Abstract
In order to investigate differences in the effects of spatial attention between the left visual field (LVF) and the right visual field (RVF), we employed a full/poor attention paradigm using stimuli presented in the LVF vs. RVF. In addition, to investigate differences in the effects of spatial attention between the Dorsal and Ventral processing streams, we obtained motion thresholds (motion coherence thresholds and fine direction discrimination thresholds) and orientation thresholds, respectively. The results of this study showed negligible effects of attention on the orientation task, in either the LVF or RVF. In contrast, for both motion tasks, there was a significant effect of attention in the LVF, but not in the RVF. These data provide psychophysical evidence for greater effects of spatial attention in the LVF/right hemisphere, specifically, for motion processing in the Dorsal stream.
Keywords: spatial attention, laterality, visual field asymmetries, dorsal/ventral, motion, orientation
INTRODUCTION
Many studies have compared performance for different visual tasks and stimuli presented in the left visual field (LVF) vs. right visual field (RVF), with the assumption that lateralized performance reflects the processing capacities of the right vs. left cerebral hemispheres, respectively (see Christman & Niewbauer, 1997; Davidson & Hugdahl, 1998 for reviews). Some of the visual field asymmetries observed in performance have been attributed to asymmetries in retinotopic organization across the visual field due to anisotropies in neural density of ganglion cell layers (Perry & Cowey, 1985), lateral geniculate nucleus (Connolly & Van, 1984), or striate cortex (Tootell, Silverman, Hamilton, Switkes, & De Valois, 1988; Van Essen, Newsome, & Maunsell, 1984), however, there is also ample evidence that the two hemispheres differ in spatial attention abilities.
The strongest evidence that the two hemispheres differ in spatial attention comes from the clinical literature. It is well documented that visual inattention and visual hemifield neglect are more commonly associated with, and more severe or persistent for, right, as compared to left, hemisphere parietal lobe lesions (Becker & Karnath, 2007; Bowen, McKenna, & Tallis, 1999; Heilman, Watson, & Valenstein, 1985; Ringman, Saver, Woolson, Clarke, & Adams, 2004; Schenkenberg, Bradford, & Ajax, 1980, and see Heilman, Pandya, & Geschwind, 1970; Lynch, 1980 for similar results from monkey lesion studies). For example, after right hemisphere damage, patients have trouble reporting the leftmost letters in a letter string and trouble with visual search in the left half of the visual field, tend to bisect horizontal lines too far to the right, and fail to copy the left half of an image (for a review, see Plummer, Morris, & Dunai, 2003). Also supporting the notion that the right hemisphere has a greater role in spatial attention are data from split-brain patients, who show poor spatial vigilance when stimuli are presented in the RVF, i.e., left hemisphere, yet intact spatial vigilance when stimuli are presented in the LVF, i.e., right hemisphere (Coslett, Bowers, Fitzpatrick, Haws, & Heilman, 1990; Dimond, 1979; Dimond & Beaumont, 1973; Heilman et al., 1985; Proverbio, Zani, Gazzaniga, & Mangun, 1994; Wilkins, Shallice, & McCarthy, 1987). One notion that has been used to explain the differential effects of right vs. left hemisphere damage is that while the right hemisphere can direct attention to both the contralateral LVF and the ispilateral RVF, the left hemisphere has a strong bias to direct attention to the contralateral RVF. By this account, after left hemisphere damage, the intact right hemisphere is able to mediate spatial attention in both visual hemifields, whereas the converse is not true after right hemisphere damage (Heilman & Van Den Abell, 1980; Mesulam, 1999, and see Kinsbourne, 1977; Szczepanski, Konen, & Kastner, 2010 for an alternative explanation based on interhemispheric competition). The evidence that these hemispheric asymmetries are attentional, and not related to detection per se, is based on studies showing that patients with right hemisphere damage can compensate for the LVF neglect when they are instructed to actively attend to the LVF (e.g., Riddoch & Humphreys, 1983).
Another avenue of research that has addressed hemispheric differences in spatial attention are brain imaging studies in healthy humans, using positron emission tomography (PET), functional magnetic resonance imaging (fMRI), and event-related potentials (ERPs). Although results are somewhat mixed, in general, like the results from clinical studies, brain imaging studies tend to support a right hemisphere dominance for spatial attention. Hemispheric asymmetries in spatial attention have been addressed by measuring left and right hemisphere activity in response to the same stimulus when it is attended vs. ignored, with larger responses in the attended condition indicating an attention effect. These studies have been conducted by using either full field, or lateralized (LVF vs. RVF), stimuli. In studies using full field stimuli, effects of manipulating spatial attention have been reported to be greater in the right, than the left, hemisphere (PET: Corbetta, Miezin, Shulman, & Petersen, 1993; Nobre et al., 1997; fMRI: Arrington, Carr, Mayer, & Rao, 2000; Gitelman et al., 1999; Husain & Rorden, 2003, and Corbetta, Kincade, Ollinger, McAvoy, & Shulman, 2000 for reorienting to unexpected stimuli). However, some studies have reported more equal hemispheric effects of spatial attention (fMRI: Hopfinger, Buonocore, & Mangun, 2000; Kastner, Pinsk, De Weerd, Desimone, & Ungerleider, 1999; Shulman et al., 2010; and see Corbetta et al., 2000 for top-down voluntary attention effects).
In line with the bulk of the studies using full field stimuli, in studies using lateralized stimuli, spatial attentional effects have been reported to be greater in the right hemisphere (in response to LVF stimuli) than in the left hemisphere (in response to RVF stimuli) (fMRI: Szczepanski et al., 2010; PET: Pardo, Fox, & Raichle, 1991; ERPs: Luck & Hillyard, 1994; Neville & Lawson, 1987; Heilman & Van Den Abell, 1980; but cf. Hillyard & Anllo-Vento, 1998). In addition, there is evidence that whereas the left hemisphere represents mainly the contralateral RVF, the right hemisphere represents both the contralateral LVF and the ipsilateral RVF. For example, fMRI studies have reported regions in the right, but not left, parietal cortex that reveal greater activation due to spatial attention (Szczepanski et al., 2010), and the right hemisphere exhibits attention effects to stimuli presented in both the contralateral LVF and the ipsilateral RVF (Siman-Tov et al., 2007). These findings are in line with theories explaining why there is greater spatial neglect after right hemisphere lesions (Heilman & Van Den Abell, 1980; Mesulam, 1999; see above).
A final avenue of research that has addressed hemispheric differences in spatial attention is visual psychophysical attention studies using lateralized stimuli in healthy humans. In contrast to the results from clinical studies in brain-damaged patients and brain imaging studies in healthy humans, the bulk of these psychophysical studies do not strongly support a right hemisphere dominance for spatial attention. One way in which hemispheric differences in spatial attention have been addressed in psychophysical studies involves measuring the ability to sustain spatial attention. In line with the notion of a right hemisphere advantage in spatial attention, several studies have reported that it is easier to maintain attentional vigilance to stimuli in the LVF than in the RVF (Dimond & Beaumont, 1973; Whitehead, 1991). By contrast, other psychophysical studies, using spatial cueing, suggest no difference in spatial attention between the right and left hemispheres. These cueing studies investigate whether visual performance is enhanced by the presence (vs. absence) of a pre-cue alerting the subject to the location of a to-be-presented stimulus, and whether this differs between the LVF vs. RVF (and across space in general). Two studies have reported that there are no differences between LVF vs. RVF (or anywhere across the visual field) in the effects of cueing on a variety of measures of orientation discrimination performance, including thresholds, accuracy, speed, and slopes of psychometric functions (Carrasco, Talgar, & Cameron, 2001, Cameron, Tai, & Carrasco, 2002). Likewise, two studies, one that measured motion coherence thresholds (Bosworth & Dobkins, 2002), and another that measured motion and orientation thresholds (Rezec & Dobkins, 2004), found no difference in the effect of a pre-cue for stimuli in the LVF vs. RVF (and no differences in the effects of pre-cue on superior vs. inferior visual field stimuli). In sum, while the results from clinical studies in brain-damaged patients and brain imaging studies in healthy humans suggest a right hemisphere bias for spatial attention, data from psychophysical studies using lateralized stimuli have been far less supportive.
In the current study, we revisited the possibility for hemispheric differences in spatial attention revealed psychophysically by using a paradigm that yields large attention effects (greater than that seen with pre-cueing, for example), which, in turn, may be more effective in revealing hemifield differences in spatial attention. To this end, we employed a dual-task paradigm, where discrimination thresholds were measured under conditions of full- vs. poor-attention. In the full attention condition, subjects performed only the main discrimination task, allowing the task to be conducted under full attentional allocation. In the poor attention condition, subjects performed a dual-task, i.e., they performed the main discrimination task while also performing another attentionally demanding task at the central fixation spot. In this dual task condition, the amount of attention allocated to the main task is expected to be reduced. We and others have previously shown that this paradigm yields large attention effects, on the order of 3- to 9-fold (Bonnel & Miller, 1994; Bonnel, Possamai, & Schmitt, 1987; Braun, 1994; Braun & Sagi, 1990, 1991; Huang & Dobkins, 2005; Lee, Koch, & Braun, 1997, 1999; Sperling & Melchner, 1978, also relevant are tasks that require concurrent discriminations of different features on the same object, Duncan, 1984).
The current study tested two main types of stimuli/tasks: motion discrimination and orientation discrimination. We chose to use these two different stimuli/tasks as a way to ask whether effects of attention, and/or the interaction between attention and hemifield, differed between tasks that are thought to rely predominantly on activity within the Dorsal visual processing stream (i.e., motion tasks) vs. those that are thought to rely predominantly on activity within the Ventral visual processing stream (i.e., orientation tasks), as effects of spatial attention have been reported in both streams (see Ungerleider, 2004 and Desimone & Duncan, 1995 for reviews).
METHODS
Subjects
Nine subjects (mean age = 22.2 years, S.D. = 1.0; four males) participated in this study. All subjects reported a dominant right hand, and normal or corrected-to-normal vision. Participants were recruited from the student population at University of California, San Diego as well as from the local San Diego community.
Apparatus
Visual stimuli were generated on a Dell PC laptop with an ATI Radeon graphics card and displayed on a 21-inch SONY monitor (refresh rate = 60 Hz). Stimuli were created using a PC version of Matlab (version 6.5), and calibrated using a Photo Research PR-650 spectrometer. For each subject, eye position was monitored using a closed couple device (CCD) infrared camera with variable focus (12.5–75 mm) lens (Model #Fc62, Image Sensor), which was focused on the left eye of the subject. The subjects’ face was lit with an infrared illuminator and an enlarged image of the eye was viewed on a 12″ Monitor (Ultrak) outside the testing room. Before beginning each block of trials, subjects were instructed to fixate a black fixation square (0.9° × 0.9°) in the center of the video display, and the outline of the pupil was drawn on transparency film that covered the monitor. Previous experiments in our laboratory have shown that this set-up allows for the easy detection of saccadic eye movements and eye drift within ± 2 degrees of fixation (Dobkins & Bosworth, 2001). Subjects were instructed to maintain fixation throughout the experiment and were informed that the experiment would be temporarily interrupted if eye movements or eye drift were detected by the experimenter (author JP), who was watching the eye camera during the experiment. Thus, subjects were highly discouraged from breaking fixation, and the experiment never needed to be interrupted. Although we cannot rule out the possibility that the experimenter did not catch a subject breaking fixation on some trials, we believe strongly that such occurrences (if they existed) cannot account for any observed visual field asymmetries. To account for visual field asymmetries, we would have to suppose that subjects fixated to the right or left of fixation (thereby making the motion/orientation stimulus less eccentric and easier to discriminate for LVF vs. RVF stimuli, respectively), there is no a priori reason to believe this would vary systematically across trials and/or subjects.
Stimuli
Each subject was tested on three main tasks, two of which were motion tasks: (1) motion coherence task, (2) fine direction-of-motion discrimination task, and (3) an orientation discrimination task. Note that the original version of our study had just a single motion task (1), but after obtaining interesting visual field attentional asymmetries on that motion task, we added another motion task to determine whether the effect generalized to other motion tasks. Stimuli for these tasks are shown in Figure 1. All stimuli were presented on a grey background (45.7 cd/m2), within a 5° circular aperture, in either the left visual field (LVF) or the right visual field (RVF), centered 5° eccentric to fixation, and for a duration of 100 msec. We chose this eccentricity based on pilot studies showing that the fine direction-of-motion task and the orientation task were too hard to do if presented further out in the periphery. For the two motion tasks (tasks 1 and 2), a stochastic motion stimulus was employed (modeled after Newsome & Paré, 1988; and see Bosworth & Dobkins, 2002). This stimulus consisted of a field of 300 white dots (each 0.04 degrees in diameter, 95.8 cd/m2, 35.4% Michelson contrast compared to the background) wherein a proportion of “signal” dots moved in a coherent direction while the remaining “noise” dots moved in a random fashion. The trajectory for each signal dot lasted for a duration, on average, of 67 msec (3 frames), after which it disappeared and then reappeared in a random location within the circular aperture, moved coherently for another 67 msec, and so on. Noise dots were positioned in a random location from frame to frame.
Figure 1.
Stimulus for each task. a) Motion coherence task, for which a proportion of the dots moved coherently (3 – 67%), and subjects discriminated the direction of these coherent dots (upward vs. downward). b) Fine direction-of-motion task, for which 67% of the dots moved coherently, and subjects discriminated their direction (tilted leftward vs. rightward of downward motion). c) Orientation task, for which a static 1% contrast sinusoidal grating was presented and subjects discriminated its orientation (tilted slightly to the left or right of vertical).
In the motion coherence task (task 1), the signal dots moved upward or downward, and the percentage of signal dots vs. noise dots varied across trials in a staircase design (see below), from 3.3% to 67% coherence, in order to obtain motion coherence thresholds. In the fine direction-of-motion discrimination task (task 2), motion coherence (i.e., percent motion signal) was maintained at 67%, while direction of motion varied within ±45 degrees to the left or right of downward motion across trials in a staircase design, in order to obtain direction discrimination thresholds. For the orientation discrimination task (task 3), the stimulus consisted of a static 0.8 cycle/degree sinusoidal grating stimulus, presented at 1% contrast (mean luminance = 45.7 cd/m2). Grating orientation varied by ±45 degrees (i.e., tilted left or right of vertical), across trials in a staircase design, in order to obtain orientation discrimination thresholds.
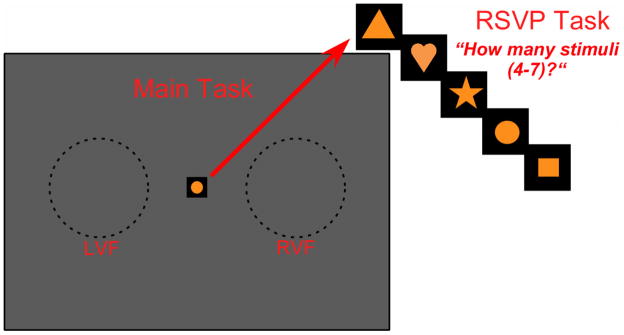
For each main task, a rapid serial visual presentation (RSVP) stimulus was simultaneously presented at fixation, the purpose of which was to modulate the amount of attention paid to the main task (see below). As shown in Figure 2, the RSVP stimulus consisted of a series of five possible orange-colored shapes (triangle, heart, star, circle and square)1 presented within the fixation square, and the order of these shapes was randomized across trials. Each shape was presented for 120 ms, separated by a blank period of 120 msec. A total of four to seven shapes were presented (for a total duration that varied from 840 msec, for four shapes, to 1560 msec, for seven shapes). The main stimulus (moving dots or grating stimulus, see above) was presented simultaneously with the last shape (in the LVF or RVF), for a duration of 100 msec.
Figure 2.
The RSVP stimuli consisted of a rapid succession of 4 to 7 shapes (triangle, heart, star, circle, and square, in random order across trials), each lasting 120 msec, with a 120 msec interstimulus interval in between each shape, presented within the black fixation square. Each main task was tested in the LVF or RVF (within the area shown here as dashed circles), concurrently with the RSVP stimuli. In the poor attention condition, on each trial, subjects counted the number of shapes in the RSVP task, and then reported on the main task in the LVF or RVF.
Procedures
Subjects were tested in a darkened room and viewed the video display binocularly from a chin rest situated 57 cm away. Subjects were instructed to maintain fixation on a small (0.9° × 0.9°) black square in the center of the monitor for the duration of each trial. The first trial was initiated by the subject with a key press, which was followed by the RSVP stimulus presented at fixation, and the main stimulus presented simultaneously with the last shape of the RSVP (see above). Upon disappearance of the last RSVP shape/main stimulus, the subject used key presses on a keyboard to enter either one (full attention condition) or two (poor attention condition) responses. The next trial automatically started 1500 msec after the subject response. In the full attention condition, subjects performed only the main task, reporting, in a 2-AFC manner, “left” vs. “right” (motion coherence task), “down-to-the-left” vs. “down-to-the-right” (fine direction-of-motion discrimination task) or “left-tilt” vs. “right-tilt” (orientation discrimination task), using two digits on their left hand. They were told to ignore the irrelevant RSVP at the center of gaze. In the poor attention condition, subjects performed a dual task on each trial. They were required to first, report how many shapes appeared in the RSVP stimulus (ranging from four to seven, so that the task was a 4-AFC), using four digits on their right hand, and second, report on the main task, using their left hand. Because the central RSVP task was very demanding, subjects paid substantially less attention to the main task in this poor attention condition, and, thus, their thresholds in the main task were expected to be higher (i.e., worse) in the poor than in the full attention condition. No feedback was provided in either attention condition.
Each subject was tested under 12 total conditions: 3 main tasks (coherent motion, fine direction-of-motion and orientation), 2 attention conditions (full vs. poor), and 2 visual field locations (LVF vs. RVF). For each main task, the two attention conditions and the two visual fields were presented in blocks, with these blocks in randomized and counterbalanced order across subjects. Subjects completed all four of these conditions for one task before proceeding with another task, and the task order was randomized and counterbalanced across subjects. Before beginning the study, subjects were given practice on the main tasks under full attention, followed by practice on the RSVP task alone, and finally, practice on the main tasks under poor attention. During this practice, subjects’ performance on the RSVP task was required to be consistent and stay above 62.5% correct (which is half way between chance, 25%, and ceiling, 100%).
Adaptive Staircase Procedure for Obtaining Thresholds
In each of the three main tasks, the variable of interest was varied across trials in an adaptive staircase procedure. Specifically, on the first trial, a highly discriminable stimulus was presented (i.e., task 1: dots moving with 56% motion coherence; task 2: dots moving 45 degrees to the left or right of downward; task 3: grating tilted 45 degrees to the left or right of vertical). The value for subsequent trials varied in a 1-down/2-up procedure, based on the Parameter Estimation and Sequential Testing (PEST) method (Taylor, 1967). The value was decreased by one step after a correct response, and was increased by two steps after an incorrect response. The maximum step size for task 1 was multiplicative, a 1.59-fold change. For tasks 2 and 3, we used a linear metric, and the maximum step size was an absolute amount of 4.0 degrees. The value of the step size was determined by an acceleration factor (AC) of 1.5 and a reversal factor (RF) of 1.0. The step size was multiplied by AC, following either two correct or two incorrect responses, and was multiplied by (1/AC)RF following a reversal in correctness. The use of a variable step size allowed more precision than a fixed step size. For each participant, at the end of the experiment, the 125 trials obtained for each task were used to obtain a threshold. (Note that for the poor attention condition, all trials were used, whether or not the subject was correct on the central RSVP task, which follows the analyses of our previous study using the full/poor attention paradigm, Huang & Dobkins, 2005). Specifically, a Weibull function (Weibull, 1951) was fit to the data, using maximum likelihood method (Watson, 1979). Threshold for each task was defined as the value yielding 75% correct performance. Thresholds were then logged, since logarithmic, but not linear, threshold data conform to normal distributions. To look at relative effects, we logged the ratio of linear thresholds, which is identical to subtracting one log threshold from another.
Data Analysis
For each task, we performed two-factor ANOVAs (2 attention conditions x 2 visual field locations). To investigate what drove main effects and interactions, post hoc comparisons were conducted using two log ratio metrics: 1) Attention ratios: calculated as Log (ThresholdPoor/ThresholdFull), with values greater than zero indicating better performance in the Full Attention condition. 2) Laterality ratios: calculated as Log (ThresholdLVF/ThresholdRVF), with values greater than zero indicating better performance in the RVF. Two-tailed t tests were used to determine if each mean ratio was significantly different from zero, and also for comparing attention effects between LVF and RVF, as well as laterality effects between full and poor attention conditions. We also performed ANOVAs on slopes of the psychometric functions and RSVP performance data, explained further in the Results.
RESULTS
Slopes of the Psychometric Functions
As discussed in our previous study of full vs. poor attention (Huang & Dobkins, 2005), because thresholds are predicted to be higher in the poor than the full attention condition, we believe it is important to show that this does not result from subjects being less engaged in the poor attention condition, or from the two tasks (as opposed to a single task) being particularly difficult on memory or motor load in the poor attention condition. In other words, it could be that the RSVP task in the poor attention condition is so difficult that, on some trials, subjects simply decide to disengage entirely from the main task. In this scenario, thresholds on the main task would be elevated without necessarily reflecting impaired discrimination per se. If this were the case, individual psychometric curves for the main task in the poor attention condition should have shallower slopes than those obtained in the full attention condition. To address this possibility, we asked whether attention condition (full vs. poor) affected slopes, by conducting a three-factor ANOVA (task x attention condition x visual field). The results of this ANOVA revealed no significant effects of attention condition on slopes (F(1,7) = 3.69; p = 0.10), nor did attention condition interact with task or visual field. All two-way interactions, p > 0.14, and the three-way interaction were also nonsignificant, p = 0.89, (see footnote 2 for effects of task and visual field on slopes)2. Thus, we can say with some certainty that different thresholds in the poor vs. full attention condition (or between visual fields) reflect mainly differences in low-level discrimination abilities. There is another, perhaps more important, reason why we feel confident that different thresholds in the poor vs. full attention condition cannot be accounted for by differences in general difficulty between the full (single-task) and poor (dual-task) conditions. This is because we found some situations (i.e., when the main task was orientation discrimination, see below) where attention condition had virtually no effect on thresholds. If there were differences in general difficulty between the full and poor attention conditions, we would expect to see significant attention effects across the board, which was not the case.
RSVP Performance
Along a similar line, we also felt it important to entertain the possibility that differences in attention effects between LVF and RVF, or between the three main tasks, could be driven by differential performance on the RSVP task across visual field or task. For example, if subjects performed better on the RSVP task when the stimulus was in the RVF, as compared to the LVF, we might assume that they paid more attention to the RSVP task when the stimulus was in the RVF, and in turn paid relatively less attention to the main task when it was in the RVF, compared to when it was in the LVF. A two-factor ANOVA (task x visual field) revealed no effect of task (F(2,16) = 1.38; p = 0.28) or visual field (F(1,8) = 1.45; p = 0.26) on RSVP accuracy, nor was there an interaction (F(2,16) = 1.42; p = 0.27). Mean accuracy was 85% (SD = 0.16). We therefore conclude that the amount of attention devoted to the RSVP task did not differ across task and visual field.
Group Mean Log Thresholds
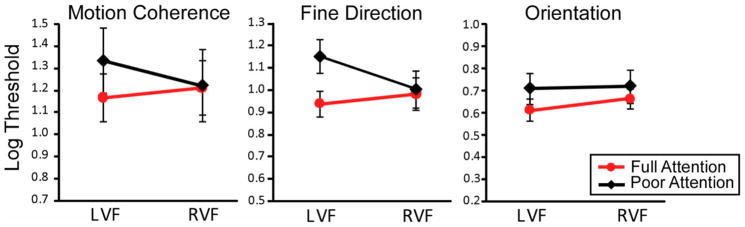
Group mean log thresholds for all 12 conditions--3 main tasks (coherent motion, fine direction-of-motion and orientation), 2 attention conditions (full vs. poor), and 2 visual field locations (LVF vs. RVF) -- are presented in Figure 3. The results of two-factor ANOVAs (attention x visual field), conducted separately for each task, revealed no main effect of visual field on any task (all p values > 0.19)3. With regard to attention, a main effect was revealed on the fine direction-of-motion task only (F(1,8) = 6.78; p = 0.03), which was driven by significantly elevated thresholds (i.e., worse performance) in the poor, than in the full, attention condition. Although the main effect of attention did not reach significance for the motion coherence task (F(1,8) = 2.73; p = 0.14), both motion tasks revealed a significant attention x visual field interaction (motion coherence: (F(1,8) = 5.58; p = 0.045, fine direction-of-motion: F(1,8) = 10.54; p = 0.01), indicating that attention affects motion performance differently for the LVF and RVF. For the orientation task, there was no main effect of attention (F(1,8) = 1.31; p = 0.28), nor was there an interaction between attention and visual field (F(1,8) < 1), which we address later in the Discussion. To investigate further what drove the attention by visual field interactions for the two motion tasks, data are presented and analyzed as attention ratios and laterality ratios, below.
Figure 3.
Group mean log thresholds for each task under full attention condition (red lines) and poor attention condition (black lines) for nine subjects. Error bars denote standard error of the mean (±SEM).
Attention and Laterality Ratios
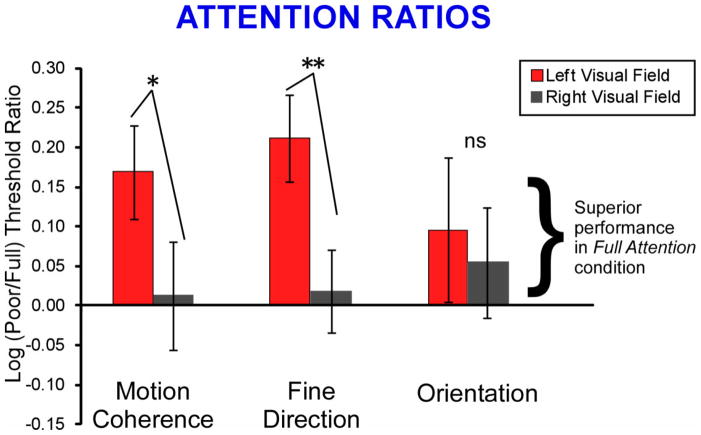
Group mean log attention ratios, for each task and each visual field, are presented in Figure 4. Values greater than zero indicate lower thresholds (i.e., better performance) in the full, than in the poor, attention condition. For both motion tasks, attention effects were significantly greater in the LVF than the RVF (2-tailed t-tests, motion coherence: p = 0.04, fine direction-of-motion: p = 0.01). And, attention ratios were significantly greater than zero only in the LVF (motion coherence, p = 0.02, fine direction-of-motion: p = 0.004), and not the RVF (motion coherence, p = 0.86, direction-of-motion: p = 0.74). No significant attention effects were seen for the orientation task in either the LVF (p = 0.32) or RVF (p = 0.45). In sum, these results indicate greater attention effects in the LVF, than the RVF, on both motion tasks, but not on the orientation task.
Figure 4.
Group Mean Log Attention Ratios. For both motion tasks, the effects of spatial attention were greater in the LVF than the RVF, and only for the LVF were the attention ratios significantly above 0. ** p < 0.01, * p < 0.05 (2-tailed t-test).
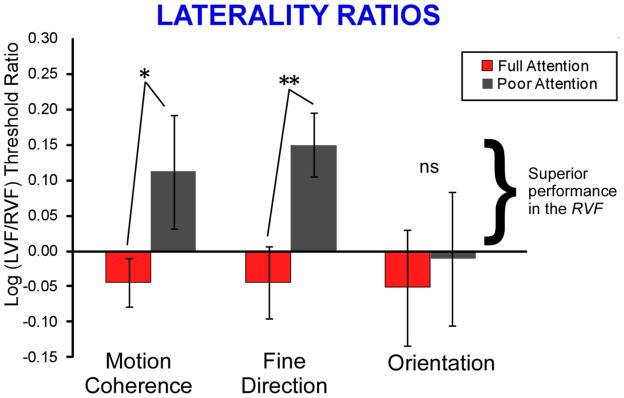
Group mean log laterality ratios, for each task and each attention condition, are presented in Figure 5. As would be predicted from the observed attention ratio differences between the LVF vs. RVF (see Figure 4), for both motion tasks, laterality effects were significantly greater in the poor than the full attention condition (motion coherence: p = 0.04; fine direction-of-motion: p = 0.01, and of course, these p values are identical to those for the comparison of attention ratios between LVF and RVF, see above). Laterality ratios were significantly greater than zero for only the fine direction-of-motion task in the poor attention condition (p = 0.01), with superior performance in the RVF. This result, which suggests a left hemisphere advantage for motion processing under poor attention conditions, is a bit surprising since some previous psychophysical studies of motion processing conducted under full attention have reported a left visual field advantage (e.g., see Bosworth & Dobkins, 1999), which is also the trend in the full attention condition of the current study. Further studies will be required to elucidate this issue. In sum, these results indicate greater laterality effects under poor, than full attention, on both motion tasks, but not on the orientation task.
Figure 5.
Group Mean Log Laterality Ratios. For both motion tasks, the effects of laterality were greater in the poor, than the full, attention condition, ** p < 0.01, * p < 0.05 (2-tailed t-test).
DISCUSSION
Using a full/poor attention paradigm, the results of the current study show that on motion tasks (motion coherence thresholds and fine direction-of-motion discrimination thresholds), effects of attention are greater in the left visual field (LVF) than in the right visual field (RVF). Although previous studies have used the same full/poor attention paradigm as in the current study, unlike the current study, their stimuli were not lateralized. Instead, these previous studies presented stimuli in both the LVF and RVF, with the target stimulus in one of the two hemifields (e.g., Huang & Dobkins, 2005; Lee et al., 1997; Morrone, Denti, & Spinelli, 2004). Most importantly, none of these previous studies reported data separately for targets in the LVF vs. RVF. Thus, to our knowledge, the current study is the first to use the full/poor attention paradigm to provide psychophysical evidence for greater effects of spatial attention in the LVF/right hemisphere. Our findings match the conclusions from much of the clinical literature from brain-damaged patients as well as results from many brain imaging studies in healthy humans, which suggest a right hemisphere bias for spatial attention. For the remainder of the Discussion, we address 1) potential differences in spatial attention between the Dorsal and Ventral processing streams, 2) neural correlates of spatial attention, and 3) alternative accounts of our results.
Effects of Attention for Orientation (Ventral Stream) vs. Motion (Dorsal Stream)
In contrast to the results from the motion tasks of the current study, the results from our orientation task yielded only small and insignificant effects of attention and no differences between hemifields, suggesting that attention has much smaller effects on orientation, than on motion, discrimination (although note that our orientation results trended in the same direction as in the motion task, i.e., greater attention effects in the LVF). In turn, these psychophysical results suggest that areas of the brain underlying orientation discrimination (primarily the ventral stream) are less affected by spatial attention than areas underlying motion discrimination (primarily the dorsal stream).4 To date, there have been many neural studies (single-unit studies in monkeys and brain imaging studies in humans, using fMRI, PET, and ERPs) showing effects of attention on neural responses. As described briefly in the Introduction, the type of neural study most directly related to the full/poor paradigm of the current psychophysical study are those that measure neural responses under conditions when a subject (human or monkey) attends vs. ignores the same visual stimulus. The general consensus from these neural studies is that there are clear effects of attention in both the ventral stream (e.g., monkeys: Chelazzi, Duncan, Miller, & Desimone, 1998; Chelazzi, Miller, Duncan, & Desimone, 1993, 2001; Luck, Chelazzi, Hillyard, & Desimone, 1997; Moran & Desimone, 1985; Moran & Desimone, 1993; Reynolds, Chelazzi, & Desimone, 1999; Reynolds & Desimone, 2003; Sheinberg & Logothetis, 2001; Spitzer, Desimone, & Moran, 1988, humans: Kastner, De Weerd, Desimone, & Ungerleider, 1998) and the dorsal stream (e.g., monkeys: Recanzone & Wurtz, 2000; Treue & Martinez-Trujillo, 1999; Treue & Maunsell, 1996, humans: Bavelier et al., 2000; Rees, Frith, & Lavie, 1997, and see Rezec & Dobkins, 2005 for a review of effects of attention on motion processing revealed psychophysically).
To our knowledge, no neural study has made direct comparisons of attentional modulation between the ventral and dorsal pathways. Unfortunately, results from neural studies that could potentially address this question would be difficult to interpret, since attention differences seen between processing streams could be due to differences in the effectiveness of the stimulus in driving activity in the two streams, and/or differences in the effectiveness of the task in the two streams (see Bosworth & Dobkins, 2002; Buracas, Fine, & Boynton, 2005; Saenz, Buracas, & Boynton, 2002). Nonetheless, the results of the current study suggest that could such a neural study be performed, it would reveal greater attention effects in the dorsal stream. There has, in fact, been speculation that this is the case. Specifically, Posner & Peterson (1990) proposed that the norepinephrine pathway from the locus coeruleus, involved in arousal and vigilance (and by extension, attention), projects more strongly to cortical areas thought to be associated with the dorsal stream (specifically, the posterior parietal lobe, as well as pulvinar, and superior colliculus) than to areas associated with the ventral stream. Interestingly, they and others also suggest that this norepinephrine pathway is strongly lateralized to the right hemisphere (Coull, Frith, Frackowiak, & Grasby, 1996; Marrocco, Witte, & Davidson, 1994; Posner & Petersen, 1990), which is in line with the apparent right hemisphere dominance of spatial attention in the current, and previous, studies.
Still, our null result for orientation is somewhat surprising given that Lee et al. (1997), using a very similar full/poor attention paradigm, did report effects of attention on discriminating small changes in orientation. There are at least three possible reasons for the lack of attention effect on orientation discrimination observed in the current study. First, it could be that, in the poor attention condition of the orientation task, our subjects did not pay sufficient attention to the central RSVP task. We think this is unlikely because the RSVP task was sufficient to impair performance on the two motion tasks, and RSVP performance did not differ between the three tasks (see Results). Thus, our RSVP task should have been sufficient to reveal an effect of attention on orientation thresholds if one existed. Second, our orientation discrimination task may not have been sensitive enough to reveal an attention effect, which could occur if attention effects are inherently small and the resolution of our orientation changes were too large. A third possibility to consider is that, because the stimulus edge of the oriented grating was fairly close (about 2.5 degrees) to the fixation point, in the poor attention condition, subjects could potentially have attended to both the central RSVP task as well as the edge of the oriented grating, with the idea that the edge has ample information. (This is less true for the motion task, where averaging across the entire random dot stimulus is expected to be much more beneficial than simply using the information at the edge.) If subjects could adequately use the information at the edge of the grating, this could result in a negligible effect of attention on orientation discrimination. Still, because we cannot rule out these alternative explanations for the small and insignificant spatial attention effects on orientation discrimination, whether spatial attention effects (and hemifield asymmetries of spatial attention) exist for orientation tasks remains an open question.
Neural Correlates of Spatial Attention
As described above, many neural studies (single-unit studies in monkeys and brain imaging studies in humans) have reported greater neural responses for attended vs. ignored stimuli. Particularly relevant are data from a study of spatial attention in area V4 (McAdams & Maunsell, 1999b). These investigators argued that attention-related increases in neural response amplitude are expected to: 1) increase the slope of the tuning function and 2) increase the signal-to-noise ratio of neurons, both of which are predicted to improve discrimination at the perceptual level. This is hypothesized to occur even if there is no change in bandwidth of neural tuning functions between the attended and ignored conditions, as is often found to be the case (orientation tuning in V4: McAdams & Maunsell, 1999a, but see Spitzer et al., 1988, direction tuning in MT: Treue & Maunsell, 1996, 1999, but see Martinez-Trujillo & Treue, 2004). (How, exactly, attention boosts the amplitude of neural responses is a topic of much interest, although outside the scope of the current discussion, see Reynolds & Heeger, 2009 for a review.) In line with the McAdams & Maunsell model, results of the current and many previous psychophysical studies that used the full/poor attention paradigm have reported improved stimulus discrimination in the full attention condition (motion thresholds: current study, orientation and vernier thresholds: Lee et al., 1997, contrast discrimination thresholds: Morrone, Denti, & Spinelli, 2002; Huang & Dobkins, 2005, detection thresholds: Huang & Dobkins, 2005; Lee et al., 1999, but see Lee et al., 1997; Morrone et al., 2002, 2004). The current study observed greater attention effects for motion discrimination in the LVF, which is in line with many human brain imaging studies showing greater attention-related increases in neural response in the right hemisphere, including those that have looked in motion processing area MT (e.g., Bavelier et al., 2000, and see Introduction).
Alternative Explanations
Above, we suggest that the full attention condition in the full/poor attention paradigm acts to boost the response amplitude of neural signals, and in turn, improve perceptual discriminability. However, others have interpreted the full/poor paradigm in a somewhat different light, specifically, as a way to measure “attentional resources” (Morrone et al. 2002; 2004, and see Pashler, 1998 for a review of attentional resources). In their use of the full/poor attention paradigm, Morrone and colleagues used two types of attentionally-demanding tasks in the poor-attention condition (a luminance or color pop-out task in the center of the visual field, asking subjects whether an oddball target was present or absent on each trial) and two types of peripheral tasks (luminance or color contrast discrimination, asking subjects to report which side, left or right, had higher contrast). They reported that attention effects were only observed when the central pop-out task and peripheral discrimination tasks were of the same domain (both luminance or both color). These results were interpreted as evidence for separate attentional resources devoted to color vs. luminance tasks. Interestingly, their view of the full/poor paradigm might lead to a conclusion about our data that is somewhat different from our own. Specifically, in their view, a large attention effect in the full/poor paradigm reflects shared and limited attentional resources between the central and peripheral task. Conversely, the lack of an attention effect reflects separate attentional resources between the central and peripheral task. Alternatively, the lack of an attention effect could reflect shared, but unlimited, attentional resources between the central and peripheral task. Based on Morrone and colleagues’ point of view, the fact that we found attention effects for motion discrimination in the LVF, and not in the RVF, would suggest that there are shared and limited attentional resources between the central RSVP task and the motion task in the LVF, yet unlimited attentional resources in the RVF. In other words, one could conclude that: 1) our central shape counting RSVP task and our peripheral motion tasks tapped the same attentional resources, and 2) there are greater attentional resources in the RVF/left hemisphere. Because this conclusion is not in line with the bulk of the human clinical and brain imaging data suggesting greater spatial attention effects in the LVF/right hemisphere, we are inclined to interpret our data in terms of enhanced amplitudes of neural signals (which are in line with the clinical and brain imaging data), as described above.
There is one last interpretation of the current data that should be considered. The greater effects of attention (on motion discrimination) in the LVF, as compared to the RVF, observed in the current study (and in human brain imaging studies) could be due to a greater ability to direct attention to the LVF than the RVF, as opposed to actual differences between hemifields (hemispheres) in the effects of attention, per se. While, of course, it is very difficult to tease apart these two possibilities, clinical and brain imaging studies have nonetheless localized effects of attention in regions in the right hemisphere. Regardless of the exact nature of the attentional asymmetry between the two hemifields, the results of the current study show that the effects of spatial attention on motion discrimination are greater in the left visual field (LVF) than in the right visual field (RVF). Such results suggest that attention-related increases in neural responses are greater in the right, than the left, hemisphere, particularly in the dorsal stream.
Examined effects of full/poor attention on lateralized motion and orientation tasks
Negligible effects of attention found on orientation task
Attention effects found in the left, but not right, visual field for motion tasks
Greater attention effects in the Dorsal stream of the right, than left, hemisphere
Acknowledgments
National Science Foundation grants (SBR- 9870897 and BCS-0241557) awarded to KRD.
Footnotes
Note that we chose shapes, rather than a more conventional stimulus, like letters (see Huang & Dobkins, 2005) because some of the subjects were tested as part of another study comparing hearing and deaf subjects. Because deaf signers may have less English reading experience than hearing subjects do, we thought it fairer to use non-orthographic stimuli, with the notion that both groups would have roughly equal experience with shapes.
There was a marginally significant effect of task (F(2,14) = 3.28; p = 0.07), and visual field (F(2,14) = 0.53; p = 0.06) on slopes, which likely reflects some differences across tasks and visual fields in amount of probability summation (see Graham, 1989; Watson, 1979).
Note that we chose to conduct ANOVAs separately for each of the three tasks because there is no need to discern if there are threshold differences across tasks, as the thresholds are not comparable across tasks.
It is worth noting that, generally speaking, motion and orientation tasks do not necessarily exclusively engage the dorsal and ventral processing streams, respectively. For example, motion sensitive area MT does contain a large percentage of orientation-tuned cells (Albright, 1984). And, masking and adaptation studies have shown influences between motion and orientation stimuli (Apthorp, Wenderoth, & Alais, 2009; Geisler 1999), which suggests an interaction between pathways.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albright TD. Direction and orientation selectivity of neurons in visual area MT of the macaque. J Neurophysiol. 1984;52(6):1106–1130. doi: 10.1152/jn.1984.52.6.1106. [DOI] [PubMed] [Google Scholar]
- Apthorp D, Wenderoth P, Alais D. Motion streaks in fast motion rivalry cause orientation-selective suppression. J Vis. 2009;9(5):10, 11–14. doi: 10.1167/9.5.10. [DOI] [PubMed] [Google Scholar]
- Arrington CM, Carr TH, Mayer AR, Rao SM. Neural mechanisms of visual attention: object-based selection of a region in space. J Cogn Neurosci. 2000;12(Suppl 2):106–117. doi: 10.1162/089892900563975. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Tomann A, Hutton C, Mitchell T, Corina D, Liu G, et al. Visual attention to the periphery is enhanced in congenitally deaf individuals. J Neurosci. 2000;20(17):RC93. doi: 10.1523/JNEUROSCI.20-17-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E, Karnath HO. Incidence of visual extinction after left versus right hemisphere stroke. Stroke. 2007;38(12):3172–3174. doi: 10.1161/STROKEAHA.107.489096. [DOI] [PubMed] [Google Scholar]
- Bonnel AM, Miller J. Attentional effects on concurrent psychophysical discriminations: investigations of a sample-size model. Percept Psychophys. 1994;55(2):162–179. doi: 10.3758/bf03211664. [DOI] [PubMed] [Google Scholar]
- Bonnel AM, Possamai CA, Schmitt M. Early modulation of visual input: a study of attentional strategies. Q J Exp Psychol A. 1987;39(4):757–776. doi: 10.1080/14640748708401812. [DOI] [PubMed] [Google Scholar]
- Bosworth RG, Dobkins KR. Visual field asymmetries for motion processing in deaf and hearing signers. Brain Cogn. 2002;49(1):170–181. doi: 10.1006/brcg.2001.1498. [DOI] [PubMed] [Google Scholar]
- Bosworth RG, Dobkins KR. Left hemisphere dominance for motion processing in deaf signers. Psychological Science. 1999;10(3):256–262. [Google Scholar]
- Bowen A, McKenna K, Tallis RC. Reasons for variability in the reported rate of occurrence of unilateral spatial neglect after stroke. Stroke. 1999;30(6):1196–1202. doi: 10.1161/01.str.30.6.1196. [DOI] [PubMed] [Google Scholar]
- Braun J. Visual search among items of different salience: removal of visual attention mimics a lesion in extrastriate area V4. J Neurosci. 1994;14(2):554–567. doi: 10.1523/JNEUROSCI.14-02-00554.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J, Sagi D. Vision outside the focus of attention. Percept Psychophys. 1990;48(1):45–58. doi: 10.3758/bf03205010. [DOI] [PubMed] [Google Scholar]
- Braun J, Sagi D. Texture-based tasks are little affected by second tasks requiring peripheral or central attentive fixation. Perception. 1991;20(4):483–500. doi: 10.1068/p200483. [DOI] [PubMed] [Google Scholar]
- Buracas GT, Fine I, Boynton GM. The relationship between task performance and functional magnetic resonance imaging response. J Neurosci. 2005;25(12):3023–3031. doi: 10.1523/JNEUROSCI.4476-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron EL, Tai JC, Carrasco M. Covert attention affects the psychometric function of contrast sensitivity. Vision Res. 2002;42(8):949–967. doi: 10.1016/s0042-6989(02)00039-1. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Talgar CP, Cameron EL. Characterizing visual performance fields: effects of transient covert attention, spatial frequency, eccentricity, task and set size. Spat Vis. 2001;15(1):61–75. doi: 10.1163/15685680152692015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelazzi L, Duncan J, Miller EK, Desimone R. Responses of neurons in inferior temporal cortex during memory-guided visual search. J Neurophysiol. 1998;80(6):2918–2940. doi: 10.1152/jn.1998.80.6.2918. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Miller EK, Duncan J, Desimone R. A neural basis for visual search in inferior temporal cortex. Nature. 1993;363(6427):345–347. doi: 10.1038/363345a0. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Miller EK, Duncan J, Desimone R. Responses of neurons in macaque area V4 during memory-guided visual search. Cereb Cortex. 2001;11(8):761–772. doi: 10.1093/cercor/11.8.761. [DOI] [PubMed] [Google Scholar]
- Christman SD, Niewbauer CL. The relation between left-right and upper-lower visual field asymmetries. In: Christman S, editor. Cerebral Asymmetries in Sensory and Perceptual Processing. Amsterdam, The Netherlands: Elsevier Science; 1997. pp. 263–296. [Google Scholar]
- Connolly M, Van ED. The representation of the visual field in parvocellular and magnocellular layers of the lateral geniculate nucleus in the macaque monkey. The Journal of Comparative Neurology. 1984;226(4):544–564. doi: 10.1002/cne.902260408. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3(3):292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET study of visuospatial attention. J Neurosci. 1993;13(3):1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coslett HB, Bowers D, Fitzpatrick E, Haws B, Heilman KM. Directional hypokinesia and hemispatial inattention in neglect. Brain. 1990;113:475–486. doi: 10.1093/brain/113.2.475. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Frackowiak RSJ, Grasby PM. A fronto-parietal network for rapid visual information processing: A PET study of sustained attention and working memory. Neuropsychologia. 1996;34:1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Hugdahl K, editors. Brain Asymmetry. Cambridge, Massachusetts: MIT Press; 1998. [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dimond SJ. Performance by split-brain humans on lateralized vigilance tasks. Cortex. 1979;15:43–50. doi: 10.1016/s0010-9452(79)80005-2. [DOI] [PubMed] [Google Scholar]
- Dimond SJ, Beaumont JG. Difference in the vigilance performance of the right and left hemispheres. Cortex. 1973;9(3):259–265. doi: 10.1016/s0010-9452(73)80003-6. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Bosworth RG. Effects of set-size and selective spatial attention on motion processing. Vision Res. 2001;41(12):1501–1517. doi: 10.1016/s0042-6989(01)00038-4. [DOI] [PubMed] [Google Scholar]
- Duncan J. Selective attention and the organization of visual information. J Exp Psychol Gen. 1984;113(4):501–517. doi: 10.1037//0096-3445.113.4.501. [DOI] [PubMed] [Google Scholar]
- Geisler WS. Motion streaks provide a spatial code for motion direction. Nature. 1999;400(6739):65–69. doi: 10.1038/21886. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, et al. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122(Pt 6):1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Graham NVS. Visual Pattern Analyzers. New York: Oxford University Press; 1989. [Google Scholar]
- Heilman KM, Pandya DN, Geschwind N. Trimodal inattention following parietal lobe abolations. Transactions of the American Neurologic Association. 1970;95:259–261. [PubMed] [Google Scholar]
- Heilman KM, Van Den Abell T. Right hemisphere dominance for attention: the mechanisms underlying hemispheric asymmetries of inattention (neglect) Neurology. 1980;30:327–330. doi: 10.1212/wnl.30.3.327. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. New York: Oxford; 1985. pp. 243–293. [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci U S A. 1998;95(3):781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3(3):284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Huang L, Dobkins KR. Attentional effects on contrast discrimination in humans: evidence for both contrast gain and response gain. Vision Res. 2005;45(9):1201–1212. doi: 10.1016/j.visres.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Husain M, Rorden C. Non-spatially lateralized mechanisms in hemispatial neglect. Nat Rev Neurosci. 2003;4(1):26–36. doi: 10.1038/nrn1005. [DOI] [PubMed] [Google Scholar]
- Itti L, Rees G, Tsotsos JK. Neurobiology of Attention. Academic Press/Elsevier; 2004. Attentional Effects on Motion Processing; pp. 490–495. [Google Scholar]
- Johnson NL, Kotz S, Balakrishnan N. Continuous univariate distributions. 2. Vol. 2. New York: John Wiley; 1995. [Google Scholar]
- Kastner S, De Weerd P, Desimone R, Ungerleider LG. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282(5386):108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22(4):751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. Hemi-neglect and hemisphere rivalry. Adv Neurol. 1977;18:41–49. [PubMed] [Google Scholar]
- Lee DK, Koch C, Braun J. Spatial vision thresholds in the near absence of attention. Vision Res. 1997;37(17):2409–2418. doi: 10.1016/s0042-6989(97)00055-2. [DOI] [PubMed] [Google Scholar]
- Lee DK, Koch C, Braun J. Attentional capacity is undifferentiated: concurrent discrimination of form, color, and motion. Percept Psychophys. 1999;61(7):1241–1255. doi: 10.3758/bf03206177. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77(1):24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31(3):291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Lynch JC. The functional organization of posterior parietal association cortex. Behavioral Brain Science. 1980;3:485–534. [Google Scholar]
- Marrocco RT, Witte EA, Davidson MC. Arousal systems. Current Opinion in Neurobiology. 1994;4:166–170. doi: 10.1016/0959-4388(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo JC, Treue S. Feature-based attention increases the selectivity of population responses in primate visual cortex. Curr Biol. 2004;14(9):744–751. doi: 10.1016/j.cub.2004.04.028. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J Neurosci. 1999a;19(1):431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Effects of attention on the reliability of individual neurons in monkey visual cortex. Neuron. 1999b;23(4):765–773. doi: 10.1016/s0896-6273(01)80034-9. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354(1387):1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229(4715):782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Morrone MC, Denti V, Spinelli D. Color and luminance contrasts attract independent attention. Curr Biol. 2002;12(13):1134–1137. doi: 10.1016/s0960-9822(02)00921-1. [DOI] [PubMed] [Google Scholar]
- Morrone MC, Denti V, Spinelli D. Different attentional resources modulate the gain mechanisms for color and luminance contrast. Vision Res. 2004;44(12):1389–1401. doi: 10.1016/j.visres.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol. 1993;70(3):909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Lawson D. Attention to central and peripheral visual space in a movement detection task: an event-related potential and behavioral study. I. Normal hearing adults. Brain Res. 1987;405(2):253–267. doi: 10.1016/0006-8993(87)90295-2. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) J Neurosci. 1988;8(6):2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RS, Frith CD. Functional localization of the system for visuospatial attention using positron emission tomography. Brain. 1997;120(Pt 3):515–533. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349(6304):61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Pashler HE. The Psychology of Attention. Cambridge, Massachusetts: MIT Press; 1998. [Google Scholar]
- Perry VH, Cowey A. The ganglion cell and cone distributions in the monkey’s retina: implications for central magnification factors. Vision Res. 1985;25(12):1795–1810. doi: 10.1016/0042-6989(85)90004-5. [DOI] [PubMed] [Google Scholar]
- Plummer P, Morris ME, Dunai J. Assessment of Unilateral Neglect. Physical Therapy. 2003;83(8):732–740. [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The Attention System of the Human Brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Zani A, Gazzaniga MS, Mangun GR. ERP and RT signs of a rightward bias for spatial orienting in a split-brain patient. Neuroreport. 1994;5(18):2457–2461. doi: 10.1097/00001756-199412000-00013. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Wurtz RH. Effects of attention on MT and MST neuronal activity during pursuit initiation. J Neurophysiol. 2000;83(2):777–790. doi: 10.1152/jn.2000.83.2.777. [DOI] [PubMed] [Google Scholar]
- Rees G, Frith CD, Lavie N. Modulating irrelevant motion perception by varying attentional load in an unrelated task. Science. 1997;278(5343):1616–1619. doi: 10.1126/science.278.5343.1616. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J Neurosci. 1999;19(5):1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Desimone R. Interacting roles of attention and visual salience in V4. Neuron. 2003;37(5):853–863. doi: 10.1016/s0896-6273(03)00097-7. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61(2):168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezec AA, Dobkins KR. Attentional weighting: a possible account of visual field asymmetries in visual search? Spat Vis. 2004;17(4–5):269–293. doi: 10.1163/1568568041920203. [DOI] [PubMed] [Google Scholar]
- Rezec AA, Dobkins KR. Attentional Effects on Motion Processing. In: Itti L, Rees G, Tsotsos JK, editors. Neurobiology of Attention. Academic Press/Elsevier; 2005. pp. 490–495. [Google Scholar]
- Riddoch MJ, Humphreys G. The effect of cuing on unilateral neglect. Neuropsychologia. 1983;21:589–599. doi: 10.1016/0028-3932(83)90056-8. [DOI] [PubMed] [Google Scholar]
- Ringman JM, Saver JL, Woolson RF, Clarke WR, Adams HP. Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology. 2004;63(3):468–474. doi: 10.1212/01.wnl.0000133011.10689.ce. [DOI] [PubMed] [Google Scholar]
- Saenz M, Buracas GT, Boynton GM. Global effects of feature-based attention in human visual cortex. Nat Neurosci. 2002;5(7):631–632. doi: 10.1038/nn876. [DOI] [PubMed] [Google Scholar]
- Schenkenberg T, Bradford DC, Ajax ET. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology. 1980;30(5):509–517. doi: 10.1212/wnl.30.5.509. [DOI] [PubMed] [Google Scholar]
- Sheinberg DL, Logothetis NK. Noticing familiar objects in real world scenes: the role of temporal cortical neurons in natural vision. J Neurosci. 2001;21(4):1340–1350. doi: 10.1523/JNEUROSCI.21-04-01340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Pope DL, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci. 2010;30(10):3640–3651. doi: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman-Tov T, Mendelsohn A, Schonberg T, Avidan G, Podlipsky I, Pessoa L, et al. Bihemispheric leftward bias in a visuospatial attention-related network. J Neurosci. 2007;27(42):11271–11278. doi: 10.1523/JNEUROSCI.0599-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling G, Melchner MJ. The attention operating characteristic: examples from visual search. Science. 1978;202(4365):315–318. doi: 10.1126/science.694536. [DOI] [PubMed] [Google Scholar]
- Spitzer H, Desimone R, Moran J. Increased attention enhances both behavioral and neuronal performance. Science. 1988;240(4850):338–340. doi: 10.1126/science.3353728. [DOI] [PubMed] [Google Scholar]
- Szczepanski SM, Konen CS, Kastner S. Mechanisms of spatial attention control in frontal and parietal cortex. J Neurosci. 2010;30(1):148–160. doi: 10.1523/JNEUROSCI.3862-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MMC, CD PEST: Efficient estimates on probability functions. Journal of the Acoustical Society of America. 1967;41:782–787. [Google Scholar]
- Tootell RB, Silverman MS, Hamilton SL, Switkes E, De Valois RL. Functional anatomy of macaque striate cortex. V. Spatial frequency. J Neurosci. 1988;8(5):1610–1624. doi: 10.1523/JNEUROSCI.08-05-01610.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S, Martinez-Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399(6736):575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382(6591):539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- Treue S, Maunsell JH. Effects of attention on the processing of motion in macaque middle temporal and medial superior temporal visual cortical areas. J Neurosci. 1999;19(17):7591–7602. doi: 10.1523/JNEUROSCI.19-17-07591.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LGaPT. Ventral and Dorsal Cortical Processing Streams. In: Werner JSaCLM., editor. The Visual Neurosciences. Vol. 1. Cambridge, Massachusetts: The MIT Press; 2004. [Google Scholar]
- Van Essen DC, Newsome WT, Maunsell JH. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vision Res. 1984;24(5):429–448. doi: 10.1016/0042-6989(84)90041-5. [DOI] [PubMed] [Google Scholar]
- Watson AB. Probability summation over time. Vision Research. 1979;19(5):515–522. doi: 10.1016/0042-6989(79)90136-6. [DOI] [PubMed] [Google Scholar]
- Weibull W. A statistical distribution function of wide applicability. Journal of Applied Mechanics. 1951;18:292–297. [Google Scholar]
- Whitehead R. Right hemisphere processing superiority during sustained visual attention. Journal of Cognitive Neuroscience. 1991;3(4):329–334. doi: 10.1162/jocn.1991.3.4.329. [DOI] [PubMed] [Google Scholar]
- Wilkins AJ, Shallice T, McCarthy R. Frontal lesions and sustained attention. Neuropsychologia. 1987;25:359–366. doi: 10.1016/0028-3932(87)90024-8. [DOI] [PubMed] [Google Scholar]