Abstract
High-mobility group box 1 (HMGB1) is a ubiquitous nuclear protein that is passively released from damaged and necrotic cells, and actively released from immune cells. In contrast, cytochrome c is released from mitochondria in apoptotic cells, and is considered a reliable biomarker of apoptosis. Thus, HMGB1 and cytochrome c may in part reflect the degree of necrosis and apoptosis present after traumatic brain injury (TBI), where both are felt to contribute to cell death and neurological morbidity. Ventricular cerebrospinal fluid (CSF) was obtained from children admitted to the intensive care unit (ICU) after TBI (n=37). CSF levels of HMGB1 and cytochrome c were determined at four time intervals (0–24 h, 25–48 h, 49–72 h, and>72 h after injury) using enzyme-linked immunosorbent assay (ELISA). Lumbar CSF from children without TBI served as controls (n=12). CSF HMGB1 levels were: control=1.78±0.29, 0–24 h=5.73±1.45, 25–48 h=5.16±1.73, 49–72 h=4.13±0.75,>72 h=3.80±0.90 ng/mL (mean±SEM). Peak HMGB1 levels were inversely and independently associated with favorable Glasgow Outcome Scale (GOS) scores at 6 mo (0.49 [0.24–0.97]; OR [5–95% CI]). CSF cytochrome c levels were: control=0.37±0.10, 0–24 h=0.69±0.15, 25–48 h=0.82±0.48, 49–72 h=1.52±1.08,>72 h=1.38±1.02 ng/mL (mean±SEM). Peak cytochrome c levels were independently associated with abusive head trauma (AHT; 24.29 [1.77–334.03]) and inversely and independently associated with favorable GOS scores (0.42 [0.18–0.99]). In conclusion, increased CSF levels of HMGB1 and cytochrome c were associated with poor outcome after TBI in infants and children. These data are also consistent with the designation of HMGB1 as a “danger signal.” Distinctly increased CSF cytochrome c levels in infants and children with AHT and poor outcome suggests that apoptosis may play an important role in this unique patient population.
Key words: abusive head trauma, child abuse, cytochrome c, high mobility group box 1
Introduction
Traumatic brain injury (TBI) accounts for significant morbidity and mortality among children in the United States. According to the Centers for Disease Control and Prevention, TBI accounts for an estimated 473,947 emergency department visits, 35,136 hospitalizations, and 2174 deaths in children 0–14 years of age annually (Faul et al., 2010). There are many obstacles to identifying severity of injury and prognostic outcome in children after TBI, including the diverse forms of primary injury and the impact of secondary cell death cascades (primarily necrosis and apoptosis), especially in cases of moderate and severe TBI.
High-mobility group box 1 (HMGB1) was originally described in 1973 as a nonhistone DNA-binding protein (Goodwin et al., 1973). Intracellularly it serves as a co-transcriptional factor with the ability to serve as a structural DNA-binding protein, stabilizing nucleosomes and regulating transcription (Agrawal and Schatz, 1997; Bustin and Reeves, 1996; Sutrias-Grau et al., 1999). HMGB1 can move extracellularly via two mechanisms. The first involves the passive release of HMGB1 from damaged or necrotic cells. Interestingly, apoptotic cells do not release HMGB1, but instead retain it within their cells (Scaffidi et al., 2002). Additionally, through its interactions with receptor for advanced glycation end (RAGE) products and members of the toll-like family of receptors (TLRs), including TLR2 and TLR4 (Klune et al., 2008), it may represent a danger signal that alerts the immune system to the presence of injured cells. The second manner in which HMGB1 mobilizes to the extracellular space involves its active secretion by immune cells. Specifically, in macrophages and monocytes, HMGB1 is hyper-acetylated, which allows it to accumulate in the cytosol and prevents it from being translocated into the nucleus. HMGB1 is then packaged into secretory lysosomes and secreted (Bonaldi et al., 2003; Gardella et al., 2002). Extracellularly, HMGB1 activates the immune system by inducing dendritic cell maturation, increasing secretion of inflammatory cytokines, and activating neutrophils, monocytes, and natural killer (NK) cells (Andersson et al., 2000; Fan et al., 2007; Klune et al., 2008; Messmer et al., 2004; Raucci et al., 2007; Rouhiainen et al., 2004). Furthermore, extracellular HMGB1 has been shown to promote tissue repair and regeneration (Bianchi and Manfredi, 2007; Palumbo and Bianchi, 2004).
In 1994 Matzinger proposed the “danger model,” the idea that antigen-presenting cells are activated by signals from injured cells (Matzinger, 1994,2002). Matzinger originally theorized that danger signals were more likely to be released during necrosis, and not by apoptosis. Fink has suggested that damage caused by trauma or ischemia can cause HMGB1 to be released from necrotic tissue, and thus serves as a danger signal that alerts the immune system to the presence of injured cells (Fink, 2007). While current studies confirm the presence of danger signals released during necrosis, evidence involving the apoptotic release of danger signals remains controversial (Rock et al., 2005).
Cytochrome c is best known for its role in the mitochondrial electron transport chain, participating in oxidative phosphorylation and ATP synthesis. Cytochrome c also has an integral role in apoptosis. In response to apoptotic stimuli it is released from the mitochondria into the cytosol. There, cytochrome c binds apoptotic protease-activating factor-1 to form the apoptosome, activating caspases responsible for the initiation of the intrinsic apoptotic pathway (Kluck et al., 1997; Liu et al., 1996). We have previously reported that increased cytochrome c in cerebrospinal fluid (CSF) is a potential biomarker of abusive head trauma (AHT; Satchell et al., 2005). Cytochrome c and other biomarkers have the potential to predict severity of injury and short- and long-term outcome, and may give insights into potential therapeutic modalities after TBI (Berger et al., 2006; Kochanek et al., 2008; Shore et al., 2007).
This study focuses on the CSF biomarkers HMGB1 and cytochrome c in infants and children after severe TBI. These biomarkers are examined with respect to relevant clinical variables and outcome after TBI.
Methods
All procedures for this study were approved by the Institutional Review Board at the University of Pittsburgh Medical Center. As part of other past and ongoing clinical studies, informed consent was obtained for collection of the CSF made available for this study.
A clinical protocol for the treatment of children with severe TBI has been used at our institution and has been previously described (Adelson et al., 2005; Exo et al., 2011). Briefly, all children with severe TBI received comprehensive neurocritical care to rapidly stabilize and assess for injuries, prevent secondary insults, and promote neurological recovery, in accordance with published guidelines (Adelson et al., 2003). Intracranial pressure (ICP) monitoring was instituted for all children with post-resuscitation Glasgow Coma Scale (GCS) scores<8 (although some children had an initial GCS score>8, but deteriorated) via an externalized ventricular drain. CSF drainage was part of standard management throughout this study. Intracranial hypertension (generally defined as ICP≥20 mm Hg) was treated with step-wise escalations of care via a protocol that included head positioning (raising the head of the bed to 30°), analgesia with fentanyl, neuromuscular blockade with vecuronium, mild hyperventilation-normoventilation (Paco2 ∼ 35 mm Hg), and hyperosmolar therapy (mannitol or 3% NaCl). Second-tier therapies include barbiturate administration, hypothermia, or decompressive craniectomy.
CSF collection
As previously described (Satchell et al., 2005), CSF was continuously drained by gravity into a sterile buretrol at the bedside at 3 cm above the mid-brain. Once drained, CSF of the study subjects was extracted from this system using sterile technique to minimize the risk of contamination. This procedure was done daily for up to 7 days after TBI, with samples analyzed at four time intervals (0–24 h, 25–48 h, 49–72 h, and>72 h after injury). Lumbar CSF from children without TBI or meningoencephalitis (controls) was collected in a single aliquot after diagnostic lumbar puncture. The CSF was immediately centrifuged to remove cellular debris and frozen at −80°C until analysis. Enzyme-linked immunosorbent assay (ELISA) kits for HMGB1 and cytochrome c were purchased from Shino-Test Corporation (Kanagawa, Japan) and R&D Systems (Minneapolis, MN), respectively. For both assays, the plates were coated with detecting antibody and washed with a buffered solution. Study samples (from both TBI subjects and healthy controls) and known control solutions were applied to individual wells followed by application of a detector antibody. Absorbances were quantified and concentrations of study samples were calculated based on standard curves generated from known solutions.
Statistical analysis
Clinical data collection included patient age, sex, mechanism of injury, admission GCS score, Glasgow Outcome Scale (GOS) score determined 6 months after injury, and mortality. GOS was defined as GOS 1=dead, 2=vegetative state, 3=severe disability, 4=moderate disability, and 5=normal. Furthermore, good outcome was defined as a GOS score of 4 or 5, and poor outcome as a GOS score of 1–3. AHT was diagnosed by the Child Protection Team at Children's Hospital of Pittsburgh independently of this study. Data are expressed as mean±standard error of the mean (SEM), or median [range], as appropriate. For threshold analyses, increased HMGB1 and cytochrome c levels were a priori defined as levels more than 2 standard deviations (SD) above the control means. Comparisons between control levels and peak and mean levels in TBI patients were made using the Mann-Whitney rank sum test. Changes over time were analyzed using repeated-measures analysis of variance (RM-ANOVA). Associations between CSF HMGB1 and cytochrome c and the clinical variables age, sex, initial GCS score, mechanism of injury (dichotomized into accidental TBI versus AHT), and GOS scores were determined using univariate models. Multivariate models were constructed using variables with a univariate p value<0.2, or with severity of injury (initial GCS score) forced into the model in the case of HMGB1. Receiver-operating characteristic (ROC) curves were carried out for peak HMGB1 and cytochrome c levels as a predictor of outcome at 6 months. Statistical calculations were performed with SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA) and Stata/IC (StataCorp LP, College Station, TX).
Results
Demographic data for the TBI patients are shown in Table 1. There were 37 TBI and 12 control patients. In brief, the control patients were younger than the TBI patients, and TBI was more common in males than females. The initial GCS scores were diverse, ranging from 3–15, but all had a GCS score<8 prior to implementation of ICP monitoring. The mechanisms of injury showed that TBI as a consequence of motor vehicle collisions was the most common etiology (35.1%), followed by AHT (18.9%), and falls (18.9%; Table 2).
Table 1.
Demographic Data of the Study Subjects
| Control patients | |
| Number | 12 |
| Age, years [range] | 2.1±1.0 [5 weeks–9 years] |
| Number of male patients (%) | 6a (66.7) |
| Traumatic brain injury patients | |
| Number | 37 |
| Age, years [range] | 6.1±0.9 [5 weeks–16 years] |
| Number of male patients (%) | 22 (59.5) |
| Initial Glasgow Coma Scale score | 7 [3–15] |
| Glasgow Outcome Scale score at 6 monthsb | 4 [1–5] |
| Presence of contusion on initial head CT scan (%) | 12c (33) |
Mean±standard error of the mean or median [range], as appropriate.
Undocumented in 3 control patients.
1=dead, 2=vegetative state, 3=severe disability, 4=moderate disability, 5=normal.
Undocumented in 1 patient.
CT, computed tomography.
Table 2.
Mechanism of Injury
| n | Percent | |
|---|---|---|
| Motor vehicle collision | 13 | 35.1 |
| Abusive head trauma | 7 | 18.9 |
| Fall | 7 | 18.9 |
| Pedestrian/automobile accident | 4 | 10.8 |
| Bicycle/automobile accident | 2 | 5.4 |
| Other | 4 | 10.8 |
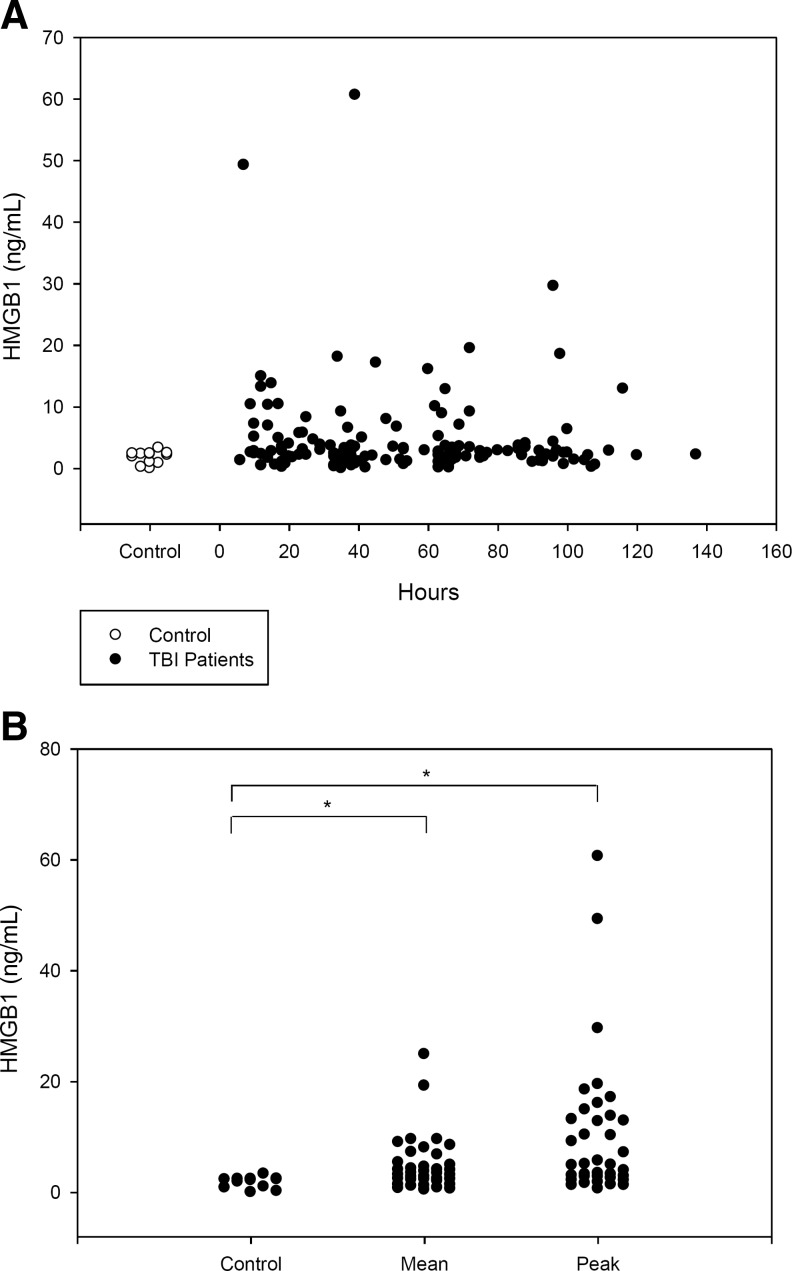
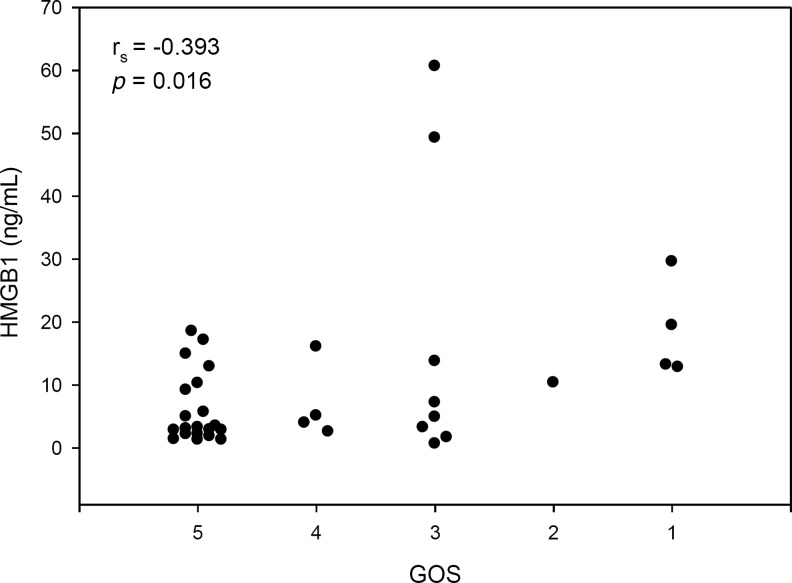
The temporal profile of CSF HMGB1 is shown in Figure 1A. CSF HMGB1 levels did not change over time when examined in epochs of 0–24 h, 25–48 h, 49–72 h, and>72 h after injury (Table 3; p>0.05 comparing all epochs). Both mean and peak CSF HMGB1 levels (4.83±0.81 and 10.17±2.11 ng/mL, respectively) were increased compared with controls (1.78±0.29 ng/mL, p<0.05; Fig. 1B). Figure 2 shows that the peak HMGB1 level is inversely proportional to the GOS score at 6 months (rs=−0.393, p=0.016). Increased CSF HMGB1, was independently associated with GOS when controlling for initial GCS to adjust for injury severity (Table 4; p=0.041).
FIG. 1.
(A) Temporal profile of CSF HMGB1 levels in children with severe TBI versus controls. (B) Mean and peak CSF HMGB1 levels among TBI patients were increased versus controls (*p<0.05; CSF, cerebrospinal fluid; HMGB1, high-mobility group box 1; TBI, traumatic brain injury).
Table 3.
Temporal Profile of Cerebrospinal Fluid HMGB1 and Cytochrome c Levels
| Control | 0–24 h | 25–48 h | 49–72 h | >72 h | |
|---|---|---|---|---|---|
| HMGB1 (ng/mL) | 1.78±0.29 | 5.73±1.45 | 5.16±1.73 | 4.13±0.75 | 3.80±0.90 |
| Cytochrome c (ng/mL) | 0.37±0.10 | 0.69±0.15 | 0.82±0.48 | 1.52±1.08 | 1.38±1.02 |
HMGB1, high-mobility group box 1.
FIG. 2.
Peak CSF HMGB1 level plotted against 6-month Glasgow Outcome Scale (GOS) score. The peak HMGB1 level was found to be inversely proportional to GOS scores (rs=−0.393, p=0.016; GOS: 1=dead, 2=vegetative state, 3=severe disability, 4=moderate disability, 5=normal; CSF, cerebrospinal fluid; HMGB1, high-mobility group box 1).
Table 4.
Associations between Increased Cerebrospinal Fluid HMGB1a and Clinical Variables
| |
Univariate |
Multivariate |
||
|---|---|---|---|---|
| p | OR | [95% CI] | p | |
| Age | 0.410 | — | — | — |
| Sex | 0.927 | — | — | — |
| Initial Glasgow Coma Scale score | 0.271 | 0.87 | [0.69,1.10] | 0.241 |
| Abusive head trauma | 0.632 | — | — | — |
| Glasgow Outcome Scale score at 6 months | 0.016 | 0.49 | [0.24,0.97] | 0.041 |
| Presence of contusion on initial head computerized tomography scan | 0.896 | — | — | — |
Increased HMGB1 defined as control mean±2 standard deviations
HMGB1, high-mobility group box 1; CI, confidence interval; OR, odds ratio.
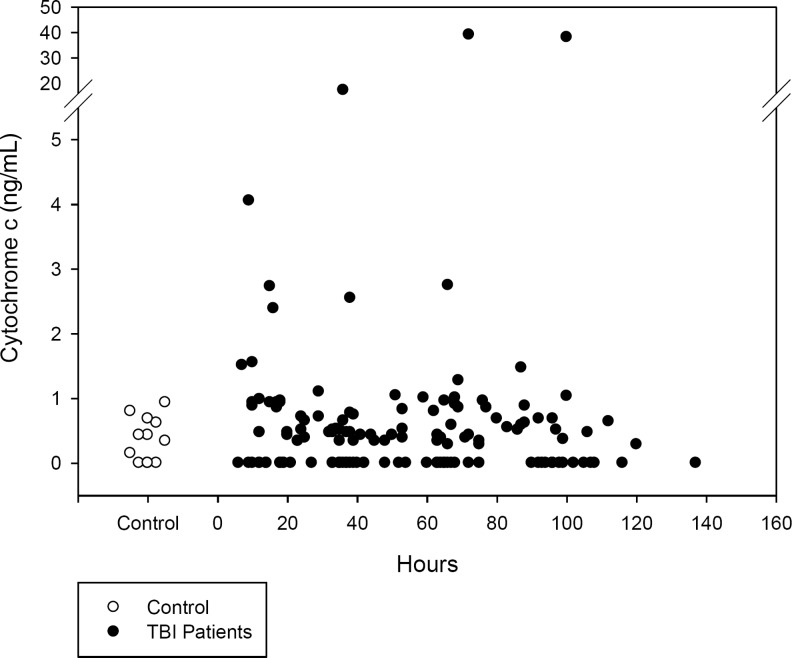
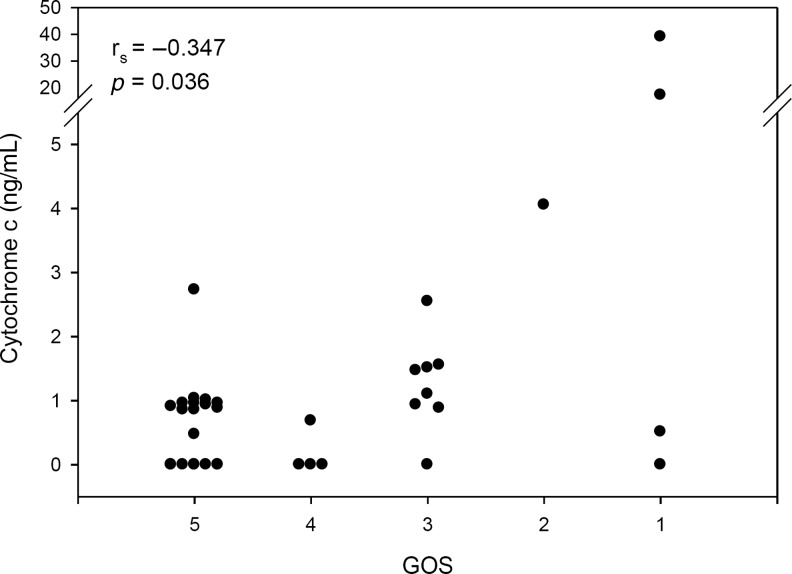
The temporal profile of CSF cytochrome c is shown in Figure 3. CSF cytochrome c did not change over time when examined in epochs of 0–24 h, 25–48 h, 49–72 h, and>72 h after injury (Table 3; p>0.05 for all epochs). Neither mean nor peak CSF cytochrome c levels (1.27±0.70 and 2.27±1.12 ng/mL, respectively) were increased compared with controls (0.37±0.10 ng/mL). However, as with HMGB1, the peak cytochrome c level was found to be inversely proportional to GOS score at 6 months (rs=−0.347, p=0.036; Fig. 4). Additionally, the peak CSF cytochrome c level was higher in patients with AHT compared with accidental TBI patients (9.13±5.46 versus 0.67±0.16 ng/mL, respectively; p<0.05). Increased CSF cytochrome c was independently associated with AHT (p=0.017) and GOS (p=0.05), when controlling for patient age, sex, and initial GCS score (Table 5).
FIG. 3.
Temporal profile of CSF cytochrome c levels in children with severe TBI versus controls (CSF, cerebrospinal fluid; TBI, traumatic brain injury).
FIG. 4.
Peak CSF cytochrome c level plotted against 6-month Glasgow Outcome Scale (GOS) score. The peak cytochrome c level was found to be inversely proportional to GOS score (rs=−0.347, p=0.036; GOS: 1=dead, 2=vegetative state, 3=severe disability, 4=moderate disability, 5=normal; CSF, cerebrospinal fluid).
Table 5.
Associations between Increased Cerebrospinal Fluid Cytochrome ca and Clinical Variables
| |
Univariate |
Multivariate |
||
|---|---|---|---|---|
| p | OR | [95% CI] | p | |
| Age | 0.476 | — | — | — |
| Sex | 0.192 | 0.28 | [0.02,3.25] | 0.310 |
| Initial Glasgow Coma Scale score | 0.890 | — | — | — |
| Abusive head trauma | 0.001 | 24.29 | [1.77,334.03] | 0.017 |
| Glasgow Outcome Scale score at 6 months | 0.036 | 0.42 | [0.18,0.99] | 0.050 |
Increased cytochrome c level defined as control mean±2 standard deviations
CI, confidence interval; OR, odds ratio.
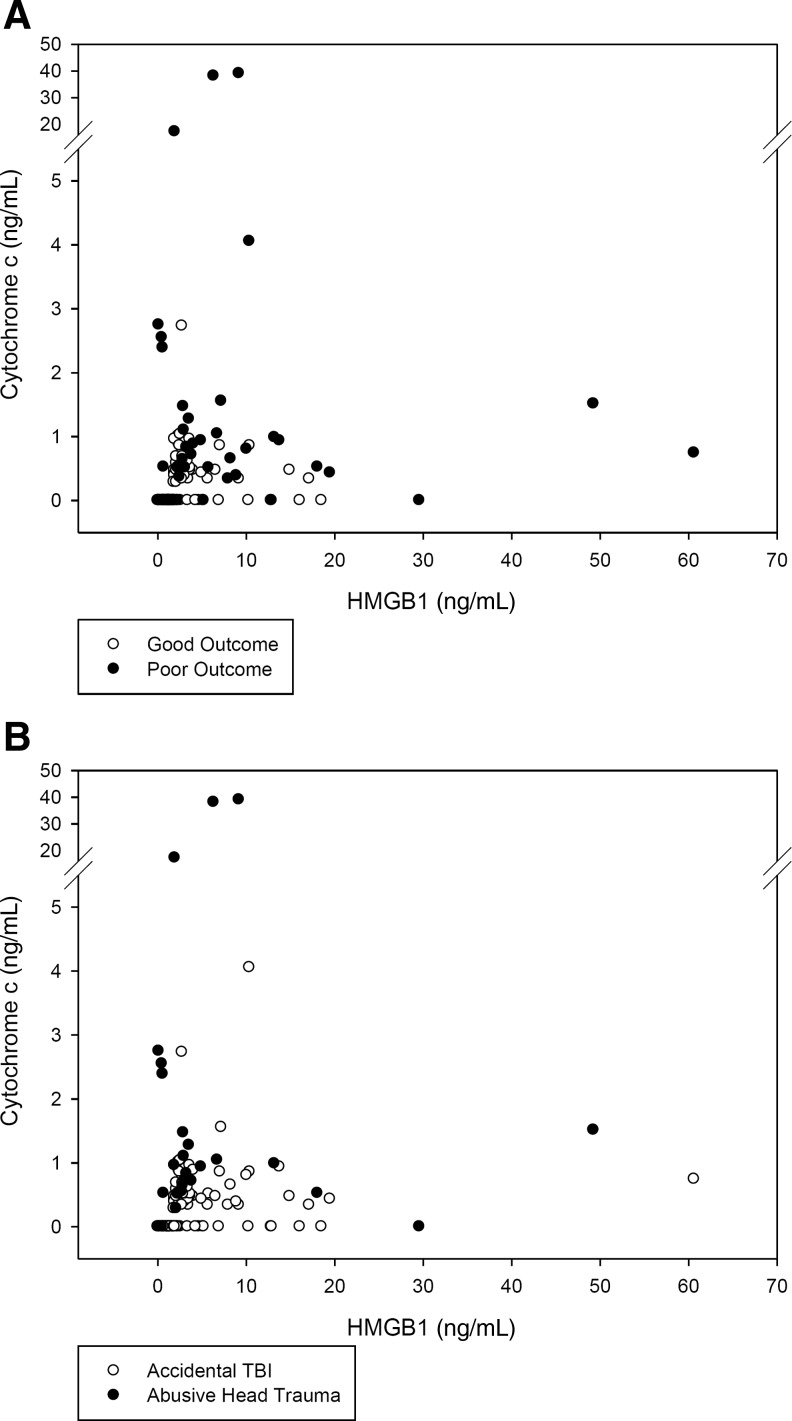
Since both biomarkers were assayed in each CSF sample, comparisons could be made to determine whether changes in CSF HMGB1 levels paralleled changes in CSF cytochrome c. CSF HMGB1 and cytochrome c in matched samples were correlated (rs=0.442, p<0.001), supporting concurrent increases in HMGB1 and cytochrome c in TBI patients. Subgroup analysis further supported parallel increases in HMGB1 and cytochrome c in both the group of patients with poor outcomes (GOS score 1–3; rs=0.320, p<0.025; Fig. 5A), and good outcomes (GOS score 4 or 5; rs=0.473, p<0.001). However, simultaneous increases in both HMGB1 and cytochrome c were not seen in patients with AHT (Fig. 5B; rs=0.122, p=0.54), raising the possibility that apoptosis may be relatively more prominent in these patients. However, the smaller sample of AHT patients (7 of 37) than poor outcome patients (13 of 37) may have influenced the statistical analysis.
FIG. 5.
Scatterplot of CSF HMGB1 versus cytochrome c in (A) patients with good outcomes (open circles; GOS score 4 or 5; rs=0.473, p<0.001), or poor outcomes (solid circles; GOS score 1–3; rs=0.320, p<0.025), and (B) patients with accidental TBI (open circles) and abusive head trauma (solid circles; rs=0.122, p=0.54; CSF, cerebrospinal fluid; HMGB1, high-mobility group box 1; GOS, Glasgow Outcome Scale).
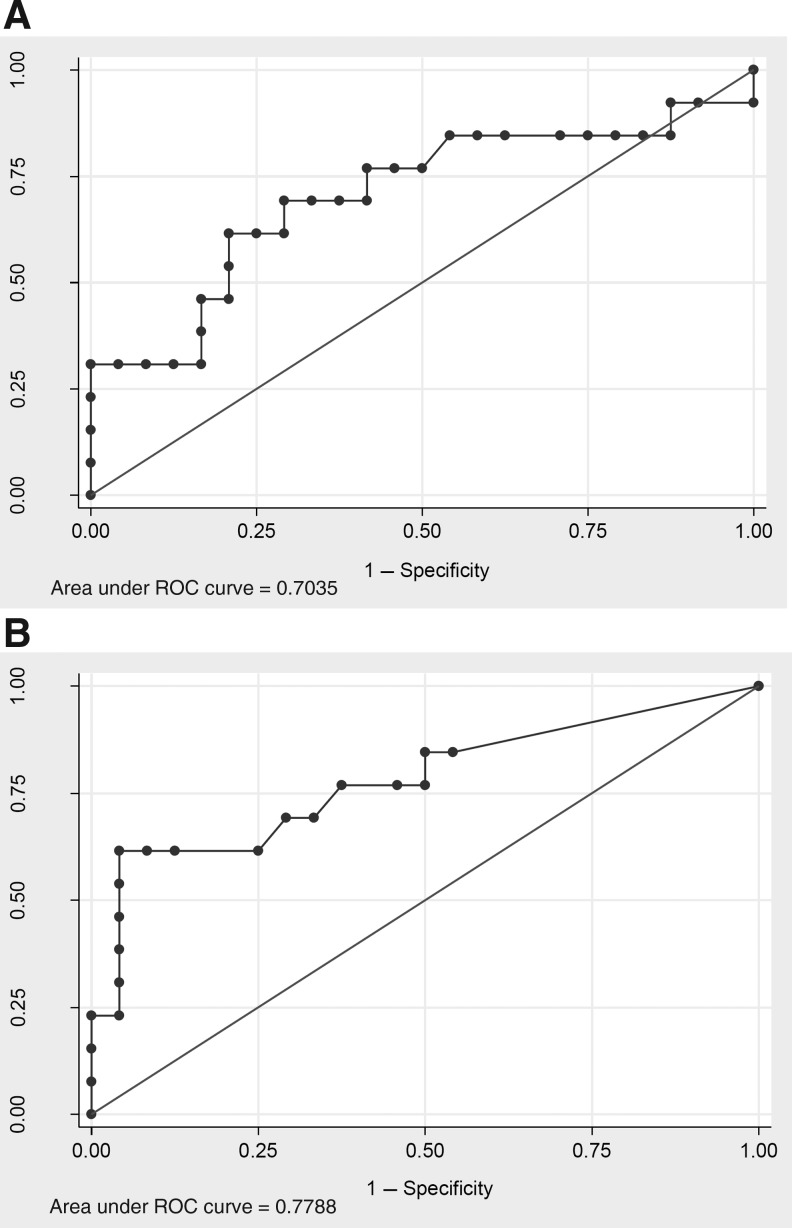
ROC analysis of the performance of peak HMGB1 level to predict outcome at 6 months had an area under the curve (AUC) of 0.70 (95% confidence interval [CI] 0.51,0.90; Fig. 6A). An HMGB1 cutoff point of 19.49 ng/mL had a sensitivity of 30.77% and specificity of 100%. ROC analysis of cytochrome c levels revealed an AUC of 0.78 (95% CI 0.61,0.95; Fig. 6B), with a cutoff point of 4.06 ng/mL with a sensitivity of 23.08% and a specificity of 100%.
FIG. 6.
Receiver-operating characteristic (ROC) analysis using (A) peak CSF HMGB1 as a predictor of outcome at 6 months (AUC=0.70, 95% CI 0.51,0.90), and (B) peak CSF cytochrome c as a predictor of outcome at 6 months (AUC=0.78, 95% CI 0.61,0.95; CSF, cerebrospinal fluid; HMGB1, high-mobility group box 1; AUC, area under the curve).
Discussion
We found that HMGB1 and cytochrome c represent CSF biomarkers that independently predict poor outcome after pediatric TBI. The cytochrome c-dependent cell death pathway again appears to be readily detectable in patients with AHT, consistent with previous reports indicating a prominent role for apoptotic cell death in this unique TBI group (Satchell et al., 2005).
Clinical studies involving HMGB1 have primarily focused on plasma levels. Peltz and associates reported increased plasma HMGB1 levels after trauma that were>30-fold higher than healthy controls within 1 h of injury, which peaked at 2–6 h post-injury (Peltz et al., 2009). However, their study involving 23 adults did not find a correlation between HMGB1 level and patient outcome. Cohen and colleagues performed a larger study in 168 adults following severe trauma (Cohen et al., 2009). They also reported that plasma HMGB1 levels were increased early (within 30 min); however, they did identify a correlation with severity of injury and survival. While both studies included trauma patients with and without brain injuries, TBI was not the main focus of their studies. In fact, the Peltz group excluded patients with isolated head injuries, while the study by Cohen and associates included only 27% patients with severe head injury. Relatively little information exists regarding CSF HMGB1 levels. CSF HMGB1 levels have been shown to be increased in pediatric patients with bacterial meningitis (Tang et al., 2008), and in adult patients after subarachnoid hemorrhage (Nakahara et al., 2009). To our knowledge, this is the first study examining CSF HMGB1 levels after pediatric or adult TBI.
HMGB1 measured in this study likely represents a combination of HMGB1 passively released during necrotic cell death and actively secreted from macrophages and/or microglia. It is generally accepted that after TBI necrosis precedes apoptosis. While mean HMGB1 levels peaked in the 0–24-h epoch, and cytochrome c levels did not peak until the 48–72-h epoch (Table 3), this was not statistically significant. This is consistent with passive HMGB1 release from necrotic cells, followed by later immune cell activation. Importantly, our study found that increased CSF HMGB1 is independently associated with poor outcome (6-month GOS score 1–3). The neurodegenerative effects of HMGB1 have been described by Goa and associates, who in a model of neuronal cell culture found that HMGB1, whether released by necrotic or secreted from inflammatory cells, acts on the microglia Mac1 receptor, resulting in persistent neuroinflammation and chronic, progressive dopaminergic neurodegeneration (Gao et al., 2011).
In contrast to HMGB1, there are several studies examining cytochrome c levels in CSF after TBI. In an adult series including 9 patients following TBI, Darwish and Amiridze (2010) detected increased cytochrome c levels in over half of their samples. A correlation with outcome was not found, possibly due to small sample size. In a pediatric study including 67 infants and children, our laboratory reported that the CSF cytochrome c level was independently associated with AHT and female sex after TBI (Satchell et al., 2005). In the present study, we found that the peak CSF cytochrome c level was also independently associated with AHT, in addition to outcome (6-month GOS score). However, in contrast to the previous study by Satchell and co-workers (2005), we did not detect an association between CSF cytochrome c and female sex. The most likely explanation for this difference (in our opinion) is related to the smaller sample size in the current versus the previous study (37 versus 67 patients, respectively). To definitively determine whether sex-based differences in CSF cytochrome c exist, either a prospectively-collected larger cohort and/or multivariate analysis using a combined data set appears necessary.
While cytochrome c is recognized intracellularly as a marker of apoptosis, there is uncertainty about the mechanisms by which it may move into the extracellular space, which is necessary for detection in the CSF. Renz and associates found that cell death induced by apoptotic, but not necrotic stimuli, resulted in the rapid release of cytochrome c from the cell, allowing detection in the extracellular medium (Renz et al., 2001). Ahlemeyer and colleagues similarly found that cytochrome c is released extracellularly after staurosporine-induced apoptosis, and suggested that extracellular cytochrome c may contribute to neuronal apoptosis (Ahlemeyer et al., 2002). Several studies have since been undertaken using the serum cytochrome c level as a marker of apoptosis (Adachi et al., 2004; Barczyk et al., 2005; Ben-Ari et al., 2003). Our data suggest that peak CSF cytochrome c levels may reflect the degree of apoptosis after TBI, possibly resulting in worse brain injury and ultimately poor outcome.
CSF αII-spectrin breakdown products (SBDPs) have been used to evaluate the timing and impact of necrosis and apoptosis after severe TBI in adult patients (Brophy et al., 2009; Mondello et al., 2010; Pineda et al., 2007). SBDP150 and SBDP145 result primarily from proteolysis by calpain, whereas SBDP120 results primarily from proteolysis by caspase-3, thus representing markers of necrosis and apoptosis, respectively. Through the use of exposure and kinetic characteristics, these studies suggest that necrosis is prominent in adults after TBI (Brophy et al., 2009). Temporally, SBDP145 peaks early after injury, while SBDP120 shows sustained elevation that persists for at least 7 days after injury (Mondello et al., 2010; Pineda et al., 2007).
There are limitations to using CSF levels of HMGB1 and cytochrome c to directly compare the degree of necrosis and apoptosis after TBI. Data regarding the release of HMGB1 and cytochrome c in response to comparable necrotic or apoptotic insults, and dose-response curves with regard to severity of injury are not available. However, our data do suggest, similarly to our previous report (Satchell et al., 2005), that elevated CSF cytochrome c levels are independently associated with AHT. Increased CSF cytochrome c in this population often occurred without a simultaneous increase in CSF HMGB1 (Fig. 5B), suggesting different cell death pathways detected among patients with accidental TBI and AHT. AHT has been commonly associated with poor outcome, and several clinical aspects differ significantly from children presenting with accidental TBI (Ewing-Cobbs et al., 1999; Keenan et al., 2004). AHT patients are younger than accidental TBI patients, primarily consisting of infants or toddlers. AHT patients often present for medical attention in a delayed fashion and with non-specific symptoms such as vomiting, seizures, or apparent life-threatening events (Guenther et al., 2010). AHT victims may undergo repeated insults prior to presentation, including violent shaking and/or blunt trauma, perhaps also with hypoxemia related to impact apnea (Adamsbaum et al., 2010; Jenny et al., 1999; Keenan et al., 2004). Furthermore, apoptosis is prominent in the developing relative to the mature mammalian brain (Yakovlev et al., 2001), consistent with cytochrome c release and apoptosis contributing to pathology after AHT.
In terms of utility as prognostic biomarkers, both CSF HMGB1 and cytochrome c appear very specific but not sensitive in predicting outcome. For HMGB1 a cutoff point of 19.49 ng/mL had a sensitivity of 30.8% and specificity of 100%. For cytochrome c a cutoff point of 4.06 ng/mL had a sensitivity of 23.1% and a specificity of 100%. Thus, having high CSF HMGB1 and cytochrome c levels were highly predictive of poor outcome, but levels below their respective cutoff values were not indicative of good outcome. As such, a panel of biomarkers appears necessary to accurately predict outcome at both ends of the spectrum with high sensitivity and specificity.
This study has other limitations. Detection of CSF HMGB1 using ELISA technology does not differentiate between HMGB1 that is passively released versus that actively secreted into the extracellular space. Thus, HMGB1 levels measured by this technique may represent necrotic cell death, immunomodulatory release from macrophages and monocytes, or a combination of both after TBI. Two-dimensional gel electrophoresis followed by immunoblotting (Kim et al., 2006) has the ability to identify the different forms of HMGB1, as actively secreted HMGB1 is hyper-acetylated; however, this capacity was not possible using ELISA. An additional limitation of our study is the difficulty in obtaining age-matched CSF controls, as the majority of children undergoing diagnostic lumbar puncture are infants and toddlers. Therefore our control group represents a younger population than our TBI group, with the exception of the subgroup with AHT. Finally, while our sample size (n=37) was sufficient to identify correlations between these CSF biomarkers and clinical outcome, our study may have been underpowered to detect associations with other clinical variables such as sex of the patient, which was previously shown using a larger patient cohort (Satchell et al., 2005).
In summary, CSF HMGB1 and cytochrome c appear to be useful biomarkers that independently predict neurological outcome after TBI, perhaps reflecting degrees of necrosis and apoptosis, respectively. In the clinical setting, HMGB1 and cytochrome c could be combined with other biomarkers to form a panel to predict neurological outcome. AHT is a unique population in whom apoptosis is readily distinguishable, perhaps implying that anti-apoptotic agents may be effective in this subgroup of TBI patients.
Acknowledgment
This work was supported by grants from the National Institute of Child Health and Human Development T32 HD040686 (AKA), and the National Institute for Neurological Diseases and Stroke R01 NS30318 (RSBC, PMK).
Author Disclosure Statement
No competing financial interests exist.
References
- Adachi N. Hirota M. Hamaguchi M. Okamoto K. Watanabe K. Endo F. Serum cytochrome c level as a prognostic indicator in patients with systemic inflammatory response syndrome. Clin. Chim. Acta. 2004;342:127–136. doi: 10.1016/j.cccn.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Adamsbaum C. Grabar S. Mejean N. Rey-Salmon C. Abusive head trauma: judicial admissions highlight violent and repetitive shaking. Pediatrics. 2010;126:546–555. doi: 10.1542/peds.2009-3647. [DOI] [PubMed] [Google Scholar]
- Adelson P.D. Bratton S.L. Carney N.A. Chesnut R.M. du Coudray H.E. Goldstein B. Kochanek P.M. Miller H.C. Partington M.D. Selden N.R. Warden C.R. Wright D.W. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Pediatr. Crit. Care Med. 2003;4:S2–S75. doi: 10.1097/01.CCM.0000066600.71233.01. [DOI] [PubMed] [Google Scholar]
- Adelson P.D. Ragheb J. Kanev P. Brockmeyer D. Beers S.R. Brown S.D. Cassidy L.D. Chang Y. Levin H. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56:740–754. doi: 10.1227/01.neu.0000156471.50726.26. discussion 740–754. [DOI] [PubMed] [Google Scholar]
- Agrawal A. Schatz D.G. RAG1 and RAG2 form a stable postcleavage synaptic complex with DNA containing signal ends in V(D)J recombination. Cell. 1997;89:43–53. doi: 10.1016/s0092-8674(00)80181-6. [DOI] [PubMed] [Google Scholar]
- Ahlemeyer B. Klumpp S. Krieglstein J. Release of cytochrome c into the extracellular space contributes to neuronal apoptosis induced by staurosporine. Brain Res. 2002;934:107–116. doi: 10.1016/s0006-8993(02)02365-x. [DOI] [PubMed] [Google Scholar]
- Andersson U. Wang H. Palmblad K. Aveberger A.C. Bloom O. Erlandsson-Harris H. Janson A. Kokkola R. Zhang M. Yang H. Tracey K.J. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barczyk K. Kreuter M. Pryjma J. Booy E.P. Maddika S. Ghavami S. Berdel W.E. Roth J. Los M. Serum cytochrome c indicates in vivo apoptosis and can serve as a prognostic marker during cancer therapy. Int. J. Cancer. 2005;116:167–173. doi: 10.1002/ijc.21037. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Z. Schmilovotz-Weiss H. Belinki A. Pappo O. Sulkes J. Neuman M.G. Kaganovsky E. Kfir B. Tur-Kaspa R. Klein T. Circulating soluble cytochrome c in liver disease as a marker of apoptosis. J. Intern. Med. 2003;254:168–175. doi: 10.1046/j.1365-2796.2003.01171.x. [DOI] [PubMed] [Google Scholar]
- Berger R.P. Adelson P.D. Richichi R. Kochanek P.M. Serum biomarkers after traumatic and hypoxemic brain injuries: insight into the biochemical response of the pediatric brain to inflicted brain injury. Dev. Neurosci. 2006;28:327–335. doi: 10.1159/000094158. [DOI] [PubMed] [Google Scholar]
- Bianchi M.E. Manfredi A.A. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol. Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- Bonaldi T. Talamo F. Scaffidi P. Ferrera D. Porto A. Bachi A. Rubartelli A. Agresti A. Bianchi M.E. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy G.M. Pineda J.A. Papa L. Lewis S.B. Valadka A.B. Hannay H.J. Heaton S.C. Demery J.A. Liu M.C. Tepas J.J., 3rd Gabrielli A. Robicsek S. Wang K.K. Robertson C.S. Hayes R.L. alphaII-Spectrin breakdown product cerebrospinal fluid exposure metrics suggest differences in cellular injury mechanisms after severe traumatic brain injury. J. Neurotrauma. 2009;26:471–479. doi: 10.1089/neu.2008.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M. Reeves R. High-mobility-group chromosomal proteins: architectural components that facilitate chromatin function. Prog. Nucleic Acid Res. Mol. Biol. 1996;54:35–100. doi: 10.1016/s0079-6603(08)60360-8. [DOI] [PubMed] [Google Scholar]
- Cohen M.J. Brohi K. Calfee C.S. Rahn P. Chesebro B.B. Christiaans S.C. Carles M. Howard M. Pittet J.F. Early release of high mobility group box nuclear protein 1 after severe trauma in humans: role of injury severity and tissue hypoperfusion. Crit. Care. 2009;13:R174. doi: 10.1186/cc8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwish R.S. Amiridze N.S. Detectable levels of cytochrome C and activated caspase-9 in cerebrospinal fluid after human traumatic brain injury. Neurocrit. Care. 2010;12:337–341. doi: 10.1007/s12028-009-9328-3. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L. Prasad M. Kramer L. Landry S. Inflicted traumatic brain injury: relationship of developmental outcome to severity of injury. Pediatr. Neurosurg. 1999;31:251–258. doi: 10.1159/000028872. [DOI] [PubMed] [Google Scholar]
- Exo J. Kochanek P.M. Adelson P.D. Greene S. Clark R.S. Bayir H. Wisniewski S.R. Bell M.J. Intracranial pressure-monitoring systems in children with traumatic brain injury: Combining therapeutic and diagnostic tools. Pediatr. Crit. Care Med. 2011;12:560–565. doi: 10.1097/PCC.0b013e3181e8b3ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J. Li Y. Levy R.M. Fan J.J. Hackam D.J. Vodovotz Y. Yang H. Tracey K.J. Billiar T.R. Wilson M.A. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J. Immunol. 2007;178:6573–6580. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- Faul M. Xu L. Wald M.M. Coronado V.G. Atlanta: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. [Google Scholar]
- Fink M.P. Bench-to-bedside review: High-mobility group box 1 and critical illness. Crit. Care. 2007;11:229. doi: 10.1186/cc6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H.M. Zhou H. Zhang F. Wilson B.C. Kam W. Hong J.S. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J. Neurosci. 2011;31:1081–1092. doi: 10.1523/JNEUROSCI.3732-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardella S. Andrei C. Ferrera D. Lotti L.V. Torrisi M.R. Bianchi M.E. Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G.H. Sanders C. Johns E.W. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur. J. Biochem. 1973;38:14–19. doi: 10.1111/j.1432-1033.1973.tb03026.x. [DOI] [PubMed] [Google Scholar]
- Guenther E. Powers A. Srivastava R. Bonkowsky J.L. Abusive head trauma in children presenting with an apparent life-threatening event. J. Pediatr. 2010;157:821–825. doi: 10.1016/j.jpeds.2010.04.072. [DOI] [PubMed] [Google Scholar]
- Jenny C. Hymel K.P. Ritzen A. Reinert S.E. Hay T.C. Analysis of missed cases of abusive head trauma. JAMA. 1999;281:621–626. doi: 10.1001/jama.281.7.621. [DOI] [PubMed] [Google Scholar]
- Keenan H.T. Runyan D.K. Marshall S.W. Nocera M.A. Merten D.F. A population-based comparison of clinical and outcome characteristics of young children with serious inflicted and noninflicted traumatic brain injury. Pediatrics. 2004;114:633–639. doi: 10.1542/peds.2003-1020-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.B. Sig Choi J. Yu Y.M. Nam K. Piao C.S. Kim S.W. Lee M.H. Han P.L. Park J.S. Lee J.K. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J. Neurosci. 2006;26:6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluck R.M. Bossy-Wetzel E. Green D.R. Newmeyer D.D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Klune J.R. Dhupar R. Cardinal J. Billiar T.R. Tsung A. HMGB1: endogenous danger signaling. Mol. Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek P.M. Berger R.P. Bayir H. Wagner A.K. Jenkins L.W. Clark R.S. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Curr. Opin. Crit. Care. 2008;14:135–141. doi: 10.1097/MCC.0b013e3282f57564. [DOI] [PubMed] [Google Scholar]
- Liu X. Kim C.N. Yang J. Jemmerson R. Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Messmer D. Yang H. Telusma G. Knoll F. Li J. Messmer B. Tracey K.J. Chiorazzi N. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J. Immunol. 2004;173:307–313. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- Mondello S. Robicsek S.A. Gabrielli A. Brophy G.M. Papa L. Tepas J. Robertson C. Buki A. Scharf D. Jixiang M. Akinyi L. Muller U. Wang K.K. Hayes R.L. AlphaII-spectrin breakdown products (SBDPs): diagnosis and outcome in severe traumatic brain injury patients. J. Neurotrauma. 2010;27:1203–1213. doi: 10.1089/neu.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara T. Tsuruta R. Kaneko T. Yamashita S. Fujita M. Kasaoka S. Hashiguchi T. Suzuki M. Maruyama I. Maekawa T. High-mobility group box 1 protein in CSF of patients with subarachnoid hemorrhage. Neurocrit. Care. 2009;11:362–368. doi: 10.1007/s12028-009-9276-y. [DOI] [PubMed] [Google Scholar]
- Palumbo R. Bianchi M.E. High mobility group box 1 protein, a cue for stem cell recruitment. Biochem. Pharmacol. 2004;68:1165–1170. doi: 10.1016/j.bcp.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Peltz E.D. Moore E.E. Eckels P.C. Damle S.S. Tsuruta Y. Johnson J.L. Sauaia A. Silliman C.C. Banerjee A. Abraham E. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock. 2009;32:17–22. doi: 10.1097/shk.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda J.A. Lewis S.B. Valadka A.B. Papa L. Hannay H.J. Heaton S.C. Demery J.A. Liu M.C. Aikman J.M. Akle V. Brophy G.M. Tepas J.J. Wang K.K. Robertson C.S. Hayes R.L. Clinical significance of alphaII-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J. Neurotrauma. 2007;24:354–366. doi: 10.1089/neu.2006.003789. [DOI] [PubMed] [Google Scholar]
- Raucci A. Palumbo R. Bianchi M.E. HMGB1: a signal of necrosis. Autoimmunity. 2007;40:285–289. doi: 10.1080/08916930701356978. [DOI] [PubMed] [Google Scholar]
- Renz A. Berdel W.E. Kreuter M. Belka C. Schulze-Osthoff K. Los M. Rapid extracellular release of cytochrome c is specific for apoptosis and marks cell death in vivo. Blood. 2001;98:1542–1548. doi: 10.1182/blood.v98.5.1542. [DOI] [PubMed] [Google Scholar]
- Rock K.L. Hearn A. Chen C.J. Shi Y. Natural endogenous adjuvants. Springer Semin. Immunopathol. 2005;26:231–246. doi: 10.1007/s00281-004-0173-3. [DOI] [PubMed] [Google Scholar]
- Rouhiainen A. Kuja-Panula J. Wilkman E. Pakkanen J. Stenfors J. Tuominen R.K. Lepantalo M. Carpen O. Parkkinen J. Rauvala H. Regulation of monocyte migration by amphotericin (HMGB1) Blood. 2004;104:1174–1182. doi: 10.1182/blood-2003-10-3536. [DOI] [PubMed] [Google Scholar]
- Satchell M.A. Lai Y. Kochanek P.M. Wisniewski S.R. Fink E.L. Siedberg N.A. Berger R.P. DeKosky S.T. Adelson P.D. Clark R.S. Cytochrome c, a biomarker of apoptosis, is increased in cerebrospinal fluid from infants with inflicted brain injury from child abuse. J. Cereb. Blood Flow Metab. 2005;25:919–927. doi: 10.1038/sj.jcbfm.9600088. [DOI] [PubMed] [Google Scholar]
- Scaffidi P. Misteli T. Bianchi M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Shore P.M. Berger R.P. Varma S. Janesko K.L. Wisniewski S.R. Clark R.S. Adelson P.D. Thomas N.J. Lai Y.C. Bayir H. Kochanek P.M. Cerebrospinal fluid biomarkers versus Glasgow Coma Scale and Glasgow Outcome Scale in pediatric traumatic brain injury: the role of young age and inflicted injury. J. Neurotrauma. 2007;24:75–86. doi: 10.1089/neu.2006.0062. [DOI] [PubMed] [Google Scholar]
- Sutrias-Grau M. Bianchi M.E. Bernues J. High mobility group protein 1 interacts specifically with the core domain of human TATA box-binding protein and interferes with transcription factor IIB within the pre-initiation complex. J. Biol. Chem. 1999;274:1628–1634. doi: 10.1074/jbc.274.3.1628. [DOI] [PubMed] [Google Scholar]
- Tang D. Kang R. Cao L. Zhang G. Yu Y. Xiao W. Wang H. Xiao X. A pilot study to detect high mobility group box 1 and heat shock protein 72 in cerebrospinal fluid of pediatric patients with meningitis. Crit. Care Med. 2008;36:291–295. doi: 10.1097/01.CCM.0000295316.86942.CE. [DOI] [PubMed] [Google Scholar]
- Yakovlev A.G. Ota K. Wang G. Movsesyan V. Bao W.L. Yoshihara K. Faden A.I. Differential expression of apoptotic protease-activating factor-1 and caspase-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury. J. Neurosci. 2001;21:7439–7446. doi: 10.1523/JNEUROSCI.21-19-07439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]