Abstract
Mushrooms collected from Deogyu mountain, Korea, in 2011, were identified as four classes, four orders, 13 families, 22 genera, and 33 species. In particular, agaricales was most abundant and comprised more than 70%. Their antioxidant activities were estimated using three different bioassay methods, the 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) radical scavenging assay, 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay, and reducing power assay. As a result, the methanol extracts of Stereum ostrea, Laetiporus sulphureus var. miniatus, and Tyromyces sambuceus exhibited potent antioxidant activity in all bioassays tested.
Keywords: Antioxidant activity, Free radical scavenging activity, Mushrooms, Reducing power activity
Mushrooms are a rich source of secondary metabolites with unprecedented structural features and remarkable bioactivities. Therefore, they exhibit a very broad spectrum of pharmacological activities, including antifungal, anti-inflammatory, antitumor, antiviral, antibacterial, antiparasitic, immunomodulating, and hepatoprotective activities [1-5].
Free radicals have been implicated in the pathogenesis of various diseases, including ischemia, arteriosclerosis, diabetes, arthritis, inflammation, and cancer, as well as in the aging process [6, 7]. According to considerable evidence, due to their capacity to quench free radicals, antioxidants could aid in prevention of these diseases [8]. Thus, the demand for antioxidants has shown a gradual increase. As part of a continuous search for new natural antioxidants [9, 10], we have assessed the antioxidant activity of mushrooms collected from Deogyu-mountain, Muju, Korea, in 2011. In this study, mushrooms collected from Deogyu-mountain and their antioxidant activity are described.
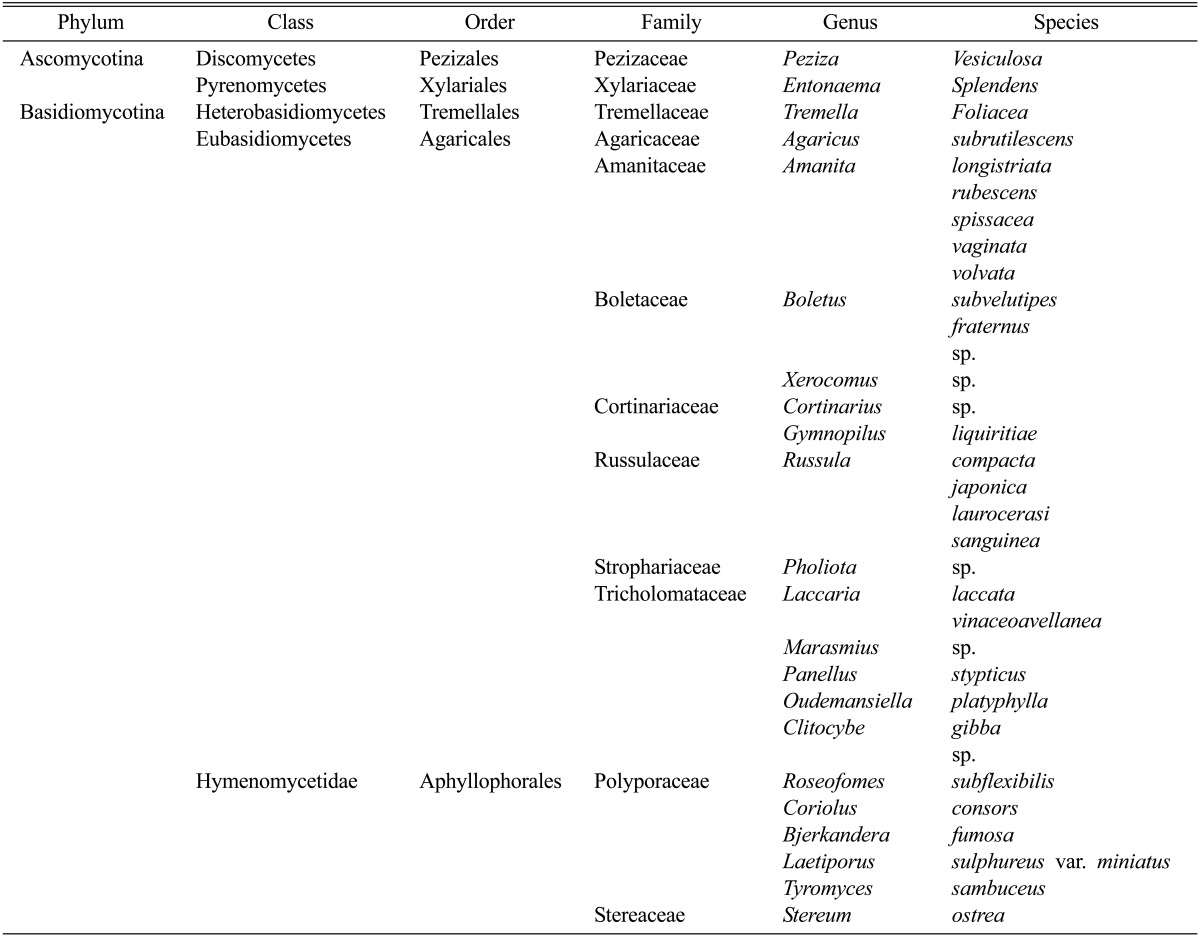
As shown in Table 1, mushrooms collected from Deogyu mountain, Muju, Korea, in 2011, were identified as 33 taxa of macrofungi belonging to 22 genera in 13 families. Among them, agaricales was most abundant and comprised more than 70%. Included are many edible species, which belong to the Genus Peziza, Tremella, Boletus, Laccaria, and Clitocybe, whereas genus Amanita is known to contain a poison that is fatal to humans.
Table 1.
Mushrooms collected at Deogyu mountain, Muju, Korea, in July 2011
Fruiting bodies of 33 species were cut into small pieces and extraction was performed using 80% aqueous MeOH at room temperature for one day. The methanolic extract was filtered and concentrated under reduced pressure. The concentrates were dissolved in dimethyl sulfoxide at a concentration of 10 mg/mL, and their antioxidant activity was estimated. Three different bioassay methods, the 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) radical scavenging assay, 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay, and reducing power assay were used for evaluation of the antioxidant capacity of the methanolic extracts of the collected mushrooms.
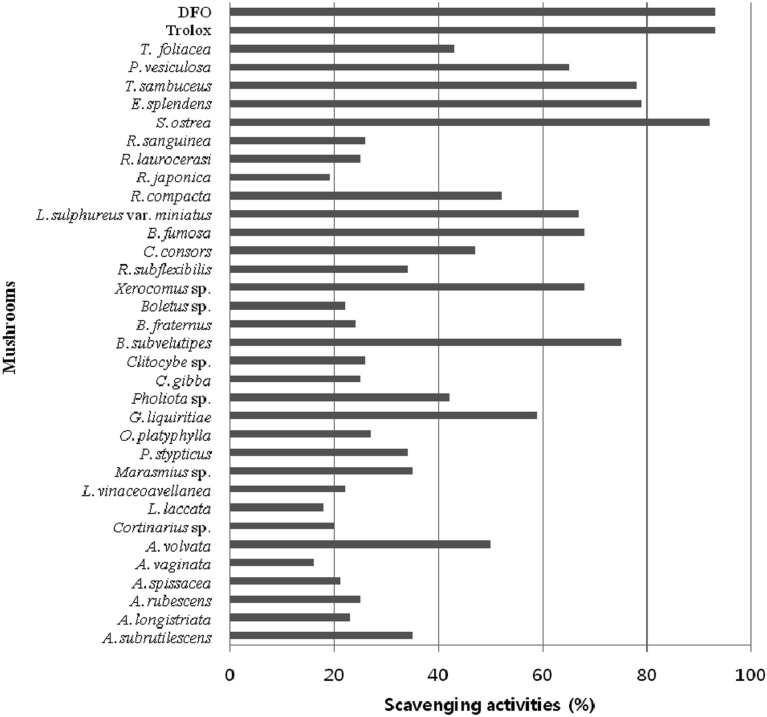
The ability to scavenge free radicals is the primary characteristic of an antioxidant. The ABTS radical cation and DPPH radical scavenging assay methods were used for evaluation of the free radical scavenging efficacies of the methanolic extracts. A method described in the literature was used for measurement of radical scavenging activity of ABTS [11]. In brief, ABTS was dissolved in water to a concentration of 7 mM. The ABTS cation radical was produced by reaction of the ABTS stock solution with 2.45 mM potassium persulfate and by allowing the mixture to stand in the dark for 12 hr. Following addition of 190 µL of ABTS radical cation solution to 10 µL of mushroom extracts, absorbance was measured using a microplate reader at 734 nm after mixing for up to 7 min. As shown in Fig. 1, among the methanol extracts tested, Stereum ostrea exhibited the most potent ABTS radical scavenging activity, with a scavenging effect of above 90%, which was comparable to that of butylated hydroxyanisole (BHA) and trolox, which were used as positive controls. Although their activity was less than that of positive controls, the methanol extracts of Boletus subvelutipes, Entonaema Splendens, and Tyromyces sambuceus exhibited potent ABTS radical scavenging activity above 70%.
Fig. 1.
2,2'-Azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) radical scavenging activities of mushroom methanolic extracts. Methanol was used for extraction of mushrooms, and 10 µL of the methanol extracts (10 mg/mL) was added to 190 µL of ABTS radical cation solution for measurement of ABTS radical scavenging activity.
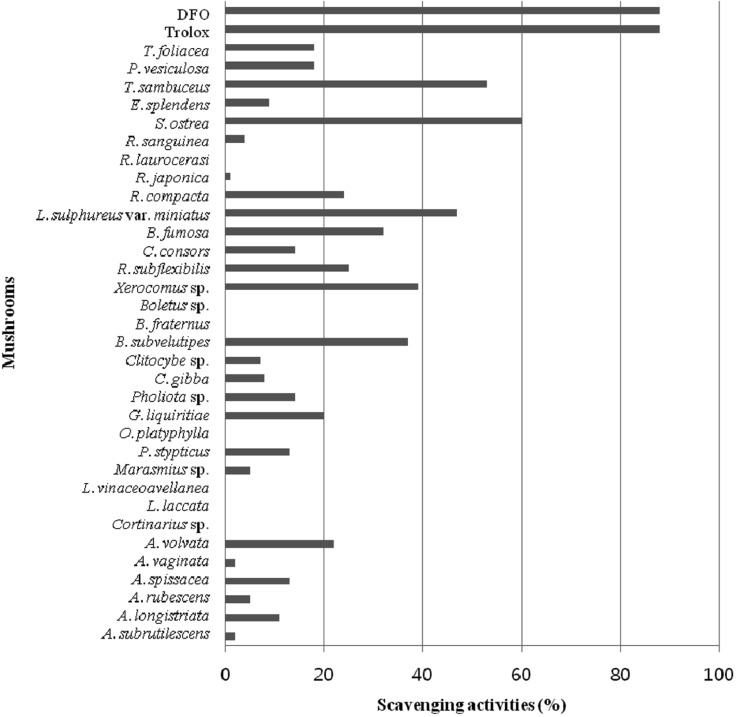
DPPH radical scavenging activity was measured using a method described in the literature [12]. Ten µL of each extract was combined with 90 µL of 150 µM methanolic DPPH. Following incubation at room temperature for 10 min, absorbance was read at 517 nm using a Molecular Devices Spectromax microplate reader (Molecular Devices Corp., Sunnyvale, CA, USA). According to the results, the methanolic extracts of Laetiporus sulphureus var. miniatus, Stereum ostrea, and Tyromyces sambuceus exhibited relatively potent activity, with DPPH radical scavenging effects of 40~60% (Fig. 2). Stereum ostrea, Tyromyces sambuceus, Boletus subvelutipes, Xerocomus sp., and Laetiporus sulphureus var. miniatus exhibited significant scavenging activity against both ABTS and DPPH radicals.
Fig. 2.
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activities of mushroom methanolic extracts. Methanol was used for extraction of mushrooms, and 10 µL of the methanol extracts (10 mg/mL) was added to 90 µL of 150 µM methanolic DPPH for measurement of DPPH radical scavenging activity.
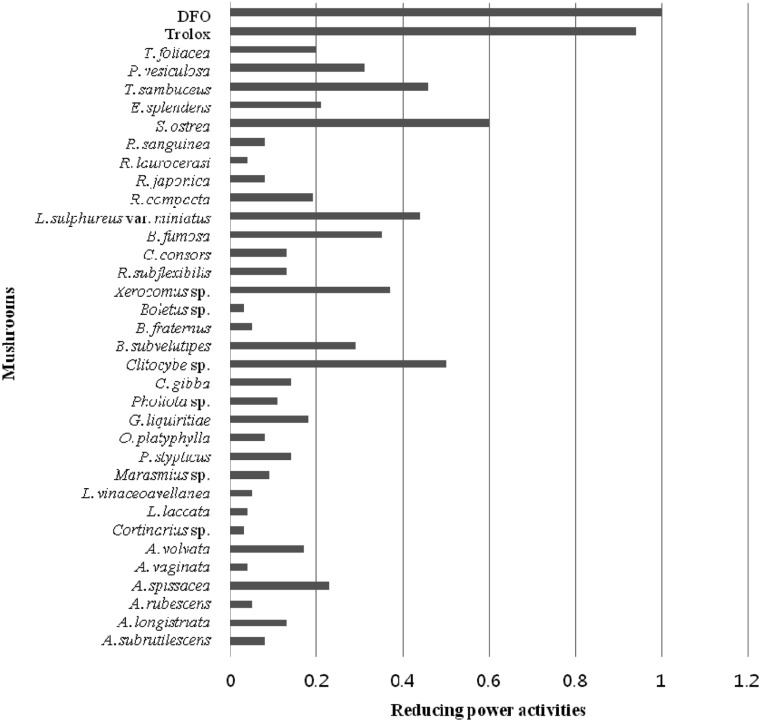
The potassium ferricyanide reduction method was used with minor modification for evaluation of reducing power activity [13]. In brief, sample (20 µL) was mixed with 50 µL of 200 mM potassium phosphate buffer (pH 6.6) and 50 µL of 1% potassium ferricyanide, followed by incubation at 50℃ for 20 min. After addition of 50 µL of 10% trichloroacetic acid (w/v), the mixture was centrifuged at 650 rpm for 10 min. The upper layer (100 µL) was mixed with 100 µL distilled water and 20 µL of 0.1% ferric chloride, followed by measurement of absorbance at 700 nm. Assays were performed in triplicate, and antioxidants BHA and trolox were used as positive controls. Results are expressed as relative activity against trolox (absorbance value of sample/absorbance value of 10,000 ppm trolox). In reducing power activity, among the mushroom extracts tested, Stereum ostrea showed the highest activity, and Clitocybe sp., Laetiporus sulphureus var. miniatus, and Tyromyces sambuceus exhibited moderate activity, although their activity was less than that of positive controls, as shown in Fig. 3.
Fig. 3.
Reducing power activities of mushroom methanolic extracts. Methanol was used for extraction of mushrooms, and 20 µL of the methanol extracts (10 mg/mL) was used for measurement of reducing power activity, as described in the text.
Acknowledgements
This work was supported by a grant from the Korea Forest Service and, in part, by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2009-353-F00020), Republic of Korea.
References
- 1.Hobbs C. Medicinal mushrooms: an exploration of tradition, healing, and culture. Portland: Botanica Press; 1986. [Google Scholar]
- 2.Ferreira IC, Barros L, Abreu RM. Antioxidants in wild mushrooms. Curr Med Chem. 2009;16:1543–1560. doi: 10.2174/092986709787909587. [DOI] [PubMed] [Google Scholar]
- 3.Zjawiony JK. Biologically active compounds from Aphyllophorales (polypore) fungi. J Nat Prod. 2004;67:300–310. doi: 10.1021/np030372w. [DOI] [PubMed] [Google Scholar]
- 4.Lee IK, Yun BS. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J Antibiot (Tokyo) 2011;64:349–359. doi: 10.1038/ja.2011.2. [DOI] [PubMed] [Google Scholar]
- 5.Quang DN, Hashimoto T, Asakawa Y. Inedible mushrooms: a good source of biologically active substances. Chem Rec. 2006;6:79–99. doi: 10.1002/tcr.20074. [DOI] [PubMed] [Google Scholar]
- 6.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Gutteridge JM. Free radicals in disease process: a compilation of cause and consequence. Free Radic Res Commun. 1993;19:141–158. doi: 10.3109/10715769309111598. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev. 2012;70:257–265. doi: 10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee IK, Cho SM, Seok SJ, Yun BS. Chemical constituents of Gymnopilus spectabilis and their antioxidant activity. Mycobiology. 2008;36:55–59. doi: 10.4489/MYCO.2008.36.1.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee IK, Han MS, Lee MS, Kim YS, Yun BS. Styrylpyrones from the medicinal fungus Phellinus baumii and their antioxidant properties. Bioorg Med Chem Lett. 2010;20:5459–5461. doi: 10.1016/j.bmcl.2010.07.093. [DOI] [PubMed] [Google Scholar]
- 11.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 12.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 13.Ferreira IC, Baptista P, Vilas-Boas M, Barros L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: individual cap and stipe activity. Food Chem. 2007;100:1511–1516. [Google Scholar]