Abstract
The ontogeny of corticotropin-releasing hormone (CRH) receptor messenger ribonucleic acid (mRNA) in rat brain, using in situ hybridization, is the focus of this study. The developmental profile of CRH receptor using binding assays and receptor autoradiography has been reported, but may be confounded by the presence of a binding protein. The recent cloning of the rat CRH receptor gene has permitted the use of in situ hybridization histochemistry to map the distribution of cells expressing CRH receptor mRNA in the developing brain. We used antisense 35S-labeled oligodeoxynucleotide probes for the two reported splice-variants of the CRH receptor mRNA, which yielded essentially identical localization patterns. CRH receptor mRNA was clearly detectable in infant brain starting on the second postnatal day. Signal in hippocampal CA1, CA2 and CA3a increased to 300–600% of adult levels by postnatal day 6 with a subsequent gradual decline. In the amygdala, in contrast, CRH receptor mRNA abundance increased steadily between the second and the ninth postnatal days, to levels twice higher than those in the adult. In the cortex, CRH receptor mRNA levels were high on postnatal day 2 and decreased to adult levels by day 12. Transient signal over the hypothalamic paraventricular nucleus, observed on the second postnatal day, was not evident at older ages. These results demonstrate robust synthesis of CRH receptor as early as on the second postnatal day and unique region-specific developmental profiles for CRH receptor gene expression.
Keywords: Corticotropin releasing hormone, Receptor, Messenger RNA, Amygdala, Hippocampus, Development, Rat
1. Introduction
Regulation of CRH receptor number may be an important mechanism for modulation of the response to stress [9,10]. Developmental regulation of stress-related [8,20] and other effects of CRH have been demonstrated [1,3]. Autoradiography and radioligand binding studies during early postnatal life in the rat have demonstrated changes in receptor number and regional distribution [5,6,11,12,15]. The two methods have yielded conflicting information regarding CRH receptor distribution in a number of brain regions [12,16,17]. A potential basis for this discrepancy derives from CRH binding protein which may interact with the CRH ligand [12,16]. Binding studies may also provide limited information on the actual site of receptor synthesis because ligands may bind to neural receptors on cell bodies and along dendrites and axons [17]. The recent cloning of cDNAs encoding the rat CRH receptor [4,14] permits mapping the distribution of cells expressing CRH receptor messenger ribonucleic acid (mRNA) [17,21]. In this study, we report on the developmental profile of CRH receptor mRNA in limbic brain regions of the rat brain.
2. Materials and methods
2.1. Animals
Timed pregnancy Sprague–Dawley rats were purchased from Zivic-Miller (Zelienople, PA). Rats were maintained in NIH-approved animal facilities and kept on a 12-h light/dark cycle with access to unlimited lab chow and water [17]. Delivery was verified at 12-h intervals and the day of birth was considered day 0. Rats were sacrificed on postnatal day 2, 4, 6, 9, 12, 16 or 90 (adult).
2.2. Tissue preparation and handling
Rats were decapitated within 45 s of disturbance. Brains were rapidly removed, frozen on powdered dry ice and stored at −80°C. They were cut into 20 μm sections in the coronal plane in a cryostat and mounted on gelatin-coated slides.
2.3. Generation and labeling of oligodeoxynucleotide probes
An antisense probe was designed, based on the published sequence of the cloned rat-CRH receptor [4,14]. The probe targeted the spliced region between the third and fourth transmembrane domains [4], and consisted of a 35 oligodeoxynucleotide encompassing bases 211–246 of the CRH-receptor cDNA, which is entirely within the ‘spliced’ region. (The putative splice sites are at the 178–179 and 223–224 codons). Probe sequence was: 5′-ACAGCCAT-TGTGCTCACGTACTCCACCGACCGTCT-3′. A ‘sense’ deoxyoligonucleotide, complementary to the sequence above was generated and labeled as a specificity control. Probes were 3′-labeled with [S35]dATP using terminyldeoxynucleotidyl transferase [23], and purified on Nuctrap columns (Stratagene, La Jolla, Ca). Specific activity was 1.3–5.4 × 108 cpm/μg.
2.4. In situ hybridization histochemistry and analysis
Details of in situ hybridization (ISH) and image analysis have been described [2,23]. Briefly, sections were brought to room temperature, air-dried and fixed for 20 min in fresh 4% buffered paraformaldehyde. After a graded ethanol treatment, sections were exposed to acetic anhy-dride-triethanolamine and dehydrated through 100% ethanol. Sections were prehybridized for 1 h, then hybridized for 20 h at 40°C in a humidity chamber. Sections were washed in 2 × SSC for 15 min four times at 40°C, followed by 1 × and 0.3 × SSC for 30 min each at room temperature. The sections were dehydrated through 70, 80, 95 and 100% ethanol, air dried and apposed to film (Hyperfilm B-Max, Amersham, IL) for 5–7 days.
Quantitative image analysis of CRH receptor mRNA was achieved using the MCID software image analysis system (Imaging Research, St. Catherine, Ont., Canada). Optical density was determined over hippocampal regions CA1, CA2, CA3, over the dentate gyrus (DG), the frontal and piriform cortex, central nucleus (CEA) and lateral nucleus (ALA) of the amygdala, and the paraventricular nucleus (PVN). Each point was derived from a minimum 4 sections from 2–3 individual rats. Brainpaste standardized values and ratio of structure/background were both obtained. For tabulation, a semiquantitative scale was used: (+) = 40–225, (+ +) = 225–500, (+ + +) = 500–1000 and (+ + + +) = 1000–1600 cpm/mg tissue.
3. Results
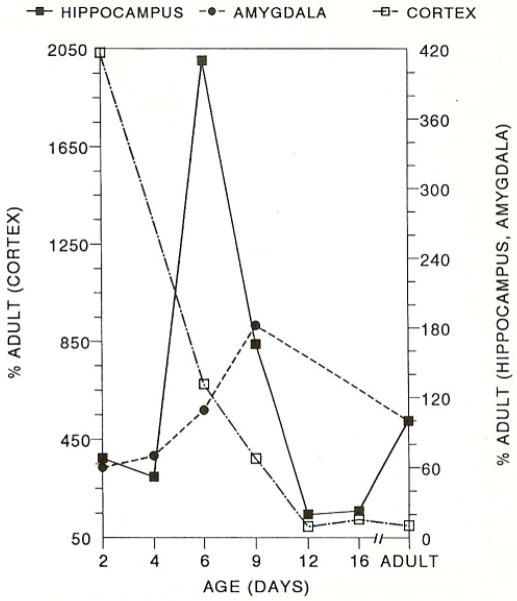
CRH receptor mRNA was clearly detectable in infant rat brain starting on the second postnatal day (Table 1). In the hippocampus, CRH receptor was expressed throughout the pyramidal cell layer including all subregions of the CA3. The signal over CA1, CA2 and CA3a increased to 300–600% of adult values by postnatal day 6 with a subsequent gradual decline (Table 1, Figs. 1 and 2). CRH receptor mRNA in both central and lateral amygdala nuclei increased steadily starting on the second postnatal day and peaked at twice adult levels by postnatal day 9. In neocortex, CRH receptor mRNA was maximal on the second postnatal day and decreased gradually, reaching adult values by the twelfth day (Table 1, Figs. 1 and 2). Transient signal over the PVN observed on the second postnatal day was not evident at older ages (Table 1).
Table 1.
Levels of CRH receptor mRNA in limbic structures as a function of age
| Age (days) | 2 | 4 | 6 | 9 | 12 | 16 | A |
|---|---|---|---|---|---|---|---|
| Hippocampus | |||||||
| CA1 | + + + | + | + | + | |||
| CA2 | + | + + + + | + + + | + | + | + | |
| CA3a | + | + | + + + + | + + + | + | + | + + |
| CA3b,c | + | + + | + | + | + + | ||
| Dentate gyrus | + | + | + | + + + | |||
| Amygdala | |||||||
| CEA | + | + | + | + | + | ||
| ALA | + | + | + | + | + | ||
| Cortex | |||||||
| Piriform | + + | + + | + | + | + | + | + + + |
| FP | + + + + | + | + | + | + | + | |
| PVN | + | − | − | − | − | − | − |
In situ hybridization of CRH receptor mRNA using oligodeoxynucleotide probes. Semiquantitative scale in cpm/mg tissue: (+) = 40–225, (+ +) = 225–500, (+ + +) = 500–1000, (+ + + +) = 1000–1600. A = adult, CEA = central nucleus of the amygdala, FP = frontoparietal cortex, PVN = paraventricular nucleus.
Fig. 1.
Graph showing distinct developmental profiles of CRH receptor mRNA in three limbic structures, cortex, hippocampus and amygdala.
Fig. 2.
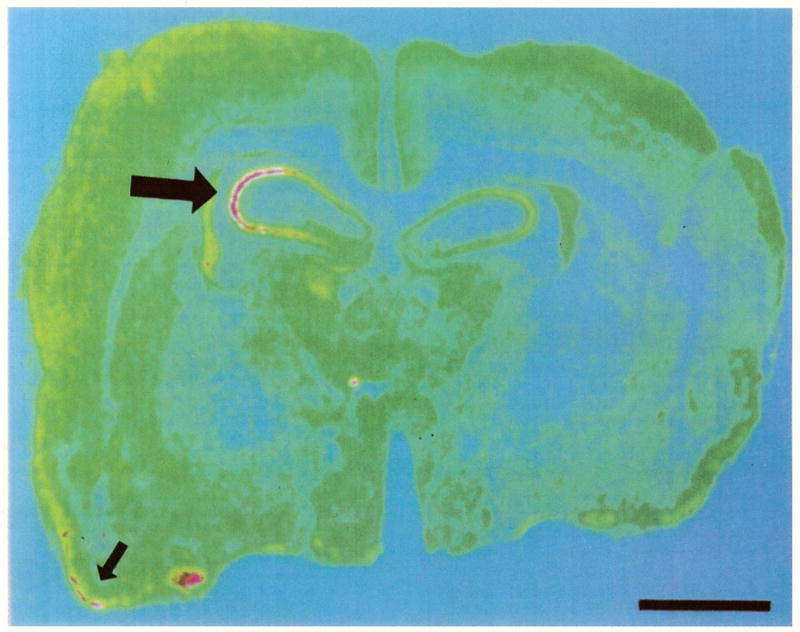
Computer-generated pseudocolor image, showing CRH receptor mRNA distribution in the nine day old rat forebrain. Large arrow points to the CA3 pyramidal layer of the hippocampus, revealing high receptor mRNA density (yellow-pink). Small arrow shows the piriform cortex. Bar = 0.18 mm.
In adult brain (Fig. 3), CRH receptor mRNA abundance was high in the granule cell layer of the dentate gyrus, especially the inferior blade (large arrow), in the piriform cortex (star) and in the pyramidal cell layer of the hippocampus. In the hippocampal CA3 region, CRH receptor was expressed in CA3a (small arrow) a as well as CA3b and c. No signal was localized over the PVN (Table 1). ISH using the ‘sense’ probe yielded no evident hybridization (Fig. 4).
Fig. 3.
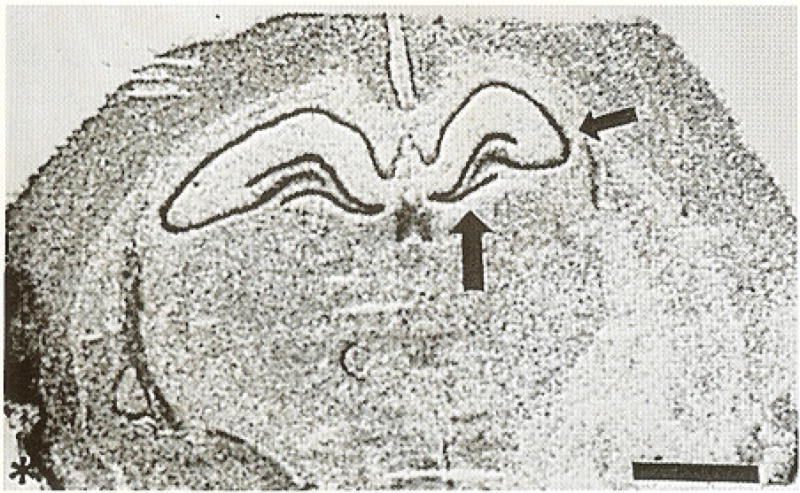
Coronal section through adult forebrain after in situ hybridization for the CRH receptor mRNA. High receptor density is seen in hippocampal CA3 (small arrow), the inferior blade of the dentate gyrus (large arrow) and the piriform cortex (star). Bar = 0.20 mm.
Fig. 4.

Coronal section of an adult rat forebrain. The section was subjected to in situ hybridization using an oligodeoxynucleotide probe complementary to that used for localization of CRH-receptor-mRNA (‘sense’ control). Little signal is visible. Bar = 0.2 mm.
4. Discussion
CRH receptor mRNA distribution during development revealed several distinctive spatial and temporal patterns. In the hippocampal CA1, CA2 and CA3a, peak CRH receptor mRNA occurred on postnatal day 6. In the amygdala, CRH receptor mRNA levels was maximal on the ninth postnatal day, but reached only 180% of adult values. Conversely, a steady decline from high postnatal day 2 levels occurred in neocortical CRH receptor mRNA. These findings demonstrate distinct, regional, age-specific control of the synthesis of CRH receptor [12,15,17].
The developmental pattern of the expression of CRH receptor may provide a mechanism for modulation of the age specific role of CRH in different regions. For example, a high ratio of hippocampus/amygdala receptors may preferentially activated negative hippocampal input into the PVN during the neonatal period [18,19,22]. Increased CRH receptor mRNA in the infant compared with the adult also provides a mechanism for the high excitatory effect of the peptide at this age [1,3]. Both in the infant [1,3] and in the adult [7], CRH-induced seizures originate in the amygdala and propagate to the hippocampus and neocortex [1]. These excitatory CRH effects are mediated via CRH receptors, since they are completely reversed by CRH receptor antagonists [3].
This study focused on the first member of the CRH receptor family, which has been demonstrated in the brain, pituitary, and other organs [13]. This receptor likely mediates the neuroendocrine effects of CRH [13]. A second member of the CRH receptor family has been recently described [13]. The novel receptor has been named CRF2, while the original CRH receptor was designated as CBF1. These two receptor cDNAs share a 71% homology [13]. GenBank data combined with analysis of homology and hybridization (MacMolly, SoftGene, Berlin, Germany) strongly indicate that the oligodeoxynucleotide used for this study interacts specifically with the CRF1.
In the adult brain, there are significantly distinct distribution patterns of the two receptors: for example, CRF2 is undetectable in neocortex, unlike published [17] and our findings (Table 1) for CRF1. Conversely, high CRF2 levels have been demonstrated in the ventromedial nucleus of the hypothalamus and in the lateral septum, both regions with little CRF1, gene expression in our hands. The developmental profile of the novel receptor, CRF2, and its mRNA are not yet known.
In summary, CRH receptor mRNA is distributed in the rat limbic system in a distinctive, developmentally regulated pattern. This suggests that receptor regulation contributes to the modulation of the effect of CRH itself.
Acknowledgments
We wish to thank Dr. J. Peters and C.J.L. Newth. This work was supported by CHLA-BRSG research fellowship award (S.A.E.)) and by NINDS NS28912 (T.Z.B.).
References
- 1.Baram TZ, Hirsch E, Snead OC, III, Schultz L. CRH induced seizures in the infant brain originate in the amygdala. Ann Neurol. 1992;31:488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baram TZ, Lerner SP. Ontogeny of corticotropin releasing hormone gene expression in rat hypothalamus-comparison with somatostatin. Int J Dev Neurosci. 1991;9:473–478. doi: 10.1016/0736-5748(91)90033-i. [DOI] [PubMed] [Google Scholar]
- 3.Baram TZ, Schultz L. CRH is a rapid and potent convulsant in the infant rat. Dev Brain Res. 1991;61:97–101. doi: 10.1016/0165-3806(91)90118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang C-P, Pearse RV, II, O’Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–1195. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- 5.De Souza EB. Corticotropin-releasing factor receptors in the rat central nervous system: characterization and regional distribution. J Neurosci. 1987;7:88–100. doi: 10.1523/JNEUROSCI.07-01-00088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Souza EB, Perrin MH, Insel TR, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors in rat forebrain: autoradiographic identification. Science. 1984;224:1449–1451. doi: 10.1126/science.6328656. [DOI] [PubMed] [Google Scholar]
- 7.Ehlers CL. CRF effects on EEG activity: implications for the modulation of normal and abnormal brain states. In: De Souza EB, Nemeroff CB, editors. Corticotropin-Releasing Factor: Basic and Clinical Studies of a Neuropeptide. CRC; Boca Raton: 1990. pp. 233–248. [Google Scholar]
- 8.Grino M, Burgunder JM, Eskay RL, Eiden LE. Onset of glucocorticoid responsiveness of anterior pituitary corticotrophs during development is scheduled by corticotropin releasing factor. Endocrinology. 1989;124:2686–2692. doi: 10.1210/endo-124-6-2686. [DOI] [PubMed] [Google Scholar]
- 9.Hauger RL, Irwin MR, Lorang M, Aguilera G, Brown MR. High intracerebral levels of CRH result in CRH receptor downregulation in the amygdala and neuroimmune desensitization. Brain Res. 1993;616:283–292. doi: 10.1016/0006-8993(93)90219-d. [DOI] [PubMed] [Google Scholar]
- 10.Hauger RL, Millan MA, Lorang M, Harwood JP, Aguillera G. Corticotropin releasing factor receptors and pituitary adrenal response during immobilization stress. Endocrinology. 1988;123:396–405. doi: 10.1210/endo-123-1-396. [DOI] [PubMed] [Google Scholar]
- 11.Insel TR. Brain corticotropin releasing factor and development. In: De Souza EB, Nemeroff CB, editors. Corticotropin Releasing Factor: Basic and Clinical Studies of a Neuropeptide. CRC; Boca Raton: 1990. pp. 91–106. [Google Scholar]
- 12.Insel TR, Battaglia G, Fairbanks DW, De Souza EB. The ontogeny of brain receptors for corticotropin-releasing factor and the development of their functional association with adenylate cyclase. J Neurosci. 1988;8:4151–4158. doi: 10.1523/JNEUROSCI.08-11-04151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. Cloning and functional expression of a rat brain corticotropin-factor (CRF) receptor. Endocrinology. 1993;133:3058–3061. doi: 10.1210/endo.133.6.8243338. [DOI] [PubMed] [Google Scholar]
- 15.Pihoker C, Cain ST, Nemeroff CB. Postnatal, development of regional binding of corticotropin-releasing factor and adenylate cyclase activity in the rat brain. Prog Neuro-Psychopharmacol & Biol Psychiatry. 1992;16:581–586. doi: 10.1016/0278-5846(92)90063-k. [DOI] [PubMed] [Google Scholar]
- 16.Potter E, Behan DP, Linton EA, Lowry PJ, Sawchenko PE, Vale WW. The central distribution of a corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF. Proc Natl Acad Sci USA. 1992;89:4192–4196. doi: 10.1073/pnas.89.9.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale WW. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci USA. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suchecki D, Mozaffarian D, Gross G, Rosenfeld P, Levine S. Effects of maternal deprivation on the ACTH response in the infant rat. Neuroendocrinology. 1993;57:204–212. doi: 10.1159/000126361. [DOI] [PubMed] [Google Scholar]
- 19.Widmaier EP. Glucose homeostasis and hypothalamic-pituitary-adreno-cortical axis during development in rats. Am J Physiol. 1990;259:601–613. doi: 10.1152/ajpendo.1990.259.5.E601. [DOI] [PubMed] [Google Scholar]
- 20.Walker C-D, Scribner KA, Casico CS, Dallman MF. The pituitary-adrenal system of neonatal rats is responsive to stress throughout development in a time-dependent and stress-specific fashion. Endocrinology. 1991;128:1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- 21.Wong M-L, Licino J, Pasternak KI, Gold PW. Localization of corticotropin-releasing hormone (CRH) receptor mRNA in adult rat brain by in situ hybridization histochemistry. Endocrinology. 1994;135:2275–2278. doi: 10.1210/endo.135.5.7956950. [DOI] [PubMed] [Google Scholar]
- 22.Yi S-J, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide’s gene expression. Endocrinology. 1994;135:2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi S-J, Masters JN, Baram TZ. Effects of a specific glucocorticoid receptor antagonist on corticotropin releasing hormone gene expression in the paraventricular nucleus of the neonatal rat. Dev Brain Res. 1993;73:253–259. doi: 10.1016/0165-3806(93)90145-z. [DOI] [PubMed] [Google Scholar]