Abstract
Multiple treatment modalities, including topical and systemic corticosteroid and phototherapy, have been used in treatment of patients with atopic dermatitis. However, long-term corticosteroid therapy may have various adverse effects. The purpose of this study was to investigate the therapeutic efficacy and safety of bath therapy using green tea extracts for treatment of patients with atopic dermatitis. A total of four patients with atopic dermatitis were enrolled in this study. A Malassezia multiplex detection kit was used in performance of multiplex PCR on clinical isolates, which confirmed Malassezia sympodialis. Subjects underwent treatment with bath therapy using green tea extracts three times per wk for a period of 4 wk. Assessment using the scoring atopic dermatitis (SCORAD) index, the visual analogue scale for pruritus, and transepidermal water loss was performed weekly. Laboratory tests were performed before and after treatment. All patients showed marked improvement on the mean SCORAD and visual analogue scale, and a significant decrease in the mean values of serum eosinophil counts was observed after treatment. Bath therapy with green tea extract is an effective, safe, and nonsteroidal therapy for treatment of patients with atopic dermatitis associated with Malassezia sympodialis.
Keywords: Atopic dermatitis, Bath therapy, Catechin, Green tea extract
Introduction
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease, with a genetic and environmental background. Its pathogenesis is complex; in most cases, the dermatitis fades during childhood; however, the course of the disease is unpredictable [1]. Multiple treatment modalities, including topical and systemic corticosteroid, and phototherapy at various wavelengths, have been used in treatment of patients with AD. However, long-term corticosteroid therapy may have various adverse effects. Many research studies have been conducted in an effort to find alternatives to steroids. Various biological activities of catechins, the main constituent of green tea, including antimutagenic, antibacterial, antioxidant, and antitumor properties, have been reported [2-4]. In this study, we assessed the effectiveness of bath therapy with green tea extracts in treatment of patients with AD associated with Malassezia sympodialis infection.
Materials and Methods
Preparation of green tea extracts
Purified green tea extract was provided by AmorePacific Corporation (Korea). Green tea leaves were taken from Jeju Island. Green leaves were steamed soon after being nipped off, which led to inactivation of tea enzymes. After primary grinding, the leaves were divided into juice and solid content. The juice was distilled for 4 hr, then, after filtration, we obtained the final product; 5% solution was prepared for clinical challenge.
Clinical experiment
We recruited four patients aged 5~9 years who had been diagnosed with extrinsic AD according to previously published definitions [5]. This clinical study was approved by the Institutional Review Board of Chung-Ang University Hospital. Prior to enrollment, patients were informed of the study procedures, as well as possible risks and benefits, and written consent was obtained from each patient. Patients were instructed to avoid the use of any other therapeutic agents during the course of treatment. The following subjects were excluded: patients who had undergone treatment with systemic corticosteroid or topical agents, including corticosteroid, calcineurin inhibitor, and any type of phototherapy or photosensitizing drugs during the three months prior to enrollment. First, a swab was rubbed over the eczematous skin lesion, and skin samples were then obtained. Yeasts were cultured in Leeming-Notman medium [6, 7]. A Malassezia multiplex detection kit was used for performance of multiplex PCR on clinical isolates [8]. The isolates were identified as M. sympodialis.
For bath therapy treatment using green tea extracts, subjects bathed in 700 mL of green tea extract solution mixed with 150 L of filtered tap water three times per wk for a period of 4 wk. Duration of bath treatment was 30 min, with a water temperature of 37℃. For evaluation of the therapeutic efficacy, assessment of the scoring atopic dermatitis (SCORAD) score and the visual analogue scale (VAS) for pruritus was performed every wk. For investigation of changes in skin barrier function during bath therapy, measurement of skin hydration and transepidermal water loss (TEWL) was performed using a Corneometer CM825 (Courage Khazaka GmbH, Cologne, Germany) and a Tewameter TM300 (Courage Khazaka GmbH), respectively. Photographs using identical camera settings, lighting, and positioning were taken during each visit. Evaluation and comparison of photographs were performed by two independent dermatologists using the quartile grading scale: 1, minimal improvement or steady state (0~25%); 2, moderate improvement (26~50%); 3, marked improvement (51~75%); and 4, excellent improvement (76~100%). In addition, laboratory tests, including serum eosinophil counts, eosinophil cationic protein (ECP) levels, and immunoglobulin E (IgE) were performed twice, at baseline and week 3.
Statistical analysis
Data are presented as the mean and standard deviation. The Wilcoxon signed rank test was used in performance of statistical analysis. SPSS software (SPSS Inc., Chicago, IL, USA) was used in performance of all analyses. A p < 0.05 was considered statistically significant.
Results
Clinical evaluation
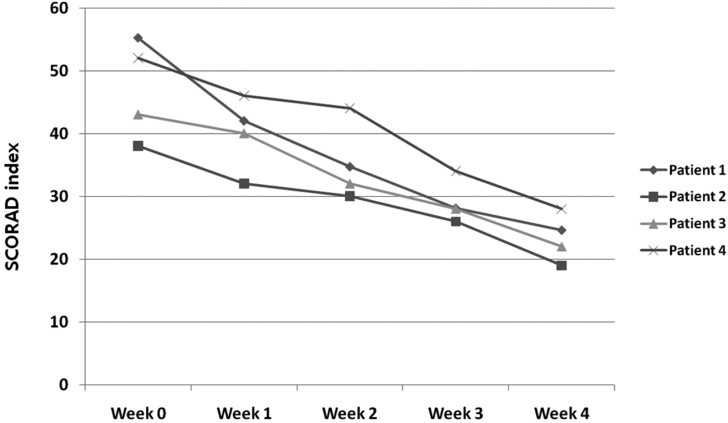
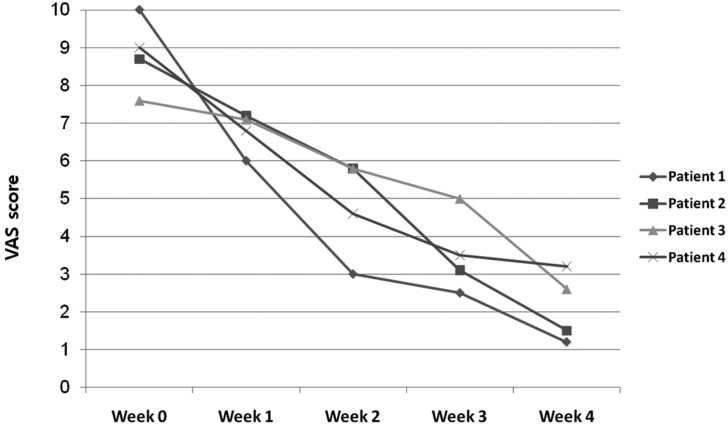
Excellent improvement was noted in one patient, and marked improvement was achieved in three patients (Fig. 1). A decrease was observed in the mean SCORAD value, from 47.05 ± 7.94 at baseline to 23.4 ± 3.83 at week 4, a reduction of 50.3% (Fig. 2). None of the patients reported a lack of change in or worsening of their AD. A decrease was observed in the mean VAS score, from 8.83 ± 0.99 at baseline, to 2.13 ± 0.94 at week 4 (Fig. 3). However, changes of SCORAD value and VAS scores were not statistically significant.
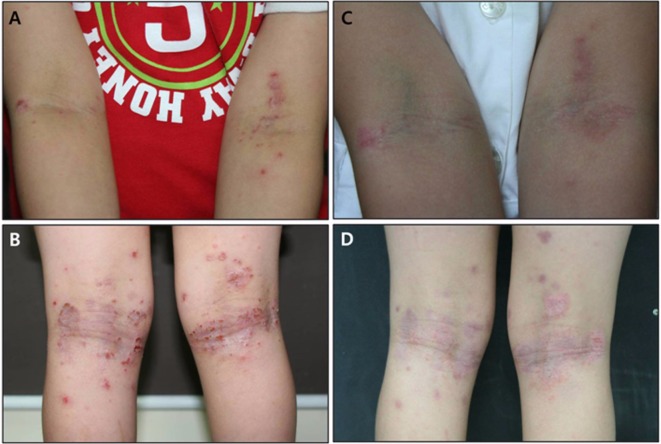
Fig. 1.
A, B, Erythematous plaques and papules with excoriation and lichenification were observed on both popliteal and cubital areas before treatment; C, D, Clinical improvement of atopic dermatitis was observed after 12 bath treatments with green tea extracts.
Fig. 2.
Changes in scoring atopic dermatitis (SCORAD) index values.
Fig. 3.
Changes in visual analogue scale (VAS) scores of patients.
Assessment of skin barrier function
No significant changes in skin hydration were observed. A decrease in TEWL was observed in two patients; however, the change was not statistically significant (data not shown).
Laboratory examination
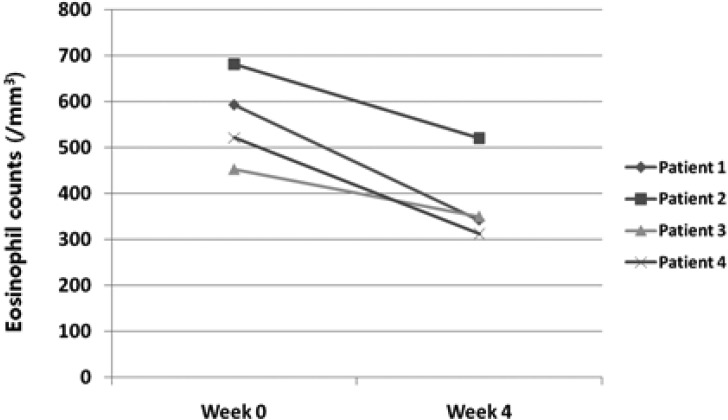
All subjects underwent laboratory tests. Compared with baseline values, the mean values of serum eosinophil counts showed a decrease at week 4 (Fig. 4). However, no significant changes in serum ECP, IgE levels were observed over the entire study period.
Fig. 4.
Variation of total eosinophil counts of patients at baseline and week 4.
Post-treatment mycological examination
After 12 sessions of bath therapy, mycological examination of swab samples from skin lesions using a Malassezia multiplex detection kit showed no identified Malassezia species.
Safety and adverse effects
In general, bath therapy was well tolerated by all patients. No serious adverse effects were reported during the study.
Discussion
Topical corticosteroid is a baseline therapy for treatment of patients with AD. When applied properly, it is a very useful tool for treatment of patients with AD, with few adverse effects; however, steroid abuse can cause adverse effects, including skin atrophy, epidermal thinning, striae distensae, and perioral dermatitis. Recently, many studies for treatment of patients with AD using non-steroidal topical agents from natural sources have been conducted. Green tea, which is widely consumed as a beverage, consists of unprocessed dried young leaves of Camellia sinensis, also known as Thea sinensis L. In recent years, green tea has been studied widely for evaluation of its beneficial effects in treatment and prevention of human disease. Green tea constituents, particularly catechins, exhibit a wide range of pharmacological effects, including anticarcinogenic activity and prevention of cardiovascular diseases [9]. Green tea catechins exhibit antithrombotic activity, which might be due to an antiplatelet effect rather than anticoagulation [10]. Staphylococcal superantigens (SsAgs) have gained attention as one of the factors aggravating AD and several potential mechanisms of AD aggravation by SsAgs have been reported. The inhibitory effect of the green tea catechin extract, Polyphenon, and epigallocatechin gallate (EGCg) on staphylococcal enterotoxin B (SEB) and its mechanisms of action, as well as the possible therapeutic benefits of tea catechin for patients with AD, have also been reported [11].
Findings from studies of the antibacterial effects of tea extracts have demonstrated that Gram-positive bacteria are more sensitive to tea extracts than Gram-negative bacteria; in addition, it has been suggested that negatively charged EGCg acts by binding to the positively charged lipids of the membrane, resulting in damage to the lipid bilayer. Antifungal effects have also been explained by similar mechanisms [11].
Although Malassezia yeasts are a part of the normal microflora, under certain conditions, they can cause superficial skin infections, such as pityriasis versicolor, seborrheic dermatitis, and Malassezia folliculitis. Lipophilic yeasts have been under consideration as major opportunistic pathogens for a very long time. Most of the yeasts have demonstrated an absolute requirement for long fatty acid chains and specific procedures are required for their isolation, conservation, and identification [12]. The causal role of Malassezia in seborrheic dermatitis and dandruff has become clear, however, the role of specific species is still being defined. In patients with AD and psoriasis, evidence for a causal relationship remains less defined. However, literature studies linking all of these disorders with Malassezia yeasts have been reported [13]. Malassezia yeasts appear to be a particularly important factor in development of AD, especially in cases where the disease is localized to the head and neck. Malassezia yeasts have been cultured from 83% of adult patients with this form of AD [14]. The yeasts are also frequently colonized from control subjects; therefore, it has been hypothesized that they function as allergens, rather than as infectious agents, in patients who are susceptible [15]. Recent results of molecular work have also elucidated the structure of some allergens derived from Malassezia yeasts [16, 17]. In the present study, M. sympodialis was the species most commonly isolated from AD patients using the Malassezia multiplex detection kit.
Use of gentle cleansers incorporating synthetic detergents with minimal irritation potential and moisturizers containing a variety of humectants, occlusive agents, and lipids have been used in management of xerosis and AD [18]. Some studies addressing skin moisturization and barrier repair therapy have reported on the adjunctive clinical benefit in treatment of active AD, and a few trials, although more limited, have reported prolonged remission between flares, as well as reduction in usage of topical corticosteroid therapy. In the present study, patients with AD associated with M. sympodialis were treated with bath therapy using green tea extract. Results of this study showed correlation with clinical severity, as measured by the SCORAD index. A decrease in SCORAD index and total eosinophil counts, as well as a marked decrease in VAS score for pruritus, was observed after treatment. Not only Malassezia, but also bacteria, particularly Staphylococcus aureus, and other yeasts and filamentous fungi, such as Candida species and Trichophyton rubrum, have shown correlation with AD [19, 20]. However, S. aureus infection is more likely to be a secondary cause of AD, whereas Malassezia yeasts are recognized as the primary causative agent for AD [21]. Based on the clinical improvement demonstrated by our patients, we suggest the antibacterial and anti-inflammatory effects of catechin against SEB and antifungal effect against Malassezia and its mechanisms, and we discuss the potential of bath therapy with green tea extract as a candidate therapy for treatment of patients with AD.
In the present study, treatment was well tolerated and no serious side effects were noted. This open and single treatment arm study is limited by the absence of randomization and a placebo control. Absence of direct comparisons with other bathing therapies and conventional treatment modalities is a significant limitation. Nevertheless, our results can be used in design of future randomized trials of bath therapy using green tea extract.
In conclusion, bath therapy with green tea extract is an effective, safe, and non-steroidal therapy for treatment of patients with AD associated with M. sympodialis. However, the effects of catechin against SsAgs and the antifungal effect of green tea extract are still not clear. Further studies of the antimicrobial effect against yeasts, using a larger sample size, are necessary; in addition, we discuss the potential of bath therapy with green tea extract as a candidate therapy for treatment of patients with AD.
Acknowledgements
This research was financially supported by the Ministry of Knowledge Economy (MKE) and the Korea Institute for Advancement of Technology (KIAT) through the Research and Development for Regional Industry.
References
- 1.James W, Berger T, Elston DM. Andrews' diseases of the skin: clinical dermatology. 10th ed. Philadelphia: W. B. Saunders; 2006. p. 789. [Google Scholar]
- 2.Hara Y, Matsuzaki T, Suzuki T. Angiotensin I converting enzyme inhibiting activity of tea components. J Agric Chem Soc Jpn. 1987;61:803–808. [Google Scholar]
- 3.Matsuzaki T, Hara Y. Antioxidative activity of tea leaf catechins. J Agric Chem Soc Jpn. 1985;59:129–134. [Google Scholar]
- 4.Xu Y, Ho CT, Amin SG, Han C, Chung FL. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res. 1992;52:3875–3879. [PubMed] [Google Scholar]
- 5.Majoie IM, Oldhoff JM, van Weelden H, Laaper-Ertmann M, Bousema MT, Sigurdsson V, Knol EF, Bruijinzeel-Koomen CA, de Bruin-Weller MS. Narrowband ultraviolet B and medium-dose ultraviolet A1 are equally effective in the treatment of moderate to severe atopic dermatitis. J Am Acad Dermatol. 2009;60:77–84. doi: 10.1016/j.jaad.2008.08.048. [DOI] [PubMed] [Google Scholar]
- 6.Kindo AJ, Sophia SK, Kalyani J, Anandan S. Identification of Malassezia species. Indian J Med Microbiol. 2004;22:179–181. [PubMed] [Google Scholar]
- 7.Guého E, Midgley G, Guillot J. The genus Malassezia with description of four new species. Antonie van Leeuwenhoek. 1996;69:337–355. doi: 10.1007/BF00399623. [DOI] [PubMed] [Google Scholar]
- 8.Kim HM, Lim YY, Park EJ, Chun YJ, Kim MN, Kim BJ, Lee DH. The development and evaluation of multiplex PCR technique for identification of Malassezia yeast. Korean J Med Mycol. 2010;15:51–60. [Google Scholar]
- 9.Yun YP, Kang WS, Lee MY. The antithrombotic effects of green tea catechins. J Food Hyg Saf. 1996;11:77–82. [Google Scholar]
- 10.Kang WS, Chung KH, Chung JH, Lee JY, Park JB, Zhang YH, Yoo HS, Yun YP. Antiplatelet activity of green tea catechins is mediated by inhibition of cytoplasmic calcium increase. J Cardiovasc Pharmacol. 2001;38:875–884. doi: 10.1097/00005344-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Hisano M, Yamaguchi K, Inoue Y, Ikeda Y, Iijima M, Adachi M, Shimamura T. Inhibitory effect of catechin against the superantigen staphylococcal enterotoxin B (SEB) Arch Dermatol Res. 2003;295:183–189. doi: 10.1007/s00403-003-0411-x. [DOI] [PubMed] [Google Scholar]
- 12.Ahn KJ. Taxonomy of the genus Malassezia. Korean J Med Mycol. 1998;3:81–88. [Google Scholar]
- 13.Gupta AK, Batra R, Bluhm R, Boekhout T, Dawson TL., Jr Skin diseases associated with Malassezia species. J Am Acad Dermatol. 2004;51:785–798. doi: 10.1016/j.jaad.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 14.Broberg A, Faergemann J, Johansson S, Johansson SG, Strannegård IL, Svejgaard E. Pityrosporum ovale and atopic dermatitis in children and young adults. Acta Derm Venereol. 1992;72:187–192. [PubMed] [Google Scholar]
- 15.Jensen-Jarolim E, Poulsen LK, With H, Kieffer M, Ottevanger V, Stahl Skov P. Atopic dermatitis of the face, scalp, and neck: type I reaction to the yeast Pityrosporum ovale? J Allergy Clin Immunol. 1992;89(1 Pt 1):44–51. doi: 10.1016/s0091-6749(05)80039-9. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt M, Zargari A, Holt P, Lindbom L, Hellman U, Whitley P, van der Ploeg I, Härfast B, Scheynius A. The complete cDNA sequence and expression of the first major allergenic protein of Malassezia furfur, Mal f 1. Eur J Biochem. 1997;246:181–185. doi: 10.1111/j.1432-1033.1997.00181.x. [DOI] [PubMed] [Google Scholar]
- 17.Yasueda H, Hashida-Okado T, Saito A, Uchida K, Kuroda M, Onishi Y, Takahashi K, Yamaguchi H, Takesako K, Akiyama K. Identification and cloning of two novel allergens from the lipophilic yeast, Malassezia furfur. Biochem Biophys Res Commun. 1998;248:240–244. doi: 10.1006/bbrc.1998.8944. [DOI] [PubMed] [Google Scholar]
- 18.Del Rosso JQ. Understanding skin cleansers and moisturizers: the correlation of formulation science with the art of clinical use. Cosmet Dermatol. 2003;16:19–31. [Google Scholar]
- 19.Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139(Suppl 53):13–16. doi: 10.1046/j.1365-2133.1998.1390s3013.x. [DOI] [PubMed] [Google Scholar]
- 20.Klein PA, Clark RA, Nicol NH. Acute infection with Trichophyton rubrum associated with flares of atopic dermatitis. Cutis. 1999;63:171–172. [PubMed] [Google Scholar]
- 21.Skov L, Baadsgaard O. The potential role of Staphylococcus aureus superantigens in atopic eczema. J Eur Acad Dermatol Venereol. 1996;7(Suppl 1):S8–S11. [Google Scholar]