Abstract
Interest in commercial cultivation and product development of Cordyceps species has shown a recent increase. Due to its biochemical and pharmacological effects, Cordyceps militaris, commonly known as orange caterpillar fungus, is being investigated with great interest. Cultivation of C. militaris has been practiced on a large scale in order to fulfill a demand for scientific investigation and product development. Isolates of C. militaris can be easily established from both spores and tissue. For isolation of spores, ascospores released from mature stromata are trapped in sterile medium. Multi-ascospore isolates, as well as combinations of single ascospore strains, are used for production of fruiting bodies. Progeny ascospore strains can be isolated from artificial fruiting bodies, thus, the cycle of fruiting body production can be continued for a long period of time. In this study, we examined fruiting body production from multi-ascospore isolates and their progeny strains for three generations. F1 progeny strains generally produced a larger number of fruiting bodies, compared with their mother multi-ascospore isolates; however, F2 and F3 progeny strains produced fewer fruiting bodies. Optimum preservation conditions could help to increase the vitality of the progeny strains. In order to retain the fruiting ability of the strains, further testing of various methods of preservation and different methods for isolation should be performed.
Keywords: Biological efficiency, Cordyceps militaris, Multi-ascospore isolate, Progeny strains, Single ascospore isolation
Introduction
Cordyceps species, which are distributed worldwide, are regarded as medicinal herbs in oriental society of Asia, including Korea. Ophiocordyceps sinensis (syn. Cordyceps sinensis) is a highly regarded medicinal herb, not only among Cordyceps species, but also among mushrooms in general [1]. It grows in alpine grasslands of Asia and has significant socio-economic impact in rural areas [2-5]. Due to their similar biochemical compositions and pharmacological properties, Cordyceps militaris has recently been viewed as a substitute for O. sinensis [6-9]. Many experimental studies for in vitro stroma production of C. militaris had been conducted [10 14]. Similarly, different insect larvae and pupae have been used for study of the infection process and stromata formation of C. militaris [15-18]. Alternatively, brown rice has been used for mass cultivation of C. militaris [19, 20].
Isolates derived from spores or tissues are used for establishment of culture and production of fruiting bodies. Among spore isolates, both ascospores and conidia are frequently used. However, for genetic analysis, single ascospore or single conidial isolates are prerequisites. Comparative studies of fruiting body production from multi-ascospore isolates and progeny strains are lacking in C. militaris. Therefore, in this study, we attempted to compare fruiting bodies produced from multi-ascospore isolates, and their progeny strains, as well as from single conidial strains. Findings of the study demonstrated that single ascospore progeny strains isolated from in vitro stromata are equally capable of fruiting body production. Single conidial strains isolated from multi-ascospore isolates were also proven as an alternative to single ascospore strains.
Materials and Methods
Fungal isolates of C. militaris and their fruiting body formation
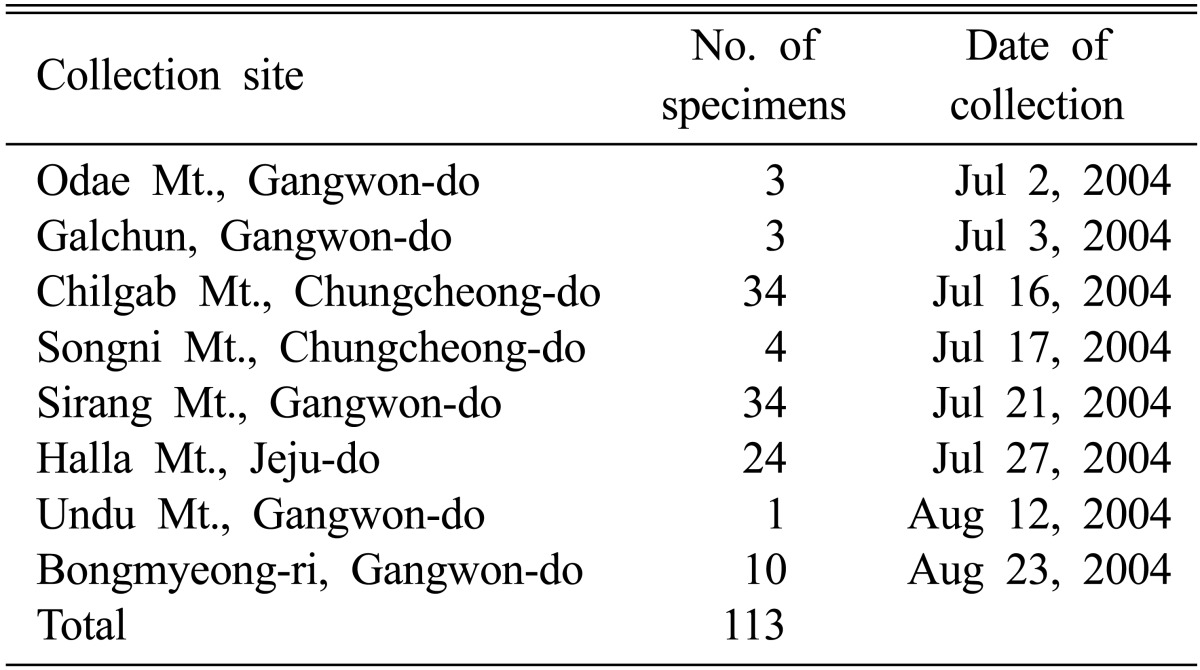
Multi-ascospore isolates were derived from all 113 wild specimens of C. militaris collected on different mountains in Korea, following the method of Sung et al. [21] (Table 1). The isolates were grown on Sabouraud dextrose agar plus yeast extract (SDAY; dextrose 20 g, yeast extract 5 g, peptone 5 g, and agar 15 g per 1,000 mL; pH 5.6) medium at 24 ± 1℃ for 3 wk for use in the experiment. The specimens have been preserved in the Cordyceps Research Institute (CRI), Mushtech, Korea. For induction of fruiting body formation, all 113 isolates were inoculated in brown rice medium, following the method of Shrestha et al. [22]. Brown rice medium was prepared by mixing 50 g of brown rice and 10 g of silkworm pupae in 70 mL of distilled water in 1,000 mL polypropylene (PP) bottles and was then sterilized. After 60 days of culture in brown rice medium, stroma length (SL) of fresh fruiting bodies was measured in mm and biological efficiency (BE) of fruiting bodies was calculated as a percentage (%). For calculation of BE, measurement of dry wt. (DW) of fruiting bodies from each PP bottle was performed by drying them at 60℃ for 24 hr. BE (%) was calculated according to the formula: [DW of fruiting body (g)/substrate wt. (60 g)] × 100. Ten specimens, C-11408, C-11445, C-11821, C-11876, C-11894, C-11913, C-12086, C-12167, C-12434, and C-12448, were selected for calculation of correlation co-efficient (r) between herbarium size of C. militaris and artificial fruiting bodies produced from their isolates, following http://easycalculation.com/statistics/correlation.php.
Table 1.
Cordyceps militaris specimens used in the experiment
Fruiting body formation from single ascospore strains
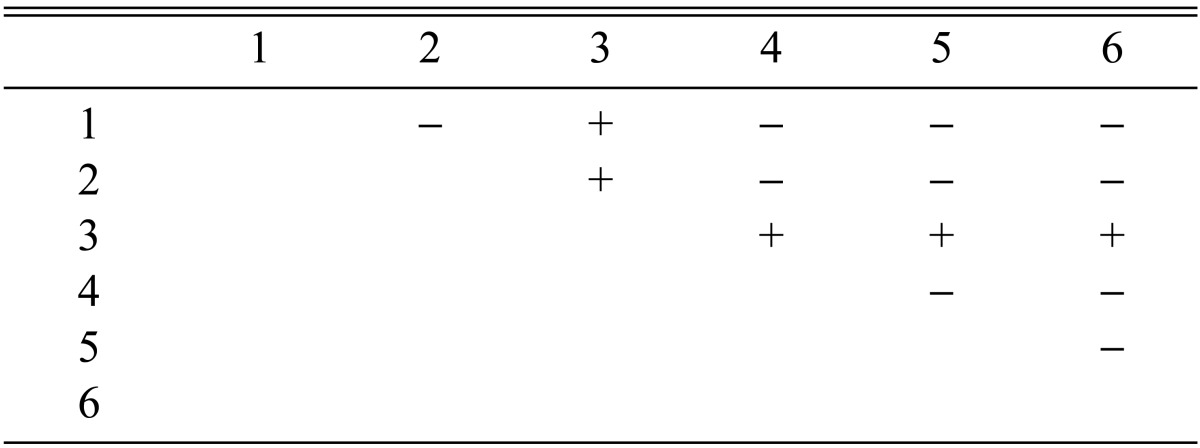
Fruiting bodies produced by the isolates CRI C-12448, CRI C-11913, CRI C-12434, CRI C-11894, and CRI C-12086 were used for isolation of progeny strains. Following the method of Shrestha et al. [23], six single ascospores were isolated from fruiting bodies produced by each isolate and were crossed among themselves in brown rice medium. Single ascospore strains derived from each isolate were numbered from 1 to 6. For example, CRI C-12448 1, CRI C-12448 2, CRI C-124483, CRI C-12448 4, CRI C-12448 5, and CRI C-12448 6 were derived from the isolate CRI C-12448. The single ascospore strains were designated as F1 progeny strains. Calculation of BE of the fruiting bodies of each crossing was performed as described above.
Among the crosses, fruiting bodies produced by the crossing CRI C-12448 4 × CRI C-12448 6 (denoted in short as CRI C-12448 4 × 6) were utilized again for further isolation of progeny strains. Six single ascospore strains isolated from that crossing were numbered as CRI C-12448 (4 × 6) 1, CRI C-12448 (4 × 6) 2, CRI C-12448 (4 × 6) 3, CRI C-12448 (4 × 6) 4, CRI C-12448 (4 × 6) 5, and CRI C-12448 (4 × 6) 6. They were designated as F2 progeny strains and crossed among themselves for fruiting body formation. BE was calculated, as described above. Six single ascospores were again isolated from the crossing CRI C-12448 (4 × 6) 3 × 4 and numbered from CRI C-12448 (4 × 6) (3 × 4) 1 to CRI C-12448 (4 × 6) (3 × 4) 6. The strains were designated as F3 progeny strains and were crossed among themselves; BE was then calculated. In all of the crosses, perithecial stroma formation was indicated as (+) and non-perithecial stroma or no stroma formation as (-).
Fruiting body production from single-conidial strains
The dilution method was used for isolation of single conidial strains from the isolates CRI C-12448 and CRI C-12086. The strains were crossed among themselves in brown rice medium and calculation for BE was performed. Six single conidial strains were also isolated from each single ascospore strain, CRI C-12448 4 and CRI C-12448 6, and crossed among themselves. In addition, crossings between single conidial strains isolated from strains CRI C-12448-4 and CRI C-12448-6 were also made, and calculation for BE was performed.
Results and Discussion
Fruiting body formation from multi-ascospore isolates
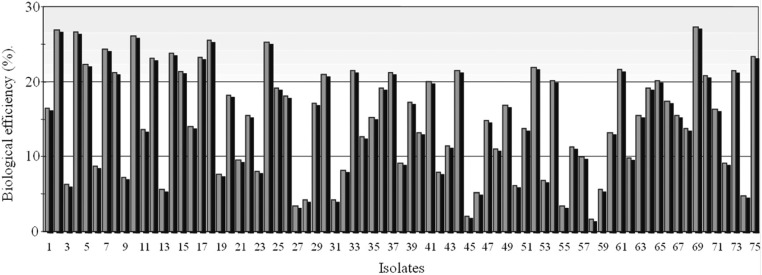
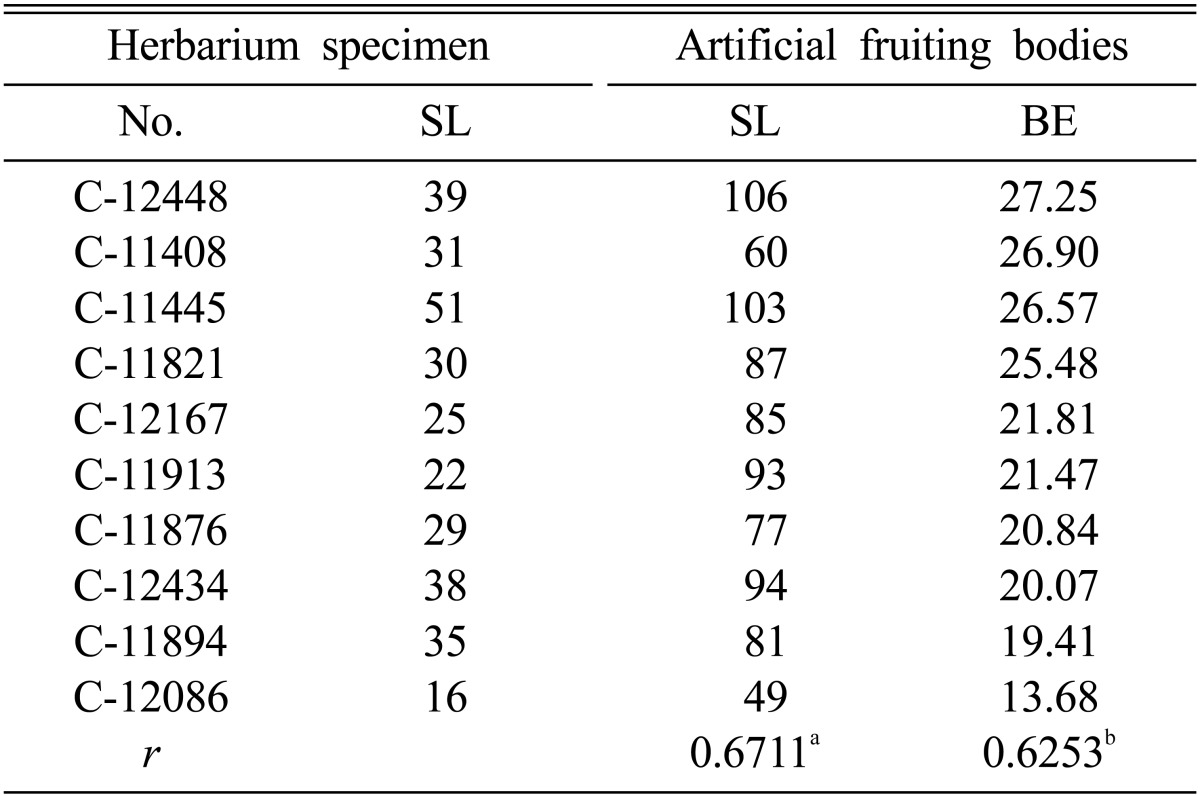
Of the 113 multi-ascospore isolates of C. militaris, only 75 isolates produced fruiting bodies (Fig. 1). The remaining isolates did not produce fruiting bodies on brown rice medium. Multi-ascospore isolates of C. militaris produce unstable fruiting bodies [22]. Multi-ascospore isolates are not favorable for stable and uniform fruiting body formation; however, they can be used for testing of fruiting ability and selection for superior isolates. In this study, we observed variation in fruiting body formation by different isolates of C. militaris. Observation of the positive correlation between herbarium specimens and their respective artificial fruiting bodies was of particular interest (Table 2, Figs. 2~4). Correlation co-efficient between SL of herbarium specimens and SL of artificial fruiting bodies, and between SL of herbarium specimens and BE of fruiting bodies was 0.6711 and 0.6253, respectively (Table 2).
Fig. 1.
Biological efficiency of fruiting bodies produced by 75 isolates of Cordyceps militaris. 1, CRI C-11255; 2, CRI C-11408; 3, CRI C-11444; 4, CRI C-11445; 5, CRI C-11559; 6, CRI C-11732; 7, CRI C-11734; 8, CRI C-11738; 9, CRI C-11740; 10, CRI C-11741; 11, CRI C-11743; 12, CRI C-11744; 13, CRI C-11745; 14, CRI C-11748; 15, CRI C-11818; 16, CRI C-11819; 17, CRI C-11820; 18, CRI C-11821; 19, CRI C-11822; 20, CRI C-11823; 21, CRI C-11825; 22, CRI C-11826; 23, CRI C-11828; 24, CRI C-11829; 25, CRI C-11831; 26, CRI C-11832; 27, CRI C-11834; 28, CRI C-11841; 29, CRI C-11845; 30, CRI C-11876; 31, CRI C-11892; 32, CRI C-11893; 33, CRI C-11894; 34, CRI C-11896; 35, CRI C-11899; 36, CRI C-11901; 37, CRI C-11902; 38, CRI C-11904; 39, CRI C-11905; 40, CRI C-11906; 41, CRI C-11907; 42, CRI C-11908; 43, CRI C-11909; 44, CRI C-11913; 45, CRI C-12019; 46, CRI C-12023; 47, CRI C-12025; 48, CRI C-12027; 49, CRI C-12028; 50, CRI C-12084; 51, CRI C-12086; 52, CRI C-12167; 53, CRI C-12169; 54, CRI C-12170; 55, CRI C-12171; 56, CRI C-12171; 57, CRI C-12176; 58, CRI C-12292; 59, CRI C-12293; 60, CRI C-12294; 61, CRI C-12295; 62, CRI C-12296; 63, CRI C-12297; 64, CRI C-12304; 65, CRI C-12434; 66, CRI C-12443; 67, CRI C-12445; 68, CRI C-12446; 69, CRI C-12448; 70, CRI C-12449; 71, CRI C-12450; 72, CRI C-12451; 73, CRI C-12452; 74, CRI C-12453; 75, CRI C-12454. CRI, Cordyceps Research Institute.
Table 2.
Correlation co-efficient between herbarium specimens of Cordyceps militaris and their artificial fruiting bodies
SL, stroma length; BE, biological efficiency; r, correlation co-efficient.
ar between stroma lengths of herbarium specimens and artificial fruiting bodies.
br between stroma lengths of herbarium specimens and BE of artificial fruiting bodies.
Fig. 2.
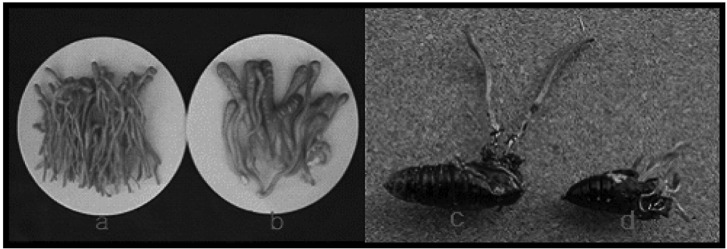
Herbarium specimens of Cordyceps militaris. a, CRI C-11408; b, CRI C-11445; c, CRI C-11821; d, CRI C-11876; e, CRI C-11913; f, CRI C-12167; g, CRI C-12434; h, CRI C-12448. CRI, Cordyceps Research Institute.
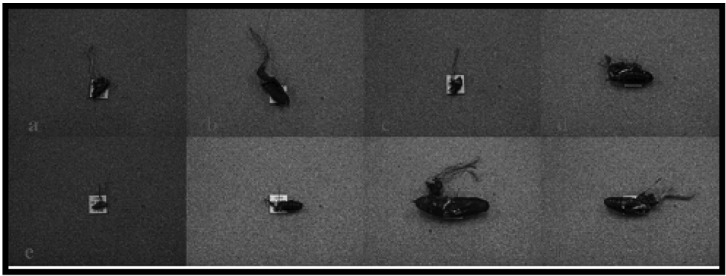
Fig. 4.
Fruiting bodies and herbarium specimens of Cordyceps militaris. a, c, CRI C-11894; b, d, CRI C-12086. CRI, Cordyceps Research Institute.
Fruiting body formation from crossing of F1 progeny strains
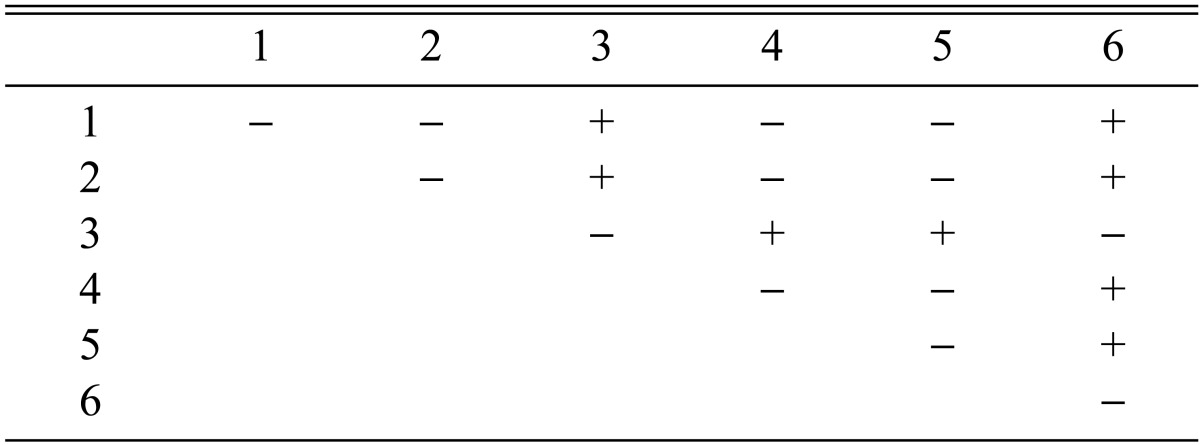
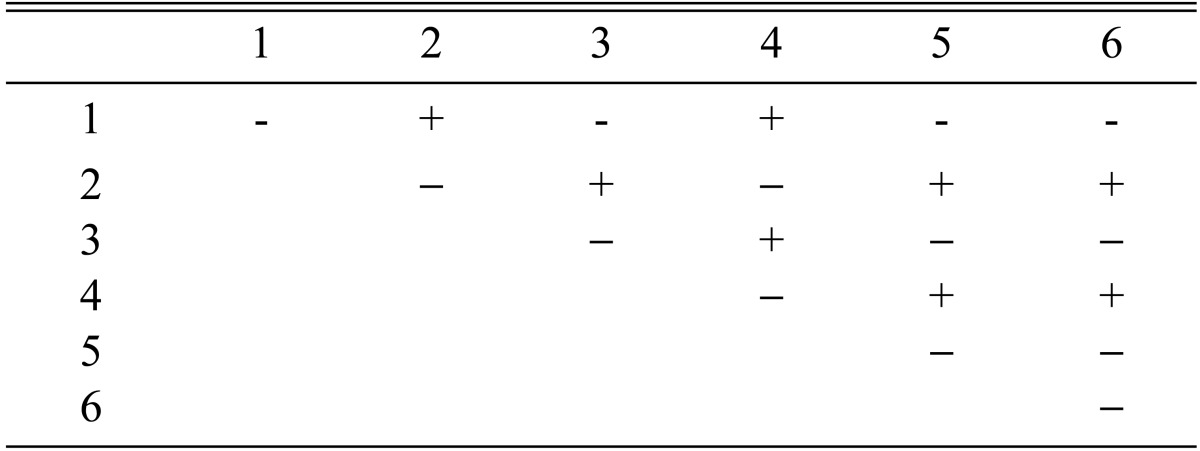
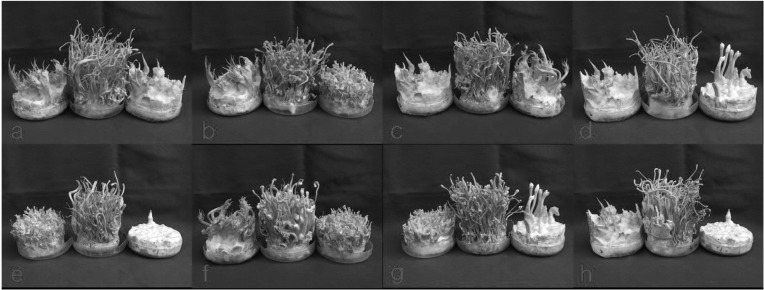
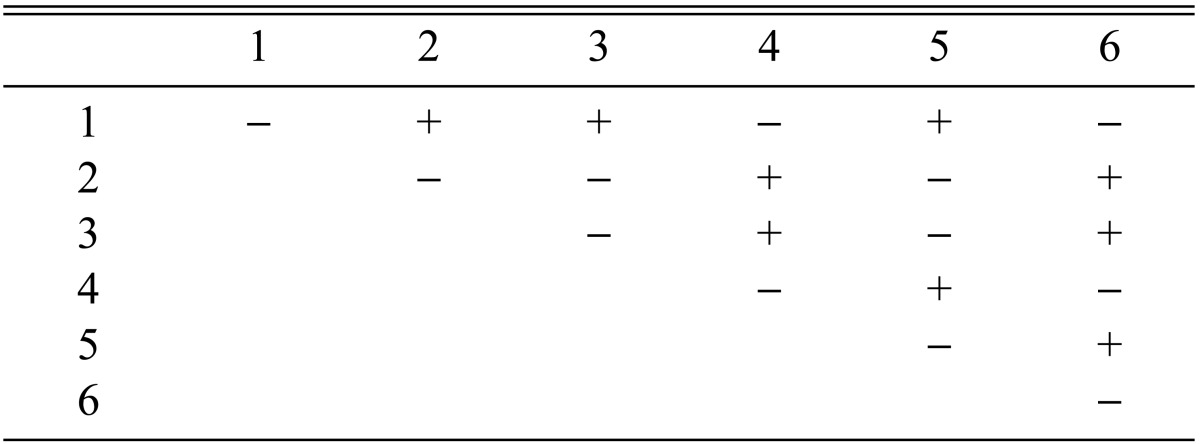
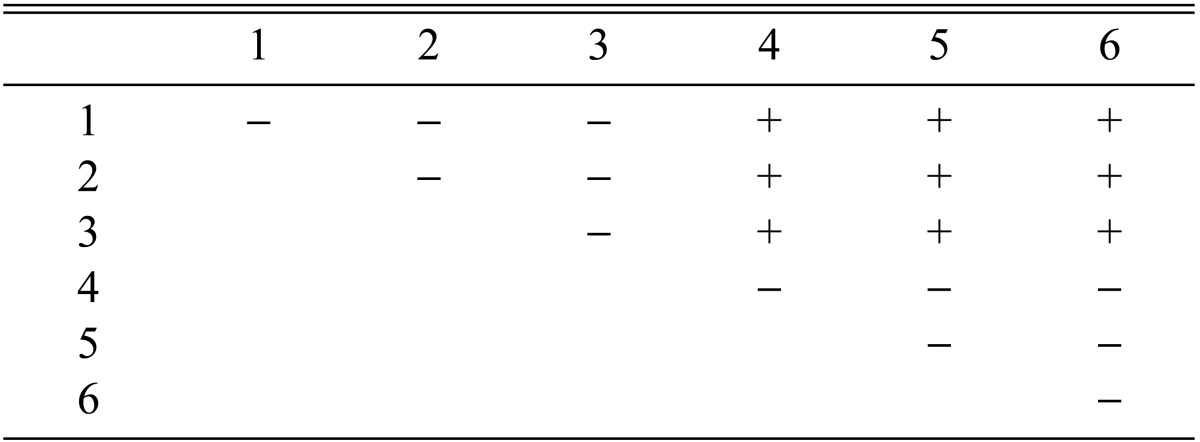
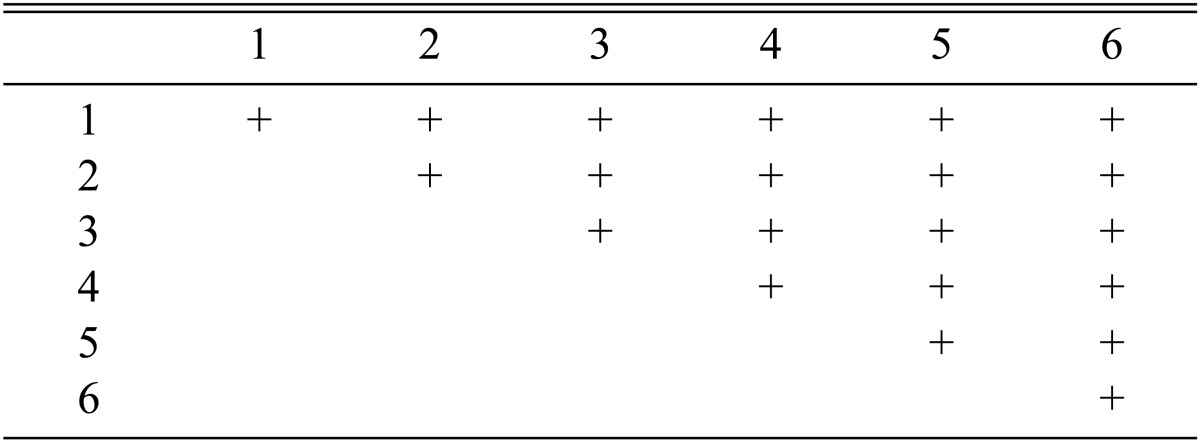
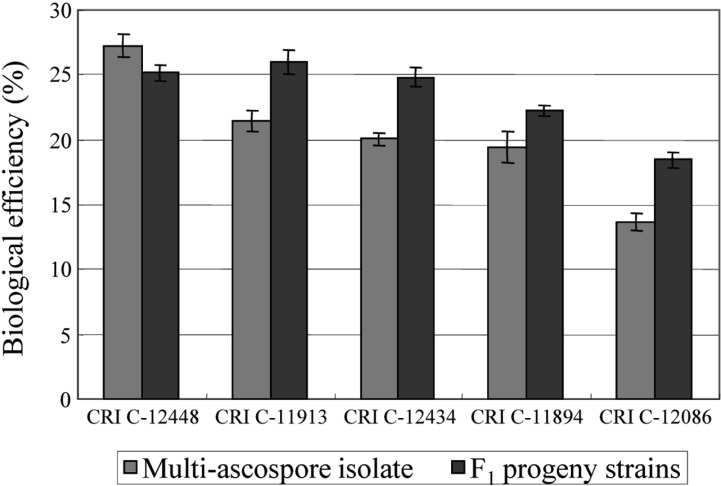
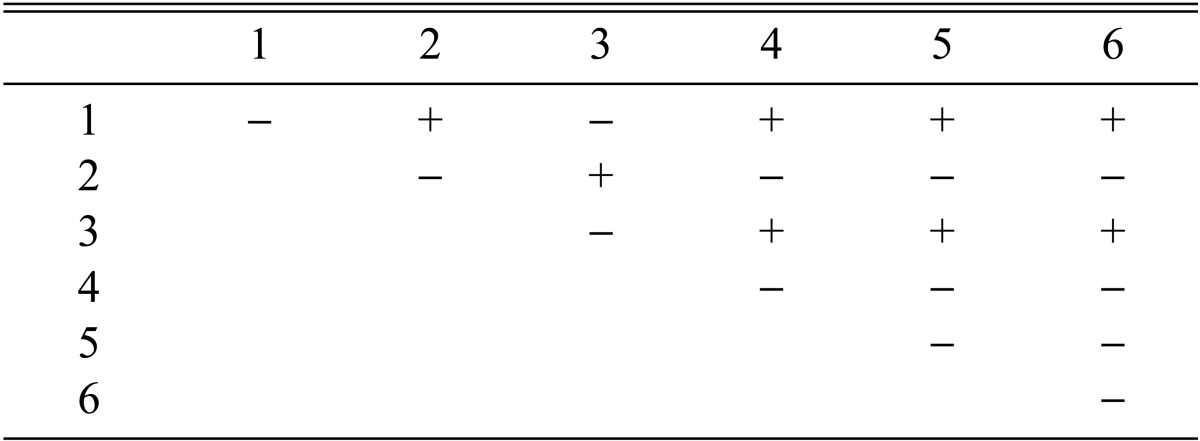
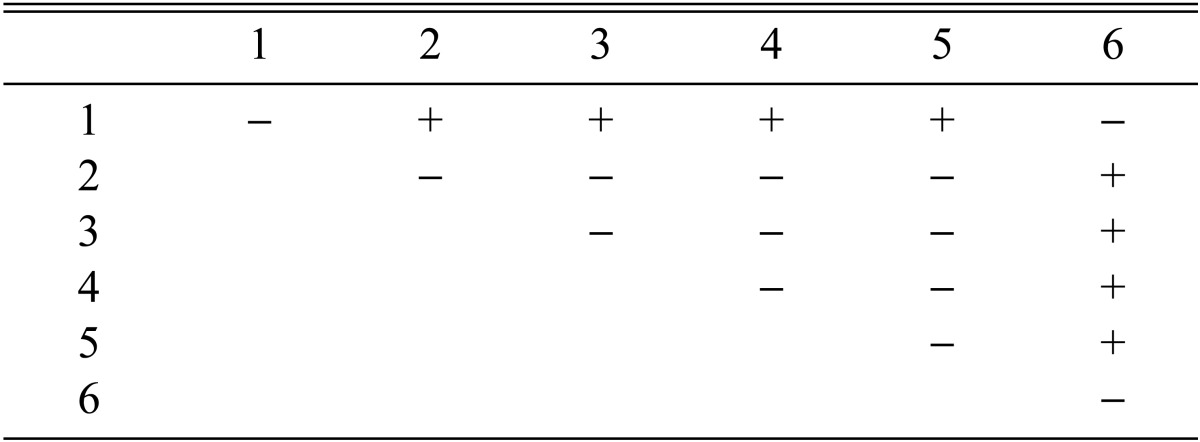
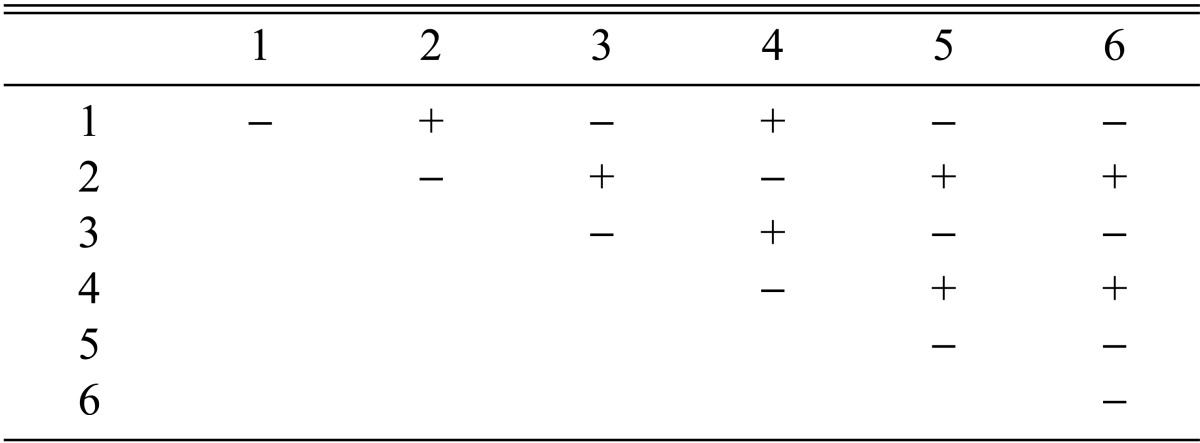
Among the 113 isolates, CRI C-12448 produced the highest BE, followed by CRI C-11408 and CRI C-11445 (Table 2, Fig. 1). In this study, F1 progeny strains were isolated from isolates that differed in fruiting ability based on BE (Table 2). Among F1 progeny strains of the isolate CRI C-12448, strains 1, 2, 4, and 5 and strains 3 and 6 were opposite in mating type (Table 3, Fig. 5). Similarly, strains 1, 3, 5, and 6 of the isolate CRI C-11913 were opposite in mating type to strains 2 and 4 (Table 4, Fig. 6). In the case of the isolate CRI C-12434, strains 1, 4, and 6 and strains 2, 3, and 5 were opposite in mating type (Table 5). In isolate CRI C-11894, strains 1, 2, and 3 were of opposite type to strains 4, 5, and 6 (Table 6). All crossings of CRI C-12086 produced perithecial stromata (Table 7). This was probably due to error in isolation of single ascospore strains or cytological abnormality in the strains. Except for the isolate C-12448, BE of crossings of F1 progeny strains were better, compared to their respective multi-ascospore isolates (Fig. 7).
Table 3.
Stromata formation from crossing of F1 progeny strains of Cordyceps militaris CRI C-12448
CRI, Cordyceps Research Institute.
Fig. 5.
Fruiting bodies produced from crossings of F1 progeny strains of Cordyceps militaris CRI C-12448. a, 1 × 3; b, 1 × 6; c, 2 × 3; d, 2 × 6; e, 3 × 4; f, 3 × 5; g, 4 × 6; h, 5 × 6. CRI, Cordyceps Research Institute.
Table 4.
Stromata formation from crossing of F1 progeny strains of Cordyceps militaris CRI C-11913
CRI, Cordyceps Research Institute.
Fig. 6.
Fruiting bodies produced from crossings of F1 progeny strains of Cordyceps militaris CRI C-11913. a, 1 × 2; b, 1 × 4; c, 2 × 3; d, 2 × 5; e, 2 × 6; f, 3 × 4; g, 4 × 5; h, 4 × 6. CRI, Cordyceps Research Institute.
Table 5.
Stromata formation from crossing of F1 progeny strains of Cordyceps militaris CRI C-12434
CRI, Cordyceps Research Institute.
Table 6.
Stromata formation from crossing of F1 progeny strains of Cordyceps militaris CRI C-11894
CRI, Cordyceps Research Institute.
Table 7.
Stromata formation from crossing of F1 progeny strains of Cordyceps militaris CRI C-12086
CRI, Cordyceps Research Institute.
Fig. 7.
Biological efficiency of multi-ascospore isolates and F1 progeny strains of Cordyceps militaris. CRI, Cordyceps Research Institute.
Cordyceps militaris is a heterothallic fungus and therefore requires a combination of two opposite mating type strains in order to form fruiting bodies [23]. Many studies have followed crossing between single ascospore strains in order to produce fruiting bodies of C. militaris [24-29]. However, isolation of a single ascospore is a time-consuming process requiring a good laboratory facility and a sterile environment. By contrast, isolation of multiple ascospores is an easy process and can also be performed in the field. Hence, isolation of multiple ascospores is a good option for establishment of culture in the lab. Once the culture has been established, single ascospore progeny strains can be isolated from fruiting bodies produced by multi-ascospore isolates, and, in turn, can be used for production of stable fruiting bodies, as demonstrated by Sung et al. [26].
Fruiting body formation from crossings of F2 and F3 single ascospore strains
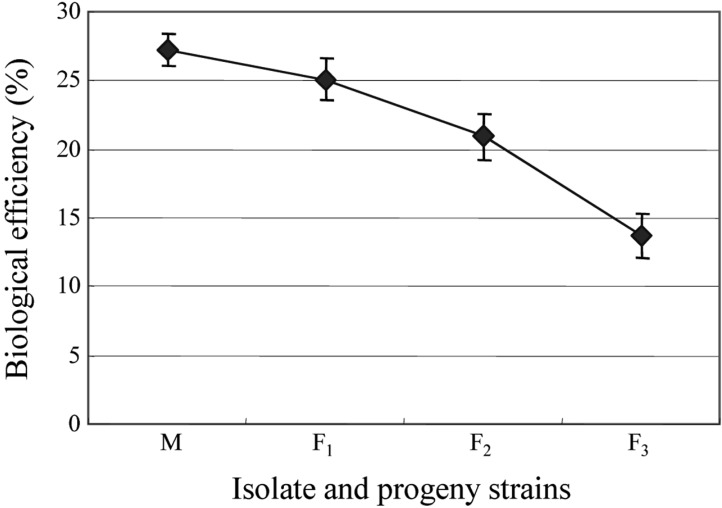
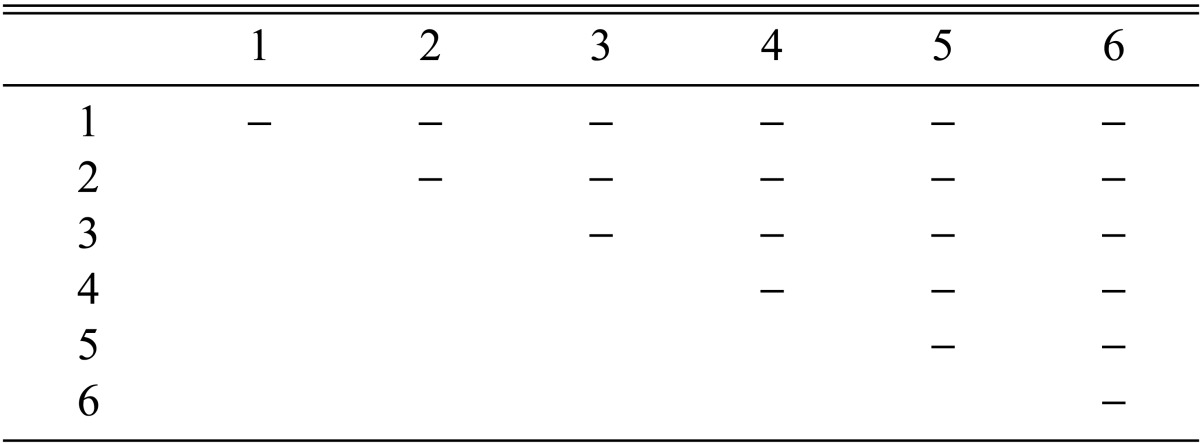
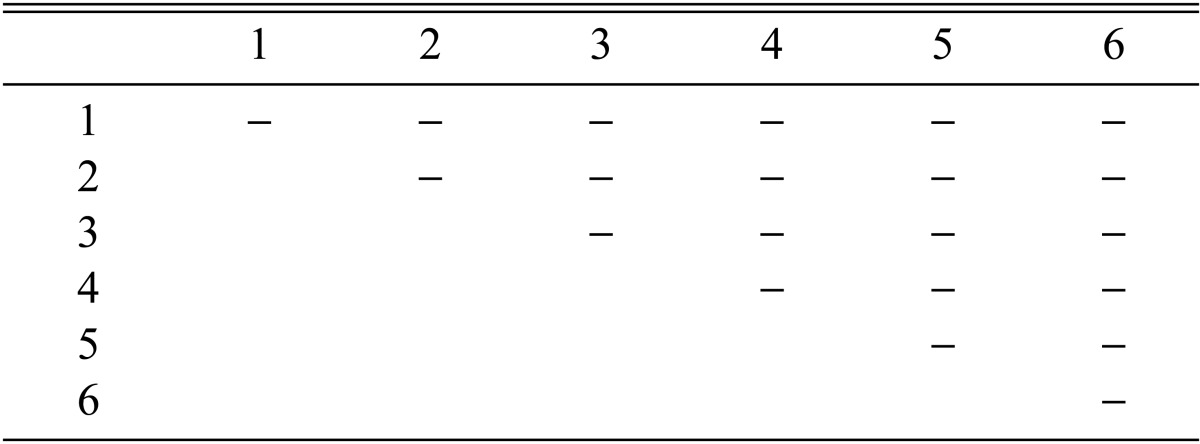
Eight combinations of F2 progeny strains isolated from a combination of CRI C-12448 4 × 6 produced fruiting bodies (Table 8). Similarly, eight combinations of F3 progeny strains isolated from CRI C-12448 (4 × 6) 3 × 4 produced fruiting bodies (Table 9). On average, isolate CRI C-12448 produced the highest BE, followed by F1, F2, and F3 progeny strains (Fig. 8).
Table 8.
Stromata formation from crossing of F2 progeny strains of Cordyceps militaris CRI C-12448 4 × 6
CRI, Cordyceps Research Institute.
Table 9.
Stromata formation from crossing of F3 progeny strains of Cordyceps militaris CRI C-12448 (4 × 6) 3 × 4
CRI, Cordyceps Research Institute.
Fig. 8.
Biological efficiency of a multi-ascospore isolate and progeny strains of Cordyceps militaris. M, multiascospore isolate CRI C-12448; F1, F1 progeny strains of CRI C-12448; F2, F2 progeny strains of C-12448 (4 × 6); F3, F3 progeny strains of C-12448 (4 × 6) (3 × 4). CRI, Cordyceps Research Institute.
Fruiting body formation from single conidial strains
Among the single conidial strains derived from the isolate CRI C-12448, strains 1, 3, 5, and 6 were opposite in mating type to remaining strains 2 and 3 (Table 10). Similarly, strains 1, 2, 4, 5, and 6, isolated from CRI C-12086, were opposite in mating type to strain 3 (Table 11). However, BE of combinations of single conidial strains were almost half or less than half of that of the isolate CRI C-12448. Single conidial strains isolated from each F1 progeny strain, C-12448-4 and C-12448-6, produced no fruiting bodies (Tables 12 and 13) that demonstrated stability of mating type loci among the conidial strains.
Table 10.
Fruiting bodies produced from crossings of single-conidium isolates of Cordyceps militaris isolate CRI C-12448
CRI, Cordyceps Research Institute.
Table 11.
Fruiting bodies produced from crossings of single-conidium isolates of Cordyceps militaris isolate CRI C-12086
CRI, Cordyceps Research Institute.
Table 12.
Crossings of single conidium isolates derived from F1 progeny strain of Cordyceps militaris CRI C-12448 4
CRI, Cordyceps Research Institute.
Table 13.
Crossings of single conidium isolates derived from F1 progeny strain of Cordyceps militaris CRI C-12448 6
CRI, Cordyceps Research Institute.
Among Cordyceps species, C. militaris is an ideal species for the study of in vitro stromata formation, as demonstrated by its successful formation [11-13]. Cordyceps militaris isolates collected from different mountains in Korea have been used for selection of superior strains [24-27]. Wen et al. [30] demonstrated variation in biochemical composition among different C. militaris isolates collected from different sites. This variation provides us an opportunity to select superior strains among the natural isolates.
In most studies, multispore isolates are used for production of fruiting bodies. Since the revelation of heterothallic mating system in C. militaris, many studies have involved isolation of single ascospores and crossing between them [23-29]. Results of genetic analysis have also demonstrated that mating type and mycelium pigmentation are independent of one another [24]. Optimum preservation of the superior strains is a persistent problem for continuous cultivation of C. militaris. Results of preservation studies conducted at low temperatures have demonstrated successful fruiting body production from successive subcultures of superior strains in C. militaris [26]. In addition, findings of this study have demonstrated that successive isolation of single ascospores from in vitro stromata is another option for preserving strains with desirable characteristics. In conclusion, subcultures of the strains and isolation of single ascospores and conidia can be applied for large scale cultivation of C. militaris and should be used complementarily.
Fig. 3.
Fruiting bodies produced by Cordyceps militaris isolates. a, CRI C-11408; b, CRI C-11445; c, CRI C-11821; d, CRI C-11876; e, CRI C-11913; f, CRI C-12167; g, CRI C-12434; h, CRI C-12448. CRI, Cordyceps Research Institute.
Acknowledgements
This work was supported by a grant from the Next-Generation BioGreen 21 Program (PJ008154 and PJ008321), Rural Development Administration, Republic of Korea. We also acknowledge the Cordyceps Research Institute (CRI), Mushtech, Republic of Korea for providing facilities
References
- 1.McKenna DJ, Jones K, Hughes K. Botanical medicines: the desk reference for major herbal supplements. 2nd ed. New York: Haworth Herbal Press; 2002. [Google Scholar]
- 2.Devkota S. Yarsagumba [Cordyceps sinensis (Berk.) Sacc.]: traditional utilization in Dolpa district, Western Nepal. Our Nat. 2006;4:48–52. [Google Scholar]
- 3.Winkler D. Yartsa Gunbu (Cordyceps sinensis) and the fungal commodification of Tibet's rural economy. Econ Bot. 2008;62:291–305. [Google Scholar]
- 4.Devkota S. The frequency and relationship of flowering plants on the distribution pattern of Ophiocordyceps sinensis (Yarchagunbu) in the highlands of Dolpa district, Nepal. Banko Janakari. 2009;19:29–36. [Google Scholar]
- 5.Shrestha B, Zhang W, Zhang Y, Liu X. What is the Chinese caterpillar fungus Ophiocordyceps sinensis (Ophiocordycipitaceae)? Mycology. 2010;1:228–236. [Google Scholar]
- 6.Huang L, Li Q, Chen Y, Wang X, Zhou X. Determination and analysis of cordycepin and adenosine in the products of Cordyceps spp. Afr J Microbiol Res. 2009;3:957–961. [Google Scholar]
- 7.Zhong S, Pan H, Fan L, Lv G, Wu Y, Parmeswaran B, Pandey A, Soccol CR. Advances in research of polysaccharides in Cordyceps species. Food Technol Biotechnol. 2009;47:304–312. [Google Scholar]
- 8.Zhou X, Gong Z, Su Y, Lin J, Tang K. Cordyceps fungi: natural products, pharmacological functions and developmental products. J Pharm Pharmacol. 2009;61:279–291. doi: 10.1211/jpp/61.03.0002. [DOI] [PubMed] [Google Scholar]
- 9.Das SK, Masuda M, Sakurai A, Sakakibara M. Medicinal uses of the mushroom Cordyceps militaris: current state and prospects. Fitoterapia. 2010;81:961–968. doi: 10.1016/j.fitote.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Pettit RH. Studies in artificial cultures of entomogenous fungi. Cornell Univ Agric Exp Stn Bot Entomol Div. 1895;97:339–378. [Google Scholar]
- 11.Sopp JO. Untersuchungen uber insektenvertilgende Pilze bei den letzten Kieferspinnerepidemien in Norwegen. Oslo: Skr VidenskSelsk Christiania; 1911. [Google Scholar]
- 12.Shanor L. The production of mature perithecia of Cordyceps militaris (Linn.) Link in laboratory culture. J Elisha Mitchell Sci Soc. 1936;52:99–105. [Google Scholar]
- 13.Kobayasi Y. The genus Cordyceps and its allies. Sci Rep Tokyo Bunrika Daigaku Sect B. 1941;5:53–260. [Google Scholar]
- 14.Basith M, Madelin MF. Studies on the production of perithecial stromata by Cordyceps militaris in artificial culture. Can J Bot. 1968;46:473–480. [Google Scholar]
- 15.Harada Y, Akiyama N, Yamamoto K, Shirota Y. Production of Cordyceps militaris fruit body on artificially inoculated pupae of Mamestra brassicae in the laboratory. Trans Mycol Soc Jpn. 1995;36:67–72. [Google Scholar]
- 16.Chen RY, Ichida M. Infection of the silkworm, Bombyx mori, with Cordyceps militaris. J Insect Biotechnol Sericol. 2002;71:61–63. [Google Scholar]
- 17.Sato H, Shimazu M. Stromata production for Crodyceps militaris (Clavicipitales: Clavicipitaceae) by injection of hyphal bodies to alternative host insects. Appl Entomol Zool. 2002;37:85–92. [Google Scholar]
- 18.Hong IP, Kang PD, Kim KY, Nam SH, Lee MY, Choi YS, Kim NS, Kim HK, Lee KG, Humber RA. Fruit body formation on silkworm by Cordyceps militaris. Mycobiology. 2010;38:128–132. doi: 10.4489/MYCO.2010.38.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sung JM. The insects-born fungus of Korea in color. Seoul: Kyohak Publishing Co., Ltd.; 1996. [Google Scholar]
- 20.Lin QY, Zhong YJ, Li TH, Shen YH, Song B. Recent research advances in Cordyceps biology. Acta Edulis Fungi. 2006;13:97–102. [Google Scholar]
- 21.Sung GH, Shrestha B, Park KB, Sung JM. Cultural characteristics of Shimizuomyces paradoxus collected from Korea. Mycobiology. 2010;38:189–194. doi: 10.4489/MYCO.2010.38.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrestha B, Park YJ, Han SK, Choi SK, Sung JM. Instability in in vitro fruiting of Cordyceps militaris. J Mushroom Sci Prod. 2004;2:140–144. [Google Scholar]
- 23.Shrestha B, Kim HK, Sung GH, Spatafora JW, Sung JM. Bipolar heterothallism, a principal mating system of Cordyceps militaris in vitro. Biotechnol Bioprocess Eng. 2004;9:440–446. [Google Scholar]
- 24.Shrestha B, Choi SK, Kim HK, Kim TW, Sung JM. Genetic analysis of pigmentation in Cordyceps militaris. Mycobiology. 2005;33:125–130. doi: 10.4489/MYCO.2005.33.3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shrestha B, Han SK, Lee WH, Choi SK, Lee JO, Sung JM. Distribution and in vitro fruiting of Cordyceps militaris in Korea. Mycobiology. 2005;33:178–181. doi: 10.4489/MYCO.2005.33.4.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sung JM, Park YJ, Lee JO, Han SK, Lee WH, Choi SK, Shrestha B. Effect of preservation periods and subcultures on fruiting body formation of Cordyceps militaris in vitro. Mycobiology. 2006;34:196–199. doi: 10.4489/MYCO.2006.34.4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung JM, Park YJ, Lee JO, Han SK, Lee WH, Choi SK, Shrestha B. Selection of superior strains of Cordyceps militaris with enhanced fruiting body productivity. Mycobiology. 2006;34:131–137. doi: 10.4489/MYCO.2006.34.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao X. Mating system of Cordyceps militaris. Acta Edulis Fungi. 2008;15:9–13. [Google Scholar]
- 29.Wen TC, Li MF, Kang JC, Lei BX. A molecular genetic study on the fruiting-body formation of Cordyceps militaris. KSM Newsl. 2009;21:76–95. [Google Scholar]
- 30.Wen L, Weng L, Zhu M, Liu S. Content comparison of active constituent in Cordyceps militaris from different forest regions. Sci Silvae Sin. 2008;44:149–151. [Google Scholar]