Abstract
We sought to assess the population-based estimates of age-standardized survival among patients with low-grade gliomas (LGG) and to determine the impact of age and time on relative survival (RS). Data from the Surveillance, Epidemiology, and End Results (SEER) program of NCI from 1973 through 2006 were analyzed to assess survival among 5037 patients. Relationships were modeled using Dickman's piecewise constant hazards RS model. The 3- and 10-year age-standardized RS were 67% and 37%, respectively. When analyzed by age group, the 10-year overall survival (OS) and RS for children (age, <16 years), young adults (age, 16–39 years), adults (age, 40–64 years), and older patients (age, ≥65 years) were 86% and 86%, 61% and 62%, 40% and 43%, and 10% and 14%, respectively. The observed difference between OS and RS was larger among older patients (4%) and smallest among children (<1%). Older patients were 30.5 times (excess hazard ratio [eHR]; 95% confidence interval [CI], 20.3–50.0) as likely as young adults to die during the first year and 18.2 times as likely to die during the second year. Adults were 5.3 (eHR; 95% CI, 3.5–8.1) times as likely to die during their first year as young adults. In the remaining years, the observed survival differences were substantially decreased, and the presence of an age-by-follow-up interaction was observed. Survival among older patients with LGG was substantially different from the one computed for young adults and children. Despite the hazards across age groups not being proportional, RS does not provide additional information, compared with OS, in patients with LGG.
Keywords: age-standardized survival, brain tumor, low-grade glioma, relative survival, standardized mortality ratio
Low-grade gliomas (LGG), including and limited to WHO grade 1 and 2 tumors, comprise <14% of all gliomas.1 This heterogeneous group of tumors is composed of low-grade astrocytomas, oligodendrogliomas, and mixed oligoastrocytomas. Male individuals are thereby usually affected more than female individuals (1.4:1). It is thought that these tumors may be decreasing in incidence, but it is unclear whether this is attributable to histological reclassification.2,3 The survival rates associated with these tumors are superior to those for high-grade gliomas, with a computed 5- and 10-year survivorship in epidemiological and clinical studies of 70%–72% and 40%–55%, respectively.4,5
The mainstay of the therapeutic management of these tumors is suggested to be a complete surgical resection, if possible, and adjuvant radiation therapy (RT) and/or chemotherapy may be added accordingly. These options are largely based on experience and very few randomized controlled trials.4,6,7 Prospective randomized trials showed a survival advantage as a function of the timing of RT or radiation dose, but this difference was found in the early years after diagnosis only.4,6
When reviewing Claus and Black's analysis of the Surveillance, Epidemiology, and End-Results (SEER) database,5 an interesting pattern was observed in the Kaplan-Meier curves comparing age groups. Younger (20–39 years) age groups have diagonal lines, which are typically associated with exponentially distributed hazard functions. This type of hazard function is often associated with chronic diseases and/or slow-growing cancers. In the older (≥65 years) age group, the survivor function decreases steeply early and then flattens, which resembles fast-growing cancers, often associated with a Weibull distribution. This is an important feature for 2 reasons. First, these 2 age groups may have different survival profiles, indicating the presence of 2 different disease entities or behaviors in each age group. Second, the hazards may not be proportional between the 2 age groups, meaning that proportional hazards may not be an appropriate assumption to make regarding these 2 age groups.
Therefore, the aim of this study was to describe the relationships between patients of different age groups with LGG while controlling for different expected mortalities across age groups. To describe the specific relationships, specialized biostatistical methods were used, namely, relative survival (RS) and RS piecewise constant hazards modeling.
Methods
Data were obtained from the SEER database, using the latest release (2008) of results.8 The following extraction codes were deployed: supratentorial gliomas with a WHO grade 1 or 2, specifically ICD-O-3 codes (mixed glioma, 9382; oligodendroglioma, 9450; and astrocytoma, 9400). Pilocytic astrocytomas were excluded from this dataset, because they are primarily found infratentorially. Relative survival measures were calculated using expected mortality tables obtained from NCI.9 The final dataset used in this analysis included only those patients who received follow-up during 2001–2006 (period method or delayed-entry modeling to ensure that these data are current). These data do not describe patterns of care, because patients who received a diagnosis before 1984 were only included if they had survived a minimum of 17 years, and only their follow-ups from 17 years onward were included. The period approach to survival analysis used and described here was developed by Herman Brenner, a German cancer survival statistician.10 Individual patient data were obtained from the SEER 17 registries and were matched to SEER expected mortality tables by year of birth, age, sex, and race using the Ederer II technique.8,9
The data were analyzed by stratification according to the Adolescent and Young Adult Oncology Progress Review Group's recommended age groups: children (0–15 years of age), young adults (16–39 years of age), adults (40–64 years of age), and older patients (≥65 years of age).11 Although an LGG is now thought to be a different disease when it is diagnosed in adults than when it is diagnosed in children, we included children in this analysis because the histological diagnosis remains the same and there is no additional way of stratification of diseases based on molecular factors in the SEER data. We controlled for this by age-standardizing our survival estimation in the estimation of the entire group (described below) and subsequently age stratifying our analysis to discuss this disease in an age group–specific manner. It should be noted that the SEER data, without Medicare linkage, does not have information about chemotherapy.
Statistical Methods
To describe the patient cohort, simple proportions were used along with logistic regression to measure the magnitude of the differences where appropriate. All survival estimates are all presented as RS estimates, and regression modeling was performed using Dickman's piecewise constant hazards RS model.12 The measure used by this model is excess hazard ratio (eHR). The eHR is a unit of measurement similar to the traditional hazard ratio used when measuring differences in overall survival (OS) using models such as the Cox proportional hazards model. Dickman's piecewise constant hazards model is superior to the Cox proportional hazards model in this situation for 2 reasons. First, it provides estimates of survival differences in a cause-specific manner because of the use of RS data. Second, this model is flexible enough to handle nonproportional hazards simply by using an interaction term. Models with and without interaction terms are tested using the likelihood ratio test, and P values <.05 are considered to be statistically significant. If a statistically significant difference is found, an interaction is considered to be present in the data, and the interaction model is deemed to be the model of best fit. This is because a nonproportional hazards model is a more detailed model and does not have proportional hazards as an assumption.
A statistical interaction occurs when a relationship of a independant variable (potential risk factor/predictor or intervention) to a dependant variable varies depending on the level of another independant variable. In this study, the relationships between 2 variables were examined, age category (independant variable #1) and survival years (independant variable #2). An age group by follow-up interaction is said to occur when the probability of survival through that time period (dependant variable) differs across different levels of a variable. If survival worsens relative to another group or hazards increase relative to this other group after a certain period, this is termed an interaction.
The finding of an age-by-follow-up interaction necessitates the use of age-standardized cumulative RS to report the survival experience of the entire group. If eHRs, the summary statistic comparing hazard rates (HRs) across age groups, vary across age groups during follow-up, the groups with the higher eHRs early will have proportionately fewer patients representing their group later during follow-up, thereby artificially overestimating the overall (entire group) RS rates. Therefore, direct standardization, as recommended by Hakulinen et al. as the gold standard of RS estimation, is used to estimate overall group RS.13
The standardized mortality ratio (SMR) is an epidemiologic measure that describes the impact that a tumor has on the likelihood of death when compared with the general population. It has similarities to a cohort study in that the likelihood of death is measured in each population and then compared using a ratio. The LGG population mortality is compared with that of the general population mortality tables (expected mortality tables). The SMR is interpreted as how much more likely a patient with an LGG is going to die, compared with a similar person in the general population.
Results
Overall, the SEER database included 11 675 patients with a diagnosis of LGG. After the application of Brenner's period approach to create the final dataset, the number of patients included was 5037, with a total of 16 493 years of follow-up and 1322 (26.2%) deaths. Children (n = 490) formed 9.7% of the included patients. Young adults (n = 2251), adults (n = 1849), and older patients (n = 447) formed 44.7%, 36.7%, and 8.9% of the overall patients included in this study, respectively.
Table 1 details the patients’ characteristics contained in the dataset used for the regression models. The period approach is used, and therefore, only patients who had follow-up data during 2001–2006 are included in the final dataset. The effect of the period approach on this dataset can be seen in the “year of diagnosis” rows. All variables in rows presented have an association with age categories (P < .001). Of particular importance, the treatments offered to patients were different across age groups (Table 1). Young adults are the most likely group to receive surgery (odds ratio [OR], 4.0; P < .001). Children were the least likely to receive radiation (at least 50% less likely compared with each other age group [OR, 0.40; P < .001]), all excluding the unknown category of patients. Because these data are observational in nature, the decisions to administer radiation treatment are highly likely to be subject to selection bias. The decision was made not to adjust, stratify, or case delete based on these covariates, but to observe the final model's stability by repeating the estimation for each histology group alone and then each treatment category alone (surgery and no surgery, then radiation and no radiation) to observe the direction of the findings.
Table 1.
Demographic and treatment characteristics
| Children (n = 490, 9.7%) |
Young adults (n = 2251, 44.7%) |
Adults (n = 1849, 36.7%) |
Elderly (n = 447, 8.9%) |
Total (n = 5037) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||||
| Male | 264 | 54% | 1257 | 56% | 1057 | 57% | 228 | 51% | 2806 | 56% |
| Female | 226 | 46% | 994 | 44% | 792 | 43% | 219 | 49% | 2231 | 44% |
| Total | 490 | 100% | 2251 | 100% | 1849 | 100% | 447 | 100% | 5037 | 100% |
| Year of Diagnosis | ||||||||||
| 1973–1985 | 76 | 16% | 157 | 7% | 29 | 2% | 0 | 0% | 262 | 5% |
| 1986–1996 | 188 | 38% | 654 | 29% | 360 | 19% | 23 | 5% | 1225 | 24% |
| 1997–2006 | 226 | 46% | 1440 | 64% | 1460 | 79% | 424 | 95% | 3550 | 70% |
| Histology | ||||||||||
| Astrocytoma | 326 | 67% | 855 | 38% | 661 | 36% | 268 | 60% | 2110 | 42% |
| Oligodendroglioma | 124 | 25% | 946 | 42% | 848 | 46% | 110 | 25% | 2028 | 40% |
| Mixed | 40 | 8% | 450 | 20% | 340 | 18% | 69 | 15% | 899 | 18% |
| Radiation | ||||||||||
| None | 354 | 72% | 1145 | 51% | 790 | 43% | 211 | 47% | 2500 | 50% |
| Radiation | 128 | 26% | 1012 | 45% | 984 | 54% | 219 | 49% | 2343 | 47% |
| Unknown | 7 | 1% | 74 | 3% | 62 | 3% | 16 | 4% | 159 | 3% |
| Surgery | ||||||||||
| No Surgery | 80 | 16% | 372 | 17% | 410 | 22% | 199 | 45% | 1061 | 21% |
| Surgery Performed | 404 | 82% | 1861 | 83% | 1426 | 77% | 244 | 55% | 3935 | 78% |
| Unknown | 6 | 1% | 18 | 1% | 13 | 1% | 4 | <1% | 41 | 1% |
| Total | 490 | 100% | 2251 | 100% | 1849 | 100% | 447 | 100% | 5037 | 100% |
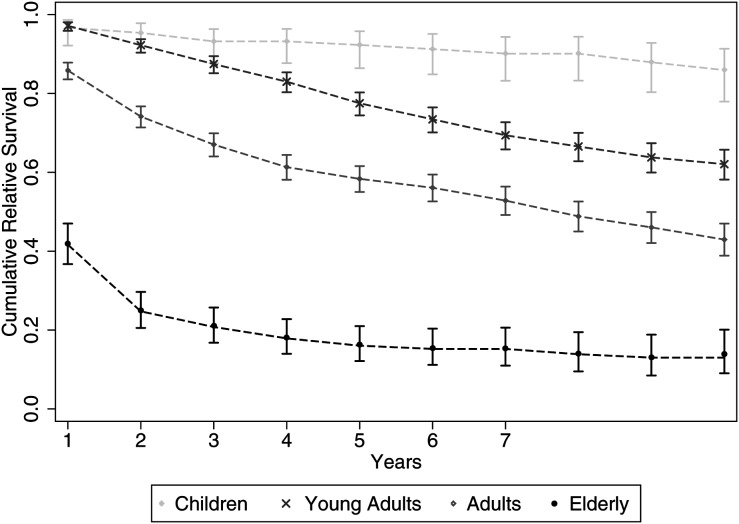
Figure 1 shows the differences and similarities between OS and RS. The 3- and 10-year age-standardized RSs were 67% and 37%, respectively. When analyzed by age group, the 10-year OS and RS for children (<16 years), young adults (16–39 years), adults (40–64 years), and elderly patients (≥65 years) were 86% and 86%, 61% and 62%, 40% and 43%, and 10% and 14%, respectively. The difference in the height of the adult OS curve, compared with the RS curve, was minimal initially; however, the use of RS estimates became useful in the later years to accurately describe cancer-specific death rates, separated from the OS rates. Older groups, however, had the greatest difference of 10% OS compared with 14% RS at 10 years, which is a small but expected difference. Of note, the incremental increase in the RS curve was an artefact because of the small patient numbers at follow-up. If no patients in the at-risk population die during a period in a RS analysis, then the at-risk population appears to have better survival than the general population. The number of patients at risk appears to be different than that of the included population (Figure 1). This is because several patients entered the study at later periods.
Fig. 1.
Kaplan-Meier curves (A) and relative survival curves (B) by age group in patients with LGG.
While reviewing Figure 1, an important difference can be found that is more than just the separation of the OS or RS curves. The survival profiles are different, with the children and young adults showing a uniphasic profile and the older patients showing a multiphasic survival profile. The adults, however, appear to be a combination of these 2 groups.
The almost straight and/or diagonal orientation of the childhood and young adult lines are noted, which implies a steady level of HRs, consistent with an exponential distribution. The HR curves are detailed in Figure 2. The curve of the elderly patients, which is higher at the start of follow-up, but decreases very steeply and then levels off, indicates high early HRs with a decrease over time (consistent with a decreasing Weibull distribution). This shows that the underlying HRs are not proportional, and any proportional hazards model used with age as a covariate will report inaccurate estimates of the variable of interest. Thus, a model capable of handling nonproportional hazards is required to model these data.
Fig. 2.
Overall smoothed hazard rates by age group, demonstrating the non-proportional hazard rates throughout the age categories. The lines start at a time point later than “0” due to the smoothing function. Note how this plot resembles a “fork” with the prongs facing left or backwards (“reverse fork”).
Survival Profile
Table 2 shows the RS among patients with LGG. The 20-year RS among children and elderly patients was 80% and 0%, respectively. The regression model used to model these data was a piecewise constant hazards model that included a covariate-by-follow-up interaction term, which markedly improved the fit of the model demonstrating that survival in age groups is clearly not proportional (likelihood ratio test P < .0001). The model demonstrated in Table 3 is parameterized so that each eHR is comparing the interval with the first year after diagnosis. The interpretation of this model is simple in that all eHR, irrespective of age categories, are compared with the baseline category, which is the first year after diagnosis in young adults. Young adult patients were chosen as baseline because there were the most patients included in this category.
Table 2.
Age-standardized relative survival rates of low grade glioma
| Year | Overall |
Children |
Young adults |
Adults |
Elderly |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RS | 95% CI | RS | 95% CI | RS | 95% CI | RS | 95% CI | RS | 95% CI | ||||||
| 1 | 81 | 79 | 84 | 97 | 92 | 99 | 97 | 96 | 98 | 86 | 84 | 88 | 42 | 37 | 47 |
| 3 | 67 | 63 | 69 | 93 | 88 | 96 | 87 | 85 | 89 | 67 | 64 | 70 | 21 | 17 | 26 |
| 5 | 59 | 55 | 62 | 92 | 86 | 96 | 78 | 74 | 80 | 58 | 55 | 62 | 16 | 12 | 21 |
| 10 | 47 | 42 | 52 | 86 | 78 | 91 | 62 | 58 | 66 | 43 | 39 | 47 | 14 | 9 | 20 |
| 15 | 37 | 32 | 42 | 85 | 77 | 91 | 50 | 45 | 54 | 31 | 26 | 35 | 5 | 1 | 15 |
| 20 | * | * | * | 84 | 75 | 90 | 40 | 36 | 45 | 19 | 14 | 24 | — | — | — |
*Age-standardized relative survival rates are not available for this category as there is no data for the elderly patients at 20 years.
Table 3.
Piecewise-constant hazards model.
| 95 Confidence interval |
|||
|---|---|---|---|
| eHR | Lower | Upper | |
| Young Adults | |||
| Year 1 | 1.00 | 1.00 | 1.00 |
| Year 2 | 1.81 | 1.12 | 2.93 |
| Year 3 | 1.85 | 1.13 | 3.03 |
| Year 4 | 1.83 | 1.10 | 3.05 |
| Year 5 | 2.38 | 1.44 | 3.94 |
| Year 6 | 1.88 | 1.08 | 3.26 |
| Year 7 | 1.98 | 1.11 | 3.52 |
| Year 8 | 1.46 | 0.77 | 2.77 |
| Year 9 | 1.48 | 0.77 | 2.84 |
| Year 10 | 0.95 | 0.44 | 2.06 |
| Children | |||
| Year 1 | 1.17 | 0.44 | 3.11 |
| Year 2 | 0.48 | 0.11 | 2.05 |
| Year 3 | 0.80 | 0.24 | 2.67 |
| Year 4 | * | — | — |
| Year 5 | 0.34 | 0.04 | 2.69 |
| Year 6 | 0.40 | 0.05 | 3.17 |
| Year 7 | 0.44 | 0.06 | 3.44 |
| Year 8 | * | — | — |
| Year 9 | 0.86 | 0.20 | 3.74 |
| Year 10 | 0.79 | 0.18 | 3.44 |
| Adults | |||
| Year 1 | 5.33 | 3.51 | 8.08 |
| Year 2 | 5.10 | 3.34 | 7.80 |
| Year 3 | 3.51 | 2.23 | 5.55 |
| Year 4 | 3.11 | 1.91 | 5.06 |
| Year 5 | 1.73 | 0.95 | 3.17 |
| Year 6 | 1.38 | 0.68 | 2.78 |
| Year 7 | 2.08 | 1.09 | 3.96 |
| Year 8 | 2.74 | 1.48 | 5.07 |
| Year 9 | 2.06 | 1.01 | 4.20 |
| Year 10 | 2.43 | 1.20 | 4.89 |
| Elderly | |||
| Year 1 | 30.53 | 20.26 | 45.99 |
| Year 2 | 18.17 | 11.42 | 28.92 |
| Year 3 | 6.08 | 2.94 | 12.56 |
| Year 4 | 5.23 | 2.17 | 12.60 |
| Year 5 | 3.81 | 1.15 | 12.63 |
| Year 6 | 1.82 | 0.17 | 19.48 |
| Year 7 | * | — | — |
| Year 8 | 3.23 | 0.50 | 20.98 |
| Year 9 | 2.24 | 0.14 | 35.58 |
| Year 10 | * | — | — |
| Constant | 0.03 | 0.02 | 0.04 |
*Indicates unstable/unrealistic excess hazard ratios due to a lack of patients/events in that category.
The model presented in Table 3 demonstrates important differences that occurred early during follow-up. When compared with the young adult group, elderly patients were 30.5 times (eHR; 95% CI, 20.3–50.0) as likely to die in the first year and 18.2 times (95% CI, 11.4–28.9) as likely to die during the second year. A similar but attenuated trend was noted in the adult population; adults were 5.3 times (eHR; 95% CI, 3.5–8.1) as likely to die in the first year. In the second to fourth years, the eHR remained elevated (above an eHR of 3) (Table 3), compared with the first year after diagnosis in young adults. The older population yielded a remarkable finding in that the first 5 years after diagnosis were the most detrimental years after diagnosis, and after this, the excess incidence density of mortality in the older population returned to that of the baseline category (young adults in year 1). Of note, after 5 years, the eHR returned to that seen in the baseline category, whereas the eHR seen in young adults remained higher than that seen in the baseline category.
The results of this model where found to be stable and accurate. When the 3 histological subtypes (mixed glioma, oligodendroglioma, and astrocytoma) and 4 treatments (surgery and no surgery, then radiation and no radiation) were modeled individually, each of the 7 submodels demonstrated similar results to the final overall model, indicating that the large effect of age demonstrated in the final model represents features seen in all the covariate groupings (data not shown). In addition, the closeness or accuracy in predicting the observed values are shown in Figure 3, which demonstrates the model's accuracy in estimating RS.
Fig. 3.
Relative survival in patients with LGG. Observed (dots with capped spikes) and estimated (dotted line) relative survival rate using the piecewise constant hazards model.
Standardized Mortality Ratio (SMR)
Patients that received follow-up from 2001 through 2006 had an overall SMR of 17 (95% CI, 16–18). This is interpreted as follows: patients with a LGG are 17 times as likely to die as a member of the matched general population during the first 20 years after diagnosis. When stratified by age group, children and young adults had SMRs of 20 (95% CI, 12–31) and 28 (95% CI, 26–31), respectively, whereas adults and older patients had SMRs of 16 (95% CI, 15–18) and 13 (95% CI, 12–15), respectively.
Discussion
Overall, patients who received a diagnosis of an LGG were 17 times more likely to die than the general population. In this analysis, the survival differences were very large in the early years, with the older population being almost 31 times as likely to die as young adults in the first year, after adjusting for expected mortality rates. After 5 years of high eHR, the likelihood of mortality among older patients decreased to a level that is close (although still elevated) to that seen in young adults. The difference between this study and others that reported increased mortality among older patients is that these data have been adjusted for background mortality before measuring differences. This finding of a survival profile in older patients resembling that of a higher grade glioma indicates that an older patient who receives a diagnosis of an LGG should be reviewed closely for imaging, histological or molecular signs of high-grade lesions. Furthermore, we describe how the differences evolved during follow-up, because after 5 years, the differences were still present but at a much lower magnitude.
The lowered prognosis demonstrated in older patients in this study may be explained by a combination of both clinical and molecular features. Kaloshi et al. reported 62 (≥60 years) patients identified in a French institutional database. Older patients were more likely to demonstrate clinical deficits, a lower KPS and a larger tumor on MRI (although there was less contrast enhancement seen on CT scans) and were less likely to achieve gross-total resection, all of which can have impacts on prognosis.14 It is unclear how long these tumors may have been present in a patient, and it is reasonable that a patient may acquire the tumor at a younger age and then present with symptoms when he or she is in the older age group.
Advances in our molecular understanding of LGG are improving and may also partially explain the differential survivorship of young and older patients observed in our study. “Pediatric LGG usually do not harbor the same molecular abnormalities as their adult counterparts, a feature also observed in medulloblastomas.”15,16 TP53 mutations are frequent (53%–88%) in adult patients but are rare (5%–10%) in children, possibly restricted to those pediatric patients with glial tumors that undergo malignant transformation.17,18 Loss of heterozygosity of chromosome 1p19q is a favorable prognostic factor in adults and is a predictive marker of response to chemotherapy,19,20 but its value in children is unproven.21 Platelet-derived growth factor (PDGF) signaling pathway has been implicated in the tumorigenesis of these tumors, and PDGF receptor immunohistochemistry expression has been shown to be substantially expressed in 50% of adult patients with LGG (n = 103) and was a negative prognosticator in multivariate analysis.22 In addition, no PDGF amplification was observed in a series of 44 pediatric astrocytomas.23 Of interest, age, with a cutoff value of 40 years, was a major prognostic factor in the analysis of data stemming from 2 large European phase III trials in adult patients with LGG.24 Only patients aged <65 years, however, were included in these prospective studies. Our results, along with the French data, keep with the commonly accepted notion that increased age is a negative prognosticator.
Covariate-by-Follow-up Interaction
In our series, we observed a follow-up-by-covariate interaction, which means that the differences between the levels of a covariate were dependent on the follow-up time after diagnosis. For example, in a SEER medulloblastoma study, there was no difference in RS for the first 4 years of follow-up, after which adults became more likely to die.25 This is visualized as a fork on a Kaplan-Meier survival plot, because the lines diverge after 4 years. In this study, a reverse fork type interaction was found on the HR plot. Instead of diverging after a period of follow-up, the HR converged after a period of follow-up (see Figure 2). Thus, because of the presence of a covariate-by-follow-up interaction, the proportional hazards assumption does not hold true, and studies using Cox-proportional hazards regression to model LGG data cause inaccurate estimates for survival differences throughout the follow-up period.
In summary, the differences in survival across age groups vary during follow-up. Therefore, the basic Cox proportional hazards model is inadequate to model HRs if age is included as an independant variable. Either the stratified Cox proportional hazards model, discrete-time survival models, or the model used here is appropriate to model hazards and survival outcomes with age as an independant variable.
Clinical Applications
Follow-up protocols have not shown to improve survival outcomes in children with brain tumors.26–28 Nonetheless, this has not been proven conclusively to not improve survival outcomes, and patients expect a follow-up plan/surveillance after the treatment of a brain tumor. Therefore, follow-up planning has generally been guided by the perceived intensity of mortality (the “lethality” of the tumor). For example, the follow-up scheduling protocols of patients with glioblastoma multiforme tend to be of a high intensity (follow-up appointment with imaging every 3 months in the beginning) because of the high intensity of mortality experienced in that disease. In LGG, the course of the disease is often more benign and often associated with follow-up protocols of a lesser intensity.
The Children's Hospital of Philadelphia follow-up protocol for cerebellar astrocytomas, for example, is an imaging study of the brain, with and without intravenously administered contrast material, at 6 months. Then a yearly follow-up imaging study continues for up to 5 years after diagnosis. Patients with a high suspicion of residual tumor after 5 years are followed up every 1 or 2 years, indefinitely.26 Others suggest a more intense follow-up schedule, but none are data-derived.29
Proposed Data-Derived Follow-up Guideline for Surveillance Scanning
We suggest that follow-up protocols should be higher in frequency in the elderly population for at least the first 5 years after diagnosis, because the intensity of mortality among adult and older patients is greater than that among children and young adults for at least 5 years after diagnosis.
The years 2–7 are the most deleterious for young adults (Table 3). This lends itself to suggest that a constant follow-up schedule should be applied to this population for the first decade after diagnosis (eHR remain significantly elevated before returning to baseline levels for 7 years after diagnosis). A similar trend is also observed with children, and therefore, it is reasonable that follow-up schedules should be kept active for at least 10 years after diagnosis.
We hereby propose a general follow-up guideline to be applied to general populations of patients with LGG (a standard guideline to be used before it is tailored to individual patient needs for surveillance, scanning, and outcomes measurement) for surveillance, and then modified by the clinician for each patient. After a patient receives his or her prescribed treatment (surgery, adjuvant therapy, or the wait and see approach), the first follow-up visit occurs at 6 months, and then yearly for 10 years in children and young adults. In adults and older patients, the follow-up visits should occur every 3 months for the first 2 years (the first 2 years have greatly increased intensities of mortality), which is similar to the protocol put forth by Kun et al. in 1985, and often offered to patients who receive a diagnosis of high-grade gliomas.29 After this first 2 years, the visits are spaced at 6 months for the next 3 years in adults and elderly patients, after which the frequency of visits returns to that of children and young adults for the next 5 years (yearly visits), until the 10-year mark.
Increased frequency of follow-up visits in the first year may be warranted from a clinical perspective. For example, increased intensity of follow-up (decreased time between visits) should be applied to patients who obtain a subtotal resection at surgery or have a brainstem lesion. Alternatively, patients who progress early require tighter follow-up schedules. These are just 2 examples amongst the many other reasons a clinician might tailor a schedule to a particular patient.
Nevertheless, we must be aware of developing a zealotry about specific follow-up recommendations as data stemming from registries may not be applied to individual patients. Each patient should have a follow-up scheduling protocol designed for his or her needs and stage of disease, including the relevant testing (of which these data cannot provide any guidance). After 10 years, these data cannot provide any guidance, because the models were built on data that were limited to 10 years of follow-up. Although this is only a first step in designing rational follow-up protocols, further studies validating and/or modifying the follow-up guides described here using progression-free/recurrence data would greatly increase the validity and applicability of this information.
Acknowledgements
We thank the epidemiologist associate professor Elmer Villanueva for his continued support and key insights.
Conflict of interest statement. None declared.
References
- 1.CBTRUS. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2007. 2011. Accessed February 25, 2012. [DOI] [PMC free article] [PubMed]
- 2.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109(1):93–108. doi: 10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- 3.Houben MP, Aben KK, Teepen JL, et al. Stable incidence of childhood and adult glioma in The Netherlands, 1989–2003. Acta oncologica. 2006;45(3):272–279. doi: 10.1080/02841860500543190. [DOI] [PubMed] [Google Scholar]
- 4.van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–990. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]
- 5.Claus EB, Black PM. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973–2001. Cancer. 2006;106(6):1358–1363. doi: 10.1002/cncr.21733. [DOI] [PubMed] [Google Scholar]
- 6.Karim AB, Afra D, Cornu P, et al. Randomized trial on the efficacy of radiotherapy for cerebral low-grade glioma in the adult: European Organization for Research and Treatment of Cancer Study 22845 with the Medical Research Council study BRO4: an interim analysis. International Journal of Radiation Oncology, Biology, Physics. 2002;52(2):316–324. doi: 10.1016/s0360-3016(01)02692-x. [DOI] [PubMed] [Google Scholar]
- 7.Karim AB, Maat B, Hatlevoll R, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. International Journal of Radiation Oncology, Biology, Physics. 1996;36(3):549–556. doi: 10.1016/s0360-3016(96)00352-5. [DOI] [PubMed] [Google Scholar]
- 8.Surveillance, Epidemiology and End Results (SEER) Program. National Cancer Institute D, Surveillance Research Program, Cancer Statistics Branch; 2011. SEER 17 Regs Research Data 1973–2007. [Google Scholar]
- 9.Surveillance, Epidemiology and End Results (SEER) Program. 2010. SEER*Stat Database: Mortality - All COD, aggregated with state, Total U.S. (1969–2006), <Katrina/Rita Population Adjustment> Underlying mortality data provided by NCHS (http://www.cdc.gov/nchs). In: National Cancer Institute D, Surveillance Research Program, Cancer Statistics Branch, ed.
- 10.Brenner H, Gefeller O. An alternative approach to monitoring cancer patient survival. Cancer. 1996;78(9):2004–2010. [PubMed] [Google Scholar]
- 11.Albritton K, Caligiurim M, Anderson B, Nichols C, Ulman D. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer. 2006. p. 108. Report of the Adolescent and Young Adult Oncology Progress Review Group.
- 12.Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004;23(1):51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 13.Hakulinen T, Seppa K, Lambert PC. Choosing the relative survival method for cancer survival estimation. Eur J Cancer. 2011;47(14):2202–2210. doi: 10.1016/j.ejca.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Kaloshi G, Psimaras D, Mokhtari K, et al. Supratentorial low-grade gliomas in older patients. Neurology. 2009;73(24):2093–2098. doi: 10.1212/WNL.0b013e3181c6781e. [DOI] [PubMed] [Google Scholar]
- 15.Korshunov A, Remke M, Werft W, et al. Adult and pediatric medulloblastomas are genetically distinct and require different algorithms for molecular risk stratification. J Clin Oncol. 2010;28(18):3054–3060. doi: 10.1200/JCO.2009.25.7121. [DOI] [PubMed] [Google Scholar]
- 16.Parsons DW, Li M, Zhang X, et al. The Genetic Landscape of the Childhood Cancer Medulloblastoma. Science. 2010;331(6016):435–439. doi: 10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broniscer A, Baker SJ, West AN, et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2007;25(6):682–689. doi: 10.1200/JCO.2006.06.8213. [DOI] [PubMed] [Google Scholar]
- 18.Felix CA, Slavc I, Dunn M, et al. p53 gene mutations in pediatric brain tumors. Med Pediatr Oncol. 1995;25(6):431–436. doi: 10.1002/mpo.2950250603. [DOI] [PubMed] [Google Scholar]
- 19.Walker C, Haylock B, Husband D, et al. Clinical use of genotype to predict chemosensitivity in oligodendroglial tumors. Neurology. 2006;66(11):1661–1667. doi: 10.1212/01.wnl.0000218270.12495.9a. [DOI] [PubMed] [Google Scholar]
- 20.Walker C, Haylock B, Husband D, et al. Genetic and metabolic predictors of chemosensitivity in oligodendroglial neoplasms. Br J Cancer. 2006;95(10):1424–1431. doi: 10.1038/sj.bjc.6603390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mariani L, Deiana G, Vassella E, et al. Loss of heterozygosity 1p36 and 19q13 is a prognostic factor for overall survival in patients with diffuse WHO grade 2 gliomas treated without chemotherapy. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2006;24(29):4758–4763. doi: 10.1200/JCO.2006.05.9238. [DOI] [PubMed] [Google Scholar]
- 22.Varela M, Ranuncolo SM, Morand A, et al. EGF-R and PDGF-R, but not bcl-2, overexpression predict overall survival in patients with low-grade astrocytomas. J Surg Oncol. 2004;86(1):34–40. doi: 10.1002/jso.20036. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura M, Shimada K, Ishida E, et al. Molecular pathogenesis of pediatric astrocytic tumors. Neuro Oncol. 2007;9(2):113–123. doi: 10.1215/15228517-2006-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pignatti F, van den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2002;20(8):2076–2084. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 25.Smoll NR. Relative survival of childhood and adult medulloblastomas and primitive neuroectodermal tumors (PNETs) Cancer. 2012;118(5):1313–1322. doi: 10.1002/cncr.26387. [DOI] [PubMed] [Google Scholar]
- 26.Sutton LN, Cnaan A, Klatt L, et al. Postoperative surveillance imaging in children with cerebellar astrocytomas. Journal of Neurosurgery. 1996;84(5):721–725. doi: 10.3171/jns.1996.84.5.0721. [DOI] [PubMed] [Google Scholar]
- 27.Torres CF, Rebsamen S, Silber JH, et al. Surveillance scanning of children with medulloblastoma. N Engl J Med. 1994;330(13):892–895. doi: 10.1056/NEJM199403313301303. [DOI] [PubMed] [Google Scholar]
- 28.Vassilyadi M, Shamji MF, Tataryn Z, Keene D, Ventureyra E. Postoperative surveillance magnetic resonance imaging for cerebellar astrocytoma. Can J Neurol Sci. 2009;36(6):707–712. doi: 10.1017/s0317167100008313. [DOI] [PubMed] [Google Scholar]
- 29.Kun LE, D'Souza B, Tefft M. The value of surveillance testing in childhood brain tumors. Cancer. 1985;56(7 Suppl):1818–1823. doi: 10.1002/1097-0142(19851001)56:7+<1818::aid-cncr2820561320>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]