Abstract
The purpose of this study is to validate the recently published Breast–Graded Prognostic Assessment (GPA) and propose a new prognostic model and nomogram for patients with brain parenchymal metastases (BM) from breast cancer (BC). We retrospectively investigated 171 consecutive patients who received a diagnosis of BM from BC during 2000–2008. We appraised the recently proposed Sperduto's BC-specific GPA in training cohort through Kaplan-Meier survival curve using log-rank test and area under the curve for the BC-GPA predicting overall survival at 1 year and developed a new nomogram to predict outcomes using multivariate Cox-regression analysis. By putting the Sperduto's Breast-GPA together with our nomogram, we developed a new prognostic model. We validated our new prognostic model with an independent external patient cohort from 2 institutes for the same period. On the basis of our Cox-regression analysis, therapeutic effect of trastuzumab and status of extracranial systemic disease control were incorporated into our new prognostic model in addition to Karnofsky performance status, age, and hormonal status. Our new prognostic model showed significant discrimination in median survival time, with 3.7 months for class I (n = 15), 7.8 months for class II (n = 82), 10.7 months for class III (n = 42), and 19.2 months for class IV (n = 32; P < .0001). The new prognostic model accurately predicted survival among patients with BC from BM in an external validation cohort (P < .0001). We propose a new prognostic model and a nomogram reflecting the different biological features of BC, including treatment effect and status of extracranial disease control, which was excellently validated in an independent external cohort.
Keywords: brain metastasis, breast cancer, HER2, prognosis, trastuzumab
Breast cancer (BC) is the second-most frequent cause of brain metastases (BM) after lung cancer. BM in patients with metastatic breast cancer (MBC) occurs in 10%–16% of patients, approaching 30% when autopsy diagnosis of BM is included.1,2 BM generally tends to occur during the late stage of MBC.3 The prognosis of patients who develop BM from BC is generally poor, and the median survival is 6–7 months (range, 2–16 months), with only approximately 20% of patients reaching 1 year of survival.4–6
Treatment decisions must take into account clinical prognostic factors to maximize survival and neurologic function while avoiding unnecessary treatment. Considering that several multimodal treatments including corticosteroids, whole brain radiotherapy (WBRT), surgery or stereotactic radiosurgery (SRS), and/or systemic chemotherapy have been used in an unorganized fashion, it is crucial to predict prognosis accurately and to determine the most appropriate treatment modality in patients with BM. The most widely accepted prognostic index for patients with BM has been the recursive partition analysis (RPA), based on the database of 1200 patients of 3 consecutive Radiation Therapy Oncology Group (RTOG) trials.7 They originally proposed 3 classes with different outcomes according to age, performance status, and extracranial primary tumor control,7 with a median survival of 7.1 months for class I, 4.2 months for class II, and 2.3 months for class III. Since then, new prognostic scores have been developed through several modifications,8–10 such as the score index for radiosurgery (SIR),9,11 basic score for brain metastases (BSBM),10 and graded prognostic assessment (GPA).8
Although several prognostic indexes have been developed, they are based on populations with BM from a combination of various primary cancers. In the original RPA proposed by Gaspar et al.,7 most of the patients had primary lung cancer, and patients with BC comprised <15% of the study population. Very few patients with BM from BC can be categorized in class I, but many patients survive beyond class II/III estimates in this RPA index system. Therefore, it remains uncertain whether the RPA classification can be safely applied to patients with BM from BC. Although a BC-specific GPA was first proposed by Sperduto et al.,12 biological features according to intrinsic subtypes of BC were not fully considered. It has been reported that BC subtypes are associated with unique patterns of metastatic spread, with notable differences in survival after relapse.13–16 In this context, a recently proposed BC-specific GPA reflecting biological subtype is a valuable tool to predict prognosis and decide further treatment options.17
The progressive neurologic disabilities both impair the quality of life and decrease survival; thus, the control of CNS lesion(s) is very important for overall disease control. However, survival depends on both intracranial disease control and extracranial systemic disease control. Causes of death in patients with BC with BM are different according to subtypes; approximately half of HER2-positive patients with BM die of CNS progression, whereas patients with triple-negative BC (TNBC) with BM rarely die due to CNS progression alone. Most cases of TNBC do not seem to respond well to systemic treatment, with the poorest survival among BC subtypes,5 and progression in extracranial systemic disease not in CNS lesion(s) is the dominant cause of morbidity and mortality. Therefore, extracranial systemic disease control is one of the most important factors for predicting outcomes. However, control of extracranial systemic disease was not a statistically significant factor in Sperduto's prognostic model system.
Systemic therapy after WBRT has been known to significantly improve survival among patients with HR-positive and/or HER2-positive BC. According to retrospective analysis reported by Dawood et al. that compared the prognosis among 3 groups of patients, women with metastatic HER2-positive BC with and without the addition of trastuzumab with women with HER2-negative disease, trastuzumab has beneficially changed the natural history of HER2-positive BC and improved the survival time among women with HER2-positive tumors beyond those of women with HER2-negative disease.18 In addition, the survival time among the patients with HER2-positive BC after BM could be prolonged with the use of anti-HER2 agents, such as trastuzumab, through extracranial systemic disease control.19,20
In the present study, we tried to validate the recently refined BC-specific GPA in our cohort, and then we developed a nomogram to comprehensively reflect tumor and host characteristics of BM from BC. Then, we proposed a new prognostic index model with a better prediction of survival among patients with BC with BM.
Materials and Methods
Patients and Methods
We retrospectively analyzed the medical records of 171 consecutive patients who received a diagnosis of BM from BC during 2000–2008 at Samsung Medical Center (SMC). All patients had histologically confirmed adenocarcinoma of the breast in the primary and/or metastatic site(s). All pathologic specimens were reviewed by 2 experienced pathologists who determined the primary tumor characteristics based on histologic and nuclear grades, tumor size, presence of lymphovascular invasion (LVI), multiplicity, axillary nodal status, and the status of immunohistochemical (IHC) staining for receptors (estrogen receptor [ER], progesterone receptor [PgR], and human epidermal growth factor receptor-2 [HER2]). ER and PgR positivity was defined as Allred scores within the range of 3–8 by IHC using antibodies to ER (Immunotech) and PgR (Novocastra), respectively. HER2 status was evaluated using an antibody (DAKO) and/or fluorescence in situ hybridization (FISH). Grades 0 and 1 for HER2 by IHC were defined as a negative result and grade 3 as a positive result. Amplification of HER2 was confirmed by FISH if HER2 was rated 2+ by IHC. Triple negativity was defined as a lack of ER, PgR, and HER2 expressions.
Treatment modalities for brain parenchymal metastases included symptomatic management with corticosteroids, WBRT, surgical resection, SRS, and systemic treatment (chemotherapy, hormonal therapy, and trastuzumab) or a combination of these modalities.
Sperduto's Breast-GPA score was calculated as the sum of each score, which included KPS, ER/PgR, HER2 status, and age. We tried to validate the Sperduto's score system using our patient cohorts. Then, we developed a nomogram to accurately predict patient outcome based on the multivariate Cox-regression model based on dichotomized variables. Using our nomogram, we developed a new prognostic model and validated it with an external patient cohort. Our study protocol was approved by the institutional review board of SMC.
For external validation of the models, we used an independent external cohort that included 199 patients with BM from BC treated at Severance Hospital of Yonsei University and Asan Medical Center from January 2004 through June 2009.
Statistical Analysis
The overall survival from BM (BM-OS) was defined from the date of BM to death or the last follow-up date. BM-OS was estimated using the Kaplan-Meier method. The log-rank test was used to compare survival rates. A cutoff P value of .05 was adopted for all statistical analyses. Statistical data were generated using the SPSS software package (SPSS/PASW, version 18.0). Receiver-operating characteristic (ROC) curves were drawn using the MedCalc software system (version 12.2.1.0). For constructing the nomogram, the open-source statistical language and platform, R, version 2.12.1 (R Development Core Team, 2010), with the package rms (version 3.1-1) was used (http://CRAN.R-project.org/package=rms).
The following data were analyzed in terms of BM-OS by log-rank test in univariate analysis: age, performance status, hormonal receptor status with HER2 expression profile, extracranial disease status at the time of diagnosis of BM, number of brain lesion(s), recursive partitioning analysis (RPA) class at the time of BM, treatment modalities such as brain surgery, WBRT, SRS, and systemic treatments including trastuzumab use. Then, the variables that were identified as risk factors in univariate analysis were examined using multivariate analysis with Cox-regression model. Differences were considered as statistically significant when P < .05.
A Cox proportional hazards regression model was used to assess the effect of each potential prognostic variable on BM-OS. All potential prognostic variables were included in the model, and variables were then removed from this model one at a time in a backward selection process using the likelihood ratio test and a significance level of .05.
To develop a well-calibrated and exportable nomogram for BM-OS, we used the Cox-regression model. The nomogram performance was quantified with respect to discrimination and calibration. Moreover, we used the bootstrapping method (1000 repetitions) to obtain relatively unbiased estimates of model performance. Discrimination was quantified with the AUC. We performed the calibration using graphic representations of the relationship between the observed outcome frequency and the predicted probability.
The ROC curve was drawn for the 1-year survival probability of all 171 patients in the nomogram. The power of the nomogram score was evaluated using the AUC with a 95% confidence interval (CI). The sensitivity and specificity were calculated for the nomogram score cutoff value to predict 1-year survival among all 171 patients. Then, ROC curves were drawn for the 199 patients of the external validation set.
Results
Patient Cohorts (Fig. 1A)
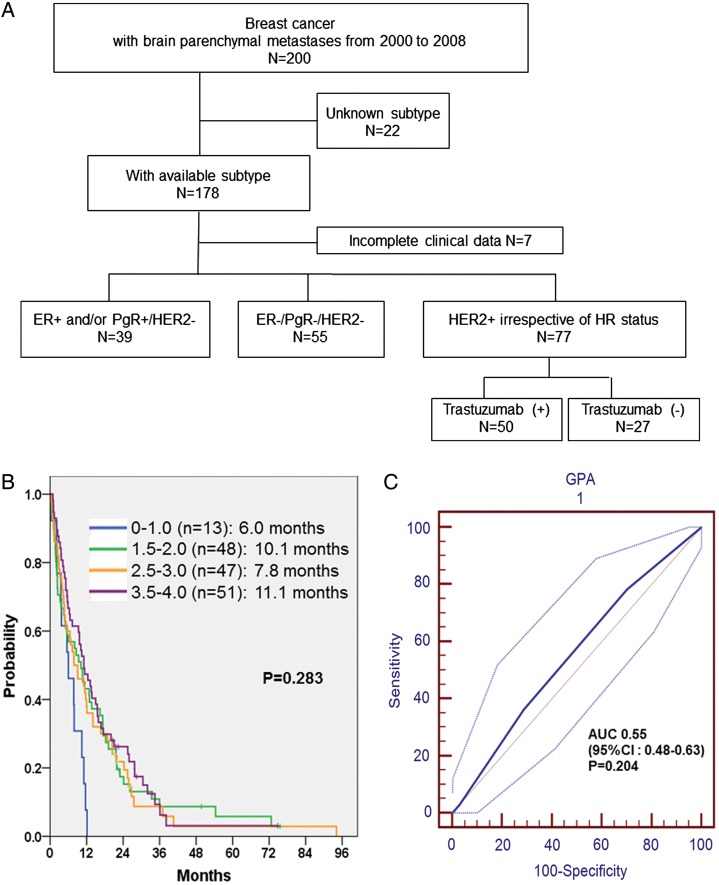
Fig. 1.
(A) Training cohort. (B) BM-OS according to Sperduto's GPA scoring system in training cohort, P = .283. (C) ROC curve on Sperduto's GPA system to predict 1-year death in training set: AUC = 0.55, P = .204, 95% CI: 0.48–0.63.
Of the 200 consecutive patients who received a diagnosis of BM, we excluded 22 patients who did not have ER, PgR, or HER2 IHC data available. An additional 7 patients whose clinical data were incomplete were excluded, leaving a final cohort of 171 patients. Of these, 39 patients were HR positive (defined as ER positive and/or PgR positive and HER2 negative), 55 were TNBC (defined as ER negative, PgR negative, and HER2 negative), and 77 were HER2 positive (38 HR positive and 39 HR negative).
Patient Characteristics and Overall Outcomes Between the Training Set and the Validation Set
Table 1 shows a comparison of the characteristics of the patients between training and validation set. The median age was not different (47 vs. 49; P = .332 by Mann-Whiney test). More patients with good PS (KPS, 70–100) were in the training set than were in the validation set, but the difference was not statistically significant (67.8% vs. 58.3%; P = .067). The subtype distributions were also similar (HR-positive/HER2-negative, 22.8% vs. 29.1%; HR-positive/HER2-positive, 22.2% vs. 21.1%; HR-negative/HER2-positive, 22.8% vs. 19.6%; TN, 32.2% vs. 30.2%; P = .865). The status of extracranial systemic disease at the time of diagnosis was not different (33.9% vs. 30.7% for controlled disease; P = .506). The percentages of trastuzumab treatment for MBC were also not different (64.9% vs. 67.9%; P = .738).
Table 1.
Patients’ characteristics between the training set and the validation set
| Patient characteristic | Training set | Validation set | P value |
|---|---|---|---|
| Total number | 171 | 199 | |
| Period | 2000–2008 | 2004–2009 | |
| Age at BM | |||
| Median years (range) | 47 (26–87) | 49 (26–80) | .332 |
| ≥70 y | 5 (2.9%) | 8 (4.0%) | .778 |
| <70 y | 166 (97.1%) | 191 (96.0%) | |
| Performance status | |||
| KPS 70–100 | 116 (67.8%) | 116 (58.3%) | .067 |
| KPS <70 | 55 (32.2%) | 83 (41.7%) | |
| Subtype of tumor | |||
| ER+ and/or PgR+/HER2− | 39 (22.8%) | 58 (29.1%) | .865 |
| ER+ and/or PgR+/HER2+ | 38 (22.2%) | 42 (21.1%) | |
| ER− and/or PgR−/HER2+ | 39 (22.8%) | 39 (19.6%) | |
| ER−/PgR−/HER2− | 55 (32.2%) | 60 (30.2%) | |
| Extracranial systemic control at BM | |||
| Controlled (CR, PR, or SD) | 58 (33.9%) | 61 (30.7%) | .506 |
| Progression | 113 (66.1%) | 138 (69.3%) | |
| Anti-HER2 treatment for metastatic BC before BM among HER2+ patients | 50/77 (64.9%) | 55/81 (67.9%) | .738 |
| Overall survival from BM (BM-OS) | |||
| Median months (range) | 9.6 (0.1–88.3) | 7.8 (0.1–57.8) | .069 |
Abbreviations: BM, brain metastasis; KPS, Karnofsky performance status.
Treatment Modalities After Diagnosis of BM in 171 Patients in the Training Set
A total of 168 patients (98.2%) were treated with WBRT. Fifty-nine patients (34.5%) received surgical resection or stereotactic radiosurgery with or without WBRT, and 110 patients (64.3%) received WBRT only. For patients who have ≤3 lesions of BM (22 patients, 12.9%), we initially performed stereotactic radiosurgery and performed WBRT when metastatic brain lesions progress or new brain parenchymal lesion(s) developed. Seventy-five patients (43.9%) received systemic chemotherapy after local control of brain metastatic lesions with local modalities as surgery, SRS, and/or WBRT. Of the HER2-positive patients, 65% of the patients received anti-HER2–directed therapy before BM (Table 1).
Assessment of Sperduto's BC-Specific GPA
For our cohort, we calculated Sperduto's BC-specific GPA, which was published recently,17 and the GPA score did not discriminate among prognostic classes in our cohort. The BM-OSs for the GPA index were 6.0 months for class I (n = 13), 10.1 months for class II (n = 48), 7.8 months for class III (n = 47), and 11.1 months for class IV (n = 51; P = .283) (Fig. 1B). In addition, the prediction model for 1-year survival probability of Sperduto's BC-specific GPA had an AUC of 0.55 in the training set (P = .204) (Fig. 1C).
Multivariate Analysis of BM-OS
To develop a prognostic index with better predicting power than previous ones, we performed a Cox-regression multivariate analysis on BM-OS. Table 2 shows the independent risk factors for BM-OS in our patient cohort. KPS, age <70 years, HER2 positivity, trastuzumab use for MBC before BM diagnosis, triple negativity, and extracranial systemic disease control were identified as independent risk factors for BM-OS (hazard ratio [HR], 0.51 [P = .0002] for KPS ≥70; HR, 0.23 [P = .002] for age <70 years; HR, 2.06 [P = .005] for HER2 positivity; HR, 0.54 [P = .017] for trastuzumab use; HR, 2.03 [P = .002] for triple negativity; HR, 0.57 [P = .002] for extracranial systemic control).
Table 2.
Multivariate Cox-regression analysis on survival time from brain metastasis to death
| Hazard Ratio (HR) | P value | 95% CI | |
|---|---|---|---|
| KPS ≥70 | 0.51 | .0002 | 0.36–0.74 |
| Age <70 | 0.23 | .002 | 0.09–0.60 |
| HER2 positivity | 2.06 | .005 | 1.24–3.45 |
| Trastuzumab use | 0.54 | .017 | 0.33–0.90 |
| Triple negativity | 2.03 | .002 | 1.29–3.18 |
| Extracranial disease control | 0.57 | .002 | 0.41–0.81 |
Abbreviations: KPS, Karnofsky performance status.
Developing a Nomogram to Predict BM Outcomes and External Validation
On the basis of the variables independently associated with BM-OS from Cox-regression multivariate analysis, we constructed a nomogram (Fig. 2A). The prediction model for 1-year survival probability had an AUC of 0.73 in the training set (P < .0001) (Fig. 2B). In the validation set, discriminations were good, with AUCs of 0.78 (P< .0001) (Fig. 2C). These results demonstrate that the predictions for an independent data set were sufficient, confirming the exportability of the model.
Fig. 2.
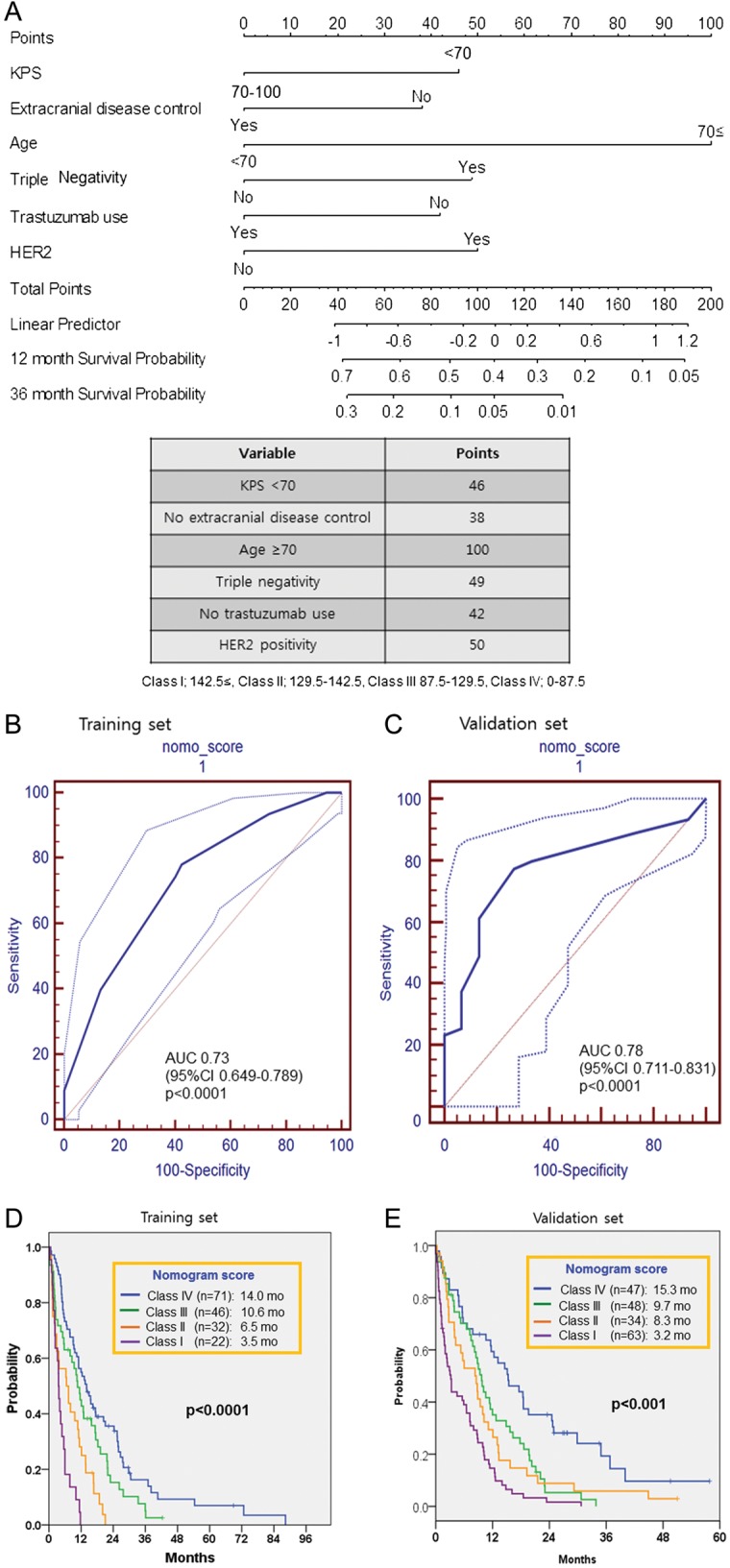
Nomogram to predict death from brain metastases (BM) in patients with BM from breast cancer (BC). (A) is nomogram. Points are translated to probability of death. Predictor points are found on the upper-most point scale that corresponds with each patient variable. The reader then manually adds up the points, and the predicted values can be read at the bottom of the nomogram. The total projected on the bottom scale indicates the probability of death at 12 and 36-month. (B) showed ROC curve on nomogram to predict 1-year death in BM breast cancers in training set. AUC = 0.73, P < .0001, 95% CI: 0.65–0.79. (C) showed ROC curve on nomogram using external validation set. AUC = 0.78, P < .0001, 95% CI: 0.71–0.83. (Thin lines for each ROC curve represent 95% CI). (D) BM-OS according to nomogram score in training set, P < .0001 by log-rank test. (E) BM-OS according to nomogram score in validation set, P < .001 by log-rank test.
According to the nomogram scores, prognostic stratification was performed. The BM-OSs for the nomogram scores were 3.5 months for class I (nomogram score, 143–188; n = 22); 6.5 months for class II (nomogram score, 130–142; n = 32), 10.6 months for class III (nomogram score, 88–129; n = 46), and 14.0 months for class IV (nomogram score, 42–87; n = 71). The nomogram scores significantly discriminated survival from BM (P < .0001) (Fig. 2D). In addition, we validated our nomogram model with an external patients’ cohort, and the BM-OS for each nomogram score was calculated. The BM-OSs for the nomogram scores in the validation set were 3.2 months for class I (nomogram score, 143–188; n = 63), 8.3 months for class II (nomogram score, 130–142; n = 34), 9.7 months for class III (nomogram score, 88–129; n = 48), and 15.3 months for class IV (nomogram score, 42–87; n = 47) for the validation set (P < .0001) (Fig. 2E). This result demonstrates excellent predictions in an independent data set and, therefore, confirmed the exportability of the model.
A New Prognostic Model Based on the Analysis of Nomogram Score to Predict BM-OS Using the Training Patient Cohort
On the basis of our previous study,21 we analyzed survival from BM according to HER2 status and administration of a trastuzumab treatment. In univariate analysis, BM-OS was significantly different according to HER2 status and trastuzumab therapy. The BM-OS was 12.4 months in HER2-positive patients who had received trastuzumab for MBC, followed by 8.6 months in HER2-negative patients and 5.7 months in HER2-positive patients who had not received trastuzumab (P = .026).
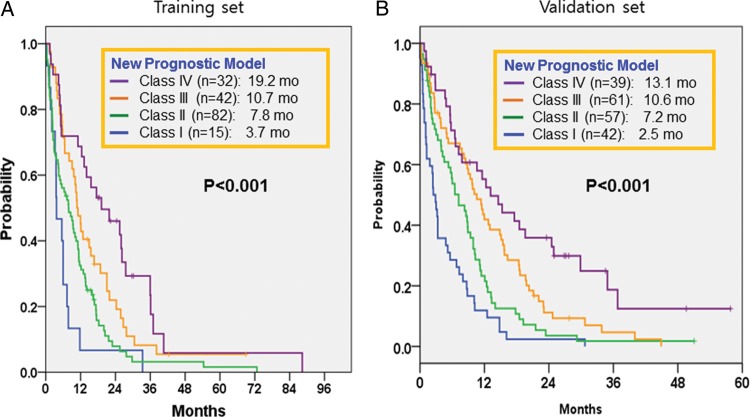
Next, we reviewed our nomogram scoring system using a Cox-regression multivariate model for BM-OS. We incorporated the status of trastuzumab use and extracranial systemic control into our new prognostic model. Considering the nomogram score used to calculate the hazard ratio for each variable, we assigned a score according to the new GPA scoring system by analyzing nomogram scores for each factor: KPS (0 for KPS <70 and 0.5 for KPS 70–100), age (0 for ≥70 years and 1.0 for <70 years), hormone receptor status (0 for ER negative and PgR negative and 0.5 for ER and/or PgR positive), HER2 status (0 for HER2 positive without trastuzumab, 0.5 for HER2 negative, 1.0 for HER2 positive with trastuzumab), extracranial disease control (0 for progressive disease and 0.5 for extracranial disease control [at least stable disease]). Our new prognostic model is displayed in Table 3. When survival analyses were performed according to our modified prognostic model, the BM-OS for each index was 3.7 months for class I (n = 15), 7.8 months for class II (n = 82), 10.7 months for class III (n = 42), and 19.2 months for class IV (n = 32; P < .0001) (Fig. 3A).
Table 3.
A new prognostic model for BM from breast cancer
| New Prognostic Model | |||
|---|---|---|---|
| Raw score | 0 | 0.5 | 1.0 |
| KPS | <70 | 70–100 | |
| Age | ≥70 | <70 | |
| ER/PgR | ER-negative & PgR-negative | ER &/or PgR-positive | |
| HER2 | HER2-positive w/o trastuzumab | HER2-negative | HER2-positive with trastuzumab |
| Extracranial disease control | No | Yes | |
Abbreviation: KPS, Karnofsky performance status; w/o, without.
Extracranial disease control: ≥SD (CR + PR + SD) of extracranial diseases at the time of diagnosis of BM.
aTotal score for each case is calculated by sum of raw scores for each variable (KPS, age, ER/PgR, HER2, extracranial disease control): Class I; 0.5–1.0, Class II; 1.5–2.0, Class III; 2.5, Class IV; 3.0-3.5.
Fig. 3.
Kaplan–Meier Survival curves from BM according to new proposed BC-specific GPA scoring system. (A) BM-OS according new proposed BC-specific GPA score in training set, P < .0001 by log-rank test. (B) BM-OS according new proposed BC-specific GPA score in validation set, P < .001 by log-rank test.
Validation of the New Prognostic Model in an External Patient Cohort (n = 199)
The new prognostic model score calculated for the validation set and the Kaplan-Meier survival curve distribution for each index are shown in Fig. 3B. The BM-OSs for our modified GPA index were 2.5 months for class I (n = 42), 7.2 months for class II (n = 57), 10.6 months for class III (n = 61), and 13.1 months for class IV (n = 39) in the validation set. The newly refined prognostic index model significantly discriminated survival among patients with BM from BC (P < .0001) (Fig. 3B).
Discussion
An accurate prognostic system for patients with BM from BC is urgently needed to appropriately select patients who would benefit from active and aggressive treatment.7,8 Obviously, the type of treatment for BM depends on several factors, including the patient's neurological condition; extracranial systemic metastases; life expectancy; number, size, and location of BM lesions; and presence of leptomeningeal disease.22,23 However, recent advances in our understanding of the biology underlying BC have brought remarkable improvements to systemic treatments for BC according to the intrinsic subtypes.24,25 Furthermore, the most prominent studies to date are primarily concerned with the biology of each metastatic organ, such as brain, lung, and bone.14,15,26–28 Therefore, harmonized disease control for extracranial systemic disease and CNS lesion(s) of each subtype may help indicate the proper treatment options in each clinical setting. Accordingly, the recently published Sperduto's BC-specific GPA is the first BC-specific prognostic score for patients with BM that includes tumor biology and is timely and seems to be appropriate. Therefore, we tried to validate Sperduto's BC-specific GPA, which was based on 400 patients incorporating KPS, ER/PR/HER2 status, and age. However, it does not include trastuzumab use, which improves BM-OS and changes the natural course of HER2-positive BC. In addition to this, the scoring system still needs to be validated, even though it has been composed of a large number of prospective cohorts. Their classification of BC included approximately 4 subtypes: basal (TN), luminal A (HER2 negative, HR positive), luminal B (HER2 positive, HR positive), and HER2 (HER2 positive, HR negative). This classification may represent intrinsic subtype using immunohistochemistry. However, according to the scoring system, the patients with HR-positive/HER2-positive (called “luminal B”) appeared to have better outcomes than those with HR-positive/HER2-negative (called “luminal A”).17,29 These may contribute to the inability to discriminate our patient cohort into prognostic groups. Therefore, we developed a nomogram to predict outcomes and used it to propose a new prognostic model (Fig. 2A). However, it would be better to develop more concise prognostic model for use in daily clinical practice, because nomogram seems to be complex to adapt and has to be calculated according to the figure for each case. For this reason, we developed the new BC-specific GPA model incorporated with the nomogram and validated excellently with an external validation cohort from 2 institutes for the same period.
The advantage of our nomogram (Fig. 2A) and the new BC-GPA model (Table 3) is that we integrated current treatment strategy and changes in disease course according to biologic subtype of BC. In other words, better extracranial systemic disease control after introduction of trastuzumab affects the policy for local control of BM. It does not result in a reduced role of systemic treatment but places the emphasis on more active systemic control and better local control. Our new BC-specific GPA could be updated in the near future to reflect the incorporation of the most current therapeutic strategies. Paradoxically, our finding is supported by the fact that TNBCs, which lack a properly targeted systemic treatment, have the worst prognosis, and that most of the deaths are associated with uncontrolled progression of systemic diseases rather than from deterioration of BM (data not shown).
Actually, Cox-regression multivariate analysis for BM-OS in our patient cohort did not find number of BM lesions as an independent prognostic factor. Instead, intrinsic subtype, use of trastuzumab, and extracranial systemic control were significant. This implies that the interactions between underlying biological factors and status of systemic disease control may have a critical role in determining tumor behavior and outcomes. Despite these strengths of the study, this is a retrospective analysis with inherent selection bias. A variety of treatment modalities had been applied during the long period (2000–2008), which implies that a lot of changes in the treatment of patients with BM from BC had occurred in terms of local modalities and imaging methods to monitor. These confounding variables may result in unacceptable results. This is the main weakness of this study.
In conclusion, we established a new prognostic model to predict BM-OS by refining the Sperduto's BC-specific GPA index through the analysis of a nomogram and through the incorporation of unique biological feature of BCs. This new model would be a valuable tool for designing innovative clinical trials and for decision making in daily clinical practice. Further prospective study is warranted.
Acknowledgments
Presented in part: 2011 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL (poster). H.K.A. and S.L. contributed equally to this work.
Conflict of interest statement. None declared.
References
- 1.Santarelli JG, Sarkissian V, Hou LC, Veeravagu A, Tse V. Molecular events of brain metastasis. Neurosurg Focus. 2007;22:E1. doi: 10.3171/foc.2007.22.3.2. doi:10.3171/foc.2007.22.3.2. [DOI] [PubMed] [Google Scholar]
- 2.Al-Shamy G, Sawaya R. Management of brain metastases: the indispensable role of surgery. J Neurooncol. 2009;92:275–282. doi: 10.1007/s11060-009-9839-y. doi:10.1007/s11060-009-9839-y. [DOI] [PubMed] [Google Scholar]
- 3.Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52:2349–2354. doi: 10.1002/1097-0142(19831215)52:12<2349::aid-cncr2820521231>3.0.co;2-b. doi:10.1002/1097-0142(19831215)52:12<2349::AID-CNCR2820521231>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Lee SS, Ahn JH, Kim MK, et al. Brain metastases in breast cancer: prognostic factors and management. Breast Cancer Res Treat. 2008;111:523–530. doi: 10.1007/s10549-007-9806-2. doi:10.1007/s10549-007-9806-2. [DOI] [PubMed] [Google Scholar]
- 5.Niwinska A, Murawska M, Pogoda K. Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT) Ann Oncol. 2010;21:942–948. doi: 10.1093/annonc/mdp407. doi:10.1093/annonc/mdp407. [DOI] [PubMed] [Google Scholar]
- 6.Altundag K, Bondy ML, Mirza NQ, et al. Clinicopathologic characteristics and prognostic factors in 420 metastatic breast cancer patients with central nervous system metastasis. Cancer. 2007;110:2640–2647. doi: 10.1002/cncr.23088. doi:10.1002/cncr.23088. [DOI] [PubMed] [Google Scholar]
- 7.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. doi:10.1016/S0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 8.Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–514. doi: 10.1016/j.ijrobp.2007.06.074. doi:10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 9.Weltman E, Salvajoli JV, Brandt RA, et al. Radiosurgery for brain metastases: a score index for predicting prognosis. Int J Radiat Oncol Biol Phys. 2000;46:1155–1161. doi: 10.1016/s0360-3016(99)00549-0. doi:10.1016/S0360-3016(99)00549-0. [DOI] [PubMed] [Google Scholar]
- 10.Lorenzoni J, Devriendt D, Massager N, et al. Radiosurgery for treatment of brain metastases: estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys. 2004;60:218–224. doi: 10.1016/j.ijrobp.2004.02.017. doi:10.1016/j.ijrobp.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Uhm JE, Park YH, Yi SY, et al. Treatment outcomes and clinicopathologic characteristics of triple-negative breast cancer patients who received platinum-containing chemotherapy. Int J Cancer. 2009;124:1457–1462. doi: 10.1002/ijc.24090. doi:10.1002/ijc.24090. [DOI] [PubMed] [Google Scholar]
- 12.Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. doi:10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. doi:10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 14.Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol. 2010;176:2958–2971. doi: 10.2353/ajpath.2010.090838. doi:10.2353/ajpath.2010.090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidler IJ, Balasubramanian K, Lin Q, Kim SW, Kim SJ. The brain microenvironment and cancer metastasis. Mol Cells. 2010;30:93–98. doi: 10.1007/s10059-010-0133-9. doi:10.1007/s10059-010-0133-9. [DOI] [PubMed] [Google Scholar]
- 16.Hinton CV, Avraham S, Avraham HK. Role of the CXCR4/CXCL12 signaling axis in breast cancer metastasis to the brain. Clin Exp Metastasis. 2010;27:97–105. doi: 10.1007/s10585-008-9210-2. doi:10.1007/s10585-008-9210-2. [DOI] [PubMed] [Google Scholar]
- 17.Sperduto PW, Kased N, Roberge D, et al. Effect of Tumor Subtype on Survival and the Graded Prognostic Assessment for Patients With Breast Cancer and Brain Metastases. Int J Radiat Oncol Biol Phys. 2011;82:2111–2117. doi: 10.1016/j.ijrobp.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. doi:10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duchnowska R, Dziadziuszko R, Czartoryska-Arlukowicz B, et al. Risk factors for brain relapse in HER2-positive metastatic breast cancer patients. Breast Cancer Res Treat. 2009;117:297–303. doi: 10.1007/s10549-008-0275-z. doi:10.1007/s10549-008-0275-z. [DOI] [PubMed] [Google Scholar]
- 20.Stemmler HJ, Heinemann V. Central nervous system metastases in HER-2-overexpressing metastatic breast cancer: a treatment challenge. Oncologist. 2008;13:739–750. doi: 10.1634/theoncologist.2008-0052. doi:10.1634/theoncologist.2008-0052. [DOI] [PubMed] [Google Scholar]
- 21.Park YH, Park MJ, Ji SH, et al. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br J Cancer. 2009;100:894–900. doi: 10.1038/sj.bjc.6604941. doi:10.1038/sj.bjc.6604941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogelbaum MA, Suh JH. Resectable brain metastases. J Clin Oncol. 2006;24:1289–1294. doi: 10.1200/JCO.2005.04.6235. doi:10.1200/JCO.2005.04.6235. [DOI] [PubMed] [Google Scholar]
- 23.Bravo Marques JM. Treatment of brain metastases in patients with HER2+ breast cancer. Adv Ther. 2009;26(suppl 1):S18–S26. doi: 10.1007/s12325-009-0047-0. doi:10.1007/s12325-009-0047-0. [DOI] [PubMed] [Google Scholar]
- 24.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. doi:10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 25.Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. doi:10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 26.Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. doi:10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bos PD, Zhang XH, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. doi:10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagani O, Price KN, Gelber RD, et al. Patterns of recurrence of early breast cancer according to estrogen receptor status: a therapeutic target for a quarter of a century. Breast Cancer Res Treat. 2009;117:319–324. doi: 10.1007/s10549-008-0282-0. doi:10.1007/s10549-008-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. doi:10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]