Abstract
Background
We studied resistance to endocrine and HER2-targeted therapies using a xenograft model of estrogen receptor positive (ER)/HER2-overexpressing breast cancer. Here, we report a novel phenotype of drug resistance in this model.
Methods
MCF7/HER2-18 xenografts were treated with endocrine therapy alone or in combination with lapatinib and trastuzumab (LT) to inhibit HER2. Archival tumor tissues were stained with hematoxylin & eosin and mucicarmine. RNA extracted from tumors at early time points and late after acquired resistance were analyzed for mucin4 (MUC4) expression by microarray and quantitative reverse transcriptase-PCR. Protein expression of the MUC4, ER and HER2 signaling pathways was measured by immunohistochemistry and Western blotting.
Results
The combination of the potent anti-HER2 regimen LT with either tamoxifen (Tam+LT) or estrogen deprivation (ED+LT) can cause complete eradication of ER-positive/HER2-overexpressing tumors in mice. Tumors developing resistance to this combination, as well as those acquiring resistance to endocrine therapy alone, exhibited a distinct histological and molecular phenotype—a striking increase in mucin-filled vacuoles and upregulation of several mucins including MUC4. At the onset of resistance, MUC4 mRNA and protein were increased. These tumors also showed upregulation and reactivation of HER2 signaling, while losing ER protein and the estrogen-regulated gene, progesterone receptor.
Conclusions
Mucins are upregulated in a preclinical model of ER-positive/HER2-overexpressing breast cancer as resistance develops to the combination of endocrine and anti-HER2 therapy. These mucin-rich tumors reactivate the HER2 pathway and shift their molecular phenotype to become more ER-negative/HER2-positive.
Keywords: Breast cancer, mucin4, mucinated phenotype, mucins, endocrine therapy, HER2 therapy, drug resistance
BACKGROUND
Found in ~70% of breast cancers, estrogen receptor-alpha (ER) generally identifies a more indolent tumor phenotype that can be targeted with endocrine therapy (either tamoxifen [Tam] or estrogen deprivation [ED] via aromatase inhibitors). Crosstalk between ER and human epidermal growth factor receptor-2 (HER2/neu/c-ErbB2) contributes to endocrine resistance in preclinical models [1,2] and combining endocrine therapy (Tam or ED) with drugs targeting the HER pathway can significantly delay resistance by inhibiting crosstalk in preclinical models [3–6]. This has been shown to be an effective treatment strategy in clinical trials [7–10]. We have modeled this phenomenon by growing MCF7 cells that stably overexpress HER2 (MCF7/HER2) as xenograft tumors. These tumors (MCF7/HER2-18) have been shown previously to be more endocrine resistant than MCF7 wild-type xenografts. Giving tamoxifen stimulates growth, exhibiting de novo resistance in these tumors. ED therapy results in transient response as tumors rapidly acquire resistance [11]. ED-resistant tumors alter their molecular phenotype by losing ER expression and upregulating HER2 signaling. These data suggest that both ER and HER2 need to be targeted therapeutically in ER-positive tumors with HER2 amplification or overexpression.
Currently two FDA-approved medications specifically target HER2: the monoclonal antibody trastuzumab and the dual kinase inhibitor lapatinib. Adding a single anti-HER2 agent to endocrine therapy can temporarily restore growth inhibition, but is inadequate to block HER2 signaling to fully eradicate tumors [3,1]. Combining both anti-HER2 drugs with endocrine therapy not only completely shuts off ER-HER2 signaling and crosstalk, but also results in complete regression of most of these xenograft tumors in mice [12]. However, a few tumors eventually acquire resistance, indicating that these tumors have reactivation of HER2 or bypass sustained HER2 inhibition with escape mechanisms are driving tumor. In this report, we present a novel and striking phenotypic shift with the model system of MCF7/HER2 tumors resistant to a combination of endocrine and anti-HER2 therapy.
MATERIALS AND METHODS
Cell-lines and antibodies
Generation and growth conditions of MUC4-positive CD18/HPAF, MCF7/HER2-18 and MCF7/HER2-11 cell lines were previously described [13]. Anti-MUC4 (8G7) was generated as previously described [14] with additional aliquots purchased along with anti-ERα (H-184) from Santa Cruz Biotechnologies (Santa Cruz, CA). Anti-phospho-HER2 (Y1248) was purchased from Millipore. Polyclonal antibodies against HER2, phospho-Akt (pThr308), total Akt, phospho-MAPK (pThr202/pTyr204), total MAPK, and β-actin were purchased from Cell Signaling Technology (Danvers, MA).
Human breast cancer xenograft tumors
Archived xenograft tumors of MCF7/HER2-18, MCF7/HER2-11, and MCF7 wild-type cell lines previously generated in nu/nu athymic nude mice treated with continued E2-supplementation (control), endocrine treatment alone (Tamoxifen or ED), or in combination with the anti-HER2 regimen (E2+LT, Tam+LT, ED+LT) for MCF7/HER2-18 only, as previously described [12]. Only two tumors developed resistance to the ED+LT regimen after >200 days. One of these tumors demonstrated a more stable phenotype and was transplantable. 2 mm3 fragments of this tumor was transplanted into an additional set of nude mice and harvested. For this study, transplants of the more stable and transplantable tumor were collected and combined with the original tumor and considered the ED+LT resistant group. These archival tumors were stored in liquid nitrogen or were formalin-fixed and paraffin-embedded (FFPE).
Histological staining, immunohistochemistry, & immunofluorescence
5 μm sections were stained with hematoxylin & eosin, mucicarmine, or were used for immunohistochemistry (IHC). Cell pellets were made by growing cells in 10 cm culture dishes at ~95% confluency, washing them with pH 7.4 phosphate buffered saline (PBS), and detaching them with Versene (Lonza, Basel, Switzerland). Cells were washed two additional times with PBS, fixed for two hours in 10% Neutral Buffered Formalin and then resuspended in PBS. Cells were pelleted to remove PBS and resuspended into 4% agar. Cells were refrigerated for 30 minutes and then embedded into paraffin. MUC4 IHC was performed with a protocol previously described [14], with the modification of using a Mouse-on-Mouse kit (Vector Labs, Burlingame, CA) to reduce background staining. Tumors were scored using Intensity scores (IS: 1–3) and Percentage Scores (PS: 0–100). A Histoscore for each tumor was calculated by multiplying IS by PS. Slides were also dual stained by combining the IHC protocol for HER2 (rabbit antibody) as before [1] and MUC4 (mouse antibody) IHC protocol. Furthermore, slides were stained for immunofluorescence (IF) by using anti-mouse-AlexaFluor568 (Invitrogen, Carlsbad, CA) and anti-rabbit-Fluorescein-Isothiocyanate (Vector Labs). Representative IF images were obtained with an SP5 confocal microscope (Leica, Bannockburn, IL) using a 63x oil immersion objective with LAS Software (Leica).
Microarray analysis
Mucin family gene expression in endocrine resistant MCF7/HER2-18 and MCF-7 wild-type tumors was extracted from a previous study [15]. Arrays were normalized and compared using DNA Chip Analyzer software (dChip, www.dChip.org). Two-sample t-tests of log-transformed data were performed as criteria for determining significant differences in mean gene mRNA levels between groups. Expression values were visualized as heatmaps using Java TreeView [16]. An additional MCF7/HER2-18 expression microarray was generated using similar methods to look specifically at MUC4 levels.
RNA isolation and Quantitative Real-time PCR assay
Tumor RNA was isolated with the EZ-1 kit (Qiagen, Germantown, MD) and the Biorobot EZ1 (Qiagen) according to manufacturer’s specifications. CD18/HPAF RNA was isolated using the RNeasy Kit (Qiagen) following manufacturer’s protocol. cDNA was made from RNA using SuperScript III Reverse Transcriptase (Invitrogen) according to manufacturer’s directions. MUC4-specific primers were designed to Entrez ID NM018406 using Primer Express (Applied Biosystems, Carlsbad, CA). β-actin specific primers were previously described [17]. Primers to MUC4 were custom designed using Primer Express Software (Applied Biosystems). The MUC4 forward primer was 5′TCACTCTGGAGATTCTAGCAAGAAGT3′ and the reverse primer was 5′ACTGTGGTCTGCCATTGCAAT3′. All primers were synthesized by Eurogentec (San Diego, CA). The qPCR was performed on Applied Biosystems 7700 using SYBR Green PCR Master Mix (Applied Biosystems) following manufacturer’s instructions. MUC4 mRNA expression was normalized to β-actin using the comparative Ct method [18], calculated as a fold change relative to a representative E2-stimulated MCF7/HER2-18 tumor, and log2-transformed.
Immunoblotting
Tumor extracts were generated by homogenizing frozen tissue in Reverse Phase Protein Array buffer [1,19]. Fifteen micrograms of protein extracts were loaded and separated on precast 8% Tris-glycine sodium dodecyl sulfate (SDS)-polyacrylamide gels (Invitrogen), before transferring to nitrocellulose membrane via iBlot (Invitrogen) per manufacturer’s directions. Membranes were blocked in 5% milk solution and then incubated with primary antibodies overnight at 4°C. After washing, membranes were incubated for an hour in appropriate horseradish peroxidase-labeled secondary antibodies at room temperature. After additional washes, chemiluminescence solution (GE Healthcare, Pittsburgh, PA) was used per manufacturer’s specifications. Membranes were exposed to film and quantified on a gel imager (Alpha Innotech, Santa Clara, CA). Protein expression was normalized to β-actin and log2-transformed.
Statistical analysis
The statistical significance of the difference between two means of data was analyzed using two-sided Student’s t-test for normally distributed samples. For the IHC data, as some data sets failed the Shapiro-Wilk normality test [20], the two-sided generalized Wilcoxon rank sum test [21] was used. All statistical analyses were performed using R [22]. Charts were plotted with mean ± SE. A p-value of <0.05 was considered significant.
RESULTS
Mucinated phenotype in drug resistant xenograft tumors
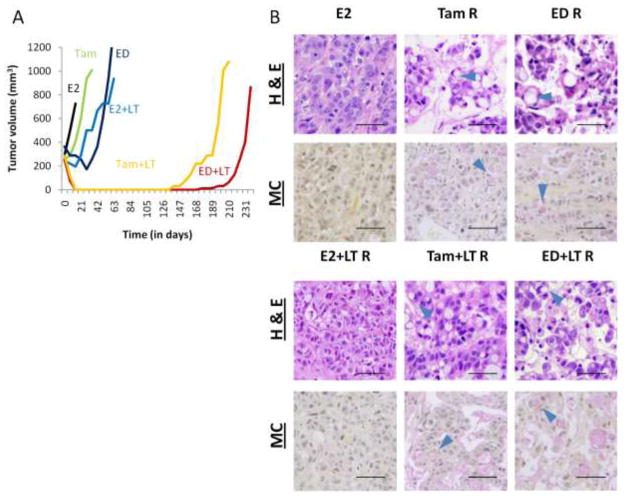
We have previously shown [11,12] that MCF7/HER2-18 xenografts are de novo resistant to Tam treatment and rapidly acquire resistance to ED, and adding potent dual-agent anti-HER2 treatment (LT) can delay this effect and even completely eradicate some tumors (Figure 1A). Importantly, we observed striking histological changes in these tumors resistant to Tam and ED, alone or in combination with LT (Figure 1B). Hematoxylin and eosin (H & E) staining of tumor sections in these groups detected the presence of multiple occupied vacuoles. This phenotype was not observed in E2-stimulated tumors alone or with LT. Mucicarmine staining confirmed the presence of mucin in these vacuoles, which were primarily intracellular, exhibiting cellular morphology similar to signet ring cells (see arrowheads) [23].
Figure 1.
MCF7/HER2-18 xenograft tumor growth curves and histological staining. (A) Growth curves of representative tumors show de novo resistance to tamoxifen (Tam, green) and temporary response to estrogen deprivation (ED, dark blue) and estrogen+lapatinib+trastuzumab (E2+LT, light blue). Tam+LT (yellow) and ED+LT (red) are successful in completely decreasing tumor size, but residual disease eventually leads to the onset of resistant tumor growth. (B) Tissue sections of MCF7/HER2-18 xenograft tumors resistant to endocrine therapy alone or with anti-HER2 therapy (LT) exhibits a vacuolar phenotype with hematoxylin & eosin (H & E) stain (top panel), that stain positive (pink) for mucin with mucicarmine (MC) stain (bottom panel). This phenotype is not seen in E2-stimulated or E2+LT-resistant tumors. Arrowheads show signet ring cells. Bar – 50 μm.
Expression of MUC4 mRNA in resistant tumors
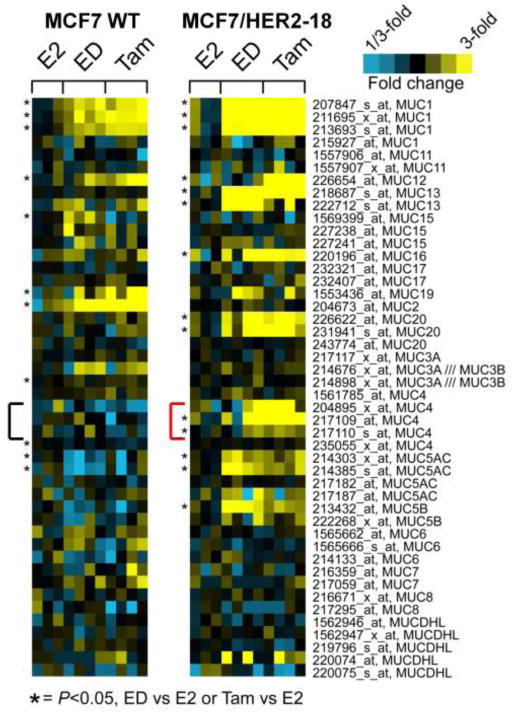
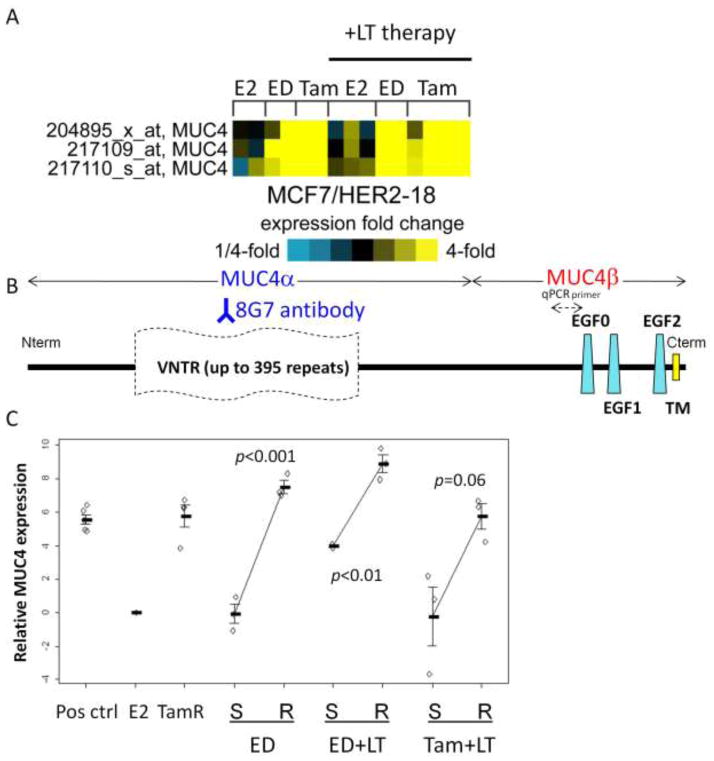
We analyzed mucin expression in previously published expression microarrays of endocrine resistant MCF7/HER2-18 and wildtype MCF7 tumors [15]. mRNA of multiple mucin genes were upregulated in endocrine resistant tumors when compared to E2-stimulated controls (Figure 2). MUC4 and MUC5AC mRNA levels were increased in Tam and ED resistant MCF7/HER2-18 tumors but not in Tam and ED resistant wild-type MCF7 tumors. Due to its reported role in HER2 stability and signaling [24,25], we next focused on MUC4 and tested for its expression in tumors treated with endocrine therapy with LT. MUC4 expression was dramatically increased in tumors resistant to endocrine therapy alone (Tam or ED) and in combination with LT when compared to E2-stimulated control tumors alone or with LT (Figure 3A). The MUC4 levels were similar in fold change to those we found in previous microarray data [15].
Figure 2.
Mucin gene expression in MCF7/HER2-18 and MCF7 wild-type tumors by microarray. Multiple mucin genes are upregulated in ED-resistant and Tam-stimulated compared to E2-stimulated controls, with MUC4 increased in endocrine resistant tumors only with HER2 overexpression. * indicates p<0.05 for expression in endocrine resistant tumors relative to E2-stimulated tumors.
Figure 3.
MUC4 mRNA expression. (A) Heatmap of MUC4 probe sets in MCF7/HER2-18 xenografts resistant to ED or Tam alone and in combination with LT compared to E2-stimulated controls. (B) MUC4 map showing 8G7 binding epitope and location of qPCR primers. VNTR – variable number tandem repeats; EGF – Epidermal growth factor-ligand-like domains; TM transmembrane domain. (C) MUC4 mRNA expression by qPCR is increased in resistant (R) MCF7/HER2-18 xenograft tumors compared to sensitive (S) tumors and MUC4+ CD18 cells (pos ctrl).
Figure 3B depicts the MUC4 gene organization including multiple EGF-ligand-like repeats in the MUC4β subunit. Quantitative real-time PCR (qPCR) using primers located near one of these EGF-ligand-like repeats revealed increased mRNA levels in the treatment resistant tumors to endocrine alone or with LT when compared to E2-stimulated controls or growth-inhibited tumors harvested after 3 days treatment with ED±LT and Tam+LT (Figure 3C). These elevated MUC4 levels in the resistant tumors were similar to those detected in MUC4-overexpressing CD18/HPAF pancreatic cancer cells (positive control). ED-resistant tumors had a striking 179-fold increase in MUC4 mRNA level compared to sensitive tumors (p<0.001). ED+LT-resistant tumors had a 34-fold increase in MUC4 mRNA compared to sensitive tumors (p<0.01), while Tam+LT-resistant tumors had a 31-fold increase compared to sensitive tumors (p=0.06).
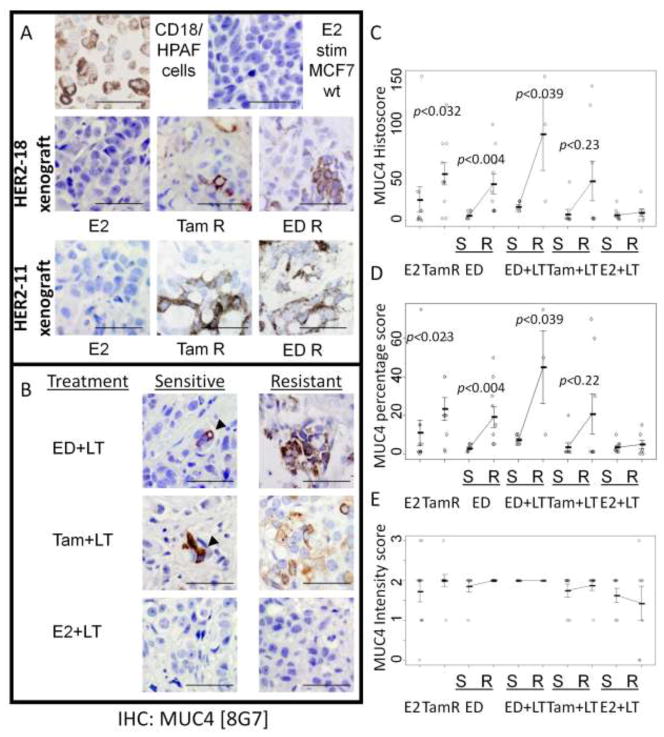
MUC4 protein expression by immunohistochemistry
Using the 8G7 clone of anti-MUC4 (Figure 3B), we detected increased MUC4 (p<0.005) in Tam-stimulated MCF7/HER2-18 tumors in comparison to little to no signal in E2-stimulated MCF7/HER2-18 or MCF7/wildtype tumors (Figure 4A). As expected, the MUC4-overexpressing CD18/HPAF control cells were also strongly IHC-positive. MUC4 expression was also increased in the ED-resistant tumors when compared to sensitive tumors. In most cases, MUC4 staining was mostly intracellular with scant membranous staining, which was similar to staining of CD18/HPAF cells. To rule out clonal effects, we assayed MUC4 expression by IHC in xenograft tumors established from a different HER2-overexpressing MCF7 clone (MCF7/HER2-11, [13]) that were Tam-stimulated, ED-resistant, or E2-stimulated. Similar to our results in the MCF7/HER2-18 clone, we detected upregulation of MUC4 protein in Tam-stimulated and ED-resistant tumors but not E2-stimulated tumors (Figure 4B). Mimicking the mRNA results, higher levels of MUC4 protein were also found in ED+LT and Tam+LT resistant MCF7/HER2-18 tumors but not in tumors in the therapy-sensitive stage. In contrast, in E2+ LT treated tumors, both sensitive and resistant tumors had little to no MUC4 expression.
Figure 4.
MUC4 immunohistochemistry. (A) Both Tam-stimulated and ED-resistant tumors have increased MUC4 protein levels by IHC when compared to E2-stimulated tumors grown from MCF7/HER2-18, MCF7/HER2-11, or MCF7 wild-type (WT) cells. The MUC4 patterns are similar to CD18 cells. (B) ED+LT and Tam+LT but not E2+LT resistance is associated with increased MUC4 protein expression compared to tumors still sensitive to therapy. Bar – 50 μm. (C) Resistant tumors have increased Histoscores versus sensitive tumors. (D) Percentage scores are increased between sensitive and resistant phases, but (E) intensity scores are similar.
Overall, the histoscores of ED and ED+LT resistant tumors were higher than sensitive tumors (p <0.01, p<0.04 respectively), but not in E2+LT treated tumors (Figure 4C). Sensitive tumors tended to have low percentages (<10%) of MUC4 staining that were observed as occasional clusters of MUC4-positive cells (Figure 4D). Interestingly, these focal regions of positivity had moderate to very intense staining similar to those found in resistant tumors (Figures 4D & 4E).
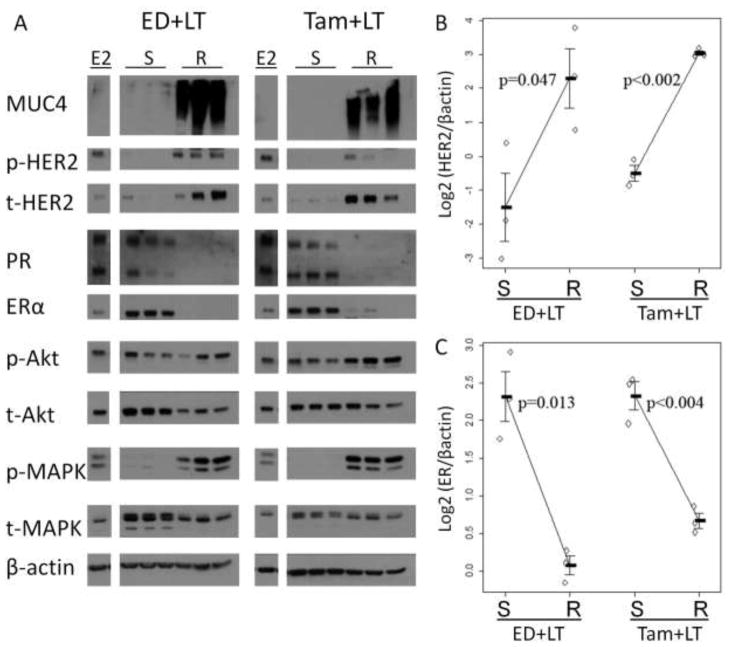
Molecular signaling of MCF7/HER2-18 resistant tumors
To further understand the signaling pathways activated in these resistant mucinated tumors, we used Western blotting analysis of protein extracts from the same Tam+LT and ED+LT tumors used for qPCR analysis (Figure 5A). Tumors in the growth-inhibited sensitive phase of both treatments showed lower MUC4, p-HER2, total HER2, and p-MAPK protein levels, but markedly increased ER levels compared to E2-stimulated controls. Protein levels of the ER-dependent gene product progesterone receptor (PR) were lower in ED±LT-sensitive tumors, an expected result in an estrogen-deprived environment.
Figure 5.
Molecular signaling of MCF7/HER2-18 resistant tumors. Western blotting (A) shows that tumors resistant to ED+LT or Tam+LT have increased MUC4, phospho- and total HER2 protein levels (B), with decreased ER protein levels (C) when compared to sensitive tumors. Downstream signaling is also affected, with increased phospho-MAPK and decreased progesterone receptor (PR) expression. All blots were run concurrently with the same exposure per marker. They are shown separately for presentation purposes.
However, once these tumors acquire resistance, their molecular profile shifted. Resistant tumors have higher HER2 protein levels (p-values=0.047 and <0.002 for ED+LT and Tam+LT respectively) (Figure 5B), and striking increases of MUC4, p-HER2, and p-MAPK. p-Akt levels were also higher in Tam+LT-resistant tumors. Resistant tumors had markedly decreased ER levels in Tam+LT resistant tumors, with ER almost undetectable in ED+LT resistant tumors (p-value=0.013 and p<0.004 respectively, Figure 5C) with PR protein expression similar to results in tumors resistant to ED alone [11].
Co-staining of MUC4 and HER2
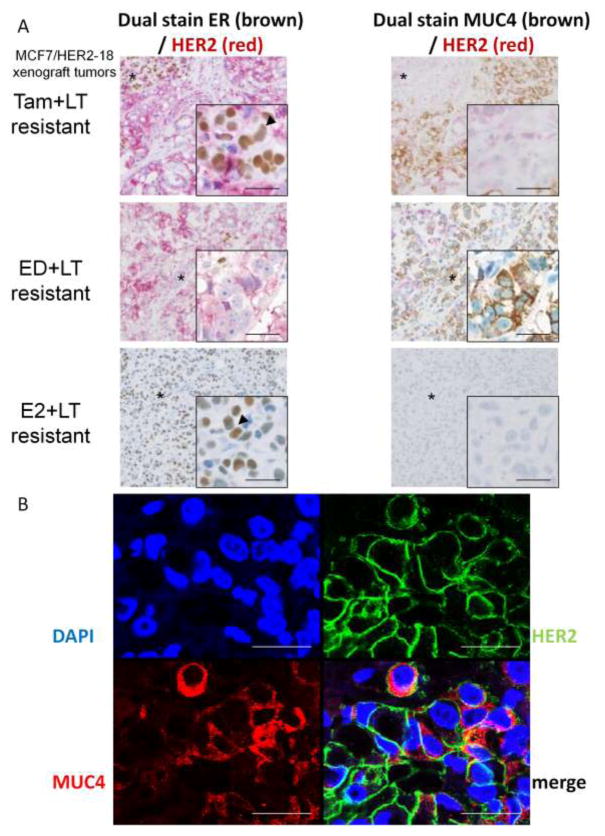
Since this molecular shift from ER-positive to ER-negative and upregulation of HER2 in resistant tumors could be associated with MUC4 expression, we investigated co-expression of MUC4 and HER2 in these tumors. We first co-stained by IHC serial sections of Tam+LT, ED+LT, and E2+LT resistant tumors for both ER/HER2 and MUC4/HER2. In Tam+LT resistant tumors, the majority of cells showed an inverse relationship between ER and MUC4. ER-negative regions were strongly HER2-positive and MUC4-positive, while a small focus of ER-positive cells in this tumor had modest HER2 expression and lacked MUC4 expression (Figure 6A). ED+LT resistant tumors, which completely lose ER expression, have more homogenous expression of MUC4 in HER2-positive regions. In contrast, E2 + LT resistant tumors, which have higher ER and lower HER2 levels, completely lack MUC4 expression (Figure 6A). We further investigated MUC4 and HER2 co-expression at the cellular level with immunofluorescence and confocal microscopy in ED+LT resistant tumors. There was a substantial fraction of HER2-positive cells that also express MUC4 (Figure 6B).
Figure 6.
Co-staining for ER/HER2 and MUC4/HER2 in serial sections of MCF7/HER2-18 xenograft tumors. (A) Tam+LT resistant tumors demonstrate heterogeneity of ER expression; MUC4 is absent in ER-positive tumor regions (arrowhead) but highly expressed in ER-negative regions. ED+LT resistant tumors, which have no ER, have more homogeneous expression of MUC4, while E2+LT resistant tumors retain ER (arrowhead) and lack MUC4. (B) Co-immunofluorescence of MUC4 (red) and HER2 (green) in ED+LT resistant xenograft tumor shows some co-expression in tumor cells. Bar – 25 μm.
DISCUSSION
In our study, resistance to ER and HER2-targeted therapies in ER-positive/HER2-overexpressing MCF7/HER2-18 xenograft tumors is associated with upregulation of mucin-filled vacuoles. This is the first breast cancer model to show endogenous upregulation of mucins, and especially MUC4, in response to therapy. The mucin family of genes has been hypothesized to be associated with drug resistance in cancer. While normally found in the epithelium of the GI tract and respiratory tree, mucin expression in common in multiple cancers [26]. One mucin in particular, mucin4 (MUC4) is overexpressed in various cancers [27–35]. There are a few studies of MUC4 in breast cancer, although recent preclinical data suggest that MUC4 regulates tumor cell survival and metastasis [36,37]. The rat form of MUC4 can form a potent signaling complex with HER2 through EGF-ligand-like domains [38,39]. MUC4 is thought to have two potential mechanisms of HER2-resistance: enhancement of HER2-HER3 signaling [40,41] or interference with trastuzumab binding [42]. Studies in pancreatic, gall bladder and melanoma cancer cell lines suggest that HER2 and MUC4 form a complex in HER2 non-overexpressing cell lines [24,39,43].
Most of these previous studies have focused on MUC4 expression in vitro. While we found MUC4 and HER2 co-expression in our xenograft tumors, MUC4 expression was mostly cytoplasmic and did not stain most mucin vacuoles. While we focused this study on MUC4, other mucins were also observed to be upregulated, including secreted mucins that may stain the mucin vacuoles. A common trigger, such as stress response or cytokine signaling, which has been previously reported to upregulate MUC4 expression in cell lines [44–48], may be responsible for the mucinated phenotype.
MUC4 regulation in breast cancer is not well understood. The MUC4-expressing cell line JIMT-1 has low levels of HER2 expression with low p-HER2. Another study suggests that MUC4 is downstream of MAPK signaling, functioning through the Ets transcription factor PEA3 [49]. Interestingly, while we did not detect an increase in PEA3 by microarray, two Ets-related factors (ELF1 and ETV6) were upregulated in resistant tumors [15]. High HER2 activity activates MAPK; Tam+LT and ED+LT-treated tumors had activation of HER2 and MAPK in resistant tumors compared to sensitive tumors. Tumors treated with E2+LT have levels of MUC4 similar to E2-stimulated controls, showing that LT alone is insufficient to induce MUC4 expression and suggesting that the mucinated phenotype is not found when estrogen signaling remains active.
Our data suggests that ER may instead have a repressive role, as loss of ER coincides with increased MUC4. Alternatively, increased growth factor signaling decreases ER expression and activity [50,51], and mucin upregulation could be related to HER2 reactivation and MAPK signaling. Although MCF7 wild-type tumors acquire endocrine resistance by upregulating HER1/HER2 signaling [3,11], they fail to upregulate MUC4. This implies that a threshold HER2 level may be needed to upregulate MUC4 as MCF7/HER2-18 stably overexpresses HER2 48-times higher than MCF7 parental cells [13].
This preclinical model has shown an interesting phenotype associated with anti-HER2 treatment and endocrine resistance. In the ED+LT and Tam+LT therapy-sensitive phase of growth, HER2 is inhibited but ER signaling is still partially active. Conversely, resistance to ED+LT and Tam+LT, similar to ED- and Tam-resistant tumors, is associated with a molecular shift away from ER signaling but with reactivation of HER2. Whether MUC4 overexpression is the cause or a contributor to reactivation of the HER2 pathway and drug resistance is unknown. Nonetheless, based on the known cellular biology of MUC4 there is a conceptual context to investigate MUC4 as a mechanism of resistance. MUC4 has been shown in pancreatic and ovarian cancer cells to increase tumor cell proliferation, motility, and tumorgenicity [52–54]. We have been unable to examine the role of MUC4 in acquired resistance in clinical samples, as resistant tumors from human trials of L+T or ED in the presence of HER2-positive disease are currently unavailable; however, these studies are warranted as samples will become available. In summary, this work describes a novel mucinated phenotype seen in conjunction with a shift in the intimate crosstalk between ER and HER2 resulting in an ER-negative/HER2-positive tumor with reactivation of HER signaling and treatment resistance [3,11,55]. Upon acquiring resistance to therapy, the molecular profile of these tumors exhibits ER plasticity as it changes its phenotype to ER-negative/PR-negative/HER2-positive; the HER2 pathway is reactivated and there is marked upregulation of several mucins, including MUC4.
Acknowledgments
The authors thank Rena Mao and the Baylor Breast Center Pathology Core for technical assistance, Dr. Susan Hilsenbeck for advice on statistical analysis, Dr. Gary Chamness for help with manuscript writing, and Dr. Kermit Carraway for scientific discussion. This work was supported by DOD grant W81XWH-08-1-0264 (to A.C.C.) and NIH SPORE grant P50CA058183 & Cancer Center grant P30CA125123, the EIF/Lee Jeans Breast Cancer Research Program, and Stand Up 2 Cancer (to C.K.O. and R.S.).
Footnotes
DISCLOSURES:
The experiments described in this study comply with the current laws of the country in which they were performed. The authors declare that they have no relevant conflicts of interest.
References
- 1.Arpino G, Gutierrez C, Weiss H, Rimawi M, Massarweh S, Bharwani L, De Placido S, Osborne CK, Schiff R. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst. 2007;99 (9):694–705. doi: 10.1093/jnci/djk151. 99/9/694 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29 (2):217–233. doi: 10.1210/er.2006-0045. er.2006-0045 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, Weiss H, Rimawi M, Schiff R. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68 (3):826–833. doi: 10.1158/0008-5472.CAN-07-2707. 68/3/826 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Britton DJ, Hutcheson IR, Knowlden JM, Barrow D, Giles M, McClelland RA, Gee JM, Nicholson RI. Bidirectional cross talk between ERalpha and EGFR signalling pathways regulates tamoxifen-resistant growth. Breast Cancer Res Treat. 2006;96 (2):131–146. doi: 10.1007/s10549-005-9070-2. [DOI] [PubMed] [Google Scholar]
- 5.Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, Barrow D, Wakeling AE, Nicholson RI. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144 (3):1032–1044. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 6.Leary AF, Drury S, Detre S, Pancholi S, Lykkesfeldt AE, Martin LA, Dowsett M, Johnston SR. Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin Cancer Res. 2010;16 (5):1486–1497. doi: 10.1158/1078-0432.CCR-09-1764. 1078-0432.CCR-09-1764 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Ellis MJ, Coop A, Singh B, Mauriac L, Llombert-Cussac A, Janicke F, Miller WR, Evans DB, Dugan M, Brady C, Quebe-Fehling E, Borgs M. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol. 2001;19 (18):3808–3816. doi: 10.1200/JCO.2001.19.18.3808. [DOI] [PubMed] [Google Scholar]
- 8.Johnston S, Pippen J, Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ, Press MF, Maltzman J, Florance A, O’Rourke L, Oliva C, Stein S, Pegram M. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27 (33):5538–5546. doi: 10.1200/JCO.2009.23.3734. JCO.2009.23.3734 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova A, Revil C, Jones A. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27 (33):5529–5537. doi: 10.1200/JCO.2008.20.6847. JCO.2008.20.6847 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Osborne CK, Neven P, Dirix LY, Mackey JR, Robert J, Underhill C, Schiff R, Gutierrez C, Migliaccio I, Anagnostou VK, Rimm DL, Magill P, Sellers M. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin Cancer Res. 2011;17 (5):1147–1159. doi: 10.1158/1078-0432.CCR-10-1869. 1078-0432.CCR-10-1869 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massarweh S, Osborne CK, Jiang S, Wakeling AE, Rimawi M, Mohsin SK, Hilsenbeck S, Schiff R. Mechanisms of tumor regression and resistance to estrogen deprivation and fulvestrant in a model of estrogen receptor-positive, HER-2/neu-positive breast cancer. Cancer Res. 2006;66 (16):8266–8273. doi: 10.1158/0008-5472.CAN-05-4045. 66/16/8266 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Rimawi MF, Wiechmann LS, Wang Y-C, Huang C, Migliaccio I, Wu M, Gutierrez C, Hilsenbeck S, Arpino G, Massarweh S, Ward RM, Soliz RD, Osborne CK, Schiff R. Reduced Dose and Intermittent Treatment with Lapatinib and Trastuzumab for Potent Blockade of the HER Pathway in HER-2/neu Overexpressing Breast Tumor Xenografts. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osborne CK. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24 (2):85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 14.Moniaux N, Varshney GC, Chauhan SC, Copin MC, Jain M, Wittel UA, Andrianifahanana M, Aubert JP, Batra SK. Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cells in humans. J Histochem Cytochem. 2004;52 (2):253–261. doi: 10.1177/002215540405200213. [DOI] [PubMed] [Google Scholar]
- 15.Creighton CJ, Massarweh S, Huang S, Tsimelzon A, Hilsenbeck SG, Osborne CK, Shou J, Malorni L, Schiff R. Development of resistance to targeted therapies transforms the clinically associated molecular profile subtype of breast tumor xenografts. Cancer Res. 2008;68 (18):7493–7501. doi: 10.1158/0008-5472.CAN-08-1404. 68/18/7493 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saldanha AJ. Java Treeview--extensible visualization of microarray data. Bioinformatics. 2004;20 (17):3246–3248. doi: 10.1093/bioinformatics/bth349. bth349 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Hammerich-Hille S, Kaipparettu BA, Tsimelzon A, Creighton CJ, Jiang S, Polo JM, Melnick A, Meyer R, Oesterreich S. SAFB1 mediates repression of immune regulators and apoptotic genes in breast cancer cells. J Biol Chem. 2010;285 (6):3608–3616. doi: 10.1074/jbc.M109.066431. M109.066431 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25 (4):402–408. doi: 10.1006/meth.2001.1262. S1046-2023(01)91262-9 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, Kornblau SM. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5 (10):2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. 5/10/2512 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52 (3–4):591–611. [Google Scholar]
- 21.Mann HB, Whitney DR. On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other. Ann Math Statist. 1947;18 (1):50–60. [Google Scholar]
- 22.Team RDC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2010. [Google Scholar]
- 23.Steinbrecher JS, Silverberg SG. Signet-ring cell carcinoma of the breast. The mucinous variant of infiltrating lobular carcinoma? Cancer. 1976;37 (2):828–840. doi: 10.1002/1097-0142(197602)37:2<828::aid-cncr2820370231>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24.Chaturvedi P, Singh AP, Chakraborty S, Chauhan SC, Bafna S, Meza JL, Singh PK, Hollingsworth MA, Mehta PP, Batra SK. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008;68 (7):2065–2070. doi: 10.1158/0008-5472.CAN-07-6041. 68/7/2065 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carraway KL, Perez A, Idris N, Jepson S, Arango M, Komatsu M, Haq B, Price-Schiavi SA, Zhang J, Carraway CA. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, in cancer and epithelia: to protect and to survive. Prog Nucleic Acid Res Mol Biol. 2002;71:149–185. doi: 10.1016/s0079-6603(02)71043-x. [DOI] [PubMed] [Google Scholar]
- 26.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4 (1):45–60. doi: 10.1038/nrc1251. nrc1251 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Karg A, Dinc ZA, Basok O, Ucvet A. MUC4 expression and its relation to ErbB2 expression, apoptosis, proliferation, differentiation, and tumor stage in non-small cell lung cancer (NSCLC) Pathol Res Pract. 2006;202 (8):577–583. doi: 10.1016/j.prp.2006.04.002. S0344-0338(06)00099-9 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Kwon KY, Ro JY, Singhal N, Killen DE, Sienko A, Allen TC, Zander DS, Barrios R, Haque A, Cagle PT. MUC4 expression in non-small cell lung carcinomas: relationship to tumor histology and patient survival. Arch Pathol Lab Med. 2007;131 (4):593–598. doi: 10.5858/2007-131-593-MEINCL. 2005-723-OA [pii] [DOI] [PubMed] [Google Scholar]
- 29.Tamada S, Shibahara H, Higashi M, Goto M, Batra SK, Imai K, Yonezawa S. MUC4 is a novel prognostic factor of extrahepatic bile duct carcinoma. Clin Cancer Res. 2006;12 (14 Pt 1):4257–4264. doi: 10.1158/1078-0432.CCR-05-2814. 12/14/4257 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Tsutsumida H, Goto M, Kitajima S, Kubota I, Hirotsu Y, Wakimoto J, Batra SK, Imai K, Yonezawa S. MUC4 expression correlates with poor prognosis in small-sized lung adenocarcinoma. Lung Cancer. 2007;55 (2):195–203. doi: 10.1016/j.lungcan.2006.10.013. S0169-5002(06)00590-3 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Chauhan SC, Singh AP, Ruiz F, Johansson SL, Jain M, Smith LM, Moniaux N, Batra SK. Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125) Mod Pathol. 2006;19 (10):1386–1394. doi: 10.1038/modpathol.3800646. 3800646 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Munro EG, Jain M, Oliva E, Kamal N, Lele SM, Lynch MP, Guo L, Fu K, Sharma P, Remmenga S, Growdon WB, Davis JS, Rueda BR, Batra SK. Upregulation of MUC4 in cervical squamous cell carcinoma: pathologic significance. Int J Gynecol Pathol. 2009;28 (2):127–133. doi: 10.1097/PGP.0b013e318184f3e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh AP, Chauhan SC, Bafna S, Johansson SL, Smith LM, Moniaux N, Lin MF, Batra SK. Aberrant expression of transmembrane mucins, MUC1 and MUC4, in human prostate carcinomas. Prostate. 2006;66 (4):421–429. doi: 10.1002/pros.20372. [DOI] [PubMed] [Google Scholar]
- 34.Miyahara N, Shoda J, Ishige K, Kawamoto T, Ueda T, Taki R, Ohkohchi N, Hyodo I, Thomas MB, Krishnamurthy S, Carraway KL, Irimura T. MUC4 interacts with ErbB2 in human gallbladder carcinoma: potential pathobiological implications. Eur J Cancer. 2008;44 (7):1048–1056. doi: 10.1016/j.ejca.2008.03.007. S0959-8049(08)00234-7 [pii] [DOI] [PubMed] [Google Scholar]
- 35.Andrianifahanana M, Moniaux N, Schmied BM, Ringel J, Friess H, Hollingsworth MA, Buchler MW, Aubert JP, Batra SK. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7 (12):4033–4040. [PubMed] [Google Scholar]
- 36.Workman HC, Miller JK, Ingalla EQ, Kaur RP, Yamamoto DI, Beckett LA, Young LJ, Cardiff RD, Borowsky AD, Carraway KL, Sweeney C, Carraway KL., 3rd The membrane mucin MUC4 is elevated in breast tumor lymph node metastases relative to matched primary tumors and confers aggressive properties to breast cancer cells. Breast Cancer Res. 2009;11(5):R70. doi: 10.1186/bcr2364. bcr2364 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Workman HC, Sweeney C, Carraway KL., 3rd The membrane mucin Muc4 inhibits apoptosis induced by multiple insults via ErbB2-dependent and ErbB2-independent mechanisms. Cancer Res. 2009;69 (7):2845–2852. doi: 10.1158/0008-5472.CAN-08-2089. 0008-5472.CAN-08-2089 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramsauer VP, Pino V, Farooq A, Carothers Carraway CA, Salas PJ, Carraway KL. Muc4-ErbB2 complex formation and signaling in polarized CACO-2 epithelial cells indicate that Muc4 acts as an unorthodox ligand for ErbB2. Mol Biol Cell. 2006;17 (7):2931–2941. doi: 10.1091/mbc.E05-09-0895. E05-09-0895 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yokoyama A, Shi BH, Kawai T, Konishi H, Andoh R, Tachikawa H, Ihara S, Fukui Y. Muc4 is required for activation of ErbB2 in signet ring carcinoma cell lines. Biochem Biophys Res Commun. 2007;355 (1):200–203. doi: 10.1016/j.bbrc.2007.01.133. S0006-291X(07)00188-X [pii] [DOI] [PubMed] [Google Scholar]
- 40.Carraway KL, 3rd, Rossi EA, Komatsu M, Price-Schiavi SA, Huang D, Guy PM, Carvajal ME, Fregien N, Carraway CA, Carraway KL. An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J Biol Chem. 1999;274 (9):5263–5266. doi: 10.1074/jbc.274.9.5263. [DOI] [PubMed] [Google Scholar]
- 41.Funes M, Miller JK, Lai C, Carraway KL, 3rd, Sweeney C. The mucin Muc4 potentiates neuregulin signaling by increasing the cell-surface populations of ErbB2 and ErbB3. J Biol Chem. 2006;281 (28):19310–19319. doi: 10.1074/jbc.M603225200. M603225200 [pii] [DOI] [PubMed] [Google Scholar]
- 42.Nagy P, Friedlander E, Tanner M, Kapanen AI, Carraway KL, Isola J, Jovin TM. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65 (2):473–482. 65/2/473 [pii] [PubMed] [Google Scholar]
- 43.Price-Schiavi SA, Jepson S, Li P, Arango M, Rudland PS, Yee L, Carraway KL. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer. 2002;99 (6):783–791. doi: 10.1002/ijc.10410. [DOI] [PubMed] [Google Scholar]
- 44.Mejias-Luque R, Peiro S, Vincent A, Van Seuningen I, de Bolos C. IL-6 induces MUC4 expression through gp130/STAT3 pathway in gastric cancer cell lines. Biochim Biophys Acta. 2008;1783 (10):1728–1736. doi: 10.1016/j.bbamcr.2008.05.020. S0167-4889(08)00193-6 [pii] [DOI] [PubMed] [Google Scholar]
- 45.Andrianifahanana M, Singh AP, Nemos C, Ponnusamy MP, Moniaux N, Mehta PP, Varshney GC, Batra SK. IFN-gamma-induced expression of MUC4 in pancreatic cancer cells is mediated by STAT-1 upregulation: a novel mechanism for IFN-gamma response. Oncogene. 2007;26 (51):7251–7261. doi: 10.1038/sj.onc.1210532. 1210532 [pii] [DOI] [PubMed] [Google Scholar]
- 46.Andrianifahanana M, Agrawal A, Singh AP, Moniaux N, van Seuningen I, Aubert JP, Meza J, Batra SK. Synergistic induction of the MUC4 mucin gene by interferon-gamma and retinoic acid in human pancreatic tumour cells involves a reprogramming of signalling pathways. Oncogene. 2005;24 (40):6143–6154. doi: 10.1038/sj.onc.1208756. 1208756 [pii] [DOI] [PubMed] [Google Scholar]
- 47.Damera G, Xia B, Ancha HR, Sachdev GP. IL-9 modulated MUC4 gene and glycoprotein expression in airway epithelial cells. Biosci Rep. 2006;26 (1):55–67. doi: 10.1007/s10540-006-9000-5. [DOI] [PubMed] [Google Scholar]
- 48.Damera G, Xia B, Sachdev GP. IL-4 induced MUC4 enhancement in respiratory epithelial cells in vitro is mediated through JAK-3 selective signaling. Respir Res. 2006;7:39. doi: 10.1186/1465-9921-7-39. 1465-9921-7-39 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez A, Barco R, Fernandez I, Price-Schiavi SA, Carraway KL. PEA3 transactivates the Muc4/sialomucin complex promoter in mammary epithelial and tumor cells. J Biol Chem. 2003;278 (38):36942–36952. doi: 10.1074/jbc.M300264200. M300264200 [pii] [DOI] [PubMed] [Google Scholar]
- 50.Guo S, Sonenshein GE. Forkhead box transcription factor FOXO3a regulates estrogen receptor alpha expression and is repressed by the Her-2/neu/phosphatidylinositol 3-kinase/Akt signaling pathway. Mol Cell Biol. 2004;24 (19):8681–8690. doi: 10.1128/MCB.24.19.8681-8690.2004. 24/19/8681 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Creighton CJ, Fu X, Hennessy BT, Casa AJ, Zhang Y, Gonzalez-Angulo AM, Lluch A, Gray JW, Brown PH, Hilsenbeck SG, Osborne CK, Mills GB, Lee AV, Schiff R. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 2010;12(3):R40. doi: 10.1186/bcr2594. bcr2594 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moniaux N, Chaturvedi P, Varshney GC, Meza JL, Rodriguez-Sierra JF, Aubert JP, Batra SK. Human MUC4 mucin induces ultra-structural changes and tumorigenicity in pancreatic cancer cells. Br J Cancer. 2007;97 (3):345–357. doi: 10.1038/sj.bjc.6603868. 6603868 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ponnusamy MP, Singh AP, Jain M, Chakraborty S, Moniaux N, Batra SK. MUC4 activates HER2 signalling and enhances the motility of human ovarian cancer cells. Br J Cancer. 2008;99 (3):520–526. doi: 10.1038/sj.bjc.6604517. 6604517 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ponnusamy MP, Lakshmanan I, Jain M, Das S, Chakraborty S, Dey P, Batra SK. MUC4 mucin-induced epithelial to mesenchymal transition: a novel mechanism for metastasis of human ovarian cancer cells. Oncogene. 2010 doi: 10.1038/onc.2010.309. onc2010309 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96 (12):926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]