Abstract
Nuruk contributes to the unique characteristics of Korean alcoholic beverages. In this study, the effects of nuruk extracts (NE) on anti-oxidant characters, melanogenesis, and anti-photoaging activity were investigated. NEs were obtained from the 70% ethanol extracts of six types of nuruk, which have been used in brewing of fermented alcohol beverages in Korea. First, various antioxidant characteristics were identified in terms of 2,2'-azino-bis(3-ethylbenzthiozoline-6-sulphonic acid) (ABTS) radical scavenging activity, superoxide dismutase (SOD) expression, and inhibition of xanthine oxidase activity. NE#4 exhibited potent ABTS radical scavenging activity (IC50 = 19.51 µg/mL). Compared with NE#4, relatively lower levels of activity were observed for NE#3 and NE#6, with IC50 values of 90.99 and 76.88 µg/mL, respectively. According to results of western blot analysis for determination of SOD expression in H2O2-treated HepG2 cells, NE#5 and NE#6 induced a dramatic increase in the expression ratio of SOD, compared to the group treated with H2O2 only. Activity of xanthine oxidase, which converts xanthine into uric acid, generating superoxide ions, was inhibited by NE#4 and NE#6 in a dose-dependent manner. NE#4 induced significant inhibition of mushroom tyrosinase activity. A reduction in cellular melanin contents of 80% was observed in B16F1 melanocytes treated with NE#5 and NE#6; these effects were similar to those of arbutin at 100 µM. In addition, gelatin zymography and reverse transcription-PCR analysis were performed for assessment of anti-photoaging activity of Nuruk. Treatment with NE#6 resulted in dramatically inhibited activities of matrix metalloproteinase (MMP)-2/-9, suppressed expression of MMP-1, and increased expression of type-1 procollagen. Results of gelatin zymography for NE#4 and NE#5 were similar, to a slightly lesser degree. These results suggest the potential of NE#4 and NE#6 as natural ingredients for use in functional foods and cosmetics.
Keywords: Anti-oxidant activity, Anti-photoaging, Melanogenesis, Nuruk
Introduction
Nuruk contributes to the unique characteristic features of Korean alcoholic beverages. Most types of of nuruk (traditional Korean fermentation starter) consist of unboiled raw wheat grains [1, 2]. Other grains, including rice, barley, millet, maize, soybean, rye, and oats are also used in different regions [3]. Nuruk, which was naturally inoculated by airborne microorganisms, is composed of useful fungi, such as Aspergillus sp., Absidia sp., Rhizopus sp., and Mucor sp., yeast, and lactic acid bacteria [1, 2]. Nuruk provides amylolytic and proteolytic enzyme sources, which have important roles in starch saccharification, dextrinogenic activity, and protein digestion. Through the process of alcoholic fermentation, it produces diverse and numerous organic acids or flavor compounds. Hence, nuruk is responsible for the deep and complex taste of Korean alcoholic beverages [3].
Many research groups have recently been involved in studies of the various aspects of nuruk, such as fermentation or enzymatic characteristics, and isolation and amelioration in microorganisms [3-5]. In addition, anti-cancer effects, including anti-metastatic and angiogenic activity, and apoptosis, have been reported [6, 7]. Extracts of nuruk have been associated with inhibitory effects on cardiovascular diseases, including hypertension and platelet aggregation [6]. Yoon et al. [8] reported that nuruk induced suppression of serum levels of cholesterol, as well as a decrease in the amount of hepatic oxygen free radicals in rats. Nuruk exerts anti-inflammatory activity by down-regulating activation of p38 mitogen-activated protein kinase [9]. However, no comparative research studies have been reported; therefore, the physiological characteristics depending on types of nuruk remain unknown.
In this study, different types of nuruk were purchased from commercial manufacturers or provided by a company that manufactures traditional Korean alcoholic beverages. We prepared the ethanol extracts from six types of nuruk and investigated the inhibitory effects of nuruk extracts (NEs) on oxidant characters, melanogenesis, and photo-aging activity.
Materials and Methods
Reagents
Mushroom tyrosinase, L-tyrosine, hydrogen peroxide, xanthine oxidase (XO), xanthine, [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT), and potassium phosphate were also purchased from Sigma-Aldrich (St. Louis, MO, USA). All other organic solvents and chemicals used in this study were of analytical grade. Fetal bovine serum (FBS), Dulbecco's modified Eagle's medium (DMEM), and cell culture reagents were purchased from Gibco-BRL (Grand Island, NY, USA). Moloney murine leukemia virus (M-MLV) reverse transcriptase and PCR premix were purchased from Rexgen Biotech Co., Ltd. (Ochang, Korea). All of the primers used for PCR were purchased from Bioneer (Daejeon, Korea). Monoclonal mouse antibody to superoxide dismutase 1 (SOD1), monoclonal rabbit to β-actin, monolclonal anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibodies, and anti-rabbit HRP-conjugated secondary antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA).
Preparation of NEs
All types of nuruk used in this study have been used in brewing of Korean alcoholic beverages. Four types of nuruk were purchased from commercial manufacturers and two were provided by Kooksoondang Brewery Co., Ltd. (Seongnam, Korea). Table 1 shows the characteristics of nuruk used in the study. Extraction of dried and powdered nuruk was performed two times using 80% (v/v) ethanol at room temperature. The ratio of sample and solvent was 1 : 10 (w/v). After extraction, the extracts were filtered through a Whatman No. 2 filter paper (Whatman International Ltd., Maidstone, England). The filtrate was dried by removal of solvents under reduced pressure on a rotary evaporator (EYELA new Rotary Vacuum Evaporator; Rikakikai Co., Tokyo, Japan) at 42℃ and then freeze-dried using a lyophilizer (Bondiro; Ilshin BioBase, Dongducheon, Korea). Each dried extract was dissolved in dimethyl sulfoxide to achieve a final concentration of 100 mg/mL. All samples were placed in a glass bottle and stored at 4℃ prior to use.
Table 1.
Summary in characteristics of applied nuruk
aSaccharogenic power was measured using the method of Shon et al. [1] with slight modification.
bNuruk#5 was inoculated with Aspergillus kwachii.
cNuruk#6 was inoculated with Rhizopus oryzae KSD-815.
Each data represents the mean ± SD of two independent experiments.
Cell culture and viability assay
Murine melanocytes, B16F1, human keratinocytes, HaCaT, and human hepatoma, HepG2, were a generous gift from Prof. Kim, DO (Kyung Hee University, Yongin, Korea). B16F1 cells were cultured in DMEM containing 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin. HaCaT cells and HepG2 cells were cultured in RPMI1640 supplemented with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin. All cell lines were incubated at 37℃ and 5% CO2. An MTT colorimetric assay was used for quantification of cell viability. Briefly, cells were seeded in a 96-well plate (2 × 104 cells/well) overnight and treated with NEs for 24 hr. Following addition of MTT solution to the wells, cells were incubated for 4 hr at 37℃, followed by measurement of optical density at 540 nm.
ABTS radical scavenging assay
Measurement of 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salts (ABTS) radical cation was performed using the method of Re et al. [10], with slight modification. ABTS was dissolved in water to a concentration of 7 mM. ABTS radical cation was produced by reacting ABTS stock solution with 2.45 mM postassium persulfate and allowing the mixture to stand in the dark at room temperature for 16 hr. ABTS•+ solution was diluted with 5mM phosphate buffer saline (PBS) to an absorbance of 0.70 ± 0.02 at 734 nm. The 0.01 mL extracts were mixed with 0.99 mL ABTS•+ solution. After reaction for 10 min at 25℃, absorbance was measured at 734 nm using a microplate reader (Gen5; BioTek Instruments, Inc., St. Charles, IL, USA).
In vitro tyrosinase inhibitory assay
Using a previously described method, with minor modifications, a spectrophotometric assay was performed using L-tyrosine as the substrate for determination of tyrosinase inhibitory activity [11]. A 10 µL sample of NEs was added to an assay mixture containing with 1 mM L-tyrosine solution, 100 mM sodium phosphate buffer, pH 6.5, and 20 µL of the aqueous solution of mushroom tyrosinase (1,000 units) was added to a 96-well microplate. Following incubation of the assay mixture at 37℃ for 20 min, the amount of dopachrome produced in the reaction mixture was determined as the optical density at 492 nm in a microplate reader and the inhibition percent of tyrosinase activity was calculated according to the following formula:
% Inhibition = [1 - (Asample (490 nm)/Acontrol (490 nm))] × 100
Melanin content assay
B16F1 cells were subsequently seeded into 24-well culture plates. After reaching confluence, they were treated with samples or with arbutin as a positive control for 72 hr. Cells were then washed with PBS and lysed with 20 mM Tris-0.1% Triton X-100 (pH 7.5). After centrifugation, the pellet was dissolved in 1 N NaOH for 30 min at 60℃. Absorbance of samples was measured at 405 nm. Cellular melanin content was adjusted according to the protein concentration of the samples. A Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA) was used for determination of protein concentration.
XO inhibitory assay
For spectrophotometric determination of XO inhibitory activity, uric acid formation was measured at 292 nm [12]. The reaction mixture, containing 1 mL substrate (2 mM xanthine) in a 0.1M potassium phosphate buffer (pH 7.5), 0.1 mL (0.2 units/mL) of enzyme solution, and 0.1 mL of extract solution, was reacted at 37℃ for 30 min. The control solution was prepared by addition of 0.1 mL 0.1M potassium phosphate buffer (pH 7.5) instead of extracts. The reaction was stopped by addition of 1 mL of 20% trichloroacetic acid. Formation of uric acid was measured at 292 nm using a microplate reader. The inhibition percent of XO was calculated as [1 - (A1/A0)] × 100, where A0 is the absorbance of control, and A1 is the absorbance of sample.
Reverse transcription (RT)-PCR
Isolation of RNA from HaCaT cells treated with or without sample was performed according to the manufacturer's instructions using Easy-Blue (Intron Biotechnology, Seongnam, Korea). For each RT-PCR reaction, 500 ng of RNA was used. Each sample was preheated to 60℃ with oligo(dT) 18 primers for 10 min, followed by addition of one unit per mL of M-MLV reverse transcriptase. The reaction was then performed at 42℃ for 60 min and was terminated by heating to 94℃ for 5 min. PCR amplification of the cDNA template was performed using PCR premix (Bioneer) and the following primer pairs: matrix metalloproteinase (MMP)-1, forward 5'-AGC GTG TGA CAG TAA GCT AA-3', reverse 5'-CAG CTG GAT GGC CAC ATC GG-3'; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward 5'-ATT GTT GCC ATC AAT GAC CC-3', reverse 5'-AGT AGA GGC AGG GAT GAT-3'. PCR was performed in a Veriti Thermal Cycler (Applied Biosystems, Foster City, CA, USA) for 25 cycles, using the following parameters: 94℃ for 30 sec, 50~52℃ for 1 min, and 72℃ for 1 min, and PCR products were separated by 1.0% agarose gel electrophoresis with ethidium bromide staining under UV illumination. GAPDH was used as an internal control.
Zymography
Gelatin zymography was used for determination of expression and activities of MMP-2 and -9 in NE-treated human keratinocytes, HaCaT cells. HaCaT cells were seeded in 100 mm plates using serum-free medium and pretreated with the indicated concentrations of NEs. After incubation for 24 hr, conditioned medium were collected and quantification of protein concentrations was performed using the Bio-Rad protein assay (Bio-Rad). Culture supernatants were subjected to electrophoresis on gelatin substrate gels (10% SDS-polyacrylamide gels containing 1 mg/mL of gelatin). Subsequently, the gels were treated with 2.5% Triton X-100 for 30 min, followed by incubation for 24 hr at 37℃ in a buffer containing 100 mM Tris-HCl, pH 7.4, 0.15 M NaCl, and 15 mM CaCl2. The gels were stained with Coomassie Blue R-250 and de-stained with water until emergence of clear zones indicative of proteolytic activity against a blue background.
Western blot analysis
Twenty micrograms of protein mixed with 5 × loading buffer [0.313 mol/L Tris-HCl (pH 6.8), 10% SDS, 0.05% bromophenol blue, 50% glycerol], and 20 × reducing agent (2 mol/L dithiothreitol; Fermentas, Hanover, MD, USA) were boiled for 5 min and loaded onto a 10% SDS-polyacrylamide gel. After electrophoresis, proteins were transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat milk in 20 mmol/L Tris-HCl (pH 7.4) containing 150 mmol/L NaCl and 0.1% Tween 20 (TBS-T), followed by incubation in non-fat milk overnight at 4℃ with primary antibodies (1 : 1,000 for SOD1, 1 : 1,000 for β-actin). The membranes were washed for 10 min in TBS-T, followed by incubation in non-fat milk at room temperature with horseradish peroxidase-conjugated anti-rabbit secondary antibody for 2 hr. An enhanced chemiluminescence detection kit (Amersham Bioscience, Pittsburgh, PA, USA) was used for visualization of bound antibodies.
Statistical analysis
One-way analysis of variance (ANOVA) followed by the Duncan's test (ver. 18.0; SPSS Inc., Chicago, IL, USA) was used for analysis of data. Data were expressed as mean ± SD. Experiments were performed in triplicate. A p < 0.05 and p < 0.01 were considered statistically significant.
Results and Discussion
Effects of NE on oxidative stress
Oxygen is absolutely necessary for life processes, however, metabolism of oxygen may generate reactive elements called free radicals, in particular the superoxide (O2-) and hydroxyl radical (OH-). Free radicals cause damage to DNA, essential cellular proteins, and membrane lipids, which may lead to cell death and heart and cardiovascular diseases [13, 14].
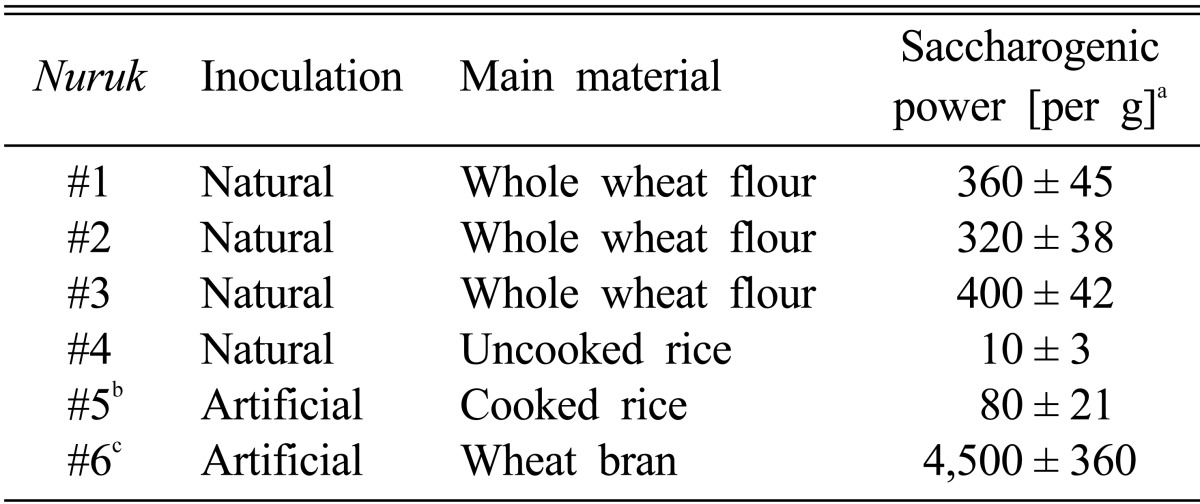
The ABTS system, which measures radical scavenging by electron donation, and due to the hydrophilic and lipophilic nature of the compounds in samples, has been used for measurement of the total antioxidative status of various biological specimens [15, 16]. As shown in Fig. 1, the highest ABTS radical scavenging activity was detected in NE#4, with a IC50 value of 19.51 µg/mL. Compared with NE#4, NE#3 and NE#6 exhibited relatively lower activities with IC50 values of 90.99 and 76.88 µg/mL, respectively.
Fig. 1.
2,2'-azino-bis(3-ethylbenzthiozoline-6-sulphonic acid) (ABTS) radical scavenging activities of nuruk extracts. Radical scavenging abilities of nuruk extracts were measured using the decolorization reaction of free ABTS radical anions at 10 min and 37℃. Values represent mean ± SD (n = 3).
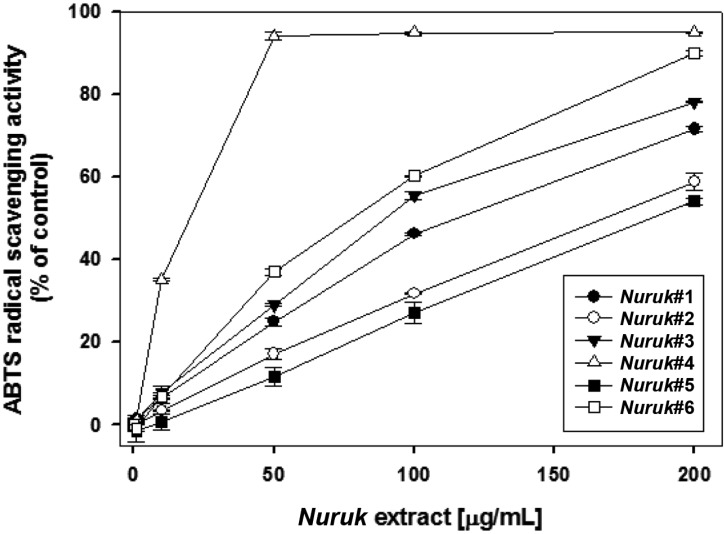
Under so-called "physiological conditions" there is a balance between production of free radicals and antioxidant endogenous defense mechanisms. These mechanisms mainly involve specific enzymes [14]. Antioxidant enzymes, including SOD, glutathione peroxidase (GPx), and catalase (CAT), are associated with these mechanisms. SOD quenches the free radical superoxide by converting it to hydrogen peroxide (H2O2). The latter is then rapidly catabolized by CAT and GPx into O2 and H2O. Western blot analysis was performed for examination of the effects of NEs on expression of SOD in HepG2 cells treated with H2O2. HepG2 cells were treated with 100 µg/mL of NEs for 24 hr, followed by exposure to 100 µM of H2O2 for 30 min. As shown in Fig. 2, expression of SOD showed a significant increase of approximately four-fold in NE#5- or NE#6-treated HepG2 cells, compared with negative control (no H2O2-treated cells). NE#4 induced an increase in expression of SOD of 50%. The powerful natural antioxidant enzyme (SOD) acts as an important source of the chain reaction resulting in reactive types of oxygen and constitutes the first and one of the main links of the defense process against free radical [14]. These results suggest the potential anti-oxidative activities of NE#4 and NE#6 and that NEs might contain compounds that directly scavenge free radicals and induce endogenous antioxidant defense mechanisms.
Fig. 2.
Effects of nuruk extracts on expression of superoxide dismutase (SOD). Cells were treated with 100 µg/mL of nuruk extracts for 24 hr and the cell lysates were used for western blot analysis with antibody against SOD and β-actin. The image data shown are representative of three independent experiments, with similar results (A) and described as relative intensity in (B). Different letters indicate a significant difference (p < 0.01) based on one-way ANOVA and the Duncan's test.
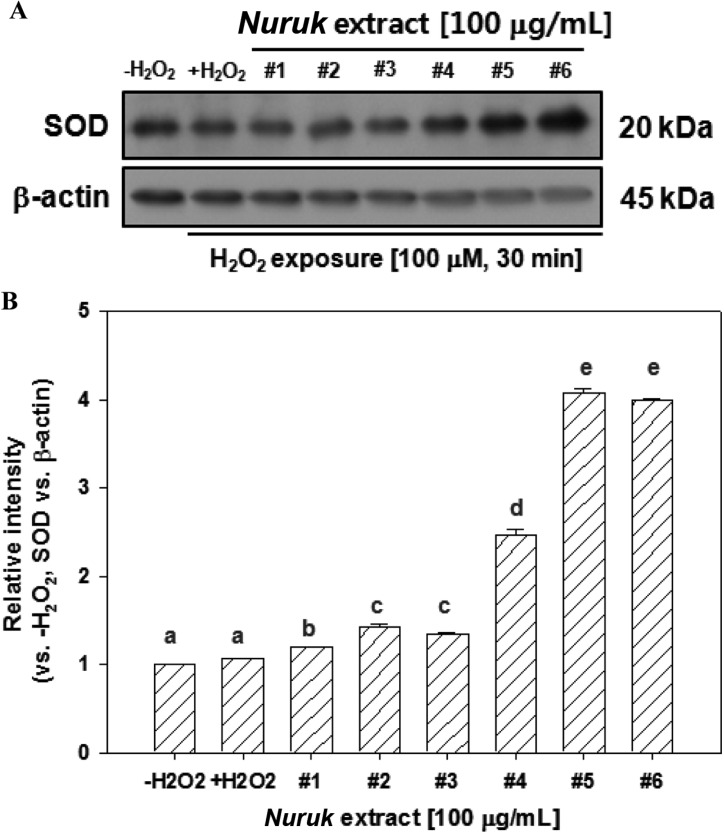
Recent research has focused on mechanisms to reduce formation of reactive oxygen species (ROS) rather than a scavenging approach to already-formed ROS [13]. The two major ROS generating systems are the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and the XO (EC 1.2.3.2) systems. The enzyme catalyzes oxidation of hypoxanthine to xanthine and xanthine to uric acid with concomitant reduction of NAD or molecular oxygen. To elucidate the relationship between NEs and the activity of XO, we performed XO inhibitory assays in the presence of NEs. As shown in Fig. 3, all of the NEs demonstrated dose-dependent inhibition of the activity of XO. Remarkably, NE#4 and NE#6 demonstrated potential inhibitory activity against XO, with IC50 values of 3.21 and 5.88 µg/mL, respectively. IC50 values of NE#1, NE#2, NE#3, and NE#5 were 12.96, 12.54, 11.60, and 10.16 µg/mL, respectively. Allopurinol, a known XO inhibitor, has been used clinically in treatment of chronic gout, which is caused by generation of uric acid and superoxide anion radical [13]. Using their assay system, Kong et al. [17] reported a IC50 value of allopurinol of 1.06 mg/mL. These results suggest that NEs exhibit anti-oxidative activity, and the potential to prevent accumulation of uric acid and inhibition of superoxide formation.
Fig. 3.
Inhibitory effects of nuruk extracts on xanthine oxidase activity. The xanthine oxidase inhibitory assay was performed for determination of activities of xanthine oxidase. Each value represents mean ± SD (n = 3).
Inhibitory effects of NE on melanogenesis
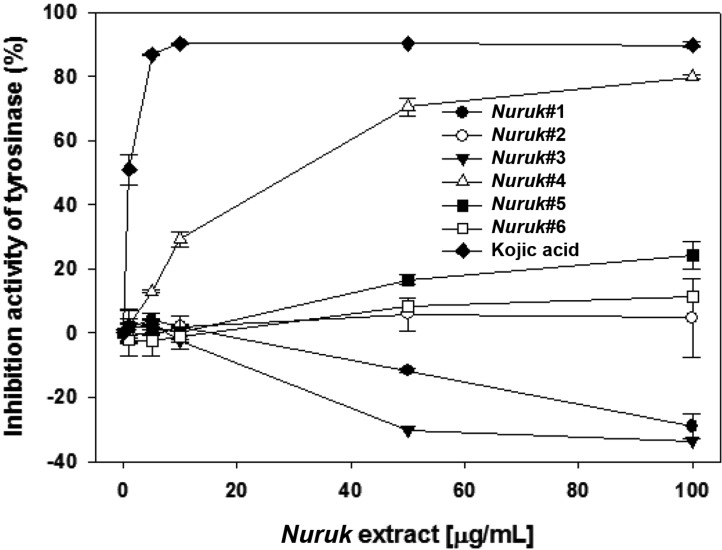
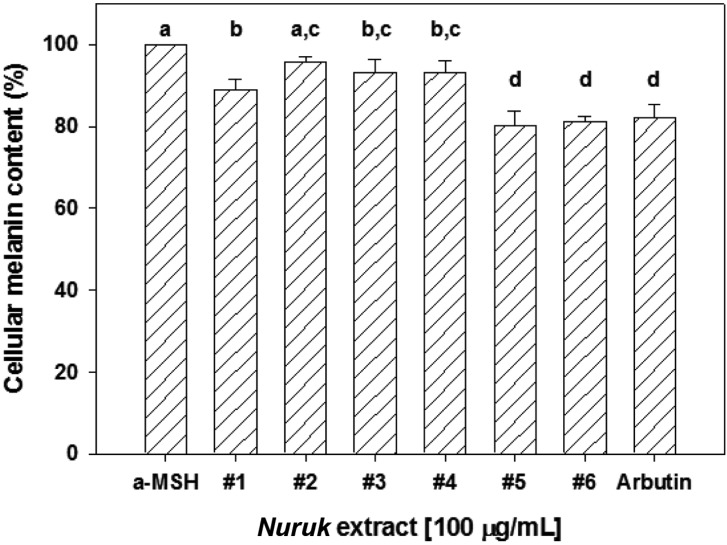
Melanin plays an essential role in defending the body against UV irradiation and other environmental challenges [18]. However, over-production of melanin could cause various dermatological disorders, including age spots, freckles, melasma, and lipid spots [19]. Kojic acid, a natural antibiotic agent isolated from koji malt (Japanese nuruk), consisting of Aspergillus oryzae, is known as a tyrosinase inhibitor and is used as a whitening agent in cosmetics [20]. In vitro tyrosinase inhibitory and melanin content assays were performed in order to examine the effect of NEs on melanogenesis. As a positive control, Kojic acid exhibited a strong inhibitory effect, with an IC50 value of 1.03 µg/mL (Fig. 4). NE#4 induced dose-dependent inhibition of mushroom tyrosinase activity, demonstrating stronger inhibitory effects of NE#4 on tyrosinase activity (IC50 = 30.318 µg/mL) in murine B16F1 melanocytes. By contrast, NE#1 and NE#3 induced a slight increase in the activity of tyrosinase. None of the other extracts was found to inhibit tyrosinase activity. To investigate the effects of NEs on cellular melanin production, B16F1 melanocytes were co-treated with a-melanin stimulating hormone and NEs for 24 hr. The MTT assay was used for measurement of cell viability. None of the NEs showed cytotoxicity in B16F1 cells at 100 µg/mL (data not shown). Arbutin was used as a positive control for quantification of cellular melanin content. As a result, inhibition of melanin production by NE#5 and NE#6 was 20 and 19%, respectively, with similar activity, compared with 100 µg/mL of arbutin (Fig. 5). These results suggest that NE#5 and NE#6, at least in part, have an inhibitory effect on hypopigmentation.
Fig. 4.
Inhibitory effects of mushroom tyrosinase by nuruk extracts. Measurement of tyrosinase activity was performed using L-tyrosine as the substrate. Kojic acid was used as a positive control. Values indicate means ± SD of n = 3 determination.
Fig. 5.
Effects of nuruk extracts on cellular melanin content. Melanin contents were determined in B16F1 cells treated with 100 µg/mL of nuruk extracts and 100 µg/mL of arbutin for 72 hr. Arbutin was used as a positive control. Values represent mean ± SD (n = 3). Different letters indicate a significant difference (p < 0.01) based on one-way ANOVA and the Duncan's test. a-MSH, alpha-melanocyte stimulating hormone.
Remarkably, NE#4, which inhibited in vitro mushroom tyrosinase activity, had no effect on melanin production in B16F1 cells. Although quercetin is known as a tyrosinase inhibitor, it has also been reported to be a strong inductor of melanogenesis in normal and malignant human melanocytes [21]. Quercetin acts as a stimulator at different steps of melanogenesis, and direct inhibition of tyrosinase or its antioxidant effect are moderate. Some medicinal plants, including Epimedium koreanum and Astragalus membraneus Bunge, which contain effective antioxidant ingredients, stimulate melanin formation in B16 melanocytes [22]. In order to use these compounds in cosmetics and dermatology, a complete understanding of the different mechanisms of antioxidants is needed.
NE protected skin photoaging
Skin aging can be classified according to two types [23]. Intrinsic aging is a basic biological process characterized as an age-dependent deterioration of skin functions and structures. Another is photoaging due to chronic exposure of skin to sunlight. MMPs are involved in remodeling of the extracellular matrix and play important roles in morphogenesis, angiogenesis, skin ulcer, tumor invasion, and metastasis [24]. In particular, MMP-1, -2, -3, and -9 are considered to be involved in photoaging, and their increase by ultraviolet (UV) irradiation in human fibroblasts and skin has been reported [23, 25, 26].
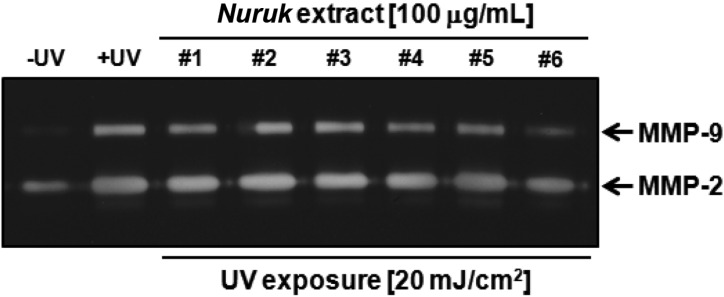
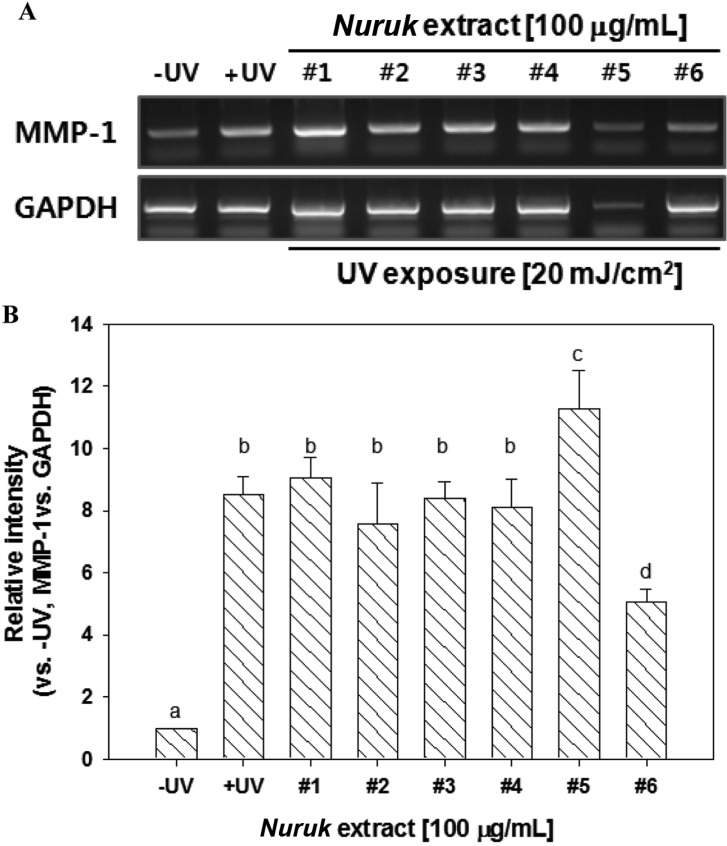
Gelatin zymography was performed in order to examine the inhibitory effects of NEs on proteolytic activities of MMP-2 and MMP-9. The UV-irradiated group showed increased activities of MMP-2 and MMP-9, compared to the negative control (Fig. 6). Treatment with NE#6 resulted in strong inhibition of the gelatinolytic activities of MMP-2 and MMP-9 in UV-irradiated HaCaT cells. Treatment with NE#1, NE#4, and NE#5 resulted in reduced activity of MMP-9 and no MMP-2 activity. In order to investigate UV-irradiated MMP-1 expression in cultured keratinocytes, HaCaT, cells were exposed to 20 mJ/cm2 of UV irradiation. As shown in Fig. 7, treatment with NE#6 resulted in significantly reduced expression of MMP-1 at the mRNA gene levels. Wang et al. [27] reported on paracrine-dependent promotion of MMP-1 production by UVB-irradiated keratinocytes in UVA-exposed fibroblasts. These results indicate a potential activity of NE#6 for protection of skin against exposure to UV irradiation.
Fig. 6.
Effects of nuruk extracts on secretion of MMP-2 and MMP-9 by gelatin zymography. HaCaT cells were treated with 100 mg/mL of NEs for 24 hr, followed by exposure to 20 mJ/cm2 of UV for 30 min prior to harvest of cell media. Gelatin zymography was performed for determination of gelatinolytic activities of nuruk extracts.
Fig. 7.
Effects of nuruk extracts on expression of matrix metalloproteinase 1 (MMP-1). HaCaT Cells were treated with 100 µg/mL of nuruk extracts for 24 hr, followed by harvest of the cells for semi-quantitative reverse transcription-PCR (RT-PCR). Semi-quantitative RT-PCR was performed for determination of mRNA expression of MMP-1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The image data shown are representative of three independent experiments, with similar results (A) and described as relative intensity in (B). Different letters indicate a significant difference (p < 0.01) based on one-way ANOVA and the Duncan's test.
Taken together, we evaluated the inhibitory effects of extracts of nuruk on oxidative stress, melanogenesis, and photoaging in skin. According to our findings, NEs exhibited free radical scavenging activity and modulation of antioxidant defense enzymes. NEs possessed biological effects, including in vitro tyrosinase and melanin biosynthesis in melanocytes. In addition, treatment with NEs in human keratinocytes resulted in suppression of MMP-1 mRNA expression and inhibition of proteolytic activities of MMP-2 and MMP-9. Consequently, NEs can be regarded as valuable sources of bioactive components with antioxidant properties and biological activity against skin aging, such as melanin accumulation and formation of wrinkles.
References
- 1.Shon SK, Rho YH, Kim HJ, Bae SM. Takju brewing of uncooked rice starch using Rhizopus koji. Korean J Appl Microbiol Bioeng. 1990;18:506–510. [Google Scholar]
- 2.Yu TS, Yeo SH, Kim HS. A new species of hyphomycetes, Aspergillus coreanus sp. nov., isolated from traditional Korean nuruk. Korean J Microbiol Biotechnol. 2004;14:182–187. [Google Scholar]
- 3.Yang S, Lee J, Kwak J, Kim K, Seo M, Lee YW. Fungi associated with the traditional starter cultures used for rice wine in Korea. J Korean Soc Appl Biol Chem. 2011;54:933–943. [Google Scholar]
- 4.Kim HR, Ahn BH. Research trend of Korean traditional alcoholic beverage. Food Ind Nutr. 2001;6:5–10. [Google Scholar]
- 5.Kim HR, Kim JH, Bai DH, Ahn BH. Identification and characterization of useful fungi with α-amylase activity from the Korean traditional nuruk. Mycobiology. 2011;39:278–282. doi: 10.5941/MYCO.2011.39.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SJ, Bae HJ, Ryu J, Lee D, Kim GW, Baek N, Kwon M, Hong S. Extracts from Rhizopus oryzae KSD-815 of Korean traditional nuruk confer the potential to inhibit hypertension, platelet aggregation, and cancer metastasis in vitro. Food Sci Biotechnol. 2009;18:1423–1429. [Google Scholar]
- 7.Yoo JG, Kim DH, Park EH, Lee JS, Kim SY, Kim MD. Nuruk, a traditional Korean fermentation starter, contains the bioactive compound 2,6-dimethoxy-1,4-benzoquinone (2,6-DMBQ) J Korean Soc Appl Biol Chem. 2011;54:795–798. [Google Scholar]
- 8.Yoon CG, Chae SN, Huh NE, Kim HS, Yu TS. Effects of nuruk or wheat bran supplemented diet on the serum levels of cholesterol and activities of hepatic oxygen free radical metabolizing enzymes in rats. J Korean Soc Food Sci Nutr. 1999;28:212–217. [Google Scholar]
- 9.Kim JE, Jung SK, Lee SJ, Lee KW, Kim GW, Lee HJ. Nuruk extract inhibits lipopolysaccharide-induced production of nitrite and interleukin-6 in RAW264.7 cells through blocking activation p38 mitogen-activated protein kinase. J Microbiol Biotechnol. 2008;18:1423–1426. [PubMed] [Google Scholar]
- 10.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 11.No JK, Soung DY, Kim YJ, Shim KH, Jun YS, Rhee SH, Yokozawa T, Chung HY. Inhibition of tyrosinase by green tea components. Life Sci. 1999;65:PL241–PL246. doi: 10.1016/s0024-3205(99)00492-0. [DOI] [PubMed] [Google Scholar]
- 12.Stirpe F, Della Corte E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O) J Biol Chem. 1969;244:3855–3863. [PubMed] [Google Scholar]
- 13.George J, Struthers AD. Role of urate, xanthine oxidase and the effects of allopurinol in vascular oxidative stress. Vasc Health Risk Manag. 2009;5:265–272. doi: 10.2147/vhrm.s4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menvielle-Bourg FJ. Superoxide dismutase (SOD), a powerful antioxidant, is now available orally. Phytothérapie. 2005;3:1–4. [Google Scholar]
- 15.You DH, Park JW, Yuk HG, Lee SC. Antioxidant and tyrosinase inhibitory activities of different parts of Guava (Psidium guajava L.) Food Sci Biotechnol. 2011;20:1095–1100. [Google Scholar]
- 16.Arnao MB. Some methodological problems in the determination of antioxidant activity using chromogen radicals: a practical case. Trends Food Sci Technol. 2000;11:419–421. [Google Scholar]
- 17.Kong LD, Cai Y, Huang WW, Cheng CH, Tan RX. Inhibition of xanthine oxidase by some Chinese medicinal plants used to treat gout. J Ethnopharmacol. 2000;73:199–207. doi: 10.1016/s0378-8741(00)00305-6. [DOI] [PubMed] [Google Scholar]
- 18.Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 19.Jun HJ, Roh M, Kim HW, Houng SJ, Cho B, Yun EJ, Hossain MA, Lee H, Kim KH, Lee SJ. Dual inhibitions of lemon balm (Melissa officinalis) ethanolic extract on melanogenesis in B16-F1 murine melanocytes: inhibition of tyrosinase activity and its gene expression. Food Sci Biotechnol. 2011;20:1051–1059. [Google Scholar]
- 20.Cabanes J, Chazarra S, Garcia-Carmona F. Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. J Pharm Pharmacol. 1994;46:982–985. doi: 10.1111/j.2042-7158.1994.tb03253.x. [DOI] [PubMed] [Google Scholar]
- 21.Nagata H, Takekoshi S, Takeyama R, Homma T, Yoshiyuki Osamura R. Quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and in normal human melanocytes. Pigment Cell Res. 2004;17:66–73. doi: 10.1046/j.1600-0749.2003.00113.x. [DOI] [PubMed] [Google Scholar]
- 22.Solano F, Briganti S, Picardo M, Ghanem G. Hypopigmentating agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006;19:550–571. doi: 10.1111/j.1600-0749.2006.00334.x. [DOI] [PubMed] [Google Scholar]
- 23.Amano S, Ogura Y, Akutsu N, Matsunaga Y, Kadoya K, Adachi E, Nishiyam T. Protective effect of matrix metalloproteinase inhibitors against epidermal basement membrane damage: skin equivalents partially mimic photoageing process. Br J Dermatol. 2005;153(Suppl 2):37–46. doi: 10.1111/j.1365-2133.2005.06968.x. [DOI] [PubMed] [Google Scholar]
- 24.Murray GI. Matrix metalloproteinases: a multifunctional group of molecules. J Pathol. 2001;195:135–137. doi: 10.1002/1096-9896(200109)195:2<135::AID-PATH939>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.Brenneisen P, Wenk J, Klotz LO, Wlaschek M, Briviba K, Krieq T, Sies H, Scharffetter-Kochanek K. Central role of ferrous/ferric iron in the ultraviolet B irradiation-mediated signaling pathway leading to increased interstitial collagenase (matrix-degrading metalloproteinase (MMP)-1) and stromelysin-1 (MMP-3) mRNA levels in cultured human dermal fibroblasts. J Biol Chem. 1998;273:5279–5287. doi: 10.1074/jbc.273.9.5279. [DOI] [PubMed] [Google Scholar]
- 26.Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Bi Z, Chu W, Wan Y. IL-1 receptor antagonist attenuates MAP kinase/AP-1 activation and MMP-1 expression in UVA-irradiated human fibroblasts induced by culture medium from UVB-irradiated human skin keratinocytes. Int J Mol Med. 2005;16:1117–1124. [PubMed] [Google Scholar]