Abstract
Animal modeling for primary brain tumors has undergone constant development over the last 60 years, and significant improvements have been made recently with the establishment of highly invasive glioblastoma models. In this review we discuss the advantages and pitfalls of model development, focusing on chemically induced models, various xenogeneic grafts of human cell lines, including stem cell–like cell lines and biopsy spheroids. We then discuss the development of numerous genetically engineered models available to study mechanisms of tumor initiation and progression. At present it is clear that none of the current animal models fully reflects human gliomas. Yet, the various model systems have provided important insight into specific mechanisms of tumor development. In particular, it is anticipated that a combined comprehensive knowledge of the various models currently available will provide important new knowledge on target identification and the validation and development of new therapeutic strategies.
Keywords: animal models, brain tumor development
Numerous animal models have been developed during the past 60 years to study brain tumor initiation and development. The models can be divided into 3 categories: (1) chemically induced models, (2) genetically engineered mouse (GEM) models, including virally induced models, and (3) xenograft models. Although such models have made significant contributions to our understanding of the mechanisms related to tumor initiation and progression, the knowledge gained from such models has only to a limited extent been translated into more effective treatment principles. In the literature, there are a vast number of reports showing the therapeutic efficacy of novel treatment modalities in various animal models, yet when these are further evaluated in the clinic, they fail in phase II/III clinical trials. The success of treating animal brain tumors in rodents and the failure in a clinical setting is most likely due to several factors: (1) The tumor models do not reflect the biological properties of the patient tumors, (2) the animals used do not display the same pharmacokinetics as humans1–3, and (3) the tumors established do not reflect the cellular heterogeneity of human tumors.
In this review, we will describe the available brain tumor model systems and set them in historical perspective. We believe this is important, since referrals to important observations made in the early days of brain tumor transplantation and animal model development are often forgotten. Then we will discuss the advantages and pitfalls of the various models used and focus on the most important challenges in an effort to delineate the model or combination of models that best reflect human disease.
Animal Models to Study Primary Brain Tumors
There are 2 main reasons for modeling brain tumors in animals. The first is to identify the genetic events and molecular mechanisms that contribute to oncogenesis within the central nervous system (CNS), and the second is to evaluate potential therapeutic strategies.4 For both applications, in vitro models are deemed insufficient. In 2000, Hanahan and Weinberg5 proposed that a series of defining hallmarks characterize cancers, including limitless replication potential, sustained angiogenesis, evasion of apoptosis, self-sufficiency in growth signals, insensitivity to antigrowth signals, and tissue invasion/metastasis. The significance of these traits has withstood the scrutiny of vigorous scientific investigation during the last 10 years, whereas additional hallmarks have emerged (including a cancer-specific metabolism and avoidance of immune destruction).6 Although, to some extent, these traits may be artificially manipulated in vitro, they all involve a complex tumor/host cellular interplay that can be modeled only in vivo. The host components involve anatomical barriers, extracellular matrix molecules, cytokines/growth factors, and cellular determinants such as endothelial cells, tissue-specific progenitor cells, and immune cells. Many in vivo brain tumor models have been established, starting with patient material transplanted into immunocompetent rodents in the 1940s and 1950s (Fig. 1).7–18 Although these early studies failed to establish reliable models due to low engraftment rates, they provided important insights into tumor biology. The experiments revealed first that the success of tumor engraftment decreases with lower histological grade19 and second that good transplantability correlates with poor clinical prognosis (thus, with malignancy).8 These early studies functionally confirmed the quality of the anterior eye chamber and the brain as immunologically privileged organs.
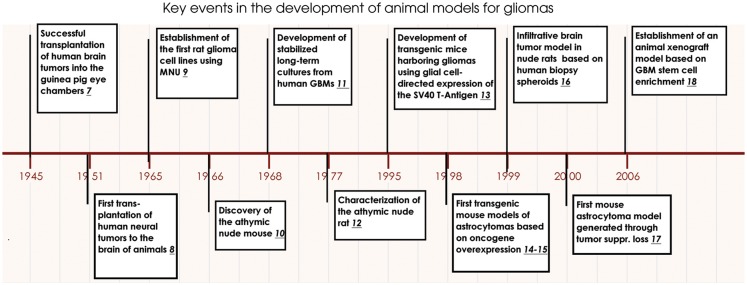
Fig. 1.
Key events in brain tumor modeling in animals. Milestones in brain tumor model development starting with transplantation of human xenografts into immunocompetent rodents in the 1940s through rodent carcinogenesis and human monolayer cell line development in the 1960s, toward the establishment of GEM models in the 1990s, and finally the establishment of xenograft models based on stem cell enrichment.
With the advent of athymic nude mice, the reliability of transplantation of different cancers, including gliomas, proved to be high.20 Starting from the mid- to late 1960s, efforts were made to evaluate both DNA- and RNA-containing viruses for experimental gliomagenesis (reviewed by Thomas and Graham21), which proved to be successful, although the application of these models has not been widespread, probably due to an uneven distribution of recombinant viruses and inconsistent tumor growth characteristics.22 Concomitantly with the development of chemical in vivo carcinogenesis (described below), glioma cell lines were established that could be passaged in culture without the risk of viral infection.23 Traditionally, both syngeneic and xenogeneic glioma models have been established as stable monolayer cell lines; however, they largely differ from human glioma biopsy material at the molecular level18,24–27 and fail to recapitulate the full range of histological traits seen in gliomas.28
Already in 1978, C. B. Wilson suggested that differences in metabolism between rats and humans could account for the inconsistencies between procarbazine sensitivity observed in a rat glioma model and in patients.29 In the same period, other authors questioned the predictive value of inadequately characterized animal models for clinical therapy,30 and it became clear that glioma cell lines growing in nude mice were unreliable in predicting clinical outcomes.31–38 Nevertheless, due to the lack of better alternatives, therapeutic studies employing cell line–based xenografts have been the mainstay of preclinical investigations up to today. However, during the last years, the in-depth characterization of genomic alterations in gliomas has led to a better understanding of the genetic mutations and alterations that underlie the initiation and progression of several glioma subtypes.39,40 Through the application of advanced genomic tools,41,42 several laboratories have created and characterized GEM models of glioma based on defined genetic alterations frequently observed in human tumors. Compared to xenotransplantation models, these in vivo brain tumor models more faithfully reflect important characteristics of several glioma subtypes and have provided insights into specific genetic events that trigger tumor initiation and progression.40
However, at present, it is clear that most if not all of the glioma models used today fail to recapitulate the full genomic and phenotypic signatures of human tumors. Despite these limitations, the last 60 years of model development have been instrumental for our current understanding of how gliomas develop.
Chemically Induced Syngeneic Models
Among the chemically induced models, the rat has been one of the most widely used,43 and since the mid-1970s several rat brain tumor models have been developed.44 Murine,45 canine,46 and feline47 models also exist but have gained less popularity. Spontaneous brain tumors are rarely reported in animals, except in dogs, where the incidence and malignancy types are similar to those seen in humans.48 About 1% of the rat strains that are commonly used for research purposes develop brain tumors spontaneously,49 but experimental tumors may be induced by local, oral, intravenous, or transplacental exposure of N-nitroso compounds into adult or pregnant rats.9,50 For instance, transplacental injection of the carcinogen N-ethylnitrosourea induces formation of various CNS tumors in the progeny, including glioma-like lesions.51–54 However, the time point for transplacental carcinogen exposure is critical, with the highest success rate observed when the fetuses are exposed 18 days after gestation.55 Other chemically induced rat brain tumor models include the syngeneic 9L, C6, F98, and CNS-1.44 In particular the cell lines 9L and C6, derived from chemically induced tumors, have frequently been used in experimental studies in vitro as well as for establishing xenografts in vivo (for review, see Barth and Kaur23). However, chemically induced brain tumors appear to be quite different from human gliomas and have frequently been referred to as “gliosarcomas” or “glioma-like tumors” (Fig. 2). It is therefore questionable how valid they are in human glioma research. In this context, it should also be emphasized that although nitrosourea induction has been very effective in inducing CNS tumors in various rat strains, induction in mice has been less successful56 but can be obtained by N-ethyl-N-nitrosourea exposure in p53 knockout mice.57 Nitrosourea-induced CNS carcinogenesis seems, therefore, also to depend on the species involved. Immunogenicity (dependent mainly on major histocompatibility [MHC] class I expression) and tumor histological traits show large variations among the different chemically induced models.23
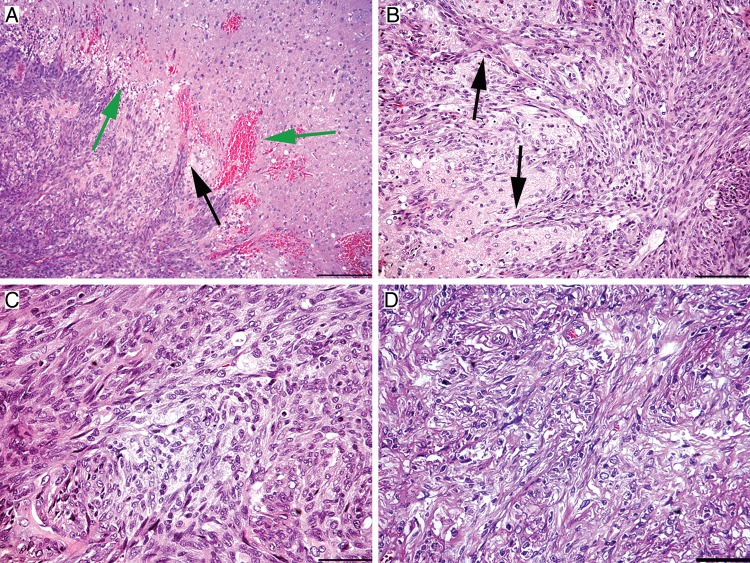
Fig. 2.
Histological features of chemically induced gliosarcoma models. (A and B) Collective infiltration of N-ethylnitrosourea–induced BT4C cells into the brain tissue (frequently referred to as mesenchymal chain movement). Black arrows indicate the leading edge of migrating tumor cells. (A) Green arrows mark peritumoral necrosis and microhemorrhages. (C) Growth pattern of BT4C cells in the tumor bed. (D) Region of a sarcomatous growth with elongated, spindle-shaped cells and extracellular matrix production. All scalebars are 20 µm, except A, which is 50 µm.
Advantages and pitfalls of chemically induced models
A possible utility of these models is that they may allow us to infer knowledge about chemically induced mutagenesis within the CNS. However, although many compounds have been shown to cause tumors in the rat nervous system, no single chemical agent has yet been implicated in human brain tumor development.58,59 This discrepancy probably relates to the fact that in carcinogenesis experiments, rodents have been deliberately exposed to toxic drug doses through a single bolus injection at an early developmental stage52 or to several months of repeated administration as adults.50,60 In contrast, in humans, exposure most likely occurs sporadically at trace levels. In rat carcinogenesis experiments, developing embryos were found to be 50–100 times more susceptible to tumor development than pregnant females.52 Thus, due to differences in chemical doses, exposure times, metabolite preferences, and kinetics among species, the chemically induced models have only provided insight into the etiology of human CNS neoplasms to a limited extent.
In our view, an advantage of chemically induced rodent cell lines grafted in syngeneic immunocompetent animals is that all arms of the immune system (innate and acquired) are present to interact with the developing tumor and also with experimental therapeutics. One drawback, however, is the observed immunogenicity of some rodent glioma cell lines, such as the C6, 9L, and T9,23 where spontaneous tumor rejection may mimic therapeutic efficacy, especially when evaluating treatment modalities that involve the immune system. In the C6 model, investigators have observed that 11% of the allegedly syngeneic Wistar rat hosts survived after intracerebral grafting of 106 cells, but when 104 cells were used, survival increased to 30%.61 Furthermore, when C6 cells were grafted simultaneously into the brain and the flank (inducing a systemic immune response), all the animals survived. In comparison, the 9L/Fischer rat model had a 100% penetrance at all the applied tumor cell numbers. Thus, in studies where the C6/Wistar model was applied to evaluate the effect of immune system–based therapies, the outcome is doubtful.61 The difficulty in this case pertains to the identity of the syngeneic strain, since some investigators claim that C6 cells are syngeneic with BDIX62,63 or with Sprague-Dawley rats64 rather than with Wistar rats. However, subsequent MHC allelotyping has revealed that none of the above-mentioned strains are syngeneic with C6 cells, suggesting that the C6 line was derived from an outbred strain.65
Another drawback regarding these models is in their histological characteristics. Often, they display some level of invasion—however, they fail to show single cell infiltration to the contralateral hemisphere and microvascular abnormalities characteristic of human glioblastoma multiforme (GBM). Although BT4C, F98, and RG-2 tumors have all been characterized as invasive xenografts, these lesions display various extents of local collective invasion (Fig. 2) rather than single cell invasion involving both hemispheres, as is frequently seen in human GBM.66,67
Another point is that most cell lines have been passaged for long time periods in culture and have therefore been subjected to a genetic drift in vitro. Thus, due to a genotypic and phenotypic homogeneity and various extents of immunogenicity, cell line–derived tumors may be overtly sensitive to certain experimental treatments, resulting in an overestimation of therapeutic efficacy.
Up to recently, syngeneic grafts were characterized only histologically and were considered appropriate if they exhibited some growth characteristics of human tumors. However, efforts to characterize these lines at the molecular level have been few.25,68,69 Such a characterization represents a crucial step to enable investigators to select the correct model to test a given drug that may interfere with a specific signal transduction pathway.
In summary, the utility of syngeneic chemically induced gliomas has been hampered by unclear documentation of their sources and by the unavailability of data relating to their genetic profile and phenotype. If one envisions the further use of these systems, a better molecular characterization is mandatory. When these results emerge, one can truly consider both the molecular signature as well as histological characteristics of the chemically induced models, and they may then be better utilized to evaluate therapeutic responses.
Xenograft Models
The establishment of tumors in animals by xenografting tumor material, mostly in the form of cell lines and biopsies, has been highly valuable in the search for mechanisms that determine tumor formation, growth, and progression. In particular, with the advent of immunodeficient animals, important insight has been obtained relating to the growth of human tumors within the CNS.
Human glioma monolayer cell lines established in serum-containing media
Transferral of patient biopsy material to tissue culture flasks and subsequent passaging in monolayers using serum-supplemented media were performed throughout the 1970s and 1980s to generate human glioma cell lines.70 In general, intracerebral inoculation of human glioma cell lines in immunodeficient animals leads to the development of tumors with typical growth characteristics, showing an expansive growth with some strands of invasion from the main tumor mass following mainly the perivascular space. However, true single cell invasion is not a common event (Fig. 3A). The advantages of cell line–based models are (1) good reproducibility with respect to engraftment rate and (2) reliable growth and disease progression. Moreover, immortalized cell lines are readily expanded for an unlimited number of passages in vitro, yielding vast numbers of tumor cells for experimental use. A major disadvantage of human cell line–based models is the genotypic and phenotypic deviation of the obtained lesions from the original patient tumor. Already in 1980, it was emphasized that when using cell lines, the nature of what determines tumorigenicity in the nude mouse is not clearly defined and may represent factors that are not pertinent to human tumors.21 Later it was shown that only 1 out of 7 human GBM cells serially transplanted in nude mice retained their chromosomal profiles,71 and the resulting lesions were histologically more homogeneous than their human origins.20 Genomic alterations in adherent serum-growing cultures often do not correspond well to the genotype of the original tumors. Even primary cultures reflect poorly the genetic imbalances seen in the tumors from which they are derived. Profiles from array comparative genomic hybridization (aCGH)27 as well as whole genome sequencing72 of commonly used GBM cell lines show profiles that are quite distinct from those typically found in primary GBM. For instance, phenotypic and gene expression alterations, clonal selection, and genetic drift occur during the adaptation of tumor cells to monolayer cultures. As an example, the prominent U87MG cell line harbors no fewer than 512 homozygously mutated genes.72
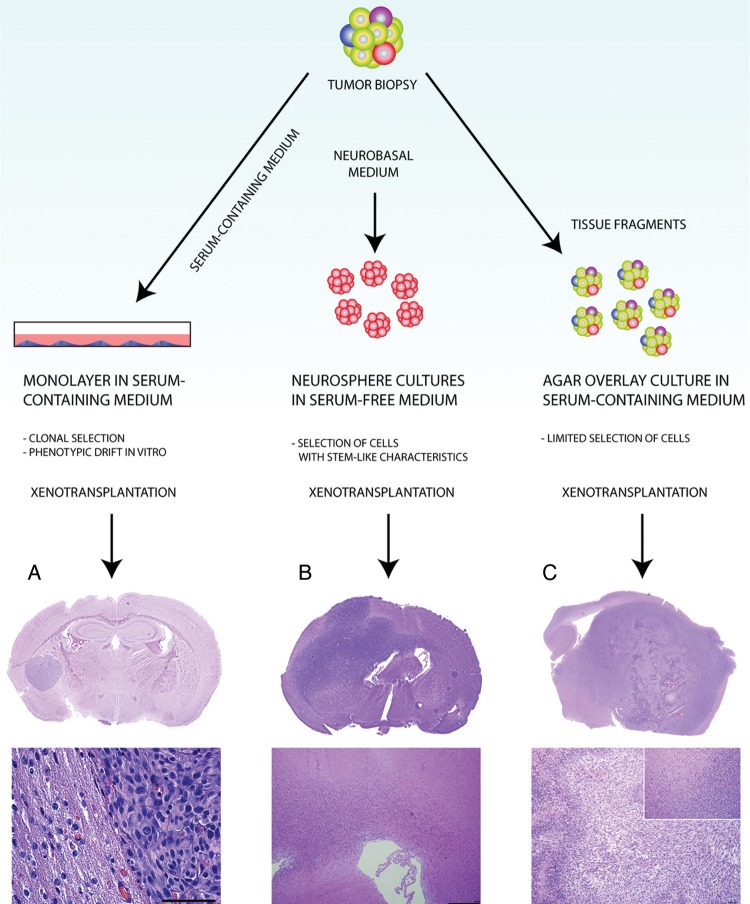
Fig. 3.
Histological features of GBM xenograft models. (A) GBM patient biopsies may be processed to yield adherent cell lines in serum-containing medium, which results in an extensive clonal selection and cellular adaptation process. Xenografts generated from such cell lines will display angiogenic growth and well-defined borders toward the brain tissue. No single tumor-cell invasion is seen (the example here is from the U-87 glioma cell line). (B) Enzymatic dissociation of patient biopsies with subsequent culture in neurobasal serum-free medium selects for a highly tumorigenic subpopulation in human GBM. In several instances, the resulting xenografts are highly infiltrative, following white matter tracts and spreading over the corpus callosum.24 (C) The biopsy xenograft model maintains several tumor cell clones from the biopsy, as well as other cell types and extracellular matrix components. When passaged extensively in immunodeficient animals, the xenografts maintain their invasive growth and develop other characteristics of human GBM, such as dilated vessels, angiogenesis, and pseudopalisading necrosis. Scale bars: 100 µm.
Most often, human cell line–derived xenografts display some levels of angiogenesis and circumscribed growth with variable extents of collective tissue invasion. They show, however, limited single cell infiltration in the brain; and necroses and microvascular abnormalities characteristic of human GBM are usually absent.28,71 Also, aberrant expression of fibrillar and basement membrane collagens occurs when GBM patient biopsies are passaged as monolayers, suggesting a certain (mesenchymal) differentiation in vitro.73,74 The cells often coexpress glial fibrillary acidic protein (GFAP) and collagens, indicating a transdifferentiation of glioma cells rather than a selection of mesenchymal, endothelial, or GFAP-negative glioma cell populations. Integrins such as β2 are similarly upregulated in primary glioma cell cultures, attributed possibly to the involvement of cytokines or growth factors in the cell culture media.75 Other differences in human GBM xenografts relate to markers of immunological significance, such as changes in MHC and FasL expression and cytokine production.76 In conclusion, comprehensive studies show that due to genomic and transcriptomic deviations from GBM in situ, established cell lines are poor models for human tumors.24
Xenografts based on human glioma–derived cell lines passaged in neurobasal medium
With the reemergence of the cancer stem cell hypothesis initially suggested more than 100 years ago,77,78 new cell culture tools have been developed for propagating cancer stem-like cells in serum-free growth media, supplemented with epidermal growth factor (EGF), fibroblast growth factor, and insulin (neurobasal media).79–81 The establishment of such spheres from gliomas has provided novel insight into tumor cells that show stem-cell characteristics82–85 and has proven to be highly successful from a variety of tumors, including pediatric gliomas83 and GBM.86
The sphere cultures have several advantages compared to monolayer cultures. First, glioma spheres have been shown to more closely reiterate the molecular makeup of the original patient tumor; furthermore, molecular profiles are more stable over time.18,87,88 At the same time, however, the derived cell populations may show considerable variations. A recent study has shown that some cell populations exhibit a stem cell–like expression profile similar to the patient tumor, while others exhibit a more mesenchymal phenotype that diverges from the original tumor.89 Furthermore, as in serum supplemented cultures, amplification of EGFR is not a common trait in the sphere cultures.87 Yet, spheres are highly tumorigenic and, more importantly, show variable amounts of angiogenesis and extensive invasion in vivo (Fig. 3B).88,89 Recently histopathological and genomic profiles were obtained from 15 orthotopic xenografts derived from sphere cultures, where a comparison was made with the respective patient tumors. It was shown that the xenografts recapitulated the diverse histology of the patient GBM to a large extent. Moreover, the xenografts could be divided into 2 groups: a discrete nodular phenotype showing little invasion and a diffusely invasive phenotype. This indicates that different courses of tumor development may occur from xenografts established from stem cell–like cultures from different patients.90
The invasive cells most likely have specific molecular signatures that are either characteristic traits of a defined subpopulation within a tumor or are due to an extensive adaptive capacity of certain cells within a tumor.91 The culture of high-grade gliomas as neurospheres is straightforward, and proliferating spheres may occur within the first week of culture. However, neurospheres do not develop successfully from all human gliomas. In fact, some controversy exists, as the success rate varies from 10% to 20% to 100% in different laboratories.79,81,92 An alternative approach has been to adjust the serum-free cell culture system to monolayer cultures.93 This system has been used for the culture of some low-grade gliomas that subsequently engrafted.94 Yet at present there is little molecular evidence indicating that adherent cultures should be preferred to sphere cultures.95 However, the format of adherent cultures may be more amenable for high throughput drug screening purposes.
Clearly, the generation of tumorigenic cell populations from human gliomas through the use of neurosphere cultures has significantly advanced our knowledge of specific subpopulations within human primary tumors. Even though their phenotypes in vivo are not necessarily predictable, they now represent, in combination with engrafting tumor tissue directly into animals, an important tool to study tumorigenicity and progression of human tumors in vivo.
Advantages and pitfalls of human cell line xenograft models
Although cell lines in serum-containing media are readily established from human GBM, it has proven difficult to establish cell lines from low-grade gliomas, including oligodendrogliomas.96–98 The reason for this discrepancy is not known. What is known is that an extensive clonal selection occurs after the transfer of glioma cell supensions into serum-containing media and that a further adaptation takes place during culture. It is therefore highly questionable to what extent extracted biological information from cell line xenografts, grown in serum-supplemented media, can contribute to our understanding of human disease, since cell lines are essentially incapable of recapitulating the complex genetic and phenotypic traits of human gliomas.
With the advent of neurobasal serum-free cultures, expandable, permanent glioma cell lines are starting to show promise, although it remains to be seen whether they retain their genetic and epigenetic profiles after years of culture. Stem cell–like spheres can be propagated and amplified to yield sufficient tumor material for tissue-demanding experimental procedures, such as setting up therapeutic studies in animals. In our mind the most striking difference, compared with cultures growing in serum-supplemented media, is the ability of stem cell–like spheres to establish extensive infiltrative lesions when transplanted into CNS. This is a major improvement in malignant glioma xenograft modeling.
Moreover, low-grade gliomas may also be developed using the neurosphere technique. Recently 2 oligodendroglioma cell lines were established from anaplastic oligodendrogliomas. These cell lines showed a codeletion of chromosomes 1p and 19q and an unbalanced translocation, t(1;19)(q10;p10). One of the cell lines (BT088) formed oligodendrogliomas in immunocompromised mice.98 Follow-up studies using the neurosphere culture method have shown that a cell line also can be established from an IDH1-mutant anaplastic oligoastrocytoma, which, as an orthotopic xenograft, showed rapid expansion in animals.99 These results clearly indicate that brain tumor stem cell lines with an endogenous R132H mutation in IDH1 can develop a tumor-initiating capacity as well as 2-hydroxygluterate production. Although it is too early to draw conclusions, neurobasal media may in the future represent a valuable tool for the development of xenografts from low-grade gliomas.
Human Biopsy Spheroid Xenograft Modeling
Biopsy spheroid xenograft models are based entirely on fresh brain tumor biopsies that are engrafted into immunodeficient animals using various techniques. The most prominent are heterotopic-to-orthotopic xenograft models and orthotopic biopsy spheroid models.
Biopsy spheroid xenograft models
In biopsy spheroid models the biopsy tissue is minced with surgical blades and transferred to flasks on agar-coated surfaces containing standard tissue culture serum-supplemented medium.100 Under these conditions, multicellular aggregates (spheroids) form within a short time. The spheroids maintain the original tissue architecture of the biopsy with endothelial outgrowths and host extracellular matrix components and resident macrophages.100 Other preserved traits include the same DNA ploidy and a similar percentage of proliferating cells as the tumor in situ.100–102 It has also been shown that such spheroids express similar aCGH profiles as the parental tumors.26
As orthotopic xenografts, the GBM biopsy spheroids display diffuse single cell infiltration both when co-cultured with fetal brain tissue in vitro and when implanted into rodent brains.16,28,102–104 The transplantation of GBM spheroids provides a reproducible tumor take rate close to 100% in immunodeficient rats. However, their growth rates and survival may vary among different tumors.16,102,104 In some instances, the initial engraftment may take up to 1 year, with 326 days being the longest period before symptoms appeared in the animals.102 The lesions grow slowly and depend largely on the host brain vasculature for oxygen and nutrients.104 Through further adaptation to the rodent brain by repeated transplantation cycles (giving rise to what is referred to as high-generation xenografts) in some of the tumors, the mitotic index increases, angiogenesis is induced, and necrotic areas and microvascular proliferations appear (Fig. 3C).102,104 Also, the biopsy xenograft model preserves the invasive characteristics of the original tumor in early generations and will, by passaging, accumulate the other histological features of human GBM. Of note, our experience is that xenografts initiated from different patients may show different morphologies. In some GBM, angiogenesis appears rapidly after xenotransplantation, whereas in others, angiogenesis appears after serial animal passages. Moreover, phenotypes may be established that show only infiltrative growth with no signs of angiogenesis, even after repeated transplantations.16 These phenotypes have also been observed in mouse models.105 The differences in morphology are due most likely to differences in the genomic makeup among tumors and thus illustrate the fact that intertumor genomic heterogeneity can lead to different tumor phenotypes in vivo.
Heterotopic-to-orthotopic xenograft models
Invasive, intracranial tumors have also been established by a heterotopic-to-orthotopic approach, where tumors are first established by direct transplantation of human biopsies into the flanks of immunodeficient mice.106 Such xenografts can easily be serially passaged in the animals. Interestingly, when the tumors were established as intracranial tumors, a strong similarity to human tumors was observed, showing highly invasive tumors frequently displaying EGF receptor amplification. Also, DNA copy number and mRNA expression of a large panel of s.c. tumors showed striking similarities to human tumors.107 In these models, the authors observed, in most instances, a lack of necrotic features, and the tumors failed to show endothelial cell proliferation. This may be due to the relatively small tumor size obtained in the mouse brain, and it remains to be shown whether angiogenesis will appear if the tumors are established in a larger brain, eg, that of the nude rat.
Advantages and pitfalls of the biopsy xenograft models
Although continued in vivo passaging may change the histological appearance and gene expression profiles of the tumor cells within the biopsy spheroid xenografts, the original chromosomal aCGH profiles are grossly maintained, including amplification of the EGF receptor.104 Furthermore, recent data in our laboratory indicate that several clonally different subpopulations within the patient tumor are maintained over several in vivo passages, thus recapitulating the genetic heterogeneity of the original tumor in the xenograft (unpublished). This is of particular relevance, as it becomes increasingly clear that the maintenance of GBM heterogeneity in model systems is of paramount importance if we want to understand better how human gliomas grow in animals and how to use such models for reliable drug testing. Thus, the strength of this model relates to the eventual recapitulation of the histological traits of human GBM and the maintenance of genetic aberrations of the original lesion. However, not all xenografts encompass all the histological traits of GBM in situ at the same passage number. Furthermore, initial engraftment may take anywhere between 2 and 11 months.102 It should also be emphasized that biopsy spheroid xenograft models are highly variable, reflecting the heterogeneity of GBM among patients. This is an advantage, since they represent a clinically relevant model system yet a disadvantage in that standardization and experimental planning may be difficult. Of note, several early passage xenograft lesions resemble gliomatosis cerebri rather than GBM, and the acquisition of GBM-like histology usually takes at least 3 repeated passages in rat brains, taking anywhere between 8 to 18 months to acquire representative lesions reflecting human GBM. In the meantime, the patient might have succumbed to the original tumor or, in the case of successful clinical treatment, harbor an extensively selected tumor cell population not representative of the original lesion, and thus of the xenograft. This makes personalized medicine studies, using this animal model, difficult. Another hurdle relates to tumor immunology. Although nude rats have a well-developed innate immune system, they lack a normally developed thymus; thus a proper education of T cells does not occur.108
Therapeutic studies with this animal model performed in our lab have shown that gene therapy and antiangiogenic therapy reveal similar results, as observed in clinical studies, when it comes to treatment effects and survival and escape mechanisms.109,110 These studies clearly highlight the clinical relevance of the biopsy-derived spheroid model.
Similar to the biopsy spheroid xenograft model, heterotopic-to-orthotopic xenografts show DNA copy numbers and mRNA expression signatures similar to those derived from the Cancer Genome Atlas.111 This includes genomic amplification and overexpression of known GBM oncogenes such as EGFR, MDM2, CDK6, and NYCN. These observations underline the value of propagating GBM tumors as subcutaneous xenografts for maintaining key molecular characteristics of human tumors and also for their use in targeting therapies. Moreover, the model may be simpler and more reliable than the spheroid biopsy model. However, when the tumors are grown at an orthotopic location, the lesions lack necrotic features and fail to show endothelial proliferation. Thus, not all histological features of human GBM are maintained.107
Genetically Engineered Mouse Models
Increased understanding of genomic alterations in primary brain tumors has led to the development of highly characterized GEM models of glioma based on specific genetic alterations observed in human tumors. In many instances, GEM models reflect the histopathology, etiology, and biology of human gliomas and represent an important experimental tool to uncover genetic alterations responsible for tumor initiation and progression, as well as for testing new therapeutic strategies.112
A number of comprehensive reviews have been published related to GEM modeling of human glioma,40,113–115 so this section will only highlight the main advances in GEM development. During the last decade GEM models have been established involving complex strategies where multiple genes are gained or lost in specific cell types and at specific developmental time points. For instance, conditional strategies have been developed that regulate gene expression. This can be done in a tissue- and/or time-specific manner.116–118 For instance, tet-regulation or cre-inducible alleles of genes have been introduced that control the timing, duration, and specific cellular compartments for gene expression or inactivation. Moreover, somatic cell gene transfer has been developed using retroviral or adenoviral vectors delivering cre recombinase, eg, the RCAS/Tva system. This GEM model is based on the somatic introduction of multiple genes into a single mouse strain by using a receptor (Tva) for subgroup-A avian sarcoma leukosis viruses (ASLVs).119 Replication-competent avian leukosis virus splice acceptor (RCAS) viral vectors were derived from ASLVs; these vectors were genetically modified to accept insertion of various oncogenes of interest.120
Several GEM models have also been developed by altering key signaling pathways known to be disrupted in human gliomas. These pathways include, among others, those of platelet-derived growth factor, EGFR, Rb, Ras, and Akt.15,121–123 A comprehensive overview of the various GEM models used for brain tumor modeling is provided in Table 1.
Table 1.
GEM models developed for the establishment of primary brain tumors
| Genes Involved | Mouse System Used/Promoter | Tumor Type Modeled | Reference |
|---|---|---|---|
| SV40 T-Ag | Transgenic mice/GFAPR | A | Danks et al.13 |
| v-src | Transgenic mice/GFAP | A, AA, schwannoma | Weissenberger et al.115 |
| PDGF-B | MoMuLV-injected C57B16 mice | GBM, PNET | Uhrbom et al.116 |
| Nf1+/− and p53+/− | Transgenic mice | A, AA, GBM | Reilly et al.17 |
| K-ras and Akt | RCAS/tv-a/nestin | GBM | Holland et al.15 |
| PDGF-B | RCAS/tv-a/ | O | Dai et al.117 |
| GFAP or nestin | OA | ||
| V12Ha-ras | Transgenic mice/GFAP | A, AA, GBM | Ding et al.118,119 |
| Ink4a-Arf −/−, K-ras and Akt | RCAS/tv-a; GFAP or nestin | Spindle cell GBM, giant cell GBM | Uhrbom et al.120 |
| Ink4a-Arf−/− and K-ras | RCAS/tv-a; GFAP or nestin | Sarcoma-like lesions | Uhrbom et al.120 |
| PTEN+/− and pRB inactivation through T121 expression | Transgenic mice/GFAPR | AA | Xiao et al.121 |
| v-erb (EGFR) and Ink4a-Arf−/− p53+/− | Transgenic mice/S100β | O, AO | Weiss et al.122 |
| V12Ha-ras and EGFRvIII | Transgenic mice/GFAP | O, OA | Ding et al.123 |
| PTEN−/− and K-ras | RCAS/tv-a; cre-lox system to delete PTEN/nestin | GBM | Hu et al.124 |
| p53−/− and Nf1−/− | GFAP-driven cre-lox system to delete Nf1 | A, AA, GBM, lymphomas, sarcomas | Zhu et al.125 |
| p53+/− and Nf1−/− | |||
| p53+/− and Nf1+/− | |||
| v-erb (EGFR) | Transgenic rats/S100β | MG, O, AO | Ohgaki et al.126 |
| V12Ha-ras and EGFRvIII (delivered by Ad) | Transgenic mice/GFAP | OA, A, AA, GBM | Wei et al.127 |
| PDGF and Ink4a−/− | RCAS/tv-a | O, AO | Tchougounova et al.128 |
| PDGF and Arf−/− | Expression from GFAP or nestin | ||
| PDGF only | |||
| p53−/− and Nf1−/− and PTEN +/− | GFAP-driven cre-lox system to delete Nf1 or PTEN | AA, GBM | Kwon et al.129 |
| PDGFB and Ink4a-Arf−/− | RCAS/tv-a; cre-lox system to delete PTEN | A, AA, GBM, OA | Hambardzumyan et al.39 |
| PDGFB and Arf−/− | GFAP or nestin | ||
| PDGFB and p53−/− | |||
| PDGFB only | |||
| PDGFB and p53−/− | Transgenic mice/GFAP | GBM, O | Hede et al.130 |
| PDGFB | Tetracycline-regulated expression/GFAP | OA, GBM | Hitoshi et al.131 |
| PDGFB | Ctv-a/CNP | O, AO, OA | Lindberg et al.132 |
| p53−/− | GFAP-driven cre-lox system to delete p53 | AA, GBM, MB | Wang Y et al.133 Wang J et al.99 |
| p53−/− and Nf1−/− | Transgenic mice/cre-lox system to remove stop casette before EGFR mini-genes | GBM | Zhu et al.134 |
| EGFRvIII and | |||
| Ink4a−/− or | |||
| PTEN−/− |
Abbreviations: SV40 T-Ag, simian vacuolating virus 40 large transforming-antigen; V12Ha-ras, constitutively active human Ras; v-src, viral-sarcoma; GFAPR, glial fibrillary acidic protein–5′ regulatory domain; RCAS/tv-a, replication competent avian leukosis virus splice acceptor/receptor for avian leukosis virus subgroup A; O, oligodendroglioma; AO, anaplastic oligodendroglioma; OA, oligoastrocytoma; MB, medulloblastoma; GBM, glioblastoma multiforme; MoMuLV, Moloney murine leukemia virus; CNP, 2′,3′-cyclic nucleotide 3′-phosphodiesterase; Ad, adenovirus.
Low-grade astrocytomas and oligodendrogliomas differ genetically from primary GBM and most other cancers in that they often sustain mutation of the isocitrate dehydrogenase 1 (IDH1) or 2 (IDH2) gene.124 The functional role of these mutations in glioma development is still unknown, but their occurrence appears to be an early mutational event, preceding 1p/19q loss in oligodendrogliomas and p53 mutation in low-grade astrocytomas. Whether IDH mutations can initiate tumor development in a transgenic model or are necessary to sustain progression remains to be shown. Additional genetic changes that are hallmarks of these tumors, such as 1p/19q loss in oligodendroglioma and p53 mutation in astrocytoma, are also missing in these models.15,125
Advantages and pitfalls of GEM models
There are important fundamental differences between GEM models and xenograft models. GEM models address specific molecular events responsible for tumor initiation and progression. Therefore, GEM models have provided important new insight into the molecular events and pathways responsible for tumor initiation, progression, and metastasis. Another major strength of GEM models is their ability to model tumor/stroma interactions that contribute to malignancy, including factors that drive angiogenesis.126 In addition, GEM models show utility for studying the M1-to-M2 transition, through which an immune system that initially works to block tumor formation is usurped to contribute to malignant progression.127 Thus, since GEM models have been established in immunocompetent animals, they have expanded our knowledge of the important role of the microenvironment in tumor biology.128
GEM models also represent an excellent tool to dissect the minimum genetic alterations that are necessary for malignant transformation and to define the interplay among different pathways involved in oncogenesis.4 These models provide insight into the sequence of events of genetic alterations that occurs in response to a specific mutation. However, it is still an open question whether the involved genes truly mirror the tumor-initiating events in human gliomas in a tissue-specific manner (see below). As an example, the loss of RB is associated with retinoblastoma development in humans, whereas RB hemizygous mice develop an array of other cancer types but no retinoblastomas.129
By engineering targeted mutations in a cell- or tissue-specific as well as temporally limited manner, embryonic lethality issues can be avoided. Thus, tools are available to evaluate the role of any oncogene or tumor suppressor gene in neuro-oncogenesis, enabling a manipulation of the molecular factors that are known to be present in human gliomas. The defined genetic alterations that are present in these models represent a double-edged sword. On the one hand, we can study the effects of a given drug on a specific dysfunctional cancer-related pathway in a controlled manner without the interference of other altered interfering genes not known to the investigator. This is a clear advantage, since transplantable cancer cell lines are often poorly characterized at the molecular level. Therefore, these models could be applicable in proof of principle studies of drug selectivity. On the other hand, since the tumors are composed of cells with a number of specific homogeneously genetic changes, they cannot reflect the complete intratumoral genomic and phenotypic heterogeneity found in human gliomas. This is reflected in the vast number of GEM models developed (Table 1), which provides a clear indication that glial tumors can be established by an array of different genetic alterations. Yet, transgenic mice have been successful in recapitulating oligodendrogliomas and low-grade astrocytomas, where transplantable cell lines until recently have not been available.130 Another point is that therapeutic studies are often difficult to perform in GEM models because tumor initiation cannot be controlled unless cell lines derived from the models are used. Thus they are not highly reproducible models in relation to the time point of tumor initiation, which is needed for controlled therapeutic studies.
Glioma Animal Model Future Perspectives
At present it is clear that none of the animal models currently available fully recapitulates human glioma development and progression. Xenografts from chemically induced models as well as normal glioma cell lines grown in serum-supplemented media do not, to a large extent, reflect the genetic background of human gliomas, whereas xenografts derived from neurosphere cultures and from biopsy spheroid cultures as well as several GEM models more faithfully reflect the genotypic and phenotypic changes seen in human GBMs and recapitulate the infiltrative growth of human gliomas (Fig. 3).
The GEM and xenograft models derived from neurosphere cultures and human xenograft biopsies provide us with excellent tools to address a very important question: What are the exact mechanisms that lead to single tumor cell infiltration into the normal brain? With the advent of immunodeficient fluorescence labeled animals expressing enhanced green fluorescent protein and dsRed,131 we are able to separate and identify the tumor/host cellular compartments and study at the single cell level how tumor cells communicate within the host microenvironment132 (Fig. 4). Next, since neurosphere cultures in serum-free media seem to select for infiltrative cell phenotypes, we should be able to determine in detail whether the infiltrative cells within a human glioma represent a single clonal selection of cells within a heterogeneous tumor or are tumor cells that show adaptive capacities to specific microenvironmental cues present in the brain/tumor interfaces, or whether both modes of action occur during tumor progression.91 In this context it should be emphasized that many research groups have focused on identifying phenotypic markers that characterize specific tumor cell phenotypes, which have then been associated with a specific biological property. Frequently, such studies ignore the fact that cells in general may change or adapt their phenotype within specific cellular microenvironments and that stochastic events may provide a phenotypic equilibrium within a heterogeneous population of cancer cells.133 Thus it should be emphasized that the phenotype is not a static entity, and it is clear that a future focus on epigenetic mechanisms will provide valuable clues as to what extent the phenotype can change based on microenvironmental factors.
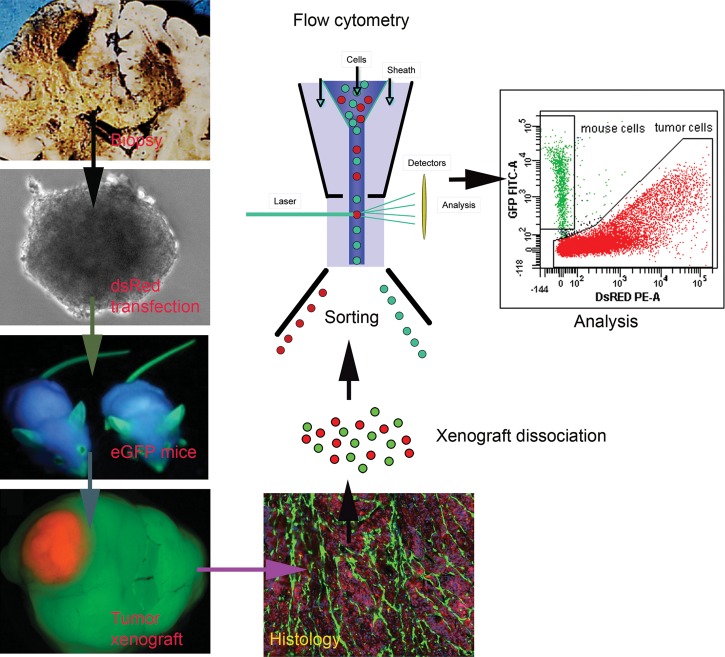
Fig. 4.
Strategy for separation and analysis of the tumor/host cellular compartments. With the development of immunodeficient mice expressing enhanced green fluorescent protein, it is at present possible to completely separate and immunophenotype the cells in the tumor/host cellular compartments.137 This technique shows considerable promise in elucidating mechanisms involved in tumor/host cell communication.
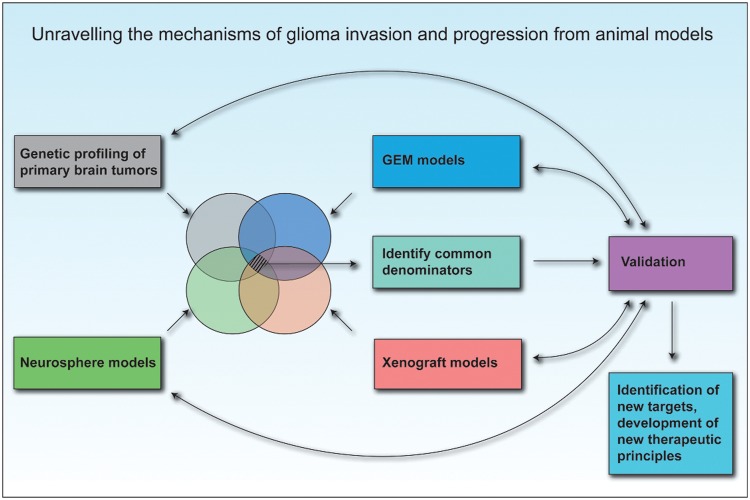
It would also be highly interesting to compare in detail the phenotypes developed in the xenograft models with those obtained in various infiltrative GEM models. This would be of particular importance in the search for the best GEM model that reflects human disease. Such information will also provide valuable clues for delineating specific mechanisms involved in human glioma initiation and progression (Fig. 5). If such comparisons are performed in detail, it could, in the coming years, lead to the identification of new and effective treatment modalities that will specifically target the tumor-initiating cells as well as the infiltrative tumor cell within human gliomas.
Fig. 5.
Unraveling the mechanisms of tumor invasion and progression from animal models. Further development in the field of neuro-oncology must integrate current molecular knowledge from human tumors as well as biological knowledge from GEM and spheroid and stem/progenitor cell–based xenotransplantation models. The search for common denominators will most likely lead to the identification of robust therapeutic targets that should eventually be validated in human GBM.
Funding
This work was supported by the Norwegian Cancer Society, the Norwegian Research Council, Innovest AS, Strategic Research Programme, Helse-Vest, Haukeland University Hospital, the Bergen Translational Research Program, the Fonds National de la Recherche (FNR) of Luxembourg, and the Centre de Recherche Public de la Santé Luxembourg.
Acknowledgments
We thank Tove Johansen and Lene Nybø for technical assistance.
Conflict of interest statement. None declared.
References
- 1.Kerbel RS. What is the optimal rodent model for anti-tumor drug testing? Cancer Metastasis Rev. 1998;17(3):301–304. doi: 10.1023/a:1006152915959. [DOI] [PubMed] [Google Scholar]
- 2.Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived—but they can be improved. Cancer Biol Ther. 2003;2(4 suppl 1):S134–S139. [PubMed] [Google Scholar]
- 3.Peterson JK, Houghton PJ. Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur J Cancer. 2004;40(6):837–844. doi: 10.1016/j.ejca.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Hesselager G, Holland EC. Using mice to decipher the molecular genetics of brain tumors. Neurosurgery. 2003;53(3):685–694. doi: 10.1227/01.neu.0000081304.57547.b5. discussion 695. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Greene HS, Arnold HL., Jr The homologous and heterologous transplantation of brain and brain tumors. J Neurosurg. 1945;2(4):315–331. [Google Scholar]
- 8.Greene HS. The significance of the heterologous transplantability of human cancer. Cancer. 1952;5(1):24–44. doi: 10.1002/1097-0142(195201)5:1<24::aid-cncr2820050106>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Druckrey H, Ivankovic S, Preussmann R. [Selective induction of malignant tumors in the brain and spinal cord of rats by N-methyl-N-nitrosourea] Z Krebsforsch. 1965;66:389–408. [PubMed] [Google Scholar]
- 10.Flanagan SP. ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet Res. 1966;8(3):295–309. doi: 10.1017/s0016672300010168. [DOI] [PubMed] [Google Scholar]
- 11.Ponten J, Macintyre EH. Long term culture of normal and neoplastic human glia. Acta Pathol Microbiol Scand. 1968;74(4):465–486. doi: 10.1111/j.1699-0463.1968.tb03502.x. [DOI] [PubMed] [Google Scholar]
- 12.Festing MF, May D, Connors TA, Lovell D, Sparrow S. An athymic nude mutation in the rat. Nature. 1978;274(5669):365–366. doi: 10.1038/274365a0. [DOI] [PubMed] [Google Scholar]
- 13.Danks RA, Orian JM, Gonzales MF, et al. Transformation of astrocytes in transgenic mice expressing SV40 T antigen under the transcriptional control of the glial fibrillary acidic protein promoter. Cancer Res. 1995;55(19):4302–4310. [PubMed] [Google Scholar]
- 14.Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12(23):3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25(1):55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 16.Engebraaten O, Hjortland GO, Hirschberg H, Fodstad O. Growth of precultured human glioma specimens in nude rat brain. J Neurosurg. 1999;90(1):125–132. doi: 10.3171/jns.1999.90.1.0125. [DOI] [PubMed] [Google Scholar]
- 17.Reilly KM, Loisel DA, Bronson RT, McLaughlin ME, Jacks T. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat Genet. 2000;26(1):109–113. doi: 10.1038/79075. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Krementz ET, Greene HS. Heterologous transplantation of human neural tumors. Cancer. 1953;6(1):100–110. doi: 10.1002/1097-0142(195301)6:1<100::aid-cncr2820060110>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Jones TR, Bigner SH, Schold SC, Jr, Eng LF, Bigner DD. Anaplastic human gliomas grown in athymic mice. Morphology and glial fibrillary acidic protein expression. Am J Pathol. 1981;105(3):316–327. [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas DGT, Graham DI. Brain Tumours: Scientific Basis, Clinical Investigation, and Current Therapy. London/Boston: Butterworth; 1980. xiv, 382 p. [Google Scholar]
- 22.Peterson DL, Sheridan PJ, Brown WE., Jr Animal models for brain tumors: historical perspectives and future directions. J Neurosurg. 1994;80(5):865–876. doi: 10.3171/jns.1994.80.5.0865. [DOI] [PubMed] [Google Scholar]
- 23.Barth RF, Kaur B. Rat brain tumor models in experimental neuro-oncology: the C6, 9L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. J Neurooncol. 2009;94(3):299–312. doi: 10.1007/s11060-009-9875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li A, Walling J, Kotliarov Y, et al. Genomic changes and gene expression profiles reveal that established glioma cell lines are poorly representative of primary human gliomas. Mol Cancer Res. 2008;6(1):21–30. doi: 10.1158/1541-7786.MCR-07-0280. [DOI] [PubMed] [Google Scholar]
- 25.Sibenaller ZA, Etame AB, Ali MM, et al. Genetic characterization of commonly used glioma cell lines in the rat animal model system. Neurosurg Focus. 2005;19(4):E1. doi: 10.3171/foc.2005.19.4.2. [DOI] [PubMed] [Google Scholar]
- 26.De Witt Hamer PC, Van Tilborg AA, Eijk PP, et al. The genomic profile of human malignant glioma is altered early in primary cell culture and preserved in spheroids. Oncogene. 2008;27(14):2091–2096. doi: 10.1038/sj.onc.1210850. [DOI] [PubMed] [Google Scholar]
- 27.Ernst A, Hofmann S, Ahmadi R, et al. Genomic and expression profiling of glioblastoma stem cell-like spheroid cultures identifies novel tumor-relevant genes associated with survival. Clin Cancer Res. 2009;15(21):6541–6550. doi: 10.1158/1078-0432.CCR-09-0695. [DOI] [PubMed] [Google Scholar]
- 28.Mahesparan R, Read TA, Lund-Johansen M, Skaftnesmo KO, Bjerkvig R, Engebraaten O. Expression of extracellular matrix components in a highly infiltrative in vivo glioma model. Acta Neuropathol. 2003;105(1):49–57. doi: 10.1007/s00401-002-0610-0. [DOI] [PubMed] [Google Scholar]
- 29.Bigner DD, Laerum OD, Rajewsky MF. Biology of Brain Tumors. Geneva: International Union Against Cancer; 1978. International Union against Cancer. 209 p. [Google Scholar]
- 30.Neuwelt EA, Barnett P, Barranger J, McCormick C, Pagel M, Frenkel E. Inability of dimethyl sulfoxide and 5-fluorouracil to open the blood-brain barrier. Neurosurgery. 1983;12(1):29–34. doi: 10.1227/00006123-198301000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Schold SC, Jr, Friedman HS, Bigner DD. Therapeutic profile of the human glioma line D-54 MG in athymic mice. Cancer Treat Rep. 1987;71(9):849–850. [PubMed] [Google Scholar]
- 32.Schold SC, Jr, Friedman HS, Bjornsson TD, Bigner DD. Treatment of human glioma and medulloblastoma tumor lines in athymic mice with diaziquone and diaziquone-based drug combinations. Cancer Res. 1984;44(6):2352–2357. [PubMed] [Google Scholar]
- 33.Schold SC, Jr, Bigner DD. Treatment of five subcutaneous human glioma tumor lines in athymic mice with carmustine, procarbazine, and mithramycin. Cancer Treat Rep. 1983;67(9):811–819. [PubMed] [Google Scholar]
- 34.Eyre HJ, Eltringham JR, Gehan EA, et al. Randomized comparisons of radiotherapy and carmustine versus procarbazine versus dacarbazine for the treatment of malignant gliomas following surgery: a Southwest Oncology Group Study. Cancer Treat Rep. 1986;70(9):1085–1090. [PubMed] [Google Scholar]
- 35.Bullard DE, Schold SC, Jr, Bigner SH, Bigner DD. Growth and chemotherapeutic response in athymic mice of tumors arising from human glioma-derived cell lines. J Neuropathol Exp Neurol. 1981;40(4):410–427. doi: 10.1097/00005072-198107000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Edwards MS, Levin VA, Wilson CB. Brain tumor chemotherapy: an evaluation of agents in current use for phase II and III trials. Cancer Treat Rep. 1980;64(12):1179–1205. [PubMed] [Google Scholar]
- 37.Chamberlain MC, Prados MD, Silver P, Levin VA. A phase II trial of oral melphalan in recurrent primary brain tumors. Am J Clin Oncol. 1988;11(1):52–54. doi: 10.1097/00000421-198802000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Dinapoli RP, Brown LD, Arusell RM, et al. Phase III comparative evaluation of PCNU and carmustine combined with radiation therapy for high-grade glioma. J Clin Oncol. 1993;11(7):1316–1321. doi: 10.1200/JCO.1993.11.7.1316. [DOI] [PubMed] [Google Scholar]
- 39.Hambardzumyan D, Amankulor NM, Helmy KY, Becher OJ, Holland EC. Modeling adult gliomas using RCAS/t-va technology. Transl Oncol. 2009;2(2):89–95. doi: 10.1593/tlo.09100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hambardzumyan D, Parada LF, Holland EC, Charest A. Genetic modeling of gliomas in mice: new tools to tackle old problems. Glia. 2011;59(8):1155–1168. doi: 10.1002/glia.21142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLendon R, Friedman A, Bigner D, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cortese Hassett AL, Locker JD, Kunz HW, Gill TJ., 3rd Molecular analysis of MHC-linked genes affecting growth and development. Transplant Proc. 1989;21(1 Pt 1):563–564. [PubMed] [Google Scholar]
- 44.Mohr U, Rittinghausen S, Takenaka S, Ernst H, Dungworth DL, Pylev LN. Pathology of tumours in laboratory animals. Tumours of the lower respiratory tract and pleura in the rat. IARC Sci Publ. 1990;(99):275–299. [PubMed] [Google Scholar]
- 45.Ausman JI, Shapiro WR, Rall DP. Studies on the chemotherapy of experimental brain tumors: development of an experimental model. Cancer Res. 1970;30(9):2394–2400. [PubMed] [Google Scholar]
- 46.Berens ME, Giese A, Shapiro JR, Coons SW. Allogeneic astrocytoma in immune competent dogs. Neoplasia. 1999;1(2):107–112. doi: 10.1038/sj.neo.7900020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ernestus RI, Wilmes LJ, Hoehn-Berlage M. Identification of intracranial liqor metastases of experimental stereotactically implanted brain tumors by the tumor-selective MRI contrast agent MnTPPS. Clin Exp Metastasis. 1992;10(5):345–350. doi: 10.1007/BF00058174. [DOI] [PubMed] [Google Scholar]
- 48.Gavin PR, Kraft SL, Wendling LR, Miller DL. Canine spontaneous brain tumors—a large animal model for BNCT. Strahlenther Onkol. 1989;165(2–3):225–228. [PubMed] [Google Scholar]
- 49.Dagle GE, Zwicker GM, Renne RA. Morphology of spontaneous brain tumors in the rat. Vet Pathol. 1979;16(3):318–324. doi: 10.1177/030098587901600305. [DOI] [PubMed] [Google Scholar]
- 50.Schmidek HH, Nielsen SL, Schiller AL, Messer J. Morphological studies of rat brain tumors induced by N-nitrosomethylurea. J Neurosurg. 1971;34(3):335–340. doi: 10.3171/jns.1971.34.3.0335. [DOI] [PubMed] [Google Scholar]
- 51.Druckrey H, Landschutz C. [Transplacental and neonatal carcinogenesis by ethylnitrosobiuret (ENBU) in BD IX-rats] Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1971;76(1):45–58. doi: 10.1007/BF00304286. [DOI] [PubMed] [Google Scholar]
- 52.Ivankovic S, Druckrey H. [Transplacental induction of malignant tumors of the nervous system. I. Ethyl-nitroso-urea (ENU) in BD IX rats] Z Krebsforsch. 1968;71(4):320–360. [PubMed] [Google Scholar]
- 53.Swenberg JA, Koestner A, Wechsler W. The induction of tumors of the nervous system in rats with intravenous methylnitrosourea (MNU) J Neuropathol Exp Neurol. 1971;30(1):122. [PubMed] [Google Scholar]
- 54.Lantos PL, Pilkington GJ. Neuroblasts in cerebral tumors induced by ethylnitrosourea in rats. A fine structrual study. Virchows Arch B Cell Pathol. 1977;25(3):243–259. doi: 10.1007/BF02889437. [DOI] [PubMed] [Google Scholar]
- 55.Laerum OD, Rajewsky MF. Neoplastic transformation of fetal rat brain cells in culture after exposure to ethylnitrosourea in vivo. J Natl Cancer Inst. 1975;55(5):1177–1187. doi: 10.1093/jnci/55.5.1177. [DOI] [PubMed] [Google Scholar]
- 56.Wechsler W, Ramadan MA, Pfeiffer SE. Morphologic and biochemical characteristics of transplantable neurogenic tumors induced by N-ethyl-N-nitrosourea in inbred BD IX rats. J Natl Cancer Inst. 1979;62(4):811–817. [PubMed] [Google Scholar]
- 57.Oda H, Zhang S, Tsurutani N, et al. Loss of p53 is an early event in induction of brain tumors in mice by transplacental carcinogen exposure. Cancer Res. 1997;57(4):646–650. [PubMed] [Google Scholar]
- 58.Samkange-Zeeb F, Schlehofer B, Schuz J, et al. Occupation and risk of glioma, meningioma and acoustic neuroma: results from a German case-control study (interphone study group, Germany) Cancer Epidemiol. 2010;34(1):55–61. doi: 10.1016/j.canep.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Samanic CM, De Roos AJ, Stewart PA, Rajaraman P, Waters MA, Inskip PD. Occupational exposure to pesticides and risk of adult brain tumors. Am J Epidemiol. 2008;167(8):976–985. doi: 10.1093/aje/kwm401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benda P, Lightbody J, Sato G, Levine L, Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968;161(839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- 61.Parsa AT, Chakrabarti I, Hurley PT, et al. Limitations of the C6/Wistar rat intracerebral glioma model: implications for evaluating immunotherapy. Neurosurgery. 2000;47(4):993–999. doi: 10.1097/00006123-200010000-00050. discussion 999–1000. [DOI] [PubMed] [Google Scholar]
- 62.Resnicoff M, Sell C, Rubini M, et al. Rat glioblastoma cells expressing an antisense RNA to the insulin-like growth factor-1 (IGF-1) receptor are nontumorigenic and induce regression of wild-type tumors. Cancer Res. 1994;54(8):2218–2222. [PubMed] [Google Scholar]
- 63.Resnicoff M, Tjuvajev J, Rotman HL, et al. Regression of C6 rat brain tumors by cells expressing an antisense insulin-like growth factor I receptor RNA. J Exp Ther Oncol. 1996;1(6):385–389. [PubMed] [Google Scholar]
- 64.Kondziolka D, Lunsford LD, Claassen D, Pandalai S, Maitz AH, Flickinger JC. Radiobiology of radiosurgery: part II. The rat C6 glioma model. Neurosurgery. 1992;31(2):280–287. doi: 10.1227/00006123-199208000-00013. discussion 287–288. [DOI] [PubMed] [Google Scholar]
- 65.Beutler AS, Banck MS, Wedekind D, Hedrich HJ. Tumor gene therapy made easy: allogeneic major histocompatibility complex in the C6 rat glioma model. Hum Gene Ther. 1999;10(1):95–101. doi: 10.1089/10430349950019228. [DOI] [PubMed] [Google Scholar]
- 66.He J, Yin Y, Luster TA, Watkins L, Thorpe PE. Antiphosphatidylserine antibody combined with irradiation damages tumor blood vessels and induces tumor immunity in a rat model of glioblastoma. Clin Cancer Res. 2009;15(22):6871–6880. doi: 10.1158/1078-0432.CCR-09-1499. [DOI] [PubMed] [Google Scholar]
- 67.Mariani CL, Kouri JG, Streit WJ. Rejection of RG-2 gliomas is mediated by microglia and T lymphocytes. J Neurooncol. 2006;79(3):243–253. doi: 10.1007/s11060-006-9137-x. [DOI] [PubMed] [Google Scholar]
- 68.Gunnersen JM, Spirkoska V, Smith PE, Danks RA, Tan SS. Growth and migration markers of rat C6 glioma cells identified by serial analysis of gene expression. Glia. 2000;32(2):146–154. [PubMed] [Google Scholar]
- 69.Schlegel J, Piontek G, Kersting M, et al. The p16/Cdkn2a/Ink4a gene is frequently deleted in nitrosourea-induced rat glial tumors. Pathobiology. 1999;67(4):202–206. doi: 10.1159/000028073. [DOI] [PubMed] [Google Scholar]
- 70.Westphal M, Meissner H. Establishing human glioma-derived cell lines. Methods Cell Biol. 1998;57:147–165. doi: 10.1016/s0091-679x(08)61576-9. [DOI] [PubMed] [Google Scholar]
- 71.Bigner SH, Schold SC, Friedman HS, Mark J, Bigner DD. Chromosomal composition of malignant human gliomas through serial subcutaneous transplantation in athymic mice. Cancer Genet Cytogenet. 1989;40(1):111–120. doi: 10.1016/0165-4608(89)90152-0. [DOI] [PubMed] [Google Scholar]
- 72.Clark MJ, Homer N, O'Connor BD, et al. U87MG decoded: the genomic sequence of a cytogenetically aberrant human cancer cell line. PLoS Genet. 2010;6(1):e1000832. doi: 10.1371/journal.pgen.1000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McKeever PE, Davenport RD, Shakui P. Patterns of antigenic expression of human glioma cells. Crit Rev Neurobiol. 1991;6(2):119–147. [PubMed] [Google Scholar]
- 74.Paulus W, Huettner C, Tonn JC. Collagens, integrins and the mesenchymal drift in glioblastomas: a comparison of biopsy specimens, spheroid and early monolayer cultures. Int J Cancer. 1994;58(6):841–846. doi: 10.1002/ijc.2910580616. [DOI] [PubMed] [Google Scholar]
- 75.Arnaout MA, Michishita M, Sharma CP. On the regulation of beta 2 integrins. Adv Exp Med Biol. 1992;323:171–179. doi: 10.1007/978-1-4615-3396-2_22. [DOI] [PubMed] [Google Scholar]
- 76.Anderson RC, Elder JB, Brown MD, et al. Changes in the immunologic phenotype of human malignant glioma cells after passaging in vitro. Clin Immunol. 2002;102(1):84–95. doi: 10.1006/clim.2001.5152. [DOI] [PubMed] [Google Scholar]
- 77.Hansemann D. Ueber asymmetrische Zelltheilung in Epithelkrebsen und deren biologische Bedeutung. Virchows Arch Pathol Anat. 1890;119:299–326. [Google Scholar]
- 78.Boveri T. Zur Frage der Enstehung maligner Tumoren. Jena: Gustav Fischer Verlag; 1914. [Google Scholar]
- 79.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12(11):4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 81.Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11(5):951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- 82.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 83.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA. 2003;100(25):15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39(3):193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 85.Tunici P, Bissola L, Lualdi E, et al. Genetic alterations and in vivo tumorigenicity of neurospheres derived from an adult glioblastoma. Mol Cancer. 2004;3:25. doi: 10.1186/1476-4598-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kelly JJ, Blough MD, Stechishin OD, et al. Oligodendroglioma cell lines containing t(1;19)(q10;p10) Neuro Oncol. 2010;12:745–755. doi: 10.1093/neuonc/noq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen R, Nishimura MC, Bumbaca SM, et al. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell. 2010;17(4):362–375. doi: 10.1016/j.ccr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 88.Gunther HS, Schmidt NO, Phillips HS, et al. Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene. 2008;27(20):2897–2909. doi: 10.1038/sj.onc.1210949. [DOI] [PubMed] [Google Scholar]
- 89.Schulte A, Gunther HS, Phillips HS, et al. A distinct subset of glioma cell lines with stem cell-like properties reflects the transcriptional phenotype of glioblastomas and overexpresses CXCR4 as therapeutic target. Glia. 2011;59(4):590–602. doi: 10.1002/glia.21127. [DOI] [PubMed] [Google Scholar]
- 90.Wakimoto H, Mohapatra G, Kanai R, et al. Maintenance of primary tumor phenotype and genotype in glioblastoma stem cells. Neuro Oncol. 2011;14:132–144. doi: 10.1093/neuonc/nor195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bjerkvig R, Johansson M, Miletic H, Niclou SP. Cancer stem cells and angiogenesis. Semin Cancer Biol. 2009;19(5):279–284. doi: 10.1016/j.semcancer.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 92.Wan F, Zhang S, Xie R, et al. The utility and limitations of neurosphere assay, CD133 immunophenotyping and side population assay in glioma stem cell research. Brain Pathol. 2010;20(5):877–889. doi: 10.1111/j.1750-3639.2010.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pollard SM, Yoshikawa K, Clarke ID, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4(6):568–580. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 94.Persson AI, Petritsch C, Swartling FJ, et al. Non-stem cell origin for oligodendroglioma. Cancer Cell. 2010;18(6):669–682. doi: 10.1016/j.ccr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reynolds BA, Vescovi AL. Brain cancer stem cells: Think twice before going flat. Cell Stem Cell. 2009;5(5):466–467. doi: 10.1016/j.stem.2009.10.017. author reply 468–469. [DOI] [PubMed] [Google Scholar]
- 96.Grippo MC, Penteado PF, Carelli EF, Cruz-Hofling MA, Verinaud L. Establishment and partial characterization of a continuous human malignant glioma cell line: NG97. Cell Mol Neurobiol. 2001;21(4):421–428. doi: 10.1023/A:1012662423863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kruse CA, Varella-Garcia M, Kleinschmidt-Demasters BK, et al. Receptor expression, cytogenetic, and molecular analysis of six continuous human glioma cell lines. In Vitro Cell Dev Biol Anim. 1998;34(6):455–462. doi: 10.1007/s11626-998-0078-x. [DOI] [PubMed] [Google Scholar]
- 98.Kelly JJ, Blough MD, Stechishin OD, et al. Oligodendroglioma cell lines containing t(1;19)(q10;p10) Neuro Oncol. 2010;12:745–755. doi: 10.1093/neuonc/noq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luchman HA, Stechishin OD, Dang NH, et al. An in vivo patient-derived model of endogenous IDH1-mutant glioma. Neuro Oncol. 2012;14(2):184–191. doi: 10.1093/neuonc/nor207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bjerkvig R, Tonnesen A, Laerum OD, Backlund EO. Multicellular tumor spheroids from human gliomas maintained in organ culture. J Neurosurg. 1990;72(3):463–475. doi: 10.3171/jns.1990.72.3.0463. [DOI] [PubMed] [Google Scholar]
- 101.Nygaard SJ, Haugland HK, Laerum OD, Lund-Johansen M, Bjerkvig R, Tysnes OB. Dynamic determination of human glioma invasion in vitro. J Neurosurg. 1998;89(3):441–447. doi: 10.3171/jns.1998.89.3.0441. [DOI] [PubMed] [Google Scholar]
- 102.Wang J, Miletic H, Sakariassen PO, et al. A reproducible brain tumour model established from human glioblastoma biopsies. BMC Cancer. 2009;9:465. doi: 10.1186/1471-2407-9-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Backlund EO, Bjerkvig R. Stereotactic biopsies as a model for studying the interaction between gliomas and normal brain tissue in vitro. J Neurosurg Sci. 1989;33(1):31–33. [PubMed] [Google Scholar]
- 104.Sakariassen PO, Prestegarden L, Wang J, et al. Angiogenesis-independent tumor growth mediated by stem-like cancer cells. Proc Natl Acad Sci USA. 2006;103(44):16466–16471. doi: 10.1073/pnas.0607668103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Claes A, Schuuring J, Boots-Sprenger S, et al. Phenotypic and genotypic characterization of orthotopic human glioma models and its relevance for the study of anti-glioma therapy. Brain Pathol. 2008;18(3):423–433. doi: 10.1111/j.1750-3639.2008.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Taillandier L, Antunes L, Angioi-Duprez KS. Models for neuro-oncological preclinical studies: solid orthotopic and heterotopic grafts of human gliomas into nude mice. J Neurosci Methods. 2003;125(1–2):147–157. doi: 10.1016/s0165-0270(03)00043-8. [DOI] [PubMed] [Google Scholar]
- 107.Giannini C, Sarkaria JN, Saito A, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 2005;7(2):164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rolstad B. The athymic nude rat: an animal experimental model to reveal novel aspects of innate immune responses? Immunol Rev. 2001;184:136–144. doi: 10.1034/j.1600-065x.2001.1840113.x. [DOI] [PubMed] [Google Scholar]
- 109.Keunen O, Johansson M, Oudin A, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci USA. 2011;108(9):3749–3754. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huszthy PC, Giroglou T, Tsinkalovsky O, et al. Remission of invasive, cancer stem-like glioblastoma xenografts using lentiviral vector-mediated suicide gene therapy. PLoS One. 2009;4(7):e6314. doi: 10.1371/journal.pone.0006314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hodgson JG, Yeh RF, Ray A, et al. Comparative analyses of gene copy number and mRNA expression in glioblastoma multiforme tumors and xenografts. Neuro Oncol. 2009;11(5):477–487. doi: 10.1215/15228517-2008-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10(5):319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- 113.Momota H, Holland EC. Mouse models of CNS embryonal tumors. Brain Tumor Pathol. 2009;26(2):43–50. doi: 10.1007/s10014-009-0253-0. [DOI] [PubMed] [Google Scholar]
- 114.Fomchenko EI, Holland EC. Mouse models of brain tumors and their applications in preclinical trials. Clin Cancer Res. 2006;12(18):5288–5297. doi: 10.1158/1078-0432.CCR-06-0438. [DOI] [PubMed] [Google Scholar]
- 115.Wee B, Charles N, Holland EC. Animal models to study cancer-initiating cells from glioblastoma. Front Biosci. 2011;17:2243–2258. doi: 10.2741/3851. [DOI] [PubMed] [Google Scholar]
- 116.Macleod KF, Jacks T. Insights into cancer from transgenic mouse models. J Pathol. 1999;187(1):43–60. doi: 10.1002/(SICI)1096-9896(199901)187:1<43::AID-PATH246>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 117.Talmadge JE, Hood KC, Zobel LC, Shafer LR, Coles M, Toth B. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int Immunopharmacol. 2007;7(2):140–151. doi: 10.1016/j.intimp.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 118.Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 2007;170(3):793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Federspiel MJ, Bates P, Young JA, Varmus HE, Hughes SH. A system for tissue-specific gene targeting: transgenic mice susceptible to subgroup A avian leukosis virus-based retroviral vectors. Proc Natl Acad Sci USA. 1994;91(23):11241–11245. doi: 10.1073/pnas.91.23.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Greenhouse JJ, Petropoulos CJ, Crittenden LB, Hughes SH. Helper-independent retrovirus vectors with Rous-associated virus type O long terminal repeats. J Virol. 1988;62(12):4809–4812. doi: 10.1128/jvi.62.12.4809-4812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guha A. Ras activation in astrocytomas and neurofibromas. Can J Neurol Sci. 1998;25(4):267–281. doi: 10.1017/s0317167100034272. [DOI] [PubMed] [Google Scholar]
- 122.Ueki K, Ono Y, Henson JW, Efird JT, von Deimling A, Louis DN. CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res. 1996;56(1):150–153. [PubMed] [Google Scholar]
- 123.Henson JW, Schnitker BL, Correa KM, et al. The retinoblastoma gene is involved in malignant progression of astrocytomas. Ann Neurol. 1994;36(5):714–721. doi: 10.1002/ana.410360505. [DOI] [PubMed] [Google Scholar]
- 124.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15(15):1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2012;60(3):502–514. doi: 10.1002/glia.21264. [DOI] [PubMed] [Google Scholar]
- 127.DeNardo DG, Barreto JB, Andreu P, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Charles N, Holland EC. The perivascular niche microenvironment in brain tumor progression. Cell Cycle. 2010;9(15):3012–3021. doi: 10.4161/cc.9.15.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse. Nature. 1992;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 130.Weiss WA, Burns MJ, Hackett C, et al. Genetic determinants of malignancy in a mouse model for oligodendroglioma. Cancer Res. 2003;63(7):1589–1595. [PubMed] [Google Scholar]
- 131.Niclou SP, Danzeisen C, Eikesdal HP, et al. A novel eGFP-expressing immunodeficient mouse model to study tumor-host interactions. FASEB J. 2008;22(9):3120–3128. doi: 10.1096/fj.08-109611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Golebiewska A, Brons NH, Bjerkvig R, Niclou SP. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell. 2011;8(2):136–147. doi: 10.1016/j.stem.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 133.Gupta PB, Fillmore CM, Jiang G, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146(4):633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]