Scholar]
- Centers for Disease Control and Prevention. National Center for Injury Prevention and Control 2010What are the Potential Long-Term Outcomes of TBI? http://www.cdc.gov/traumaticbraininjury/outcomes.html Accessible at: http://www.cdc.gov/ncipc/pub-res/mtbireport.pdf
- Corrigan J.D. Harrison-Felix C. Bogner J. Dijkers M. Terrill M.S. Whiteneck G. Systematic bias in traumatic brain injury outcome studies because of loss to follow-up. Arch. Phys. Med. Rehabil. 2003;84:153–160. doi: 10.1053/apmr.2003.50093. [DOI] [PubMed] [Google Scholar]
- DeKosky S.T. Ikonomovic M.D. Gandy S. Traumatic brain injury, football, warfare, and long-term effects. N. Eng. J. Med. 2010;363:1293–1296. doi: 10.1056/NEJMp1007051. [DOI] [PubMed] [Google Scholar]
- Esposito D. Bagchi A.D. Verdier J.M. Bencio D.S. Kim M.S. Medicaid beneficiaries with congestive heart failure: association of medication adherence with healthcare use and costs. Am. J. Manag. Care. 2009;15:437–445. [PubMed] [Google Scholar]
- Faul M. Xu L. Wald M.M. Coronado V.G. Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths 2002–2006. Atlanta: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- Finkelstein E. Corso P. Miller T. The Incidence and Economic Burden of Injuries in the United States. Oxford University Press; New York: 2006. [Google Scholar]
- Flaada J.T. Leibson C.L. Mandrekar J.N. Diehl N. Perkins P.K. Brown A.W. Malec J.F. Relative risk of mortality after traumatic brain injury: a population-based study of the role of age and injury severity. J. Neurotrauma. 2007;24:435–445. doi: 10.1089/neu.2006.0119. [DOI] [PubMed] [Google Scholar]
- Glance L.G. Osler T.M. Mukamel D.B. Meredith W. Wagner J. Dick A.W. TMPM-ICD9: a trauma mortality prediction model based on ICD-9-CM codes. Ann. Surg. 2009;249:1032–1039. doi: 10.1097/SLA.0b013e3181a38f28. [DOI] [PubMed] [Google Scholar]
- Hall K.M. Karzmark P. Stevens M. Englander J. O'Hare P. Wright J. Family stressors in traumatic brain injury: a two-year follow-up. Arch. Phys. Med. Rehabil. 1994;75:876–884. doi: 10.1016/0003-9993(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Harwood H. The Lewin Group. Updating Estimates of the Economic Costs of Alcohol Abuse in the United States: Estimates, Update Methods, and Data. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2002. [Google Scholar]
- H-ICDA. Hospital Adaptation of the International Classification of Diseases. 2nd. Commission on Professional and Hospital Activities (CPHA); Ann Arbor, MI: 1973. [Google Scholar]
- Johns Hopkins Bloomberg School of Public Health. The Johns Hopkins ACG System. 2010. http://www.acg.jhsph.org/ http://www.acg.jhsph.org/
- Johns Hopkins Bloomberg School of Public Health. Update of the Johns Hopkins ACG Case-Mix System 4, 1–4. 2002. http://www.acg.jhsph.edu/ACGDocuments/newsletter_f2002.pdf http://www.acg.jhsph.edu/ACGDocuments/newsletter_f2002.pdf
- Kalsbeek W.D. McLaurin R.L. Harris B.S. Miller J.D. The National Head and Spinal Cord Injury Survey: Major Findings. J. Neurosurg. Suppl. 1980:S19–S31. [PubMed] [Google Scholar]
- Kayani N.A. Homan S. Yun S. Zhu B.P. Health and economic burden of traumatic brain injury: Missouri, 2001–2005. Public Health Rep. 2009;124:551–560. doi: 10.1177/003335490912400412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D. Hume P.A. Milburn P.D. Gianotti S. Women's rugby league injury claims and costs in New Zealand. Br. J. Sports Med. 2010;44:1016–1023. doi: 10.1136/bjsm.2009.064683. [DOI] [PubMed] [Google Scholar]
- Leibson C.L. Brown A.W. Ransom J. Diehl N. Perkins P. Mandrekar J. Malec J.F. The incidence of traumatic brain injury (TBI) across the full spectrum of disease: A population-based medical record review study. Epidemiology. 2011;22:836–844. doi: 10.1097/EDE.0b013e318231d535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson C.L. Hu T. Brown R.D. Hass S.L. O'Fallon W.M. Whisnant J.P. Utilization of acute care services in the year before and after first stroke: a population-based study. Neurology. 1996;46:861–869. [PubMed] [Google Scholar]
- Leibson C.L. Katusic S.K. Barbaresi W.J. Ransom J. O'Brien P.C. Utilization and costs of medical care for children and adolescents with and without attention deficit hyperactivity disorder. JAMA. 2001;285:60–66. doi: 10.1001/jama.285.1.60. [DOI] [PubMed] [Google Scholar]
- Leibson C.L. Long K.H. Maraganore D.M. Bower J.H. Ransom J.E. O'Brien P.C. Rocca W.A. Direct medical costs associated with Parkinson's disease: a population-based study. Mov. Disord. 2006;21:1864–1871. doi: 10.1002/mds.21075. [DOI] [PubMed] [Google Scholar]
- Long K.H. Rubio-Tapia A. Wagie A.E. Melton L.J., III Lahr B.D. Van Dyke C.T. Murray J.A. The economics of coeliac disease: A population-based study. Aliment. Pharmacol. Ther. 2010;32:261–269. doi: 10.1111/j.1365-2036.2010.04327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malec J.F. Brown A.W. Leibson C.L. Flaada J.T. Mandrekar J.N. Diehl N.N. Perkins P.K. The Mayo classification system for traumatic brain injury severity. J. Neurotrauma. 2007;24:1417–1424. doi: 10.1089/neu.2006.0245. [DOI] [PubMed] [Google Scholar]
- Manning W.G. Morris C.N. Newhouse J.P. Orr L.L. Duan N. Keeler E.B. Leibowitz A.L. Marquis K.H. Marquis M.S. Phelps C.E. A two-part model of the demand for medical care: preliminary results from the Health Insurance Study. In: van der Gaag J., editor; Perlman M., editor. Health, Economics, and Health Economics. North Holland, Amsterdam: 1981. pp. 103–123. [Google Scholar]
- Max W. MacKenzie E.J. Rice D.P. Head injuries: costs and consequences. J. Head Trauma Rehabil. 1991;6:76–91. [Google Scholar]
- McGarry L.J. Thompson D. Millham F.H. Cowell L. Snyder P.J. Lenderking W.R. Weinstein M.C. Outcomes and costs of acute treatment of traumatic brain injury. J. Trauma. 2002;53:1152–1159. doi: 10.1097/00005373-200212000-00020. [DOI] [PubMed] [Google Scholar]
- Mellick D. Gerhart K.A. Whiteneck G.G. Understanding outcomes based on the post-acute hospitalization pathways followed by persons with traumatic brain injury. Brain Inj. 2003;17:55–71. doi: 10.1080/0269905021000010159. [DOI] [PubMed] [Google Scholar]
- Melton L.J., III History of the Rochester Epidemiology Project. Mayo Clin. Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- Melton L.J., III The threat to medical-records research. N. Engl. J. Med. 1997;337:1466–1470. doi: 10.1056/NEJM199711133372012. [DOI] [PubMed] [Google Scholar]
- Miller T.R. Douglass J.B. Galbraith M.S. Lestina D.C. Pindus N.M. Head and Neck Injury. Society of Automotive Engineers, Inc.; Warrendale, PA: 1994. Costs of head and neck injury and a benefit-cost analysis of bicycle helmets; pp. 211–240. [Google Scholar]
- Mittenberg W. DiGiulio D.V. Perrin S. Bass A.E. Symptoms following mild head injury: expectation as aetiology. J. Neurol. Neurosurg. Psychiatry. 1992;55:200–204. doi: 10.1136/jnnp.55.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullahy J. Much ado about two: reconsidering retransformation and two-part model in health econometrics. J. Health Econ. 1998;17:247–281. doi: 10.1016/s0167-6296(98)00030-7. [DOI] [PubMed] [Google Scholar]
- Nemetz P.N. Leibson C. Naessens J.M. Beard M. Kokmen E. Annegers J.F. Kurland L.T. Traumatic brain injury and time to onset of Alzheimer's disease: a population-based study. Am. J. Epidemiol. 1999;149:32–40. doi: 10.1093/oxfordjournals.aje.a009724. [DOI] [PubMed] [Google Scholar]
- Ruff R. Two decades of advances in understanding of mild traumatic brain injury. J. Head Trauma Rehabil. 2005;20:5–18. doi: 10.1097/00001199-200501000-00003. [DOI] [PubMed] [Google Scholar]
- Ryu W.H. Feinstein A. Colantonio A. Streiner D.L. Dawson D.R. Early identification and incidence of mild TBI in Ontario. Can. J. Neurol. Sci. 2009;36:429–435. doi: 10.1017/s0317167100007745. [DOI] [PubMed] [Google Scholar]
- Schneier A.J. Shields B.J. Hostetler S.G. Xiang H. Smith G.A. Incidence of pediatric traumatic brain injury and associated hospital resource utilization in the United States. Pediatrics. 2006;118:483–492. doi: 10.1542/peds.2005-2588. [DOI] [PubMed] [Google Scholar]
- Schootman M. Buchman T.G. Lewis L.M. National estimates of hospitalization charges for the acute care of traumatic brain injuries. Brain Inj. 2003;17:983–990. doi: 10.1080/0269905031000110427. [DOI] [PubMed] [Google Scholar]
- Selassie A.W. Zaloshnja E. Langlois J.A. Miller T. Jones P. Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States. J. Head Trauma Rehabil. 2008;23:123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- Setnik L. Bazarian J.J. The characteristics of patients who do not seek medical treatment for traumatic brain injury. Brain Inj. 2007;21:1–9. doi: 10.1080/02699050601111419. [DOI] [PubMed] [Google Scholar]
- Shi J. Xiang H. Wheeler K. Smith G.A. Stallones L. Groner J. Wang Z. Costs, mortality likelihood and outcomes of hospitalized US children with traumatic brain injuries. Brain Inj. 2009;23:602–611. doi: 10.1080/02699050903014907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomine B.S. McCarthy M.L. Ding R. MacKenzie E.J. Jaffe K.M. Aitken M.E. Durbin D.R. Christensen J.R. Dorsch A.M. Paidas C.N. CHAT Study Group. Health care utilization and needs after pediatric traumatic brain injury. Pediatrics. 2006;117:e663–e674. doi: 10.1542/peds.2005-1892. [DOI] [PubMed] [Google Scholar]
- Sosin D.M. Sniezek J.E. Thurman D.J. Incidence of mild and moderate brain injury in the United States, 1991. Brain Inj. 1996;10:47–54. doi: 10.1080/026990596124719. [DOI] [PubMed] [Google Scholar]
- St. Sauver J.L. Grossardt B.R. Leibson C.L. Yawn B.P. Melton L.J., III Rocca W.A. Generalizability of epidemiologic findings and public health decisions: An illustration from the Rochester Epidemiology Project. Mayo Clin. Proc. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.M. Luedtke C.A. Oh T.H. Shah N.D. Long K.H. King S. Branda M. Swanson R. Direct medical costs in patients with fibromyalgia: Cost of illness and impact of a brief multidisciplinary treatment program. Am. J. Phys. Med. Rehabil. 2011;90:40–46. doi: 10.1097/PHM.0b013e3181fc7ff3. [DOI] [PubMed] [Google Scholar]
- Thurman D.J. Sniezek J.E. Johnson D. Greenspan A. Smith S.M. Guidelines for Surveillance of Central Nervous System Injury. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 1995. [Google Scholar]
- Thurman D. The epidemiology and economics of head trauma. In: Miller L., editor; Hayes R., editor. Head Trauma: Basic, Preclinical, and Clinical Directions. John Wiley & Sons; New York: 2001. pp. 327–347. [Google Scholar]
- U.S. Department of Health amd Human Services. International Classification of Diseases, 9th Revision, Clinical Modification. 3rd. (ICD-9-CM). United States Government Printing Office; Washington, DC: 1989. [Google Scholar]
- Vangel S.J., Jr. Rapport L.J. Hanks R.A. Black K.L. Long-term medical care utilization and costs among traumatic brain injury survivors. Am. J. Phys. Med. Rehabil. 2005;84:153–160. doi: 10.1097/01.phm.0000154896.55045.e7. [DOI] [PubMed] [Google Scholar]
- Vassallo J.L. Proctor-Weber Z. Lebowitz B.K. Curtiss G. Vanderplofg R.D. Psychiatric risk factors for traumatic brain injury. Brain Inj. 2007;21:567–573. doi: 10.1080/02699050701426832. [DOI] [PubMed] [Google Scholar]
- Ventura T. Harrison-Felix C. Carlson N. DiGuiseppi C. Gabella B. Brown A. DeVivo M. Whiteneck G. Mortality after discharge from acute care hospitalization with traumatic brain injury: a population-based study. Arch. Phys. Med. Rehabil. 2010;91:20–29. doi: 10.1016/j.apmr.2009.08.151. [DOI] [PubMed] [Google Scholar]
- Welch C.A. Czerwinski D. Ghimire B. Bertsimas D. Depression and costs of health care. Psychosomatics. 2009;50:392–401. doi: 10.1176/appi.psy.50.4.392. [DOI] [PubMed] [Google Scholar]
- Whitlock J.A. Hamilton B.B. Functional outcome after rehabilitation for severe traumatic brain injury. Arch. Phys. Med. Rehabil. 1995;76:1103–1112. doi: 10.1016/s0003-9993(95)80117-0. [DOI] [PubMed] [Google Scholar]
- Wilcoxon F. Individual comparisons by ranking methods. Biometrics. 1945;1:80–83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.