Abstract
Craniopharyngioma is a rare primary central nervous system neoplasm. Our objective was to determine factors associated with incidence, treatment, and survival of craniopharyngiomas in the United States. We used the surveillance, epidemiology and end results program (SEER) database to identify patients who received a diagnosis of craniopharyngioma during 2004–2008. We analyzed clinical and demographic information, including age, race, sex, tumor histology, and treatment. Age-adjusted incidence rates and age, sex, and race-adjusted expected survival rates were calculated. We used Cox proportional hazards models to determine the association between covariates and overall survival. We identified 644 patients with a diagnosis of craniopharyngioma. Black race was associated with an age-adjusted relative risk for craniopharyngioma of 1.26 (95% confidence interval [CI], 0.98–1.59), compared with white race. One- and 3-year survival rates of 91.5% (95% CI, 88.9%–93.5%), and 86.2% (95% CI, 82.7%–89.0%) were observed for the cohort; relative survival rates were 92.1% (95% CI, 89.5%–94.0%) and 87.6% (95% CI, 84.1%–90.4%) for 1- and 3-years, respectively. In the multivariable model, factors associated with prolonged survival included younger age, smaller tumor size, subtotal resection, and radiation therapy. Black race, on the other hand, was associated with worse overall survival in the final model. We demonstrated that >85% of patients survived 3 years after diagnosis and that subtotal resection and radiation therapy were associated with prolonged survival. We also noted a higher incidence rate and worse 1- and 3-year survival rates in the black population. Future investigations should examine these racial disparities and focus on evaluating the efficacy of emerging treatment paradigms.
Keywords: benign tumor, craniopharyngioma, epidemiology, racial disparity, SEER
Craniopharyngiomas are rare benign tumors of the central nervous system (CNS) that are thought to arise from remnants of Rathke's pouch, near the pituitary gland. They represent <1% of all primary CNS tumors, but are the most common intracranial non-glial tumor in children.1,2
Craniopharyngiomas often present a significant challenge to neurosurgeons, because their location and degree of invasion into surrounding neural structures, namely the hypothalamus, pituitary gland, and optic apparatus, make gross total resection (GTR) difficult, leading to a high rate of recurrence.3 Even after complete resection, these lesions have a propensity to recur, but the rate of recurrence increases dramatically when resection is subtotal.4–6 Significant controversy exists in the literature regarding optimal treatment strategies, with some groups pursuing conservative subtotal resection (STR) with either upfront or salvage fractionated radiotherapy or, more recently, radiosurgery to mitigate the risks of aggressive surgical resection.7–10
Most descriptive studies of craniopharyngioma are based on single-center experiences6,11 or represent reported incidence rates as part of comprehensive CNS tumor analyses.12,13 Although a few international studies have examined the incidence and treatment patterns on a population level, only one such study has been conducted in the United States.10,14–19 In this 1998 study, Bunin et al. surveyed a variety of cancer registries and reported an overall craniopharyngioma incidence rate of 1.3 per 1 000 000 person-years. The incidence in this study did not vary by sex or race, and a bimodal age distribution was noted, with peaks in children aged 5–14 years and in adults aged 50–74 years. In the National Cancer Database, craniopharyngioma 5-year survival was noted to be 80%, and decreased with increased age.19
In the current study, we used a large population-based database to estimate incidence, treatment, and prognostic factors for patients with craniopharyngiomas in the United States.
Materials and Methods
Patient Identification
We identified patients with a diagnosis of craniopharyngioma who were registered in the Surveillance, Epidemiology and End Results (SEER) Program of the NCI. SEER is a population-based tumor registry containing data on approximately 26% of the United States population. In 2004, the SEER Program added nonmalignant CNS tumors to case definitions and began collecting information concerning primary tumor type, patient demographic characteristics, initial cancer treatments, and survival into a database. We used data from the most recent SEER data set (2004–2008) to identify patients with International Classification of Diseases for Oncology (ICD-O-3) codes for craniopharyngioma (9350/0, 9350/1, 9350/3, 9351/0, 9352/1, 9351/3, 9352/1). Individuals with >1 primary tumor were excluded from analysis. Likewise, patients who received a diagnosis at death (with a reporting source of autopsy only or death certificate only) were not included in this analysis. Lastly, 2 individuals had malignant histology coded and were excluded from analysis.
Variable Selection
Patients were divided into the following age-related categories: <20, 20–39, 40–59, 60–79, and >80 years. Race was coded as white, black, and other. Histology was obtained from the ICD-O-3 code and collapsed into 3 categorical variables: craniopharyngioma not otherwise specified (NOS), adamantinomatous, and papillary. Lesion size was treated as a dichotomous variable with a cutoff at the median value: ≤28 mm and ≥28 mm. Lesion size was recorded from either pathologic reports or radiologic imaging. Initial surgical therapy, treated as a categorical variable, consisted of the following categories: observation or biopsy, subtotal resection, and gross total resection. Radiation treatment was dichotomized into treated versus untreated groups.
Outcomes
Age-adjusted incidence rates and expected survival rates adjusted for age, sex, and race, were obtained using SEER*Stat (Surveillance Research Program, National Cancer Institute SEER*Stat software [seer.cancer.gov/seerstat], version 7.0.5). Age adjustment was based on the 2000 US standard population. Expected survival was computed relative to the US 1970–2006 standard population by individual year. Patients with death certificate only and those alive without survival times recorded were excluded from survival analysis. Relative survival is defined as the observed survival rate as a percentage of expected survival. The date of death or censoring was also obtained from the SEER data set. The initial analysis included descriptive statistics of clinical and demographic information using means, proportions, and standard deviations. Univariate analysis of differences between surgical treatment groups was achieved using Pearson's χ2 test or Fisher's exact test. One- and 3-year estimates of survival and 95% confidence intervals (CIs) were calculated using the Kaplan-Meier method. Cox proportional hazards models were used to determine univariate and multivariable effects of covariates on survival times. A P value <.05 was considered to be statistically significant. Analyses were performed using R environment for statistical computing (R Development Core Team).
Results
We identified 644 patients with a diagnosis of craniopharyngioma. The overall age-adjusted incidence rate was 1.7 cases per 1 000 000 person-years and was stable over the 5-year period of the study. A bimodal age distribution was observed, with a first peak seen in children aged 0–19 years (1.9 cases per 1 000 000 person-years) and a second peak in adults aged 40–79 (2.1 cases per 1 000 000 person-years). Although the majority of patients were white (76.6%), the population-adjusted incidence rates suggest that black patients may have a higher incidence rate than white patients (2.1 cases per 1 000 000 person-years vs 1.7 cases per 1 000 000 person-years; relative risk [RR] = 1.26; 95% CI, 0.98–1.59). This difference in rate was driven by a difference in the incidence between black and white adults aged 40–79 years (3.1 cases per 1 000 000 person-years vs 2.0 cases per 1 000 000 person-years; RR = 1.54; 95% CI, 1.1–2.13; P = .0129). There was no statistically significant difference in population-adjusted incidence rates between white and other races. There was an equal distribution between male and female patients (50.3% and 49.7%, respectively), and incidence rates were nearly identical between the sexes (1.8 cases per 1 000 000 person-years for male patients and 1.7 cases per 1 000 000 person-years for female patients).
Approximately one-third of patients had tumors with adamantinomatous histology, whereas only 8.2% were papillary in nature. More than half of the patients had no specified histologic subtype. Overall, 134 patients (20.8%) did not undergo surgical resection or radiation therapy. Of the 462 patients who underwent surgical resection (71.7%), 216 (33.5%) received GTR and 246 (38.2%) received STR. Patients who received radiation therapy totaled 142 (22.0%). While 101 patients (21.9%) who underwent surgical resection received adjuvant radiation therapy, only 41 patients (6.4%) underwent radiation therapy as the primary and only initial treatment modality (Table 1).
Table 1.
Demographic and clinical characteristics for 644 patients who received a diagnosis of craniopharyngioma during 2004–2008
| Variable | n (%) |
|---|---|
| Age | |
| 0–19 years | 201 (31.2) |
| 20–39 years | 107 (16.6) |
| 40–59 years | 206 (32.0) |
| 60–79 years | 115 (17.9) |
| 80+ years | 15 (2.3) |
| Sex | |
| Male | 324 (50.3) |
| Female | 320 (49.7) |
| Race | |
| White | 493 (76.6) |
| Black | 86 (13.4) |
| Other | 65 (10.1) |
| Tumor Size | |
| <28 mm | 198 (30.7) |
| ≥28 mm | 235 (36.5) |
| Unknown | 211 (32.8) |
| Histology | |
| Papillary | 53 (8.2) |
| Adamantinomitous | 191 (29.7) |
| Craniopharyngioma NOS | 400 (62.1) |
| Surgical treatment | |
| Observation or biopsy | 176 (27.3) |
| Subtotal resection | 246 (38.2) |
| Gross total resection | 216 (33.5) |
| Unknown | 6 (0.9) |
| Radiation treatment | |
| No radiation | 490 (76.1) |
| Radiation | 142 (22.0) |
| Unknown | 12 (1.9) |
Abbreviations: NOS, not otherwise specified.
Univariate analyses of characteristics associated with the surgical treatment arm are presented in Table 2. Younger patients and those with larger lesions (≥28 mm) were more likely to undergo resection, whereas older patients and patients with smaller lesions (<28 mm) were more likely to be observed or have a biopsy without further surgical intervention (P = .007 and P = .019 for age and tumor size, respectively). Neither race nor sex affected a patient's likelihood of having GTR/STR versus observation/biopsy. Histologic subtype was found to be significantly associated with resection, as patients with a papillary or adamantinomatous subtype were more likely to undergo GTR/STR versus observation/biopsy than were those with unspecified histology (P = .004) (Table 2). When we examined the characteristics of patients with either adamantinomatous or papillary histology, we noted that the adamantinomatous histology was found more frequently in younger patients, and these patients were less likely to undergo radiation therapy (Table 3).
Table 2.
Univariate associations of demographic and clinical variables with surgical treatment (measured by Pearson's χ2-test or Fisher's exact test)
| Variable | Observation or biopsy (n = 176) | Subtotal resection (n = 246) | Gross total resection (n = 216) | P |
|---|---|---|---|---|
| Age | 0.007 | |||
| 0–19 years | 56 (31.8) | 78 (31.7) | 64 (29.6) | |
| 20–39 years | 17 (9.7) | 44 (17.9) | 45 (20.8) | |
| 40–59 years | 55 (31.2) | 76 (30.9) | 74 (34.3) | |
| 60–79 years | 39 (22.2) | 45 (18.3) | 31 (14.4) | |
| 80+ years | 9 (5.1) | 3 (1.2) | 2 (0.9) | |
| Sex | 0.651 | |||
| Male | 85 (48.3) | 129 (52.4) | 106 (49.1) | |
| Female | 91 (51.7) | 117 (47.6) | 110 (50.9) | |
| Race | 0.772 | |||
| White | 131 (74.4) | 188 (76.4) | 172 (79.6) | |
| Black | 27 (15.3) | 32 (13.0) | 25 (11.6) | |
| Other | 18 (10.2) | 26 (10.6) | 19 (8.8) | |
| Tumor Size | 0.019 | |||
| <28 mm | 65 (36.9) | 63 (25.6) | 69 (31.9) | |
| ≥28 mm | 62 (35.2) | 86 (35.0) | 87 (40.3) | |
| Unknown | 49 (27.8) | 97 (39.4) | 60 (27.8) | |
| Histology | 0.004 | |||
| Papillary | 11 (6.2) | 19 (7.7) | 23 (10.6) | |
| Adamantinomitous | 37 (21.0) | 76 (30.9) | 77 (35.6) | |
| Craniopharyngioma NOS | 128 (72.7) | 151 (61.4) | 116 (53.7) | |
| Radiation treatment | 0.620 | |||
| No radiation | 134 (76.1) | 194 (78.9) | 160 (74.1) | |
| Radiation | 41 (23.3) | 49 (19.9) | 52 (24.1) | |
| Unknown | 1 (0.6) | 3 (1.2) | 4 (1.9) |
Abbreviations: NOS, not otherwise specified.
Table 3.
Univariate associations of demographic and clinical variables with histology in 244 patients with adamantinomitous or papillary craniopharyngioma (measured by Pearson's χ2-test)
| Variable | Adamantinomatous (n = 191) | Papillary (n = 53) | P |
|---|---|---|---|
| Age | <0.001 | ||
| 0–19 years | 61 (31.9) | 3 (5.7) | |
| 20–39 years | 33 (17.3) | 5 (9.4) | |
| 40–59 years | 64 (33.5) | 29 (54.7) | |
| 60–79 years | 32 (16.8) | 13 (24.5) | |
| 80+ years | 1 (0.5) | 3 (5.7) | |
| Sex | 0.290 | ||
| Male | 101 (52.9) | 33 (62.3) | |
| Female | 90 (47.1) | 20 (37.7) | |
| Race | 0.088 | ||
| White | 139 (72.8) | 45 (84.9) | |
| Black | 28 (14.7) | 2 (3.8) | |
| Other | 24 (12.6) | 6 (11.3) | |
| Surgical treatment | 0.906 | ||
| Observation or biopsy | 37 (19.4) | 11 (20.8) | |
| Subtotal resection | 77 (40.3) | 23 (43.4) | |
| Gross total resection | 76 (39.8) | 19 (35.8) | |
| Unknown | 1 (0.5) | 0 (0) | |
| Radiation treatment | 0.071 | ||
| No radiation | 145 (75.9) | 33 (62.3) | |
| Radiation | 42 (22.0) | 20 (37.7) | |
| Unknown | 4 (2.1) | 0 (0) |
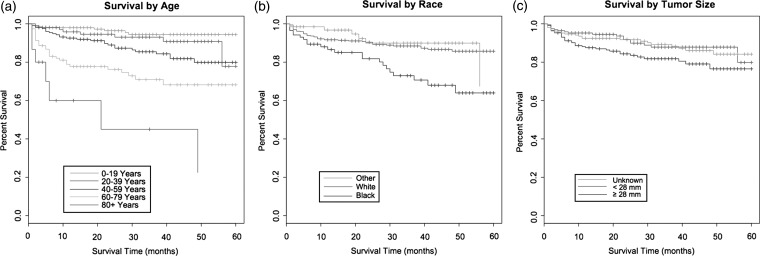
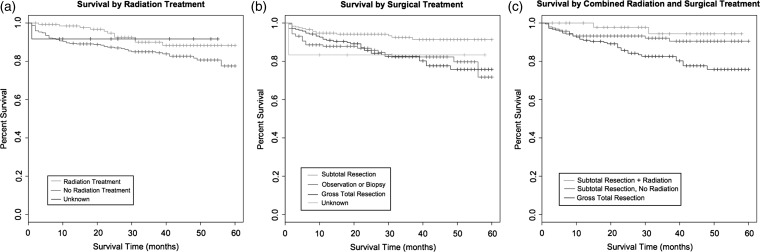
Observed overall survival rates at 1 and 3 years were 91.5% (95% CI, 88.9%–93.5%) and 86.2% (95% CI, 82.7%–89.0%), respectively. Relative overall survival rates were 92.1% (95% CI, 89.5%–94.0%) and 87.6% (95% CI, 84.1%–90.4%) at 1 and 3 years, respectively. Table 4 provides the results of univariate (unadjusted) and multivariate (adjusted) Cox proportional hazards models based on overall survival. Kaplan-Meier survival curves showing differences in survival rates by age, race, and tumor size can be seen in Figure 1a–c. Survival curves examining the effects of surgical resection and radiation therapy can be seen in Figure 2a–c. Factors associated with increased survival in both the univariate and multivariable models include younger age, smaller tumor size, subtotal resection, and radiation therapy. Alternatively, black race was associated with worse survival relative to both white and other races.
Table 4.
Results of univariate and multivariate Cox proportional hazards models for demographic and clinical variables
| Variable | Unadjusted HR | P | Adjusted HR | P |
|---|---|---|---|---|
| Age | ||||
| 0–19 years | 0.05 (0.02–0.13) | <0.001 | 0.04 (0.01–0.12) | <0.001 |
| 20–39 years | 0.09 (0.03–0.24) | <0.001 | 0.09 (0.03–0.27) | <0.001 |
| 40–59 years | 0.16 (0.07–0.37) | <0.001 | 0.16 (0.06–0.40) | <0.001 |
| 60–79 years | 0.35 (0.15–0.81) | 0.014 | 0.46 (0.19–1.12) | 0.088 |
| 80+ years | Reference | Reference | ||
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 1.14 (0.73–1.77) | 0.569 | 1.17 (0.74–1.86) | 0.500 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 2.37 (1.44–3.91) | <0.001 | 1.84 (1.08–3.15) | 0.026 |
| Other | 0.82 (0.35–1.91) | 0.645 | 0.96 (0.41–2.27) | 0.928 |
| Tumor Size | ||||
| <28 mm | Reference | Reference | ||
| ≥28 mm | 1.86 (1.06–3.25) | 0.031 | 2.64 (1.47–4.75) | 0.001 |
| Unknown | 1.11 (0.60–2.06) | 0.743 | 1.13 (0.60–2.14) | 0.710 |
| Histology | ||||
| Papillary | 1.49 (0.73–3.04) | 0.276 | 1.31 (0.61–2.81) | 0.483 |
| Adamantinomatous | 1.13 (0.69–1.86) | 0.619 | 1.39 (0.83–2.34) | 0.207 |
| Craniopharyngioma NOS | Reference | Reference | ||
| Surgical treatment | ||||
| Observation or biopsy | Reference | Reference | ||
| Subtotal resection | 0.39 (0.21–0.73) | 0.003 | 0.45 (0.23–0.85) | 0.014 |
| Gross total resection | 0.96 (0.58–1.59) | 0.877 | 1.22 (0.71–2.08) | 0.475 |
| Unknown | 1.01 (0.137–7.45) | 0.991 | 2.06 (0.25–17.17) | 0.505 |
| Radiation treatment | ||||
| No radiation | Reference | Reference | ||
| Radiation | 0.50 (0.26–0.94) | 0.032 | 0.45 (0.23–0.86) | 0.016 |
| Unknown | 0.55 (0.08–3.99) | 0.557 | 0.57 (0.08–4.28) | 0.588 |
Abbreviations: NOS, not otherwise specified.
Fig. 1.
(a) Kaplan-Meier survival curve (months) by age, (b) race, and (c) tumor size.
Fig. 2.
(a) Kaplan-Meier survival curve (months) by radiation treatment, (b) surgical treatment, and (c) combined radiation and surgical treatment.
An increase in age is associated with a worse outcome in both the univariate and multivariate models. Risk of worse survival time increased incrementally as age increased, from the youngest age group to the oldest age groups. Blacks had worse survival than did whites in both proportional hazards models, with hazard ratios (HRs) of 2.37 (95% CI, 1.44–3.91; P < .001) in the univariate model and 1.84 (95% CI, 1.08–3.15; P = .026) in the multivariable model (Table 4). White patients also had better relative survival, with 1- and 3-year relative survival rates of 92.2% (95% CI, 89.2%–94.4%) and 89.9% (95% CI, 86.1%–92.7%), compared with 1- and 3-year relative survival rates of 87.4% (95% CI, 77.5%–93.1%) and 74.8% (95% CI, 61.8%–83.9%) among black patients (Table 5). Men and women had similar survival rates. Larger tumors were associated with worse survival in both the univariate and multivariable models (HR, 1.86 [95% CI, 1.06–3.25; P = .031] and HR, 2.64 [95% CI, 1.47–4.75; P = .001] in patients with tumors ≥28 mm, compared with those with tumors <28 mm, in the univariate and multivariate models, respectively) (Table 4). Histologic subtype was not significantly associated with survival in the final adjusted model.
Table 5.
Race, sex, age, and date of coding adjusted overall survival estimates at 1- and 3- years. Relative survival the percentage of observed survival relative to expected survival. Expected survival is calculated from standard US population estimates
| Variable | Observed overall 1-year survival (%) | Relative overall 1-year survival (%) | Observed overall 3-year survival (%) | Relative overall 3-year survival (%) |
|---|---|---|---|---|
| Age | ||||
| 0–19 years | 98.0 (94.7–99.2) | 98.0 (94.7–99.2) | 94.4 (88.8–97.3) | 94.5 (88.8–97.3) |
| 20–39 years | 95.8 (89.1–98.4) | 95.9 (89.1–98.5) | 93.0 (84.9–96.8) | 93.3 (85.0–97.0) |
| 40–59 years | 92.5 (87.7–95.5) | 92.9 (88.0–95.9) | 85.4 (78.6–90.2) | 86.5 (79.5–91.3) |
| 60–79 years | 77.7 (68.5–84.5) | 79.4 (69.9–86.3) | 70.9 (60.0–79.4) | 75.6 (63.4–84.2) |
| 80+ years | 60.0 (27.5–81.7) | 62.1 (27.6–83.9) | 45.0 (13.4–72.8) | 50.8 (13.4–79.7) |
| Sex | ||||
| Male | 91.9 (88.1–94.5) | 92.6 (88.7–95.2) | 86.3 (81.2–90.1) | 88.1 (82.7–91.8) |
| Female | 91.0 (87.2–93.8) | 91.5 (87.5–94.2) | 86.1 (81.0–89.9) | 87.2 (82.0–91.0) |
| Race | ||||
| White | 91.7 (88.7–93.9) | 92.2 (89.2–94.4) | 88.4 (84.8–91.3) | 89.9 (86.1–92.7) |
| Black | 86.6 (77.0–92.3) | 87.4 (77.5–93.1) | 73.0 (60.5–82.0) | 74.8 (61.8–83.9) |
| Other | 96.7 (87.5–99.2) | 97.1 (86.9–99.4) | 89.9 (77.2–95.8) | 90.6 (77.3–96.3) |
| Tumor Size | ||||
| <28 mm | 95.2 (91.0–97.5) | 95.7 (91.3–97.9) | 87.8 (80.7–92.4) | 89.5 (81.9–94.0) |
| ≥28 mm | 87.6 (82.3–91.3) | 88.0 (82.7–91.7) | 81.8 (75.3–86.8) | 82.7 (76.1–87.7) |
| Unknown | 92.4 (87.7–95.4) | 93.0 (88.2–95.9) | 89.3 (83.5–93.1) | 91.1 (84.9–94.8) |
| Histology | ||||
| Papillary | 89.9 (77.4–95.7) | 90.6 (77.5–96.2) | 81.2 (65.4–90.3) | 82.8 (66.1–91.8) |
| Adamantinomatous | 91.3 (85.9–94.7) | 91.8 (86.3–95.1) | 85.6 (78.3–90.5) | 86.8 (79.3–91.8) |
| Craniopharyngioma NOS | 91.7 (88.4–94.1) | 92.3 (89.0–94.7) | 87.1 (82.8–90.4) | 88.6 (84.1–91.9) |
| Surgical treatment | ||||
| Observation or biopsy | 87.8 (81.7–92.0) | 88.5 (82.2–92.6) | 82.3 (74.4–87.9) | 83.8 (75.6–89.4) |
| Subtotal resection | 94.7 (90.9–97.0) | 95.2 (91.2–97.4) | 92.5 (87.6–95.5) | 94.3 (88.8–97.1) |
| Gross total resection | 91.0 (86.0–94.2) | 91.4 (86.4–94.7) | 82.6 (75.8–87.6) | 83.6 (76.7–88.6) |
| Unknown | 83.3 (27.3–97.5) | 83.4 (27.1–97.5) | 83.3 (27.3–97.5) | 83.4 (27.1–97.5) |
| radiation treatment | ||||
| No radiation | 89.6 (86.3–92.1) | 90.1 (86.9–92.7) | 85.2 (81.1–88.4) | 86.6 (82.4–89.8) |
| Radiation | 98.5 (94.0–99.6) | 98.9 (92.8–99.8) | 90.0 (82.0–94.5) | 91.4 (82.8–95.8) |
| Unknown | 84.6 (51.2–95.9) | 84.9 (50.9–96.1) | 84.6 (51.2–95.9) | 84.9 (50.9–96.1) |
Abbreviations: NOS, not otherwise specified.
Both STR and radiation therapy were independently associated with increased survival. With use of observation or biopsy as the reference value, there was no survival advantage associated with GTR. Conversely, subtotal resection decreased the risk of worse survival time, compared with observation/biopsy by nearly two-thirds, with an HR of 0.39 (95% CI, 0.21–0.73; P = .003) in the univariate model and 0.45 (95% CI, 0.23–0.85; P = .014) in the multivariable model. In addition, patients who received radiation treatment were half as likely to experience worse survival time, compared with those not receiving radiation treatment, with an HR of 0.50 (95% CI, 0.26–0.94; P = .032) in the univariate model and 0.45 (95% CI, 0.23–0.86; P = .016) in the multivariable model, when adjusted for surgical intervention (Table 4).
Discussion
We demonstrated that >85% of patients with craniopharyngioma survived 3 years after diagnosis and that STR and radiation therapy were associated with prolonged survival. We also noted a higher incidence rate and worse 1- and 3-year survival rates in the black population.
Craniopharyngioma is a histologically benign but locally aggressive sellar/suprasellar tumor characterized by a propensity to involve vital surrounding neurologic structures. Although GTR remains the therapeutic mainstay for select patients, a growing body of literature suggests that conservative surgical strategies, followed by adjuvant fractionated or targeted radiotherapy, may achieve similar levels of tumor control while minimizing visual, neurologic, and endocrine complications.20–24 Although a number of small case series have evaluated various treatment paradigms, few studies have comprehensively assessed the epidemiology of craniopharyngioma at the population level.
The overall incidence rate of 1.7 cases per 1 000 000 person-years is slightly higher than the 1.3 cases per 1 000 000 person-years noted by Bunin et al. in their 1998 study. Although statistical comparison between these 2 studies is not possible, we can surmise that the variation may be a result of superior case ascertainment in SEER or perhaps may reflect an increased rate of diagnosis because of the significant advances in neuroimaging over the past decade. Of note, the incidence rate did not vary over the 5-year span of our study.
Consistent with previous studies, a bimodal age distribution was noted for patients in the SEER database with a diagnosis of craniopharyngioma.19 The incidence among children aged 0–19 years and among adults aged 40–79 years was significantly higher than among young adults and the older population. In addition, tumors of the adamantinomatous subtype occurred with a higher frequency in children, whereas tumors of the papillary subtype occurred with a higher frequency in the adult population. This breakdown has been previously noted and is likely a result of the variable origin of these craniopharyngioma subtypes. Adamantinomatous tumors are thought to originate from aberrant odontogenic tissue from the primitive oral cavity, whereas papillary tumors develop from anterior pituitary cells, suprasellar epidermoid cysts, or Rathke cleft cysts associated with remnants of the craniopharyngeal duct and squamous metaplasia.25 As a generalization, adamantinomatous craniopharyngiomas contain both solid and cystic elements, whereas papillary craniopharyngiomas tend to be solid.26
Of interest, black race was associated with a trend towards increased relative risk for craniopharyngioma of 1.26 (95% CI, 0.98–1.59; P = .07) when compared with white patients. The difference reached statistical significance when stratified by age among adults aged 40–79 years in which black race was associated with an incidence rate of 3.0 cases per 1 000 000 person-years, compared with 2.0 cases per 1 000 000 person-years among white patients (RR = 1.54; P = .0129). The vast majority of previous studies have noted virtually identical incidence rates among black persons.19,27,28 Many of these studies, however, are limited by underreporting of benign brain tumors.28 In a subset of the data analyzed by Bunin et al., the rate among African-American children was approximately twice that among white children, a statistically significant difference.19 The remainder of their analysis found similar rates, and the discrepant incidence rates were written off as chance because of the small number of African-American cases. Our study provides additional information in support of their finding that there may be an increase in the relative risk for craniopharyngioma among black patients. This was driven by a statistically significant difference in the incidence rates between black and white adults aged 40–79 years. The incidence rates among black and white patients did not differ on the basis of sex or histologic subtype. Although prior reports have not included craniopharyngioma, most investigation indicate that white persons are at significantly elevated risk for malignant brain tumor development with relative risks of 1.86–2.5.29–31 Two older studies, one from Los Angeles and one from Washington DC, disclosed higher proportions of meningiomas in black patients than in white patients.30,32 More recent, large-scale studies have not replicated these results and most often observe no difference in the incidence rates for meningiomas between white and black individuals. These results are unlikely to be related to a detection bias, because craniopharyngioma rarely evade clinical detection given their propensity to cause deficits related to vision, endocrinologic abnormalities, and/or hydrocephalus.
Craniopharyngiomas are notoriously difficult to treat. These histologically benign tumors are located in and around the sella turcica, a region with intimate connection to the pituitary gland, hypothalamus, and optic apparatus. As such, patients frequently present with endocrine abnormalities, visual dysfunction, or symptoms of hydrocephalus because of extension into the nearby third ventricle. Despite refinement of microsurgical and endoscopic resection of these lesions, GTR may still be associated with a significant risk of endocrinopathy and neurologic morbidity.22 To avoid irreversible surgery-related morbidities, conservative surgical strategies, including planned STR followed by adjuvant radiation therapy, are increasingly being pursued.10,21 Nevertheless, consistent with the prevailing treatment paradigm, the vast majority of patients with a diagnosis of craniopharyngioma are initially treated surgically, often with the aim of GTR.
The selection of surgical approach and degree of resection are tailored based on anatomic location and neurologic, visual, and endocrinologic symptoms. Tumors that are adherent to the pituitary stalk, hypothalamus, and cerebral vessels or those that appear to have invaded into the surrounding parenchyma may be best served by an incomplete tumor resection followed by upfront or salvage irradiation.26 Of surprise, only 49 patients (19.9%) who underwent STR received adjuvant radiotherapy. A limitation of our study is the incomplete information on treatment at the time of recurrence, which is not available in SEER. It is possible that a number of patients received radiotherapy as part of a salvage protocol, which may influence outcome. There were also a small proportion of patients who received upfront radiation without surgical resection. These patients were less likely to have had adamantinomatous histology and were older, which speaks to their fitness to undergo a major intracranial operation.
Accordingly, age was the only demographic factor associated with the likelihood of undergoing tumor resection. Although the SEER database does not include comorbidity data, which are likely to affect the decision of whether or not to operate, one can assume that, on average, as age increases, patients are increasingly poor surgical candidates. Large tumor size was also found to be a positive predictor of surgical intervention. Because of the precarious anatomic location of craniopharyngiomas, tumor expansion will almost certainly compress vital structures, a factor which would favor surgical decompression/resection.
Because of the histologically benign nature of craniopharyngiomas and excellent short-term survival, data on overall survival is scant in the literature. Because these lesions often exhibit aggressive local recurrence, progression-free survival is typically used as the benchmark when evaluating various treatment paradigms. However, craniopharyngiomas are associated with substantially decreased overall survival, with mortality rates 3-fold to 6-fold higher than that of the general population.26,33 In addition to deaths directly attributable to the tumor and related interventions, cardiac, cerebrovascular, and respiratory mortality are increased. This is hypothesized to be related to hormonal deficiencies, particularly of growth hormone and gonadotropins.26 Nevertheless, 10-year survival rates range from 80% to 90% in recent investigations.33,34 At a median of 25.5 months (interquartile range, 10–43 months) follow-up, we found an observed 1-year survival rate of 91.5% and 3-year survival rate of 86.2%. These rates are consistent with the unadjusted rate determined by Bunin et al., which was 80% at 5 years.19
Of no surprise, younger patients and those with smaller tumors experienced improved 1- and 3- year survival rates (Table 5). Of interest, we noted substantially reduced overall prognosis in black patients, with 1- and 3-year relative survival rates of 87.4% (95% CI, 77.5%–93.1%) and 74.8% (95% CI, 61.8%–83.9%), compared with 1- and 3- year relative survival rates of 92.2% (95% CI, 89.2%–94.4%) and 89.9% (95% CI, 86.1%–92.7%) for white patients. There is no evidence that this difference in survival rates is the result of treatment differences, because there was not a significant difference between the proportions of white and black patients who underwent observation/biopsy as opposed to STR or GTR. Furthermore, relative survival rates are adjusted by age, sex, and year of diagnosis and should not represent an artifact of worse all-cause survival in the black population. Differential survival for craniopharyngioma based on race has not been noted in the literature, although previous studies have demonstrated worse survival and disease severity at presentation in black patients for other brain tumor subtypes.35
GTR, when it can be completed with minimal risk to vital neurologic and endocrine structures, is the current standard of care for craniopharyngioma. This dogma has recently been challenged by a number of groups because of the high rates of post-surgical mono-hormonal and pan-hormonal hypothalamic/pituitary endocrinopathy that often accompany attempts at GTR.22 Furthermore, advances in radiation therapy, including the progression of stereotactic radiosurgery, have improved radiation conformality in this critical region, thus providing more targeted treatment with minimization of exposure to nearby critical structures.10,26 These advances and realizations have ushered in a new paradigm in which subtotal resection, combined with adjuvant radiation therapy, is being considered as a viable alternative. In fact, a recent meta-analysis has shown that STR with adjuvant irradiation has similar rates of long-term disease control, compared with GTR.21 Although we are unable to analyze rates of progression-free survival in our cohort, these results suggest that such a paradigm may indeed be associated with equivalent or, perhaps, improved outcomes. Patients who received GTR had worse relative 3-year survival (83.6%; 95% CI, 76.7–88.6%), compared with those who underwent STR with or without adjuvant therapy (96.0% [95% CI, 71.5–99.5] and 93.6% [95% CI, 87.5%–96.8%], respectively). We cannot ascertain which, if any, patients underwent salvage radiation therapy. Although some studies suggest that the timing of initiation of radiation treatment may be of little consequence with regard to survival,10 other series have noted that earlier initiation of radiation therapy may be associated with improved visual acuity relative to salvage regimens.36 Ultimately, a randomized controlled trial would be critical in evaluating the relative efficacies of these various modalities and their effects on progression-free survival, overall survival, and quality of life metrics.
Although this analysis aims to provide a comprehensive overview of the demographic and clinical characteristics of patients with a diagnosis of craniopharyngioma, several limitations of this study must be recognized and taken into consideration. First, the analysis is limited by the data available in the SEER database. The database provides limited clinical information, no data on chemotherapy, and only information about the initial course of treatment, without information about tumor residual or recurrence. Although benign tumors, such as craniopharyngioma, are unlikely to be treated with chemotherapy, recurrence and subsequent treatment may play an important role in determining long-term survival, especially in those patients undergoing subtotal resection. Furthermore, performance status and medical comorbidities are likely to play an important role in deciding which patients undergo surgical resection, an intervention associated with increased rates of survival. Finally, although analyzing data from multiple sites allows for a larger cohort of patients and results that can be more reliably generalized to the patient population at large, there are limitations resulting from the potential for inaccurate coding, varying treatment patterns, and differences in clinical decision making at individual centers, which could not be accounted for in this analysis.
This study represents the first SEER-based analysis of incidence, treatment, and survival among patients with craniopharyngioma. We demonstrated that >85% of patients survived 3 years after diagnosis and that STR and radiation therapy were associated with prolonged survival in this population-based cohort. We also noted a higher incidence rate and worse 1- and 3- year survival rates in the black population. Future investigations should examine these racial disparities and focus on evaluating the efficacy of emerging treatment paradigms.
Acknowledgements
We thank Dr. Dawn Hershman for her critical review of the manuscript.
Conflict of interest statement. None declared.
References
- 1.Hoffman HJ. Surgical management of craniopharyngioma. Pediatr Neurosurg. 1994;21(Suppl 1):44–49. doi: 10.1159/000120861. [DOI] [PubMed] [Google Scholar]
- 2.States, C. B. T. R. O. T. U. 2011. CBTRUS Statistical Report. 2011 [Google Scholar]
- 3.Komotar RJ, Roguski M, Bruce JN. Surgical management of craniopharyngiomas. J Neurooncol. 2009;92:283–296. doi: 10.1007/s11060-009-9841-4. [DOI] [PubMed] [Google Scholar]
- 4.Tuniz F, Soltys SG, Choi CY, et al. Multisession cyberknife stereotactic radiosurgery of large, benign cranial base tumors: preliminary study. Neurosurgery. 2009;65:898–907. doi: 10.1227/01.NEU.0000359316.34041.A8. discussion 907. [DOI] [PubMed] [Google Scholar]
- 5.Chakrabarti I, Amar AP, Couldwell W, Weiss MH. Long-term neurological, visual, and endocrine outcomes following transnasal resection of craniopharyngioma. J Neurosurg. 2005;102:650–657. doi: 10.3171/jns.2005.102.4.0650. [DOI] [PubMed] [Google Scholar]
- 6.Weiner HL, Wisoff JH, Rosenberg ME, et al. Craniopharyngiomas: a clinicopathological analysis of factors predictive of recurrence and functional outcome. Neurosurgery. 1994;35:1001–1010. doi: 10.1227/00006123-199412000-00001. discussion 1010–1. [DOI] [PubMed] [Google Scholar]
- 7.Tomita T, Bowman RM. Craniopharyngiomas in children: surgical experience at Children's Memorial Hospital. Childs Nerv Syst. 2005;21:729–746. doi: 10.1007/s00381-005-1202-9. [DOI] [PubMed] [Google Scholar]
- 8.Stripp DCH, Maity A, Janss AJ, et al. Surgery with or without radiation therapy in the management of craniopharyngiomas in children and young adults. Int J Radiat Oncol Biol Phys. 2004;58:714–720. doi: 10.1016/S0360-3016(03)01570-0. [DOI] [PubMed] [Google Scholar]
- 9.Chiou SM, Lunsford LD, Niranjan A, Kondziolka D, Flickinger JC. Stereotactic radiosurgery of residual or recurrent craniopharyngioma, after surgery, with or without radiation therapy. Neuro-oncology. 2001;3:159–166. doi: 10.1093/neuonc/3.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon C, Kim S, Shin HJ, et al. The therapeutic efficacy of fractionated radiotherapy and gamma-knife radiosurgery for craniopharyngiomas. J Clin Neurosci. 2011;18:1621–1625. doi: 10.1016/j.jocn.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Crotty TB, Scheithaucer BW, Young WF, et al. Papillary craniopharyngioma: a clinicopathological study of 48 cases. J Neurosurg. 1995;83:206–214. doi: 10.3171/jns.1995.83.2.0206. [DOI] [PubMed] [Google Scholar]
- 12.Kramer S, Meadows AT, Jarrett P, Evans AE. Incidence of childhood cancer: experience of a decade in a population-based registry. J Natl Cancer Inst. 1983;70:49–55. [PubMed] [Google Scholar]
- 13.Radhakrishnan K, Mokri B, Parisi JE, et al. The trends in incidence of primary brain tumors in the population of Rochester, Minnesota. Ann Neurol. 1995;37:67–73. doi: 10.1002/ana.410370113. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann BM, Hollig A, Strauss C, et al. Results after treatment of craniopharyngiomas: further experiences with 73 patients since 1997. J Neurosurg. 2011;116(2):373–384. doi: 10.3171/2011.6.JNS081451. doi:10.3171/2011.6.JNS081451. [DOI] [PubMed] [Google Scholar]
- 15.Karavitaki N, Brufani C, Warner JT, et al. Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol (Oxf) 2005;62:397–409. doi: 10.1111/j.1365-2265.2005.02231.x. [DOI] [PubMed] [Google Scholar]
- 16.Pinho RS, Andreoni S, Silva NS, et al. Pediatric central nervous system tumors: a single-center experience from 1989 to 2009. J Pediatr Hematol Oncol. 2011;33:605–609. doi: 10.1097/MPH.0b013e31822031d9. [DOI] [PubMed] [Google Scholar]
- 17.Rajan B, Ashley S, Gorman C, et al. Craniopharyngioma–a long-term results following limited surgery and radiotherapy. Radiother Oncol. 1993;26:1–10. doi: 10.1016/0167-8140(93)90019-5. [DOI] [PubMed] [Google Scholar]
- 18.Van Effenterre R, Boch A.-L. Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg. 2002;97:3–11. doi: 10.3171/jns.2002.97.1.0003. [DOI] [PubMed] [Google Scholar]
- 19.Bunin GR, Surawicz TS, Witman PA, et al. The descriptive epidemiology of craniopharyngioma. J Neurosurg. 1998;89:547–551. doi: 10.3171/jns.1998.89.4.0547. [DOI] [PubMed] [Google Scholar]
- 20.Veeravagu A, Lee M, Jiang B, Chang SD. The role of radiosurgery in the treatment of craniopharyngiomas. Neurosurg Focus. 2010;28:E11. doi: 10.3171/2010.2.FOCUS09311. [DOI] [PubMed] [Google Scholar]
- 21.Yang I, Sughrue ME, Rutkowski MJ, et al. Craniopharyngioma: a comparison of tumor control with various treatment strategies. Neurosurg Focus. 2010;28:E5. doi: 10.3171/2010.1.FOCUS09307. [DOI] [PubMed] [Google Scholar]
- 22.Sughrue ME, Yang I, Kane AJ, et al. Endocrinologic, neurologic, and visual morbidity after treatment for craniopharyngioma. J Neurooncol. 2011;101:463–476. doi: 10.1007/s11060-010-0265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minniti G, Esposito V, Amichetti M, Enrici RM. The role of fractionated radiotherapy and radiosurgery in the management of patients with craniopharyngioma. Neurosurg Rev. 2009;32:125–132. doi: 10.1007/s10143-009-0186-4. discussion 132. [DOI] [PubMed] [Google Scholar]
- 24.Xu Z, Yen C-P, Schlesinger D, Sheehan J. Outcomes of Gamma Knife surgery for craniopharyngiomas. J Neurooncol. 2011;104:305–313. doi: 10.1007/s11060-010-0494-0. [DOI] [PubMed] [Google Scholar]
- 25.Okada T, Fujitsu K, Ichikawa T, et al. Coexistence of adamantinomatous and squamous-papillary type craniopharyngioma: Case report and discussion of etiology and pathology. Neuropathology. 2011;32:171–173. doi: 10.1111/j.1440-1789.2011.01235.x. doi:10.1111/j.1440-1789.2011.01235.x. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Miranda JC, Gardner PA, Snyderman CH, et al. Craniopharyngioma: A pathologic, clinical, and surgical review. Head Neck. 2011 doi: 10.1002/hed.21771. doi:10.1002/hed.21771. [DOI] [PubMed] [Google Scholar]
- 27.Stiller CA, Nectoux J. International incidence of childhood brain and spinal tumours. Int J Epidemiol. 1994;23:458–464. doi: 10.1093/ije/23.3.458. [DOI] [PubMed] [Google Scholar]
- 28.Surawicz TS, McCarthy BJ, Kupelian V, et al. Descriptive epidemiology of primary brain and CNS tumors: results from the Central Brain Tumor Registry of the United States, 1990–1994. Neuro-oncology. 1999;1:14–25. doi: 10.1093/neuonc/1.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2006;20:E1. doi: 10.3171/foc.2006.20.4.E1. [DOI] [PubMed] [Google Scholar]
- 30.Heshmat MY, Kovi J, Simpson C, Kennedy J, Fan KJ. Neoplasms of the central nervous system. incidence and population selectivity in the Washington DC, metropolitan area. Cancer. 1976;38:2135–2142. doi: 10.1002/1097-0142(197611)38:5<2135::aid-cncr2820380543>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 31.McLendon RE, Robinson JS, Chambers DB, Grufferman S, Burger PC. The glioblastoma multiforme in Georgia, 1977–1981. Cancer. 1985;56:894–897. doi: 10.1002/1097-0142(19850815)56:4<894::aid-cncr2820560432>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 32.Preston-Martin S. Descriptive epidemiology of primary tumors of the brain, cranial nerves and cranial meninges in Los Angeles County. Neuroepidemiology. 1989;8:283–295. doi: 10.1159/000110196. [DOI] [PubMed] [Google Scholar]
- 33.Pereira AM, Schmid EM, Schutte PJ, et al. High prevalence of long-term cardiovascular, neurological and psychosocial morbidity after treatment for craniopharyngioma. Clin Endocrinol (Oxf) 2005;62:197–204. doi: 10.1111/j.1365-2265.2004.02196.x. [DOI] [PubMed] [Google Scholar]
- 34.Karavitaki N, Cudlip S, Adams CBT, Wass JAH. Craniopharyngiomas. Endocr Rev. 2006;27:371–397. doi: 10.1210/er.2006-0002. [DOI] [PubMed] [Google Scholar]
- 35.Curry WT, Carter BS, Barker FG. Racial, ethnic, and socioeconomic disparities in patient outcomes after craniotomy for tumor in adult patients in the United States, 1988–2004. Neurosurgery. 2010;66:427–437. doi: 10.1227/01.NEU.0000365265.10141.8E. discussion 437–8. [DOI] [PubMed] [Google Scholar]
- 36.Moon SH, Kim IH, Park SW, et al. Early adjuvant radiotherapy toward long-term survival and better quality of life for craniopharyngiomas–a study in single institute. Childs Nerv Syst. 2005;21:799–807. doi: 10.1007/s00381-005-1189-2. [DOI] [PubMed] [Google Scholar]