Abstract
Ten strains of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana were evaluated to find the most effective strain for optimization studies. The first criterion tested for strain selection was the mortality (> 50%) of Spodoptera litura larvae after inoculation of the fungus for 4 days. Results on several bioassays revealed that B. bassiana BNBCRC showed the most virulence on mortality S. litura larvae (80% mortality). B. bassiana BNBCRC also showed the highest germination rate (72.22%). However, its conidia yield (7.2 × 108 conidia/mL) was lower than those of B. bassiana B 14841 (8.3 × 108 conidia/mL) and M. anisopliae M6 (8.2 × 108 conidia/mL). The highest accumulative radial growth was obtained from the strain B14841 (37.10 mm/day) while the strain BNBCRC showed moderate radial growth (24.40 mm/day). M. anisopliae M6 possessed the highest protease activity (145.00 mU/mL) while M. anisopliae M8 possessed the highest chitinase activity (20.00 mU/mL) during 96~144 hr cultivation. Amongst these criteria, selection based on virulence and germination rate lead to the selection of B. bassiana BNBCRC. B. bassiana B14841 would be selected if based on growth rate while M. anisopliae M6 and M8 possessed the highest enzyme activities.
Keywords: Beauveria bassiana, Enzyme activities, Germination rate, Metarhizium anisopliae, Radial growth, Spodoptera litura, Virulence
Introduction
The cutworm, Spodoptera litura Fabricius (Lepidoptera: Noctuidae), is a polyphagous insect that has very wide host range of over 150 host species including vegetable and ornamental plants [1]. It is one of the most economically important insect pests in Southeast Asia and some specific problematic pest population reports occurring in Cambodia, Hong Kong, India, the Pacific islands, Guam, American Samoa, and Hawaii [2]. Using chemicals for pest control has disadvantages that the survival pest can develop resistance to many chemical insecticides. Problems with synthetic chemical insecticides have given rise to a sense of urgency in the development of biological control agents as supplements to these chemicals. Integrated control is thought to be an attractive alternative to effective control and efforts are being made to develop such control methods [3, 4]. Entomopathogenic fungi (EPF) have been widely used against a number of insect pests, the only efficacy study of fungi against pupae of S. litura has been reported [5]. But no such report is available on the efficacy of fungi against larvae of S. litura. EPF are considered to be very promising biological control agents and the popular EPF in pest management are Metarhizium anisopliae and Beauveria bassiana. Recently, the full potential and the many advantages of this practice reached application on a commercial scale using M. anisopliae and B. bassiana with capability to synthesize antagonistic compounds [6]. They cause infection by growing through the body of insect and release extracellular cuticle-hydrolyzing enzymes (protease and chitinases) [7]. Moreover, these fungi release toxins (M. anisopliae produced destruxin and B. bassiana produced beauvericin and bassianolide) which are correlated with their entomopathogenesis. Thus, various fungal strains differ in their host range, necessitating selection of the most virulent strain against insect.
The first step in developing a microbial control program and may improve the efficiency of mycoinsecticides are laboratory evaluation of the effectiveness of potential microbial agents. Therefore, the objective of this study was to select relevant characteristic to the development of M. anisopliae and B. bassiana, based on virulence of fungal strains to the target host revealed by laboratory bioassays, germination rate, spore production, radial growth and enzyme activity. The information obtained could be used for selection of the most efficient strain to control S. litura and could reveal the potential of the candidate strain to be further improved its efficiency through biotechnology.
Materials and Methods
Microorganisms
The two strains of EPF, Metarhizium anisopliae (M6) and Beauveria bassiana (BPMC) were obtained from Pest Management Center, Songkhla Province. Three strains of B. bassiana (B14532, B14841, and B16041) were purchased from National Center for Genetic Engineering and Biotechnology Bangkok Thailand. M. anisopliae (MNBCRC) and B. bassiana (BNBCRC) were obtained from National Biological Control Research Center (BNBCRC), Bangkok, Thailand. M. anisopliae M8, M33 and M36 were kindly provided by Dr. Narit Thaochan from Department of Pest Management, Faculty of Natural Resources, Prince of Songkla University. These fungal strains were cultured on potato dextrose agar (PDA) at room temperature for 14 days for use as an inocula [8, 9]. Each stock culture was stored at 4℃ until use.
Synthetic media
The basal salts medium used for production of enzyme contained g/L: 0.1% KH2PO4, 0.05% MgSO4·H2O, 0.001% FeSO4·H2O, 0.05% KCl, then were sterilized at 121℃ for 15 min.
Insects (S. litura)
S. litura larvae was collected from vegetable garden at Bangriang, Bangglum district, Songkhla Province, and kept in plastic cages (30 × 22 × 6 cm) at 25℃ with a 16-hr photoperiod until pupation. The larvae were fed with cabbage leave. Individual adults were transferred into paper bags (8 × 15 × 20 cm) with a 10% sugar solution as a food source as well as water. After female adults laid their eggs and these eggs hatched to larvae so called the F1 larvae, the larvae of 3rd instar (5 days old) that was used for the insecticidal activity study [10, 11].
Virulence of fungal strain on S. litura
The larvae of 3rd instar (5 days old) of S. litura were dipped for 5 seconds in conidia suspension (108 conidia/mL) of M. anisopliae and B. bassiana (7 days from PDA plate). A control was maintained by applying 0.05% Tween 80. Four hundred fifty samples of the larvae samples (15 samples per replication) were kept in the sterilized petri dish, which contain sterilized damp cotton (for keeping moisture). The dead of larvae was counted and recorded everyday till 7 days, then calculated for percent of dead larvae and cultured in water agar plates to confirm the mycosis. This method was described by Lezama-Gutiérrez et al. [12].
Conidia production and radial growth of M. anisopliae and B. bassiana
For solid cultivation, one piece (10 mm diameter cork borer) of each strain of M. anisopliae and B. bassiana were placed in the center on Czapeck Dox agar (CDA) plates. These inoculated plates were incubated at room temperature (29 ± 3℃) that the optimum temperature for growth of these fungi [13] and determined the surface radial growth after 3 days intervals for 15 days. The surface colonies were measured by using two diameters (mm) of fungus and calculate radial growth (mm/day). Conidia from each plate were harvested after 15 days incubation by scrapping with 0.05% Tween 80 to ensure maximum conidial harvesting. Conidial yield was determined by suspending the conidia from the whole colony in 50 mL of 0.05% Tween 80, and counting the number of spores using an haemacytometer and a light microscope at a 400× magnification. The procedure was as described by Soundarapandian and Chandra [8].
Conidia germination test
For each fungal strain, CDA plates were inoculated at the centre with 3 µL suspension (7 days old at room temperature) of conidia from a micropipette. Fifty such inoculated plates of each strain were placed at room temperature. At nine intervals during an 18 hr period following inoculation, the conidia from each of four petri dishes of each strain were fixed by adding lactophenol to the inoculated plates. Percentage germination was then determined by randomly counting 300 spores for each plate. Conidia were considered to have germinated if it had a germ tube at least as long as the smallest diameter of the conidia [14].
Enzyme assay
Assay of general proteolytic activity was performed with casein as a substrate. A 1 g of casein was dissolved in 10 mL of 0.01 M Tris HCl at pH 8.0. A 0.4 mL of casein substrate, 0.2 mL crude enzyme and 0.2 mL of 0.01 M Tris HCl at pH 8.0 were added and incubated for 10 min at 37℃. Later 1.0 mL of 1.2M TCA was added to terminate the reaction. The contents were centrifuged at 5,720 ×g at 4℃ for 5 min and the resulting supernatants were measured at 280 nm. One unit of protease activity was defined as the amount of enzyme that produced 1.0 mM of tyrosine per minute under the above condition, the experiment was done in 3 replication [15].
Assay of chitinase activity was performed with colloidal chitin as a substrate. A 1.0 mL crude enzyme was incubated at 37℃ for 1 hr with 1.0 mL of 1% colloidal chitin in 0.2M acetate buffer (pH 5). The reaction was terminated by boiling in water bath for 20 min followed by cooling in ice-cold water. 0.2 mL of reaction product N-acetylglucosamine (GlcNAc) was determined by using Somogyi-Nelson Method [16]. Absorbance at 585 nm was taken against water as blank. One unit of chitinase activity was defined as the amount of enzyme that produced 1 µmol of GlcNAc per min under the above conditions [17].
Results
Virulence of fungal strain on S. litura
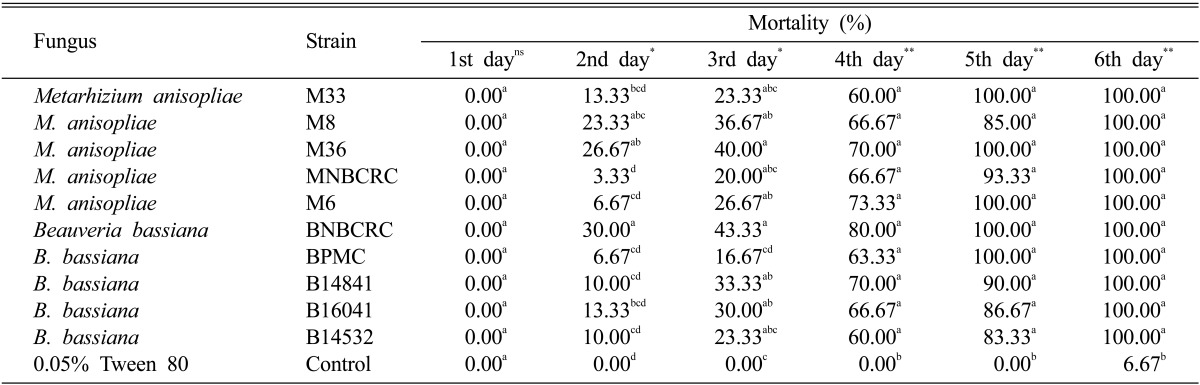
In bioassay experiment, all five strains each of M. anisopliae and B. bassiana were pathogenic to S. litura larvae. These virulences were significantly different as compared to untreated or treated by 0.05% Tween 80 (control) after 4th-6th day (p < 0.01, df = 32, F = 8.40), (p < 0.01, df = 32, F = 35.37), (p < 0.01, df = 32, F = 121.00), respectively. Percentage mortality started after 2 days incubation and reached over 50% mortality at 4th day and total death (100%) occurred at 5th day for M. anisopliae (M33, M36, M6) and B. bassiana (BNBCRC, BPMC) and 6th day for M. anisopliae (M8, MNBCRC) and B. bassiana (B14841, B16041, B14532) (Table 1). It was observed that half of them showed 100% mortality on the 5th day. The highest larvae mortality rate was obtained from B. bassiana BNBCRC while the lowest efficiency belonged to from M. anisopliae MNBCRC. Under one tested concentration (108 conidia/mL) lead to focused on pathogenicity of each fungus which caused the different mortality rate.
Table 1.
Mean percentage mortality of Spodoptera litura treated with 108 conidia/mL of 10 pathogenic fungi
Measurements were made on 1 till 6 days incubation at room temperature. Each point represents the mean of three replicates. Values in the same column followed by the different letters were significant different at the p < 0.05 level according to Duncan's multiple range test tests.
Ns, non significant different.
*Highly significant different (p < 0.05). **Highly significant different (p < 0.01).
Conidia production and radial growth
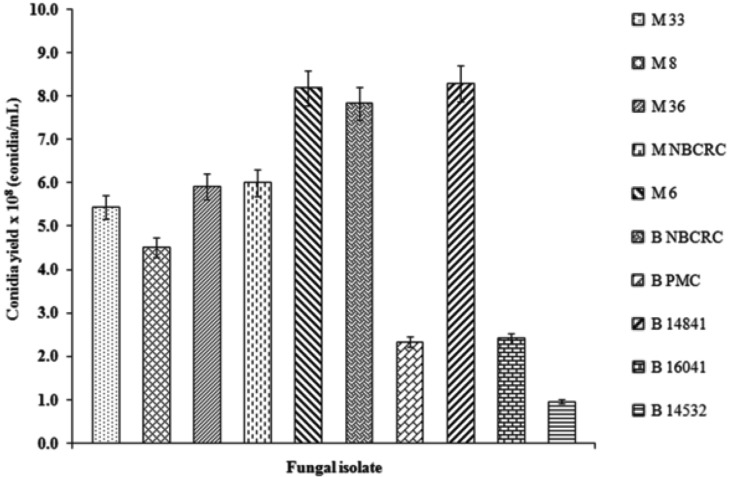
Production of aerial conidia was highly dependent on the strain used. These numbers of conidia were significantly different on CDA media (p < 0.01, df = 29, F = 288.32). The highest mean number of conidia yield (8.30 × 108 conidi/mL) was obtained from B. bassiana B14841 (Fig. 1), followed by group of M. anisopliae M6 and B. bassiana B NBCRC (8.20 × 108 and 7.8 × 108 conidia/mL, respectively).
Fig. 1.
Conidia yield of five Metarhizium anisopliae (Metsch.) Sorokin (M33, M8, M36, MNBCRC, and M6) and five Beauveria bassiana (BNBCRC, B PMC, B14841, B16041, and B14532). Each data represents the average (± SD). Measurements were made on 15 days post incubation at room temperature. Each point represents the mean of three replicates.
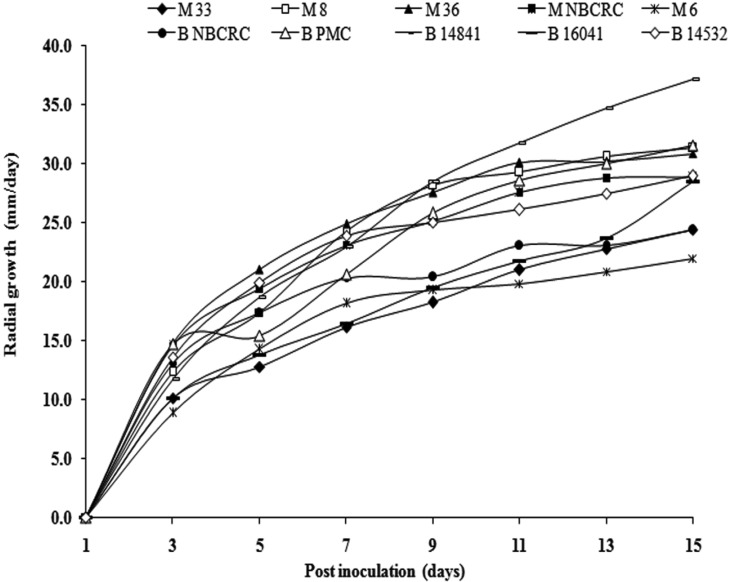
The radial growth was highly affected by incubation at room temperature (29 ± 3℃) in all tested strains. Over all, the accumulative growth rate revealed the highest radial growth rate from B. bassiana B14841 (37.10 mm/day) which was significantly (p < 0.01) higher than those obtained from all strains of M. anisopliae (Fig. 2).
Fig. 2.
Cumulative radial growth of five Metarhizium anisopliae (Metsch.) Sorokin (M33, M8, M36, MNBCRC, and M6) and five Beauveria bassiana (BNBCRC, BPMC, B14841, B16041, and B14532). Measurements were made after 3 days interval until 15 days post incubation at room temperature. Each point represents the mean of three replicates.
Conidia germination test
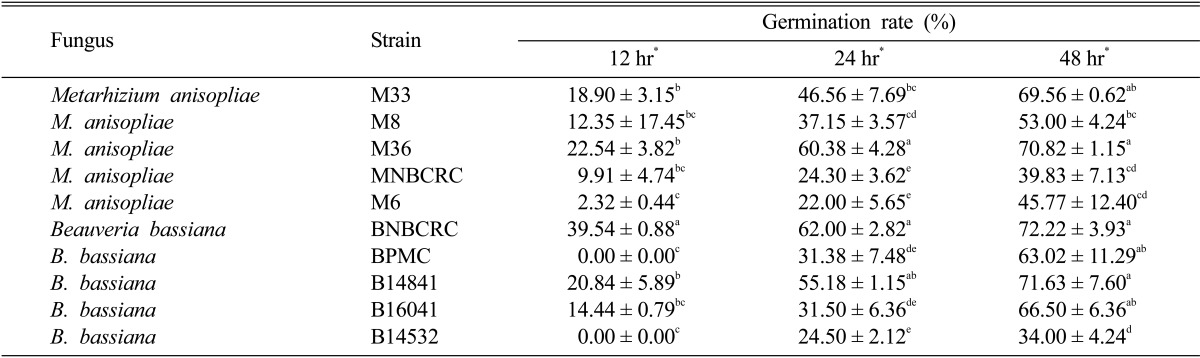
The percentage of germination varied significantly according to the strain tested. These germination rates were significantly different on CDA media after 12 hr (p < 0.01, df = 29, F = 3.53), 24 hr (p < 0.01, df = 29, F = 45.29) and 48 hr (p < 0.01, df = 29, F = 21.36). The highest germination rate on the CDA media was obtained from B. bassiana BNBCRC and significantly higher than those of the other nine strains at 2 days incubation (39.54%) (Table 2). Its germination rate after 24 hr incubation (62.00%) was not significantly higher than that of the M. anisopliae M36 (60.38%). This also occurred at 48 hr incubation with the highest conidia germination rate of B. bassiana BNBCRC (72.22%) followed by the strain B14841 (71.63%) and M. anisopliae M36 (70.82%).
Table 2.
Conidia germination (%) of five Metarhizium anisopliae (Metsch.) Sorokin (M33, M8, M36, MNBCRC, and M6) and five Beauveria bassiana (BNBCRC, BPMC, B14841, B16041, and B14532)
Each data represents the average (± SD). Measurements were made on 12, 24, and 48 days incubation at room temperature. Each point represents the mean of three replicates. Values in the same column followed by the different letters were significant different at the p < 0.05 level according to Duncan's multiple range test tests.
*Highly significant different (p < 0.05).
Enzyme assay
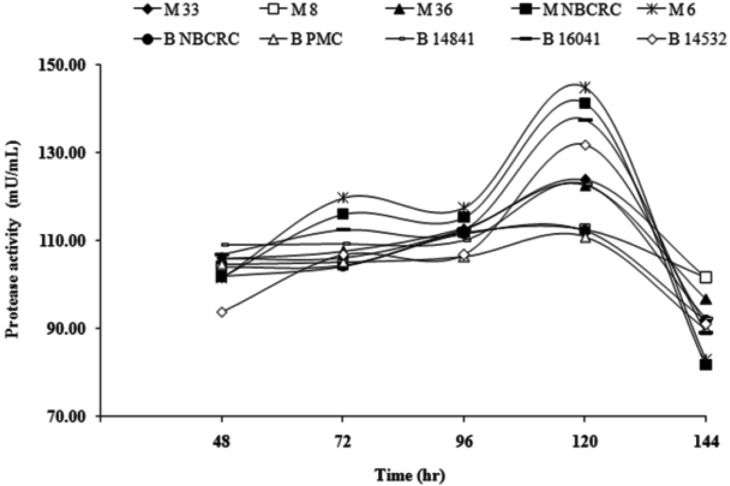
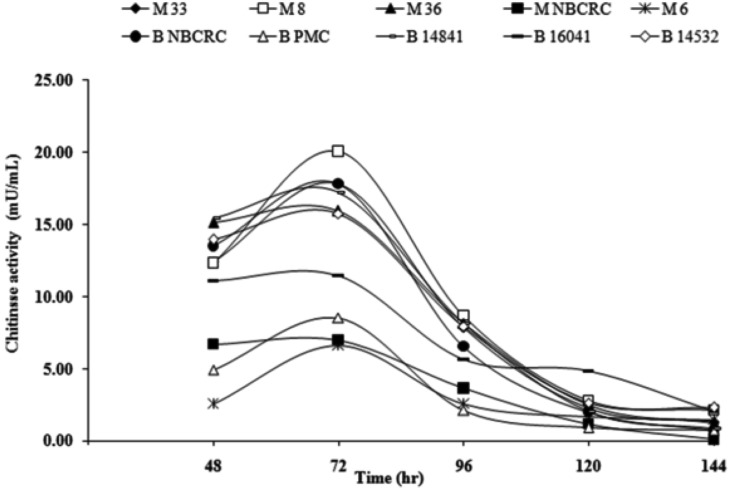
All ten fungal strains produced protease and chitinase and their activities were dependent on the strains (Figs. 3 and 4). Protease activities reached maximum after 120 hr incubation and decreased thereafter. Most of M. anisopliae showed higher protease activity (83.00~145.00 mU/mL) than those of B. bassiana (89.00~137.00 mU/mL) (Fig. 3). M. anisopliae M6 possessed the highest protease activity (145 mU/mL). All fungal strains showed the same pattern of increasing of chitinase activity during 144 hr incubation and reached the maximum values after 72 hr incubation (Fig. 4) and decreased thereafter. Most of M. anisopliae showed higher chitinase activity (0.10~20.00 mU/mL) than B. bassiana (0.70~18.00 mU/mL). The highest chitinase activity at 72 hr was achieved from M. anisopliae M8 (20.00 mU/mL).
Fig. 3.
The time course of protease activity from five Metarhizium anisopliae (Metsch.) Sorokin M33, M8, M36, MNBCRC and MPMC and five Beauveria bassiana (BNBCRC, B PMC, B14841, B16041 and B14532). The cultivation was set at room temperature and measured after 48 hr till 144 hr. Each point represents the mean of three replicates.
Fig. 4.
The time course of chitinase activity from five Metarhizium anisopliae (Metsch.) Sorokin M33, M8, M36, MNBCRC, and MPMC) and five Beauveria bassiana (BNBCRC, BPMC, B14841, B16041, and B14532). The cultivation was set at room temperature and measured after 48 hr till 144 hr. Each point represents the mean of three replicates.
Discussion
Virulence of the ten fungal strains on S. litura was similar to that reported by Swe et al. [18]. The concentration of M. anisopliae at 108 conidia/mL reached 100% mortality of S. litura after 7 days incubation. The larvae mortality of S. littoralis after 4~10 days incubation was 7.74~30.57% for M. anisopliae and 5.17~41.15% for B. bassiana [19].
Conidia production may be related to inherent trait of strain used and nutritional composition of the media, carbon sources, concentration and carbon: nitrogen ratio are known to affect conidia yield and other physical characteristics [20, 21]. Many researchers reported that PDA is the best medium for culture of fungi [22, 23]. While many researchers successfully used Sabouraud dextrose agar (SDA) medium for mass culture of M. anisopliae and B. bassiana [24]. In this study, the conidia production from both B. bassiana and M. anisopliae on CDA medium (1.0 × 108~8.3 × 108 conidia/mL) showed (Fig. 1) were higher than on PDA (2.8 × 107) and SDA (3.0 × 106), respectively [8].
The fungal strain had different radial growth rate when tested on CDA medium (Fig. 2). The highest radial growth rate was obtained from B. bassiana B14841 (37.10 mm/day). CDA medium also gave the high value of conidia production and radial growth rate. This may be due to its component such as sucrose, sodium nitrate, and minerals which are needed for growth of fungi [25]. Sabbour et al. [26] reported that the growth of B. bassiana on each basal salt medium mixed with sucrose and sodium nitrate showed the highest radial growth at 87.25 mm/day and 50.50 mm/day, respectively. This may be the reason for good performance of this media.
In this study, germination rate on CDA medium containing sucrose and sodium nitrate showed poor germination rate at 12 hr (9.91~39.54%) while Jarrold et al. [27] reported that the germination rate on locust wing, which contained fatty acids, ester, glucose, amino acid and peptide, was 90% at 12 hr. The poor germination may be due to the lack of a complex mixed nutrient, and suggest that the carbon source was a more effective trigger for germination than the nitrogen source.
Most of the strains showed high protease activity during 96~144 hr cultivation of culture which was comparable with the observations by other researchers that high protease activity was obtained at fifth day of culture in both M. anisopliae and B. bassiana [28]. Protease activity of fungal strains decreased with increase in culture age probably due to nutrient limitation or autolysis of the culture.
Chitinase activity of the ten fungal strains appeared after 72 hr and increased to 1.00~10.00 mU/mL at 96 hr and kept constant to 120 hr [29]. The low level of chitinase in the induction medium may be due to the possible reliance on other enzyme like chitin deacetylase [30]. For instance, chitin deacetylase deacetylates the cell wall chitin of the penetrating fungal hypha for protection against plant endochitinases. Insects produce chitinase to degrade old cuticle during moulting, which can also act on M. anisopliae cell wall chitin. Composite cell wall analysis of M. anisopliae mycelium content was 21.45%, chitin and chitosan composed of 53.62% and 46.38% of total hexosamines, respectively [29]. This suggests that chitin deacetylase activity could play a dual role, cuticle softening as well as self-defense.
In this study, use of colloidal chitin in the induction medium could induce chitinase production to consume chitin for growth while chitinase from fungi may also act on fungal cell wall. This may be cause of the low production of chitinase activity. Most of the 10 strains in the present study produced an appreciable amount of protease and chitinase useful in the degradation of organic substrate. Entomopathogenesis by fungi reflects that the enzyme system of the EPF is unique and is of great interest as potential criteria for mycoinsecticide improvement. The selection of a potent strain from fungal population forms the basis for their genetic optimization and prospective use.
Acknowledgements
The authors would like to thank the Office of the Higher Education Research Promotion and National Research University Project of Thailand, and the Office of the Higher Education Commission for grant made to support for this work to Miss Wanida Petlamul under the CHE-PhD Scholarship Program, Prince of Songkla University.
References
- 1.Rao GV, Wightman JA, Rao DV. World review of the natural enemies and diseases of Spodoptera litura (F.) (Lepidoptera: Noctuidae) Insect Sci Appl. 1993;14:273–284. [Google Scholar]
- 2.Wada K, Manakata K. Naturally occurring insect control cheive measurement of heptachlor in the soil and certain products of animal and plant orgin.ang. J Agric Food Chem. 1968;16:471–474. [Google Scholar]
- 3.Honda Y. Current status of integrated pest control Shokubutsu Boeki. Plant Prot. 2000;54:213–216. [Google Scholar]
- 4.Faria M, Wraight SP. Biological control of Bemisia tabaci with fungi. Crop Prot. 2001;20:767–778. [Google Scholar]
- 5.Anand R, Prasad B, Tiwary BN. Relative susceptibility of Spodoptera litura pupae to selected entomopathogenic fungi. BioControl. 2009;54:85–92. [Google Scholar]
- 6.Cloyd RA. The entomopathogenic fungus Metarhizium anisopliae. Midwest Biological Control News Vol. 6 [Internet] Madison (WI): University of Wisconsin-Masdison; 2008. [cited 2012 May 7]. Available from: http://www.entomology.wisc.edu/mbcn/kyf607.html. [Google Scholar]
- 7.St. Leger RJ, Charnley AK, Cooper RM. Cuticle-degrading enzymes of entomopathogenic fungi: mechanisms of interaction between pathogen enzymes and insect cuticle. J Invertebr Pathol. 1986;47:295–302. [Google Scholar]
- 8.Soundarapandian P, Chandra R. Mass production of entomopathogenic fungus Metarhizium anisopliae (Deuteromycota; Hyphomycetes) in the laboratory. Res J Microbiol. 2007;2:690–695. [Google Scholar]
- 9.Pham TA, Kim JJ, Kim SG, Kim K. Production of blastospore of entomopathogenic Beauveria bassiana in a submerged batch culture. Mycobiology. 2009;37:218–224. doi: 10.4489/MYCO.2009.37.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takatsuka J, Okuno S, Nakai M, Kunimi Y. Genetic and biological comparisons of ten geographic isolates of a nucleopolyhedrovirus that infects Spodoptera litura (Lepidoptera: Noctuidae) Biol Control. 2003;26:32–39. [Google Scholar]
- 11.Park SH, Yu YS, Park JS, Choo HY, Bae SD, Name MH. Biological control of tobacco cutworm, Spodoptera litura Fabricius with entomopathogenic nematodes. Biotechnol Bioprocess Eng. 2001;6:139–143. [Google Scholar]
- 12.Lezama-Gutiérrez R, Trujillo-de La Luz A, Molina-Ochoa J, Rebolledo-Dominguez O, Pescador AR, López-Edwards M, Aluja M. Virulence of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) on Anastrepha ludens (Diptera: Tephritidae): labolatory and field trials. J Econ Entomol. 2000;93:1080–1084. doi: 10.1603/0022-0493-93.4.1080. [DOI] [PubMed] [Google Scholar]
- 13.Thaochan N, Chinajariyawong A. Spore germination and mycelia growth of Metarhizium anisopliae (Metsch.) Sorokin effected by different temperature regimes. Agric Sci J. 2008;39:21–25. [Google Scholar]
- 14.Yeo H, Pell JK, Alderson PG, Clark SJ, Pye BJ. Laboratory evaluation of temperature effects on the germination and growth of entomopathogenic fungi and on their pathogenicity to two aphid species. Pest Manag Sci. 2003;59:156–165. doi: 10.1002/ps.622. [DOI] [PubMed] [Google Scholar]
- 15.Bhagya Lakshmi S, Gurvinder Kuar S, Padmini Palem PC. Isolation and purification of cuticle degradating extracellular proteases from entomopathogenic fungal species Beauveria bassiana and Metarhizium anisopliae. Int J Appl Biol Pharmaceut Technol. 2010;1:1150–1156. [Google Scholar]
- 16.Somogyi M. Notes on sugar determination. J Biol Chem. 1952;195:19–23. [PubMed] [Google Scholar]
- 17.Yanai K, Takaya N, Kojima N, Horiuchi H, Ohta A, Takagi M. Purification of two chitinases from Rhizopus oligosporus and isolation and sequencing of the encoding genes. J Bacteriol. 1992;174:7398–7406. doi: 10.1128/jb.174.22.7398-7406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swe TM, Nway WN, Thu MK, Han T. Biocontrol potential of entomopathogenic Fungus, Metarhizium anisopliae against Spodoptera Litura; The Third GMSARN International Conference on Sustainable Development: Issues and Prospects for the Greater Mekong Subregion; 2008 Nov 12-14; Kunming, China. [Google Scholar]
- 19.Amer MM, El-Sayed TI, Bakheit HK, Moustafa SA, El-Sayed YA. Pathogenicity and genetic variability of five entomopathogenic fungi against Spodoptera littoralis. Res J Agric Biol Sci. 2008;4:354–367. [Google Scholar]
- 20.Leland JE, Mullins DE, Vaughan LJ, Warren HL. Effects of media composition on submerged culture spores of the entomopathogenic fungus, Metarhizium anisopliae var. acridum. Part2: effects of media osmolality on cell wall characteristics, carbohydrate concentrations, drying stability, and pathogenicity. Biocontrol Sci Technol. 2005;15:393–409. [Google Scholar]
- 21.Shah FA, Wang CS, Butt TM. Nutrition influences growth and virulence of the insect-pathogenic fungus Metarhizium anisopliae. FEMS Microbiol Lett. 2005;251:259–266. doi: 10.1016/j.femsle.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Sulz M, Bailey KL. Growth and spore production of Plectosporium tabacinum. Can J Bot. 2001;79:1297–1306. [Google Scholar]
- 23.Zurek L, Watson DW, Schal C. Synergism between Metarhizium anisopliae (Deuteromycota: Hyphomycetes) and boric acid against the German cockroach (Dictyoptera: Blattellidae) Biol Control. 2002;23:296–302. [Google Scholar]
- 24.Hallsworth JE, Magan N. Culture age, temperature, and pH affect the polyol and trehalose contents of fungal propagules. Appl Environ Microbiol. 1996;62:2435–2442. doi: 10.1128/aem.62.7.2435-2442.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altomare C, Norvell WA, Björkman T, Harman GE. Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl Environ Microbiol. 1999;65:2926–2933. doi: 10.1128/aem.65.7.2926-2933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabbour MM, Ragei M, Abd-El Rahman A. Effect of some ecological factors on the growth of Beauveria bassiana and Paecilomyces fumosoroseus against Corn Borers. Aust J Basic Appl Sci. 2011;5:228–235. [Google Scholar]
- 27.Jarrold SL, Moore D, Potter U, Charnley AK. The contribution of surface waxes to pre-penetration growth of an entomopathogenic fungus on host cuticle. Mycol Res. 2007;111(Pt 2):240–249. doi: 10.1016/j.mycres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Dhar P, Kaur G. Production of cuticle-degrading proteases by Beauveria bassiana and their induction in different media. Afr J Biochem Res. 2010;3:65–72. [Google Scholar]
- 29.Nahar P, Ghormade V, Deshpande MV. The extracellular constitutive production of chitin deacetylase in Metarhizium anisopliae: possible edge to entomopathogenic fungi in the biological control of insect pests. J Invertebr Pathol. 2004;85:80–88. doi: 10.1016/j.jip.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Tsigos I, Martinou A, Kafetzopoulos D, Bouriotis V. Chitin deacetylases: new, versatile tools in biotechnology. Trends Biotechnol. 2000;18:305–312. doi: 10.1016/s0167-7799(00)01462-1. [DOI] [PubMed] [Google Scholar]