Abstract
Psychological or neurocognitive impairment is often seen in medulloblastoma survivors after craniospinal radiation; however, significant variability in outcomes exists. This study investigated the role of antioxidant enzyme polymorphisms in moderating this outcome and hypothesized that patients who had polymorphisms associated with lower antioxidant enzyme function would have a higher occurrence of impairment. From the Childhood Cancer Survivor Study (CCSS) cohort, 109 medulloblastoma survivors and 143 siblings were identified who completed the CCSS Neurocognitive Questionnaire (NCQ) and the Brief Symptom Inventory-18 (BSI-18) and who provided buccal DNA samples. Real-time polymerase chain reaction (PCR) allelic discrimination was used for SOD2 (rs4880), GPX1 (rs1050450), and GSTP1 (rs1695 and rs1138272) genotyping and PCR for GSTM1 and GSTT1 gene deletions. Outcomes on NCQ and BSI-18 subscale scores were examined in association with genotypes and clinical factors, including age at diagnosis, sex, and radiation dose, using univariate and multivariate analysis of variance. Patients <7 years of age at diagnosis displayed more problems with task efficiency (P < .001) and fewer problems with somatic complaints (P = .004) than did patients ≥7 years of age. Female patients reported more organization problems than did male patients (P = .02). Patients with homozygous GSTM1 gene deletion reported higher anxiety (mean null genotype = 47.3 ± 9.2, non-null = 43.9 ± 7.8; P = .04), more depression (null = 51.0 ± 9.8, non-null = 47.0 ± 9.4; P = .03), and more global distress (null = 50.2 ± 9.7, non-null = 45.2 ± 9.9; P = .01). All associations for the GSTM1 polymorphism remained statistically significant in a multivariate model controlling for age, sex, and radiation dose. Homozygous GSTM1 gene deletion was consistently associated with greater psychological distress in medulloblastoma survivors across multiple domains, suggesting that this genotype may predispose patients for increased emotional late effects.
Keywords: Childhood Cancer Survivor Study, glutathione S-transferase polymorphisms, medulloblastoma, neuropsychological impairment, radiation therapy
Medulloblastoma is the most common malignant central nervous system (CNS) tumor seen in children and adolescents, accounting for approximately 20% of all new CNS tumor diagnoses.1 With advances in chemotherapy and radiation therapy, 60–80% of patients can now achieve long-term survivorship.2,3 However, a substantial number have significant late effects, including endocrinopathies, hearing loss, renal failure, second malignancies, and neuropsychological impairment.3–6 Children with CNS tumors, particularly those treated with cranial radiation therapy (CRT) are at risk for development of serious neuropsychological problems. Progressive deficits in overall cognitive functioning (IQ), attention, memory, processing speed, and executive function are seen in 50%–60% of survivors.7,8 Younger age at the time of treatment, radiation dose, volume of brain irradiated, longer time since treatment, and female sex are associated with increased risk for poor neuropsychological outcome.7–9 In addition, survivors of childhood CNS tumors demonstrate higher levels of depression and psychological distress than do sibling control subjects.10
These residual neurocognitive and psychological effects can have a profound impact on a survivor's quality of life, school performance, and achievement of major adult milestones. For example, recent reports from the Childhood Cancer Survivor Study (CCSS) found that long-term survivors of a CNS tumor are at increased risk for lower educational attainment, unemployment, lower income, dependent living status (e.g., living with parents), and fewer marriages during adulthood.11–15
Among patients treated with similar protocols, there is significant variation in the occurrence and severity of neuropsychological late effects. We hypothesized that genetic polymorphisms affecting a patient's ability to respond to radiation-induced oxidative stress and subsequent tissue injury may explain, in part, differences in susceptibility to these late effects. In this study, we investigated several common polymorphisms that may affect antioxidant enzyme activity and have been implicated in increased risk for treatment toxicities due to presumed increased oxidative damage. Manganese superoxide dismutase (encoded by SOD2) acts as one of the most effective intracellular antioxidants by converting superoxide anions to hydrogen peroxide and oxygen.16,17 A single nucleotide polymorphism (SNP) affecting the targeting sequence of the gene leads to greater enzyme activity (rs4880, 47T > C).18,19 Glutathione peroxidase 1 (encoded by GPX1) is a major enzyme in the metabolism of hydrogen peroxide generated by superoxide dismutase. A well-characterized SNP (rs1050450, 599C > T) results in an amino acid change from proline to leucine, which is associated with greater enzyme activity.20,21 Glutathione-S-transferases (GSTs) are a group of enzymes involved in the metabolism of free radicals, products of lipid oxidation, and chemotherapy agents used in the treatment of medulloblastoma.22–25 In the case of GSTM1 and GSTT1, deletion of the gene is common, leading to no functional enzyme. GSTP1 is characterized by 2 different SNPs resulting in amino acid substitutions that lead to steric changes at the substrate-binding site of the enzyme (rs1695, 1404A > G [exon 5], rs1138272, 2294C > T [exon 6]).26 On the basis of the function of these enzymes, we aimed to examine associations between these antioxidant polymorphisms and neuropsychological impairment and psychological distress, measured by 2 validated questionnaires in childhood medulloblastoma survivors participating in the CCSS.
Methods
Participants
Subjects for the current study are participants in the Childhood Cancer Survivor Study (CCSS). The complete cohort and study design have been previously described.27,28 In brief, the CCSS is a multi-institutional retrospective cohort of children and adolescents treated for cancer from 1970 through 1986 who have been followed up longitudinally since 1992. Eligibility criteria included diagnosis of childhood cancer prior to age 21 years and survival of at least 5 years after diagnosis. The CCSS protocol was reviewed and approved by the institutional review boards of the participating institutions. Participants provided informed consent for survey data collection, medical record abstraction, and banking of DNA. The current study was approved by the Baylor College of Medicine Institutional Review Board, where the genotyping was performed.
The current study was limited to CCSS participants who met the following eligibility criteria: (1) diagnosis of medulloblastoma; (2) availability of buccal cell DNA sample; (3) receipt of craniospinal radiation therapy, with treatment records available for radiation dosimetry; (4) no history of relapse or second malignancy requiring additional cranial radiation therapy; and (5) completion of the neurocognitive and psychological outcome measures as described below and included in the 2003 follow-up survey. Participants in the CCSS sibling cohort, selected from a sample of participating survivors, who had (1) provided buccal cell DNA samples and (2) completed the 2003 follow-up survey, were also included in this study as a comparison group. On the basis of the aforementioned criteria, 109 medulloblastoma survivors and 143 siblings were eligible to be included in the current study.
Demographic and Treatment Information
Sex, race, age at survey completion, employment status, and highest educational level were obtained from participant self-report. Medical records were abstracted using a standardized protocol for treatment information including: age at diagnosis, chemotherapy agents, and radiation therapy. Radiation dosage was quantified specifically for 4 segments of the brain: (1) posterior fossa, (2) temporal lobes, (3) frontal lobes, and (4) parietal/occipital lobes. Maximum radiation to each segment was determined by central review of radiation oncology records from the treating facility.29 All questionnaires and data abstraction forms are available on the CCSS Web site (http://ccss.stjude.org).
Buccal Cell DNA Collection, Extraction, and Storage
Methods for DNA collection, extraction, and storage have been described previously.30,31 Collection kits were mailed to eligible participants, who were instructed on how to collect and return mouthwash samples in the provided container. DNA was extracted using the Gentra Puregene kit (Qiagen). Extracted DNA was stored in Qiagen AE buffer at −80°C until used for genotyping.
Genotyping
The polymorphisms investigated are listed in Table 1. For SOD2, GPX1, and GSTP1 genotyping, real-time polymerase chain reaction (PCR) TaqMan-based 5′nuclease allelic discrimination assays (Applied Biosystems) were used. Commercially predesigned assays were used for the genotyping of SOD2 (Assay ID C_8709053_10) and GSTP1 (Assay IDs C_3237198_20 and C_1049615_20). For the GPX1 assay, validated primer and minor groove binder probe sequences available on the National Cancer Institute's SNP500Cancer Database (http://variantgps.nci.nih.gov/cgfseq/pages/snp500project.do) were custom manufactured (Applied Biosystems). Sequences of the primers and probes used were as follows: 5′-CATCGAAGCCCTGCTGTCT-3′ (forward primer), 5′-CACTGCAACTGCCAAGCA-3′ (reverse primer), 5′ VIC- ACAGCTGGGCCCTT-MGB-3′ (C-specific allele probe), and 5′ FAM- ACAGCTGAGCCCTT-MGB-3′ (T-specific allele probe). Each 25 μL reaction included 5 ng of genomic DNA, 12.5 μL of 2× TaqMan Genotyping Master Mix, and 1.25 μL of the respective 20× genotyping assay mix (primer concentration 18 µM and probe concentration 4 µM), and DNase-free water. All DNA samples were diluted to a uniform concentration prior to genotyping. Genotyping was performed according to manufacturer-recommended thermocycling conditions on a CFX96 Real-Time PCR Detection System (Bio-Rad). Samples were run in duplicate with no template, positive, and negative controls on each 96-well plate. Genotype for each sample was determined by allelic discrimination with review of the scatter plots of major allele relative fluorescent units versus minor allele relative fluorescent units.
Table 1.
Study polymorphisms
| Gene | SNP rs number | Polymorphism | Amino Acid Change | Predicted At-Risk Genotype |
|---|---|---|---|---|
| SOD2 | rs4880 | 47T > C | Val16Ala | C47T and T47T |
| GPX1 | rs1050450 | 599C > T | Pro198Leu | C599T and T599T |
| GSTM1 | N/A | Gene Deletion | GSTM1 Null (homozygous deletion) | |
| GSTT1 | N/A | Gene Deletion | GSTT1 Null (homozygous deletion) | |
| GSTP1 | rs1695 | 1404A > G (exon 5) | Ile105Val | GSTP1 Not AAa |
| rs1138272 | 2294C > T (exon 6) | Ala114Val |
aGSTP1 characterized by 2 SNPs with 4 recognized alleles, GSTP1*A is Ile at position 105 and Ala at position 114.
Multiplex PCR was used to amplify GSTM1 and GSTT1 simultaneously.32 In brief, 50 μg of DNA was amplified using GSTM1 primers 5′-GAA CTC CCT GAA AAG CTA AAG C-3′ and 5′-GTT GGG CTC AAA TAT ACG GTG G-3′ and GSTT1 primers corresponding to the 3′ coding region of human cDNA: 5′-TTC CTT ACT GGT CCT CAC ATC TC-3′ and 5′-TCA CCG GAT CAT GGC CAG CA-3′. As an internal control, the dihydrofolate reductase gene (DHFR) was co-amplified using the primers 5′-GCA TGT CTT TGG GAT GTG GA-3′ and 5′-GGA ATG GAG AAC CAG GTC TT-3′. These PCRs were performed on a GeneAmp2700 system (Applied Biosystems). The PCR conditions consisted of an initial melting temperature of 95°C (5 min), followed by 35 cycles of melting (95°C, 30 s), annealing (58.3°C, 45 s), and extension (72°C, 1 min), followed by a final extension step at 72°C for 10 min. We then viewed the PCR products from co-amplification of GSTT1 (480 bp), DHFR (280 bp), and GSTM1 (215 bp) with ethidium bromide–stained 10% polyacrylamide gels for the presence or absence of GSTM1 and GSTT1 genes. DHFR was used as an internal control to ensure that there was amplifiable DNA in the sample in the case of a double-null genotype.
Genotyping was inconclusive in 1 subject for SOD2, GSTM1, and GSTT1 and 10 subjects for GPx1. For all assays, a 10% random sample of subjects were repeated for quality control with agreement between the initial results and the results of the samples repeated for quality control with the exception of 1 subject for GSTM1 and GSTT1.
Neuropsychological Functioning
Two validated, self-report questionnaires administered in the 2003 follow-up survey to survivors and siblings were used to assess level of neurocognitive impairment and psychological distress.
The CCSS Neurocognitive Questionnaire (CCSS-NCQ) consists of 25 questions designed to assess self-report of neurocognitive function. Participants rated the extent to which they had experienced problems over the previous 6 months using a Likert scale ranging from 1 (“never a problem”) to 3 (“often a problem”). Recent analysis of the CCSS-NCQ reveals it to reliably assess 4 neurocognitive factors: task efficiency (attention and processing speed), memory (working and long-term), emotional regulation (emotional lability), and organization (organization of one's environment).33 These 4 factors accurately discriminated survivors who were considered to be at high risk for neurocognitive dysfunction, from healthy low-risk survivors and siblings in a prior CCSS study33 and have been shown to be sensitive to cranial radiation in a dose-dependent fashion.34
The Brief Symptom Inventory–18 (BSI-18) is an 18-item screening questionnaire designed to assess acute symptoms of depression, anxiety, and somatic complaints. Participants described the extent to which they have been distressed or bothered by each symptom in the previous 7 days using a Likert scale ranging from 1 (“not at all”) to 5 (“extremely”). Responses to all 18 items were summed to yield the Global Severity Index (GSI).35
Statistical Analysis
For each polymorphism, the allele associated with decreased enzyme activity in the literature was considered to be the at-risk allele. With use of a dominant model, the genotypes predicted to be at risk are shown in Table 1. Outcome measures were the continuous subscale scores from the CCSS-NCQ and BSI-18. Analysis of variance (GLM procedure, SAS, version 9.2; SAS Institute) was used for univariate and multivariate analyses, comparing mean CCSS-NCQ subscale scores, BSI-18 subscale scores, or GSI T scores. Independent variables included genotype, age at diagnosis (<7 years of age vs. ≥7 years of age), sex, and segment dose level (<36 Gy vs. ≥36 Gy for temporal, frontal, and parietal segments). Because segment exposures were highly correlated, we fitted the multivariable model for one segment at a time. Posterior fossa radiation doses were not included in these multivariate analyses because of the homogeneity of exposure doses, with only 5 survivors (4.9%) receiving <36 Gy to the posterior fossa. A 2-sided P value of <.05 was considered to be statistically significant in all analyses.
Results
Characteristics of the Study Population
Demographic, treatment, and genotypic characteristics of the childhood medulloblastoma survivors and their siblings are shown in Table 2. The subjects were predominantly white. The sibling group was older (P < .001) and more educated (P = .03) than the survivor group. Genotype frequencies for SOD2, GPx1, GSTM1, GSTP1, and GSTT1 polymorphisms were not different between survivors and siblings.
Table 2.
Descriptive characteristics of the medulloblastoma survivors and sibling participants
| Characteristic | Medulloblastoma Survivors (n = 109) | Siblings (n = 143) | P valuea |
|---|---|---|---|
| Gender | |||
| Male | 56 (51.4%) | 65 (45.5%) | NS |
| Female | 53 (48.6%) | 78 (54.5%) | |
| Age at time of questionnaire completion (yrs) | |||
| Mean (SD) | 30.9 (6.06) | 34.3 (9.00) | .0004 |
| Range | 18.6–43.7 | 18.9–54.6 | |
| Ethnicity | |||
| Caucasian | 98 (89.9%) | 131 (91.6%) | NS |
| Other | 11 (10.1%) | 5 (3.5%) | |
| Missing | 0 (0%) | 7 (4.9%) | |
| Highest education level | |||
| Less than 12th grade | 7 (6.4%) | 5 (3.5%) | .0002 |
| High school graduate | 32 (29.4%) | 15 (10.5%) | |
| Further education | 68 (62.4%) | 123 (86.0%) | |
| Unknown | 2 (1.8%) | 0 (0%) | |
| Age at diagnosis (yrs) | |||
| Mean (SD) | 8.0 (0.41) | N/A | |
| Range | 0.6–18.3 | ||
| Time since diagnosis at time of questionnaire completion (yrs) | |||
| Mean (SD) | 22.8 (4.22) | N/A | |
| Range | 16.5–32.5 | ||
| Received Chemotherapy | |||
| Yes | 53 (48.6%) | N/A | |
| No | 51 (46.8%) | ||
| Unknown | 5 (4.6%) | ||
| Radiation Doses (Gy) Mean (SD) | |||
| Posterior Fossa (Segment 1) | 50.4 (6.01) | N/A | |
| Temporal (Segment 2) | 43.1 (9.53) | ||
| Frontal (Segment 3) | 36.9 (7.64) | ||
| Parietal/Occipital (Segment 4) | 40.1 (8.21) | ||
| SOD2 (47T > C) | |||
| TT | 21 (19.3%) | 34 (23.8%) | NS |
| CT | 50 (45.9%) | 78 (54.5%) | |
| CC | 37 (33.9%) | 31 (21.7%) | |
| Missing | 1 (0.9%) | 0 | |
| GPX1 (599C > T) | |||
| CC | 50 (45.9%) | 71 (49.7%) | NS |
| CT | 47 (43.1%) | 58 (40.5%) | |
| TT | 8 (7.3%) | 8 (5.6%) | |
| Missing | 4 (3.7%) | 6 (4.2%) | |
| GSTP1(1404A > G and 2294C > T) | |||
| AA | 45 (41.3%) | 60 (42.0%) | NS |
| Non AA | 64 (58.7%) | 83 (58%) | |
| GSTM1 (Gene deletion) | |||
| Null | 51 (46.8%) | 71 (49.6%) | NS |
| Non-null | 57 (52.3%) | 72 (50.4%) | |
| Missing | 1 (0.9%) | 0 | |
| GSTT1 (Gene deletion) | |||
| Null | 19 (17.4%) | 21 (14.7%) | NS |
| Non-null | 89 (81.7%) | 122 (85.3%) | |
| Missing | 1 (0.9%) | 0 |
aP-value was based on those participants without missing data.
Neurocognitive Impairment
Data for the CCSS-NCQ were available for 108 survivors and for 142 siblings. Survivors had significantly higher mean scores (worse functioning) on both Task Efficiency and Memory subscales (P < .001), compared with siblings, but no difference was found in Emotional Regulation or Organization subscales between survivors and siblings. There were no statistically significant differences in mean scores between genotypes on any of the CCSS-NCQ subscales for the survivors. However, survivors <7 years of age at diagnosis had higher mean scores (worse functioning) on the Task Efficiency subscale than did survivors ≥7 years of age at diagnosis (mean ± SD: 18.6 ± 4.32 vs. 14.1 ± 4.49, P < .001). This association was still significant (P < .001) in multivariate models controlling for genotype, sex, and radiation dose to each segment. Female survivors reported worse functioning on the Organization subscale, compared with male survivors (5.0 ± 1.87 vs. 4.3 ± 1.41, P = .02). In multivariate models, sex was still significant (P < .01) after controlling for age, genotype, and radiation dose to each segment.
For the sibling group, those with the GSTM1 null genotype had higher scores on the Emotional Regulation subscale than did those with GSTM1 non-null genotype (5.4 ± 1.77 vs. 4.8 ± 1.46, P = .03). There were no statistically significant differences in mean scores between any other genotypes on any of the CCSS-NCQ subscales for the siblings. In addition, there were no genotype variables associated with scores on the other 3 CCSS-NCQ subscales.
Psychological Impairment
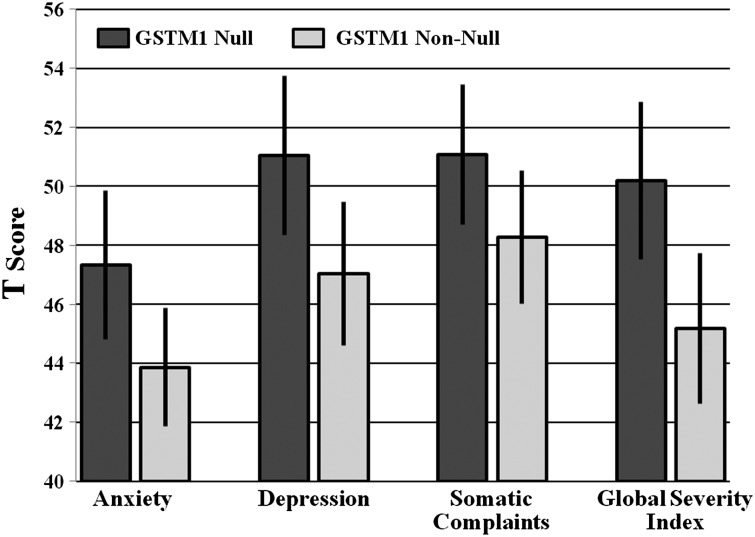
Data for the BSI-18 were available for all 109 survivors and for 143 siblings. No statistically significant difference was identified in depression, anxiety, and somatic complaints or global severity between survivors and siblings. Results from the univariate analyses are shown in Fig. 1. Survivors with the GSTM1 null genotype had higher mean scores for Anxiety (Mean ± SD: 47.3 ± 9.17 vs. 43.9 ± 7.76, P = .04), Depression (51.0 ± 9.83 vs. 47.0 ± 9.36, P = .03), Global Severity (50.2 ± 9.73 vs. 45.2 ± 9.87, P = .01), and Somatic Complaints (51.1 ± 8.64 vs. 48.3 ± 8.69, P = .1), compared with GSTM1 non-null survivors.
Fig. 1.
BSI-18 subscale scores by GSTM1 genotype.
The results of the multivariate model including radiation exposure to the temporal lobe, which was the only significant segment in univariate analyses among all segments, are shown in Table 3. Radiation doses to the other segments were not significant predictors of BSI-18 subscale scores at the univariate level and were, therefore, not examined in multivariate models. The GSTM1 null genotype remained a significant predictor of increased anxiety, depression, and global distress scores after controlling for age, sex, and radiation dose to the temporal lobes in a multivariate model. There were no interactions found between GSTM1 genotype and age, sex, or radiation dose. No other genotypes demonstrated statistically significant differences in mean scores on any BSI-18 subscale. For the sibling group, there were no statistically significant associations found between any of the polymorphisms and the BSI-18 subscales.
Table 3.
Multivariate results for selected domains for BSI-18 scores and radiation dose to the temporal lobes
| Variable | BSI-18 Scale |
|||||
|---|---|---|---|---|---|---|
|
Anxiety |
Depression |
Global Severity Index |
||||
| Estimate | P | Estimate | P | Estimate | P | |
| GSTM1 | ||||||
| Non-null | −3.29 | .05 | −3.85 | .04 | −4.53 | .02 |
| Null | Referent | Referent | Referent | |||
| Age | ||||||
| <7 years | −0.64 | .71 | 0.51 | .79 | −2.61 | .20 |
| ≥7 years | Referent | Referent | Referent | |||
| Gender | ||||||
| Male | 1.00 | .56 | 0.55 | .77 | 0.10 | .96 |
| Female | Referent | Referent | Referent | |||
| Radiation dose | ||||||
| ≥36 Gy | −2.21 | .26 | −4.50 | .04 | −3.19 | .16 |
| <36 Gy | Referent | Referent | Referent | |||
For the Somatic Complaints subscale, survivors aged ≥7 years at diagnosis had higher T scores than did those aged <7 years (51.5 ± 8.62 vs. 46.6 ± 8.13, P = .004). This association between Somatic Complaints and age remained statistically significant (P < .01) across models controlling for sex and radiation dose to each segment.
Discussion
Currently, age at the time of radiation therapy, radiation dose, volume of brain irradiated, longer time since treatment, and female sex are the main identified risk factors for neuropsychological impairment following radiation therapy in survivors of CNS malignancies. However, even among patients with these known risk factors, significant variation in levels of impairment remains and little is known to more precisely identify patients at greater risk for radiation-induced late effects at the time of diagnosis when treatment modifications or early interventions might reduce or prevent such effects. Prior studies have found that genetic polymorphisms leading to enzyme functioning differences in GSTs36 and catechol-O-methyltransferase37 are associated with neurocognitive changes following cancer therapy, suggesting that enzymes that clear reactive species or are involved in neurotransmitter metabolism may explain some of this variation in occurrence of late effects.
The most notable finding from this study was a consistent association of GSTM1 null genotype with increases in psychological distress across the domains of anxiety, depression, and global distress scores, compared with patients with the GSTM1 non-null genotype. Although the effects were modest, GSTM1 null genotype remained a significant independent predictor of increased distress in a multivariate model controlling for age at diagnosis, sex, and radiation dosage. This association is particularly interesting because it complements the findings of Barahmani et al., which demonstrated that GSTM1 null genotype was associated with significant declines in full-scale, performance, and verbal intelligence quotient (IQ) scores, compared with GSTM1 non-null genotype in childhood medulloblastoma patients following radiation therapy.36 These results taken together suggest that GSTM1 null genotype may potentially be a predictor for increased risk of neuropsychological impairment resulting from medulloblastoma therapy.
In contrast to the findings of Barahmani et al., we did not find significant associations between genetic polymorphisms and neurocognitive functioning on the CCSS-NCQ. However, we feel that it would be premature to conclude that there are no effects from the investigated polymorphisms on neurocognitive functioning, given the relatively small sample size and homogeneity of cranial radiation doses. In the prior study, patients received risk-adapted radiotherapy and global IQ was used as the outcome measure. In this study, all patients received high doses of craniospinal irradiation and neurocognitive functioning was assessed on a self-report measure that assesses attention, memory, and executive functions (CCSS-NCQ). The specific neurocognitive functions may demonstrate lower thresholds for radiation-induced impairment compared to global IQ. These factors could have overshadowed any effect of the polymorphisms on neurocognitive functioning. Thus, such associations should be further explored in a contemporary sample of medulloblastoma patients receiving risk-adapted radiotherapy, and outcomes should include variables of global and specific abilities.
It is unknown which antioxidant enzymes are most significant in the metabolism of reactive species generated by radiation. Glutathione S-transferases (GSTs) belong to a family of enzymes that catalyze the glutathione conjugation of a variety of compounds, including cytotoxic drugs, their metabolites, and reactive species generated by radiation.38 In particular, these enzymes catalyze the detoxification of alkylating agents and platinum compounds that are used in medulloblastoma chemotherapy.39–42 In 42%–60% of the white population, GSTM1 is deleted and affected individuals do not have expression of the GSTM1 enzyme.32,43 It is possible that this enzyme is of greater importance in the protection of the brain against damage from free radicals, compared with the other investigated enzyme systems, because of its function in processing both radiation- and chemotherapy-induced free radicals. Previous studies have suggested a neuroprotective role of GSTM1, such as a direct antioxidant effect against reactive metabolites from endogenous and exogenous toxins, which damage dopaminergic cells, leading to neurodegenerative processes.44–46 These findings offer support for GSTM1 null genotype as a marker of increased susceptibility to neurotoxins, suggesting that functional GSTM1 enzyme is important in the protection of the brain, whether the toxins are generated from radiation, chemotherapy, or a combination of the 2.
Results from this study were consistent with prior studies demonstrating that younger age and female sex are risk factors for worse functioning. On the CCSS-NCQ, patients who were younger at diagnosis reported greater difficulties with task efficiency and female patients reported greater difficulties with organization.
In a larger sample of all CNS tumor survivors from the CCSS cohort, Armstrong et al. demonstrated that radiation exposure to the temporal lobe is related to neurocognitive and social deficits.34 Therefore, our finding that only radiation dosage to the temporal lobes was associated with differences in Depression scores on the Brief Symptom Inventory was expected. Although radiation dose to the temporal lobes was significantly associated with Depression scores, there was considerable homogeneity in radiation dosages in this small sample, which limits the conclusions that can be drawn from this finding. However, it does suggest that exposure of the temporal lobes to radiation may affect one's functioning.
There are limitations of this present study, which may affect interpretation of the findings. In 22% of survivors, the questionnaires were completed by a proxy. Second, there are no data on the survivors’ neuropsychological functioning prior to diagnosis, which would allow evaluation of the patients’ cognitive reserve prior to therapy and change in functioning after therapy. Third, survivors in the CCSS with a college education were more likely to return DNA specimens; therefore, it is possible that our sample was biased towards more educated survivors who most likely did not have as significant impairment following therapy.31 In addition, factors such as neurological deficits from surgery and post-operative cerebellar mutism, could be important risk factors, but such information is not available from the CCSS data.
Although limited by small sample size, findings from this pilot study, in association with prior findings, suggest the GSTM1 null genotype may be a significant risk factor for neuropsychological impairment in patients being treated for medulloblastoma. A validation study is needed to examine the antioxidant pathways in a larger sample of a more recently treated cohort of patients who underwent formal neuropsychological testing. After at-risk polymorphisms are identified, testing at the time of diagnosis could provide an opportunity to intervene starting at the time of diagnosis. Through the identification of markers of increased susceptibility, it will hopefully be possible to prevent some of the late effects affecting medulloblastoma survivors through individualized treatment approaches, including possible radiation dose reductions, treatment modifications, or prevention strategies, such as treatment with free radical scavenging agents,47 cognitive-behavioral therapy, or educational interventions.
Funding
This work was supported by a Thrasher Research Fund Early Career Award to J.B, National Cancer Institute Cancer Prevention Fellowship to J.B. (R25T CA57730 to Robert M. Chamberlain, Ph.D., Principal Investigator), National Cancer Institute (U24 CA55727 to L.L.R. and Cancer Center Support grant CA21765 to St. Jude Children's Research Hospital), and the American Lebanese Syrian Associated Charities to St. Jude Children's Research Hospital.
Acknowledgments
We thank Linna Zhang and Deborah Marquez-Do, for their assistance in genotyping, and Cindy Power, for administrative assistance. Presented in part: 42nd Congress of the International Society of Pediatric Oncology, Boston, MA, 21–24 October 2010.
Conflict of interest statement. None declared.
References
- 1.Packer RJ, Rorke-Adams LB, Lau C, et al. Embryonal and Pineal Region Tumors. In: Pizzo PA, Poplack D, editors. Principles and Practice of Pediatric Oncology. Philadelphia, PA: Lippincott Williams and Wilkins; 2011. pp. 772–808. [Google Scholar]
- 2.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 3.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 4.Duffner PK. Long-term effects of radiation therapy on cognitive and endocrine function in children with leukemia and brain tumors. Neurologist. 2004;10:293–310. doi: 10.1097/01.nrl.0000144287.35993.96. [DOI] [PubMed] [Google Scholar]
- 5.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 6.Palmer SL, Reddick WE, Gajjar A. Understanding the cognitive impact on children who are treated for medulloblastoma. J Pediatr Psychol. 2007;32:1040–1049. doi: 10.1093/jpepsy/jsl056. [DOI] [PubMed] [Google Scholar]
- 7.Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23:5511–5519. doi: 10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- 8.Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children's Cancer Group study. J Clin Oncol. 2001;19:3470–3476. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- 9.Palmer SL, Gajjar A, Reddick WE, et al. Predicting intellectual outcome among children treated with 35–40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology. 2003;17:548–555. doi: 10.1037/0894-4105.17.4.548. [DOI] [PubMed] [Google Scholar]
- 10.Zebrack BJ, Gurney JG, Oeffinger K, et al. Psychological outcomes in long-term survivors of childhood brain cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2004;22:999–1006. doi: 10.1200/JCO.2004.06.148. [DOI] [PubMed] [Google Scholar]
- 11.Pang JW, Friedman DL, Whitton JA, et al. Employment status among adult survivors in the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2008;50:104–110. doi: 10.1002/pbc.21226. [DOI] [PubMed] [Google Scholar]
- 12.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705–717. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunin-Batson A, Kadan-Lottick N, Zhu L, et al. Predictors of independent living status in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2011;57:1197–1203. doi: 10.1002/pbc.22982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janson C, Leisenring W, Cox C, et al. Predictors of marriage and divorce in adult survivors of childhood cancers: a report from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2009;18:2626–2635. doi: 10.1158/1055-9965.EPI-08-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirchhoff AC, Krull KR, Ness KK, et al. Occupational outcomes of adult childhood cancer survivors: A report from the childhood cancer survivor study. Cancer. 2011;117:3033–3044. doi: 10.1002/cncr.25867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80:251–259. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 17.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Sutton A, Khoury H, Prip-Buus C, et al. The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics. 2003;13:145–157. doi: 10.1097/01.fpc.0000054067.64000.8f. [DOI] [PubMed] [Google Scholar]
- 19.Rosenblum JS, Gilula NB, Lerner RA. On signal sequence polymorphisms and diseases of distribution. Proc Natl Acad Sci USA. 1996;93:4471–4473. doi: 10.1073/pnas.93.9.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu YJ, Diamond AM. Role of glutathione peroxidase 1 in breast cancer: loss of heterozygosity and allelic differences in the response to selenium. Cancer Res. 2003;63:3347–3351. [PubMed] [Google Scholar]
- 21.Ravn-Haren G, Olsen A, Tjonneland A, et al. Associations between GPX1 Pro198Leu polymorphism, erythrocyte GPX activity, alcohol consumption and breast cancer risk in a prospective cohort study. Carcinogenesis. 2006;27:820–825. doi: 10.1093/carcin/bgi267. [DOI] [PubMed] [Google Scholar]
- 22.Zhao W, Diz DI, Robbins ME. Oxidative damage pathways in relation to normal tissue injury. Br J Radiol. 2007;80(Spec No 1):S23–S31. doi: 10.1259/bjr/18237646. [DOI] [PubMed] [Google Scholar]
- 23.McIlwain CC, Townsend DM, Tew KD. Glutathione S-transferase polymorphisms: cancer incidence and therapy. Oncogene. 2006;25:1639–1648. doi: 10.1038/sj.onc.1209373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho AY, Atencio DP, Peters S, et al. Genetic predictors of adverse radiotherapy effects: the Gene-PARE project. Int J Radiat Oncol Biol Phys. 2006;65:646–655. doi: 10.1016/j.ijrobp.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 26.Ali-Osman F, Akande O, Antoun G, Mao JX, Buolamwini J. Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants. Evidence for differential catalytic activity of the encoded proteins. J Biol Chem. 1997;272:10004–10012. doi: 10.1074/jbc.272.15.10004. [DOI] [PubMed] [Google Scholar]
- 27.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 28.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003;21:3255–3261. doi: 10.1200/JCO.2003.01.202. [DOI] [PubMed] [Google Scholar]
- 30.Mertens AC, Mitby PA, Radloff G, et al. XRCC1 and glutathione-S-transferase gene polymorphisms and susceptibility to radiotherapy-related malignancies in survivors of Hodgkin disease. Cancer. 2004;101:1463–1472. doi: 10.1002/cncr.20520. [DOI] [PubMed] [Google Scholar]
- 31.Ness KK, Li C, Mitby PA, et al. Characteristics of responders to a request for a buccal cell specimen among survivors of childhood cancer and their siblings. Pediatr Blood Cancer. 2010;55:165–170. doi: 10.1002/pbc.22513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arand M, Muhlbauer R, Hengstler J, et al. A multiplex polymerase chain reaction protocol for the simultaneous analysis of the glutathione S-transferase GSTM1 and GSTT1 polymorphisms. Anal Biochem. 1996;236:184–186. doi: 10.1006/abio.1996.0153. [DOI] [PubMed] [Google Scholar]
- 33.Krull KR, Gioia G, Ness KK, et al. Reliability and validity of the Childhood Cancer Survivor Study Neurocognitive Questionnaire. Cancer. 2008;113:2188–2197. doi: 10.1002/cncr.23809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong GT, Jain N, Liu W, et al. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro Oncol. 2010;12:1173–1186. doi: 10.1093/neuonc/noq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Derogatis LR. The Brief Symptom Inventory-18 (BSI-18): Administration, scoring and procedures manual. Minneapolis, MN: National Computer Systems; 2000. [Google Scholar]
- 36.Barahmani N, Carpentieri S, Li XN, et al. Glutathione S-Transferase M1 and T1 polymorphisms may predict adverse effects after therapy in children with medulloblastoma. Neuro Oncol. 2009;11:292–300. doi: 10.1215/15228517-2008-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Small BJ, Rawson KS, Walsh E, et al. Catechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;117:1369–1376. doi: 10.1002/cncr.25685. [DOI] [PubMed] [Google Scholar]
- 38.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 39.Dirven HA, van Ommen B, van Bladeren PJ. Involvement of human glutathione S-transferase isoenzymes in the conjugation of cyclophosphamide metabolites with glutathione. Cancer Res. 1994;54:6215–6220. [PubMed] [Google Scholar]
- 40.Goto S, Iida T, Cho S, et al. Overexpression of glutathione S-transferase pi enhances the adduct formation of cisplatin with glutathione in human cancer cells. Free Radic Res. 1999;31:549–558. doi: 10.1080/10715769900301121. [DOI] [PubMed] [Google Scholar]
- 41.Smith MT, Evans CG, Doane-Setzer P, et al. Denitrosation of 1,3-bis(2-chloroethyl)-1-nitrosourea by class mu glutathione transferases and its role in cellular resistance in rat brain tumor cells. Cancer Res. 1989;49:2621–2625. [PubMed] [Google Scholar]
- 42.Weber GF, Waxman DJ. Denitrosation of the anti-cancer drug 1,3-bis(2-chloroethyl)-1- nitrosourea catalyzed by microsomal glutathione S-transferase and cytochrome P450 monooxygenases. Arch Biochem Biophys. 1993;307:369–378. doi: 10.1006/abbi.1993.1602. [DOI] [PubMed] [Google Scholar]
- 43.Garte S, Gaspari L, Alexandrie AK, et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev. 2001;10:1239–1248. [PubMed] [Google Scholar]
- 44.Harada S, Fujii C, Hayashi A, Ohkoshi N. An association between idiopathic Parkinson's disease and polymorphisms of phase II detoxification enzymes: glutathione S-transferase M1 and quinone oxidoreductase 1 and 2. Biochem Biophys Res Commun. 2001;288:887–892. doi: 10.1006/bbrc.2001.5868. [DOI] [PubMed] [Google Scholar]
- 45.Santt O, Baranova H, Albuisson E, Bignon YJ, Lucotte G. Interaction between GSTM1-null and CYP2D6-deficient alleles in the pathogenesis of Parkinson's disease. Eur J Neurol. 2004;11:247–251. doi: 10.1046/j.1468-1331.2003.00756.x. [DOI] [PubMed] [Google Scholar]
- 46.Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem J. 1997;324(Pt 1):25–28. doi: 10.1042/bj3240025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishii J, Natsume A, Wakabayashi T, et al. The free-radical scavenger edaravone restores the differentiation of human neural precursor cells after radiation-induced oxidative stress. Neurosci Lett. 2007;423:225–230. doi: 10.1016/j.neulet.2007.07.029. [DOI] [PubMed] [Google Scholar]